Abstract
People living at sea level experience intermittent hypoxia (IH) as a consequence of sleep apnea, which is a highly prevalent respiratory disorder. Sleep apnea patients and rodents exposed to IH exhibit autonomic dysfunction manifested as increased sympathetic nerve activity and hypertension. This article highlights physiologic basis of autonomic disturbances by IH, which involves abnormal activation of the carotid body (CB) chemo reflex by reactive oxygen species (ROS).We further evaluate major molecular mechanisms underlying IH-induced ROS generation including transcriptional activation of genes encoding pro-oxidant enzymes by hypoxia-inducible factor (HIF)-1 and transcriptional repression of anti-oxidant enzyme genes by DNA methylation. Lastly, evidence is presented for CB neural activity as a major regulator of HIF-1 activation and DNA methylation by IH in the chemo reflex pathway.
Keywords: Obstructive sleep apnea, blood pressure, HIFs, DNA methylation, catecholamines
Introduction
The circulatory and respiratory systems adapt to sustained hypoxia occurring at high altitude sojourns. Molecular basis of cardio-respiratory adaptations to altitude hypoxia has been well studied [1*–4]. People living at sea level often experience intermittent hypoxia (IH) as a consequence of sleep apnea (SA), which is a highly prevalent respiratory disorder. IH leads to mal-adaptations of autonomic functions manifested as heightened sympathetic nerve activity and hypertension [5–7]. Recent development of experimental models provided important insights into the molecular mechanisms underlying mal-adaptations to IH. This article presents the emerging role of hypoxia-inducible factors (HIFs) and epigenetic regulation of gene expression by DNA methylation in mal-adaptations of cardio-respiratory functions by IH.
Physiological basis of sympathetic activation and hypertension by IH
Arterial blood O2 levels are continuously monitored by the carotid bodies (CB) [8]. Hypoxia increases the CB sensory nerve activity and the sensory information is transmitted to brainstem neurons in the nucleus tractus solitarii (nTS), and rostral ventrolateral medulla (RVLM) and then translated to sympathetic neurons in the spinal cord. Thus, the CB chemo reflex is a major regulator of sympathetic tone under hypoxia. Sleep apnea patients and rodents exposed to IH exhibit augmented CB chemo reflex [9–11]. IH produces two major effects on the CB: a) enhanced response to acute hypoxia; and b) progressive increase in baseline sensory activity following repetitive hypoxia, a phenomenon termed as sensory long-term facilitation (sLTF) [11–13]. IH also facilitates processing of the sensory information from the CB in the nTS, and RVLM by altering glutamatergic neurotransmission [14,15]. Adrenal medullary chromaffin cells (AMC), which are innervated by the sympathetic nervous system, are the major source of catecholamines. Hypoxia by activating sympathetic nerves release catecholamines from AMC, and this effect is markedly potentiated in IH exposed rodents [16]. Chronic ablation of CB prevents sympathetic activation by IH [17–19**]. Treating rats with a sympatholytic agent [17] or by ablating sympathetic innervation to the adrenal medulla [19] prevent IH-evoked hypertension. These findings demonstrate that the CB chemo reflex is a major driver of IHevoked sympathetic excitation and the ensuing hypertension.
Cellular mechanism (s) underlying chemo reflex activation by IH
Reactive oxygen species (ROS)-signaling is a major cellular mechanism mediating the augmented CB chemo reflex by IH. ROS generation is increased in all three major components of the chemo reflex pathway including the CB, nTS, RVLM and the adrenal medulla in IH treated rodents [19]. IH evoked CB hypersensitivity is due to ROS-dependent inactivation of heme oxygenase-2 and reduced CO production resulting in increased hydrogen sulfide (H2S) generation in the chemoreceptor tissue [21**]. The augmented catecholamine secretion from AMC of IH treated rodents involve ROS-dependent activation of Ca2+ channels and the ensuing Ca2+ influx [22] as well as protein kinase-C dependent increase in readily releasable pool of secretory vesicles [23]. ROS scavengers prevent IH-induced hyperactive CB chemo reflex and the increased sympathetic nerve activity [11,16,20].
Increased ROS generation by IH involves activation of pro-oxidant enzymes and reduced anti-oxidant enzymes activities. As short as few cycles of IH activate xanthine oxidase (XO), a pro-oxidant enzyme in rat pheochromocytoma (PC)12 cells, and this effect is mediated by increased proteolytic processing of XO [24,25**]. ROS generated by XO in turn, activates NADPH oxidase (Nox) 2 by facilitating the translocation of p47phox and p67phox subunits to the plasma membrane and their interaction with membrane bound gp91phox subunit [24]. ROS generated by Nox leads to further increase in ROS generation by inhibiting the Complex I of the mitochondrial electron transport chain through increased Ca2+ influx in to the mitochondria and S-glutathionylation of 75- and 50-kDa subunits of the Complex I [26]. ROS generated by XO and Nox return to baseline within couple of hours after terminating IH, whereas ROS generated by the Complex I inhibition recovers 24 hours after terminating IH [24]. Thus, IH leads to progressive recruitment of pro-oxidant mechanisms resulting in ROS-induced ROS generation (positive feed-forward mechanism) (Fig. 1). In addition, anti-oxidant enzyme activities of superoxide dismutase-2 (Sod2) and catalase are reduced by IH [27].
Figure 1:
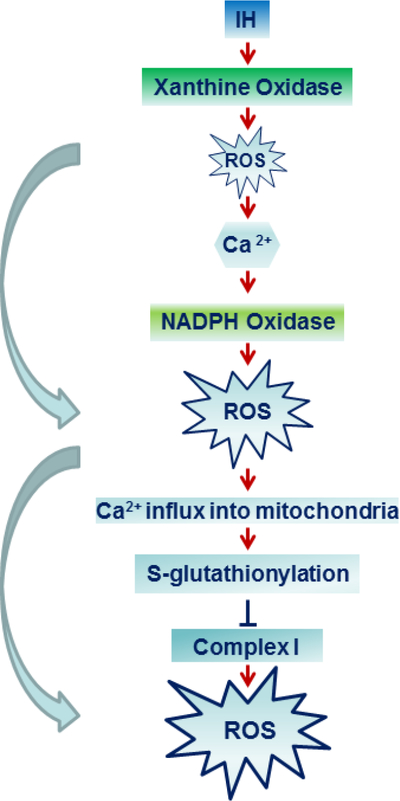
Progressive recruitment of pro-oxidant mechanisms by IH results in reactive oxygen species (ROS)-induced ROS generation (positive feed-forward) in the carotid body chemo reflex pathway.
Molecular mechanisms of redox regulation by IH
Transcriptional regulation of genes encoding pro-and anti-oxidant enzymes by hypoxiainducible factors (HIFs)-
Genes encoding pro-oxidant enzymes are upregulated and anti-oxidant enzyme genes are down regulated in the CB, nTS, RVLM and adrenal medulla of IH exposed rodents [19]. Emerging evidence suggests that HIF-1 and HIF-2, the major members of the HIF family of transcriptional activators play a major role in regulation of genes encoding pro-and antioxidant enzymes by IH [28]. IH increases HIF-1α and decreases HIF-2α, the O2-regulated alpha subunits of the HIF complex [13,19,27]. The IH evoked increase in HIF-1α protein is due to increased protein synthesis by Ca2+-dependent activation of mammalian target of rapamycin (mTOR) and decreased proline hydroxylation [25,29]. HIF-2α degradation by IH is mediated by Ca2+-dependent protease, calpain [27].
Complete HIF-1α deficiency results in embryonic lethality at mid-gestation, whereas Hif1a+/− heterozygous (het) mice, which are partially deficient in HIF-1α, develop normally and are indistinguishable from wild-type (WT) littermates [30,31]. IH treated Hif1a+/− het mice exhibit remarkable absence of increased ROS generation, CB hyperactivity, sympathetic excitation and hypertension [13]. IH increases Nox2 mRNA abundance and this response was abolished by blocking HIF-1 activity either with RNA interference or by pharmacologic inhibition of HIF-1α protein by digoxin or YC-1 in cell cultures [29]. IH-evoked increase in Nox2 mRNA was absent in Hif1a+/− mice [29]. These findings demonstrate that HIF-1 mediates transcriptional upregulation of Nox2 by IH.
HIF-2α, which also known as endothelial PAS domain protein-1 (EPAS-1) shares~50% amino acid sequence identity with HIF-1α and interacts with HIF-1β, the constitutively expressed subunit of the HIF complex [32,33]. In contrast to Hif1a+/− mice, Hif-2α+/− het mice exhibit elevated ROS levels, heightened CB response to hypoxia, elevated plasma catecholamines, higher incidence of apnea and hypertension under basal conditions, similar to responses seen in IH treated wild type mice [34]. Cell culture studies showed that HIF-2α protein degradation by IH contributes to decreased transcription of genes encoding anti-oxidant enzymes [27]. Systemic administration of ALLM, a calpain inhibitor, which prevents HIF-2α degradation, restores Sod2 enzyme activity and protein and prevents elevated ROS levels in the chemo reflex pathway of rats exposed to IH [27].
Neural regulation of HIFs during CIH-
Prolonged hypoxia is an established physiological activator of HIFs [35]. However, the duration of hypoxia occurring during each episode of IH (simulating sleep apnea) is brief (15 seconds) and the magnitide is modest. A recent study showed that arterial pO2 decreases from 100 to 80 mmHg (range between 15–20 mmHg) during each episode of IH, in unsedated rats [19**]. Because CB receives highest blood flow and is exquisitly sensitive to changes in arterial blood O2, it is likely that changes in HIF-α isoforms are due to direct effects of IH on glomus tissue. On the other hand, basal pO2 levels of most tissues including the brain and peripheral organs range between 30 and 60 mmHg [36], which are much below the level of hypoxia seen during each episode of IH. Thus, it is unlikely that altered HIF-α isoform expressions in neurons of nTS and RVLM and adrenal medulla are due to direct effects of IH. Neural activity is a potent regulator of gene expression in the nervous system [37–39]. A recent study showed that CB ablated rats exhibit remarakable absence of IH-evoked increase in HIF-1α protein and Nox2 activity and decreased HIF-2α protein and Sod2 activity in the nTS, RVLM [19**]. Likewise, either CB ablation or chronic sectioning of sympathetic nerves eliminated IH-evoked changes in HIFα isoform proteins, pro-and anti oxidant enzymes in the adrenal medulla [19**].
Sympathetic nerves innervating AMCs release acetyl choline (ACh), which binds to muscarinic and/or nicotinic ACh receptors. Treating rats with atropine, a muscarinic receptor blocker, during IH treatment blocked increased HIF-1α and decreased HIF-2α proteins, upregulation of Nox and reduced Sod-2 activities as well as increased ROS production. Further analysis revealed that stimulating muscarinic receptors elevate [Ca2+]i, which by activating mTOR increases HIF-1α protein and decreases HIF-2α protein through activation of Ca2+dependent protease, calpain [19**]. The mechanisms underlying CB neural activity-dependent changes in HIF-α proteins in the nTS and RVLM neurons by IH remain to be investigated. Nonetheless, these findings demonstrate that regulation of HIFα isoforms by CB neural activity mediate transcriptional changes of pro-and anti oxidant enzyme genes in the central and efferent components of the CB chemo reflex (Fig 2).
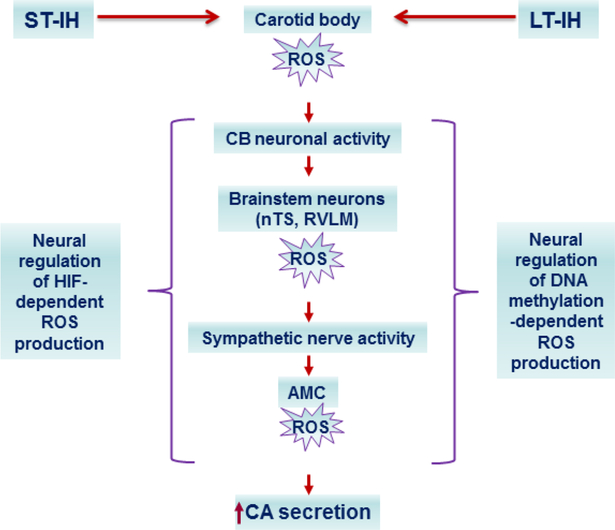
Figure 2:
Neural regulation of molecular mechanisms in the carotid body (CB) chemo reflex pathway under short-term (ST; 10 days) and log-term (LT; 30 days) exposure to intermittent hypoxia (IH). ST-IH increases HIF-1α and decreases HIF-2α proteins leading increased transcription of genes encoding pro-oxidant enzymes and decreased transcription of genes encoding anti-oxidant enzymes (AOE) in the CB chemo reflex pathway, respectively. The effects of ST-IH on the CB are likely to be direct, whereas in nucleus tractus solitarri (nTS) and rostral ventrolateral medulla (RVLM) and adrenal medullary chromaffin cells (AMC) are indirect and require CB neural activity and ensuing increase in sympathetic nerve activity, respectively. LTIH triggers long-lasting suprresion of genes encoding AOE and persistent elevation of ROS. The effects of LT-IH on nTS, RVLM and AMC require CB neural activity and the ensuing increase in sympathetic nerve activity, respectively.
Prolonged CIH activates DNA methylation
The reversal of redox- dependent CB-reflex activation depends on the duration of IH exposure. The effects produced by ten day exposure to IH are completely reversed during recovery in room air, whereas the effects produced by long-term (30 days)-exposure to IH (LTIH) persist despite recovery in room air for 30-days [40**]. What molecular mechanisms mediate the persistent effects of long-term IH? Emerging evidence suggests that epigenetic regulation of gene expression leads to long-lasting changes in physiological functions. Epigenetic changes are heritable modifications of DNA including DNA methylation and histone modifications [41,42]. DNA methylation occurs predominantly on cytosine bases that are part of a 5’-CpG-3’ dinucleotide [43]. DNA hypermethylation repress gene transcription, whereas hypomethylation activates transcription [44].
Rats exposed to LT-IH showed increased DNA methylation and repression of genes encoding several anti-oxidant enzymes including superoxide dismutase 1 and 2 (Sod1, Sod2), catalase (Cat), thioredoxin reductase 2 (Txnrd2), peroxiredoxin 4 (Prdx4), and glutathione peroxidase 2 (Gpx2) in the CB, nTS, RVLM and adrenal medulla [40**]. Bisulphite sequencing of the promoter region of the Sod2 gene showed methylation of a single CpG dinucleotide at +157 bp (relative to the transcription site), out of the 25 CpG sites analyzed [40**]. LT-IH evoked DNA methylation was tissue- and cell- selective and was not seen in brainstem regions that do not participate in the CB reflex [40**]. Brainstem regions that showed absence of DNA methylation showed unaltered anti-oxidant enzyme gene expression and ROS levels in LT-IH exposed rats [40**]. On the other hand, ten day exposure to IH had no effect on DNA methylation of anti-oxidant enzyme genes [40**], suggesting that prolonged exposure to IH is necessary to trigger DNA methylation.
DNA methyl transferases (Dnmts) catalyze DNA cytosine methylation. Several Dnmts have been identified, including Dnmt1, Dnmt2, Dnmt3a, and Dnmt3b [46]. Dnmt1 is responsible for the maintenance of pre-existing DNA methylation, whereas Dnmt3a and Dnmt3b are de novo methyltransferases [47]. LT-IH increased the aundances of Dnmt1 and Dnmt3b proteins and elevated Dnmt enzyme activity. The increased Dnmt protein expression was due to posttranslational rather than transcriptional activation [40**]. Systemic administration of decitabine, an inhibitor of DNA methylation, either during exposure to LT-IH or during recovery from LTIH, prevented DNA methylation; normalized anti-oxidant enzyme gene expression, ROS levels, CB chemo reflex, and blood pressure [40**]. These findings suggest that DNA methylationdependent repression anti-oxidant enzyme genes and the resulting increase in ROS levels contribute to persistent CB chemo reflex activation by LT-IH. CB ablation blocked LT-IHinduced DNA methylation in the nTS, RVLM, and adrenal medulla and normalized the expressions of genes encoding anti-oxidant enzymes as well as ROS levels [45**]. These observations demonstrate that CB neural activity mediates LT-IH-induced DNA methylation in the central (nTS and RVLM) and efferent (adrenal medulla) components of the chemo reflex (Fig.2).
Highlights.
Intermittent hypoxia (IH) is a hallmark manifestation of sleep apnea
Patients with sleep apnea and IH exposed rodents exhibit increased sympathetic nerve activity and hypertension
This article presents molecular mechanisms mediating the autonomic dysfunction in IH exposed rodent models.
Acknowledgments
Sources of funding
Studies in authors laboratory are supported by Public Health Service grant P01-HL-90554 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors do not have have any conflicts of interest to declare.
References and recommended reading
Papers of particular interest have been highlighted as:
* of special interest
** of outstanding interest
References
- 1. *.Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, et al. : Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci U S A 2010, 107:11459–11464.This paper shows genetic variations in HIF-2α are linked to low hemoglobin levels in tibetans residing at high altititude.
- 2.Simonson TS, McClain DA, Jorde LB, Prchal JT: Genetic determinants of Tibetan highaltitude adaptation. Hum Genet 2012, 131:527–533. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzo FR, Huff C, Myllymäki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain DA, et al. : A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet 2014, 46:951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonson TS, Wagner PD: Oxygen transport adaptations to exercise in native highland populations. Exp Physiol 2015, 100:1231–1232. [DOI] [PubMed] [Google Scholar]
- 5.Narkiewicz K, Somers VK: The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens 1997, 15:1613–1619. [DOI] [PubMed] [Google Scholar]
- 6.Iturriaga R, Moya EA, Del Rio R: Inflammation and oxidative stress during intermittent hypoxia: the impact on chemoreception. Exp Physiol 2015, 100:149–155. [DOI] [PubMed] [Google Scholar]
- 7.Prabhakar NR: Carotid body chemoreflex: a driver of autonomic abnormalities in sleep apnoea. Exp Physiol 2016, 101:975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar P, Prabhakar NR: Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2012, 2:141–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK: Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 1999, 99:1183–1189. [DOI] [PubMed] [Google Scholar]
- 10.Lesske J, Fletcher EC, Bao G, Unger T: Hypertension caused by chronic intermittent hypoxia--influence of chemoreceptors and sympathetic nervous system. J Hypertens 1997, 15: 1593–1603. [DOI] [PubMed] [Google Scholar]
- 11.Peng YJ, Prabhakar NR: Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol 2004, 96:1236–1242; discussion 1196. [DOI] [PubMed] [Google Scholar]
- 12. *.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR: Induction of sensory longterm facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A 2003, 100:10073–10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR: Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 2006, 577:705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman CG, Wang G, Park L, Anrather J, Delagrammatikas GJ, Chan J, Zhou J, Iadecola C, Pickel VM: Chronic intermittent hypoxia induces NMDA receptor-dependent plasticity and suppresses nitric oxide signaling in the mouse hypothalamic paraventricular nucleus. J Neurosci 2010, 30:12103–12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva AQ, Schreihofer AM: Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol 2011, 589:1463–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR: Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol 2006, 575:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fletcher EC: Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol 2001, 90:1600–1605. [DOI] [PubMed] [Google Scholar]
- 18.Iturriaga R: Carotid Body Ablation: a New Target to Address Central Autonomic Dysfunction. Curr Hypertens Rep 2018, 20:53. [DOI] [PubMed] [Google Scholar]
- 19. **.Peng YJ, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD, Vasavda C, Kumar GK, Semenza GL, Prabhakar NR: Regulation of hypoxia-inducible factor-α isoforms and redox state by carotid body neural activity in rats. J Physiol 2014, 592:38413858.This paper shows that CIH leads to increased HIF-1α protein, Nox2 mRNA, protein and decreased HIF-2α protein, Sod2 mRNA, protein in the CB, neurons of nTS and RVLM and adrenal medulla and absence of changes in HIF-α isoforms, pro-and anti-oxidant enzymes following chronic CB ablation in in nTS, RVLM and adrenal medulla. This paper further shows sympathetic activation through muscuranic receptor signaling leads to dysregulation of HIF-α isoforms and pro-anti oxidant enzymes in the adrenal medulla.
- 20.Souvannakitti D, Kumar GK, Fox A, Prabhakar NR: Neonatal intermittent hypoxia leads to long-lasting facilitation of acute hypoxia-evoked catecholamine secretion from rat chromaffin cells. J Neurophysiol 2009, 101:2837–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. **.Yuan G, Peng YJ, Khan SA, Nanduri J, Singh A, Vasavda C, Semenza GL, Kumar GK, Snyder SH, Prabhakar NR: H2S production by reactive oxygen species in the carotid body triggers hypertension in a rodent model of sleep apnea. Sci Signal 2016, 9:ra80.This paper evaluated how ROS activates the carotid body activity during CIH. Authors show that ROS inactivates heme oxygenase (HO)-2 resulting in reduced carbon monoxide (CO) generation. The decreased CO, in turn activates cystathionine ϒ-lyase (CSE) via PKG-dependent mechanism thereby increases H2S generation in the CB. Pharmacological or genetic blockade of CSE prevents carotid body activation by CIH as well as sympathetic activation and hypertension.
- 22.Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, Prabhakar NR: NADPH oxidasedependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia-treated neonatal rat chromaffin cells. J Neurosci 2010, 30:10763–10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuri BA, Khan SA, Chan SA, Prabhakar NR, Smith CB: Increased secretory capacity of mouse adrenal chromaffin cells by chronic intermittent hypoxia: involvement of protein kinase C. J Physiol 2007, 584:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nanduri J, Vaddi DR, Khan SA, Wang N, Makerenko V, Prabhakar NR: Xanthine oxidase mediates hypoxia-inducible factor-2alpha degradation by intermittent hypoxia. PLoS One 2013, 8:e75838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. **.Nanduri J, Vaddi DR, Khan SA, Wang N, Makarenko V, Semenza GL, Prabhakar NR: HIF-1α activation by intermittent hypoxia requires NADPH oxidase stimulation by xanthine oxidase. PLoS One 2015, 10:e0119762.This paper shows IH initially activates xanthine oxidase (XO). The ROS generated by XO facilitates translocation of NADPH oxidase subunits to the membrane, which interacts with gp91 phox to activate Nox2. Thus, IH leads to ROS-induced ROS through progressive recruitment of pro-oxidant enzymes. Nox2 generated ROS inturn leads to HIF-1α accumulation and increases Nox2 transcription.
- 26.Khan SA, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, Prabhakar NR: NADPH oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal 2011, 14:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR: Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A 2009, 106:1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR: Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol 2008, 164:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR: Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 2008, 217:674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al. : Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 1998, 12:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, et al. : Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 1999, 103:691696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y: A novel bHLHPAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A 1997, 94:4273–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian H, McKnight SL, Russell DW: Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 1997, 11:72–82. [DOI] [PubMed] [Google Scholar]
- 34.Peng YJ, Nanduri J, Khan SA, Yuan G, Wang N, Kinsman B, Vaddi DR, Kumar GK, Garcia JA, Semenza GL, et al. : Hypoxia-inducible factor 2alpha (HIF-2alpha) heterozygousnull mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc Natl Acad Sci U S A 2011, 108:3065–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prabhakar NR, Semenza GL: Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev 2012, 92:967–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C: Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 2011, 15:1239–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fields RD, Lee PR, Cohen JE: Temporal integration of intracellular Ca2+ signaling networks in regulating gene expression by action potentials. Cell Calcium 2005, 37:433–442. [DOI] [PubMed] [Google Scholar]
- 38.Carulli D, Foscarin S, Rossi F: Activity-dependent plasticity and gene expression modifications in the adult CNS. Front Mol Neurosci 2011, 4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganguly K, Poo MM: Activity-dependent neural plasticity from bench to bedside. Neuron 2013, 80:729–741. [DOI] [PubMed] [Google Scholar]
- 40. **.Nanduri J, Peng YJ, Wang N, Khan SA, Semenza GL, Kumar GK, Prabhakar NR: Epigenetic regulation of redox state mediates persistent cardiorespiratory abnormalities after long-term intermittent hypoxia. J Physiol 2017, 595:63–77.This paper shows that sympathetic activation, ROS generation, chemo rflex activation elicited by short-term exposure to IH for tendays are reversed during recovery in room air, whereas those evoked by long-term IH exposure persisted despite thirty day recovery in room air. The authors further show that persistent changes caused by long-term IH are due to repressed transcription of anti-oxidant enzyme genes in by DNA methylation in chemo reflex pathway. Treating rats with decitabine reverses the longterm effects of IH.
- 41.Feinberg AP: Phenotypic plasticity and the epigenetics of human disease. Nature 2007, 447:433–440. [DOI] [PubMed] [Google Scholar]
- 42.Jones PA, Baylin SB: The epigenomics of cancer. Cell 2007, 128:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bird AP: DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res 1980, 8:1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma S, Kelly TK, Jones PA: Epigenetics in cancer. Carcinogenesis 2010, 31:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. **.Nanduri J, Peng YJ, Wang N, Khan SA, Semenza GL, Prabhakar NR: DNA methylation in the central and efferent limbs of the chemoreflex requires carotid body neural activity. J Physiol 2018, 596:3087–3100.This paper demonstrates that the carotid body neural activity contributes to increased DNA methylation by long-term IH in the central and peripheral components of carotid body chemo reflex.
- 46.Bird A: DNA methylation patterns and epigenetic memory. Genes Dev 2002, 16:6–21. [DOI] [PubMed] [Google Scholar]
- 47.Jin B, Li Y, Robertson KD: DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer 2011, 2:607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]