Abstract
Purpose
We assess the efficacy of two next-generation biologic therapies in treating experimental autoimmune uveitis.
Methods
Variable binding domains from shark immunoglobulin novel antigen receptors (VNARs) were fused with a mouse IgG2a constant domain (Fc) to generate VNAR-Fc molecules with binding specificity to tumor necrosis factor alpha (TNFα) or inducible T-cell costimulatory ligand (ICOSL). Treatment with VNAR-Fc fusion proteins was compared to treatment with dexamethasone or vehicle in the Lewis rat model of experimental autoimmune uveitis (EAU). Inflammation control was determined by comparing OCT clinical and histologic scores, and aqueous humor protein concentration. The concentration of 27 inflammatory cytokines in the aqueous humor was measured using a multiplex enzyme-linked immunosorbent assay platform.
Results
Administration of S17-Fc significantly decreased clinical, histologic, and aqueous protein levels when compared to vehicle treatment. Inflammation scores and aqueous protein levels in A5-Fc–treated animals were decreased compared to vehicle treatment, but not significantly. The concentration of vascular endothelial growth factor (VEGF), regulated on activation, normal T cell expressed and secreted (RANTES), macrophage inflammatory protein 1 alpha (MIP-1α), interleukin (IL)-1β, LPS-induced CXC chemokine (LIX), monocyte chemoattractant protein-1 (MCP-1), and interferon (IFN)-γ were significantly decreased in the eyes of animals treated with dexamethasone. VNAR treatment demonstrated a trend towards decreased cytokine concentrations, but only VEGF and RANTES were significantly decreased by S17-Fc.
Conclusions
Treatment with the anti-TNFα VNAR S17-Fc ameliorates EAU as effectively as treatment with corticosteroids.
Translational Relevance
VNAR-Fc molecules are a next-generation therapeutic biologic that overcome the limitations of classical biologic monoclonal antibodies, such as complex structure, large size, and limited tissue penetration. This is a novel drug modality that could result in the development of new therapy options for patients with noninfectious uveitis.
Keywords: uveitis, treatment, TNF-alpha, experimental autoimmune uveitis, Lewis rat
Introduction
The term uveitis encompasses a number of diseases that feature intraocular inflammation as their primary manifestation. The incidence of all forms of uveitis is approximately 50/100,000 person-years, and the prevalence is approximately 100/100,000 person-years.1 This means that, at any given time in the United States, approximately 300,000 individuals have active uveitis. Uveitis remains a major cause of visual disability, with studies suggesting that over 50% of patients with the chronic forms of the disease will suffer significant vision loss, many quite severe.2,3 Uveitis is considered the fifth or sixth leading cause of blindness in the working age population in the United States and Europe, predominantly from noninfectious etiologies.4–6 Worldwide impact varies by location and proportion of cases caused by infectious disease or autoimmunity.7–11
Corticosteroids remain the mainstay of treatment for uveitis.12 Topical preparations are used for relatively mild, anterior cases, while periocular, intraocular, and oral administration is used for more advanced cases. Although highly efficacious for most noninfectious forms of uveitis, long-term local corticosteroid use can induce ocular complications, including cataract formation and glaucoma.13 Systemic use is associated with a host of undesirable complications, including diabetes, hyperlipidemia, osteoporosis, and cushingoid body habitus.14 Steroid sparing medications borrowed from the rheumatologic armamentarium are used for long-term treatment,12 but the efficacy of these medications is suboptimal; these medications additionally are associated with many significant systemic side effects.15,16
The tumor necrosis factor alpha (TNFα) inhibitor adalimumab (Humira) is the first nonsteroidal therapy to achieve United States Food and Drug Administration approval for the treatment of noninfectious uveitis.17,18 Adalimumab is a human monoclonal antibody that is delivered by subcutaneous injection every 2 weeks. While it is effective in preventing relapse and controlling inflammation in a percentage of patients, in the VISUAL I and Sycamore trials, there was a substantial failure rate at endpoint (∼50%) suggesting the continued need for alternative therapies.19 One possible alternative is switching from adalimumab to a different anti-TNFα agent. Infliximab has been recommended by a consensus panel as a treatment option for patients with noninfectious uveitis due to the well-established body of literature supporting efficacy.20 Other TNFα inhibitors, such as golimumab and certolizumab pegol, also have demonstrated efficacy with one report indicating that some patients can be treated successfully with golimumab after failing with adalimumab.21,22 Another alternative is using a dosing strategy or agent that provides more complete TNFα blockade. Some small studies reporting on the off label use of the anti-TNFα monoclonal antibody, infliximab (Remicade) in the treatment of uveitis, found that higher doses of infliximab and shorter dosing intervals could improve control in patients who were not controlled on other steroid-sparing immune modulating medications or on lower dose anti-TNFα therapy.23–25
Rather than increasing dosage of existing monoclonal antibodies, next-generation therapeutic biologics offer the opportunity to increase effective TNFα depletion using novel binding domains linked to immunoglobulin scaffolds. One such novel binding domain, the variable region of shark IgG novel antigen receptors (VNARs), are the smallest naturally occurring binding domains in the vertebrate kingdom.26,27 They demonstrate exquisite selectivity for target and much higher inherent solubility and stability than traditional monoclonal antibodies. This makes them ideal candidates for therapeutic drug development.28–30
Experimental autoimmune uveitis (EAU)31 is a well-established model of human uveitis that is induced by immunization with specific retinal proteins or peptide and disease is mediated by Th1 and Th17 mechanisms.31,32 Inhibition of TNFα is known to be efficacious in experimental uveitis.33–35 Therefore, we sought to demonstrate the efficacy anti-TNFα therapy with a new VNAR-based platform using this well-established model. Additionally, the inducible T-cell co-stimulator ligand (ICOSL) has been implicated in the pathogenesis of EAU,36,37 and a VNAR targeting ICOSL is effective in controlling EAU in mice.38 Thus, in this work, the efficacy of two VNAR-based therapies to control ocular inflammation was tested and compared using the Lewis rat model of EAU.
Methods
Animals, EAU Induction, and VNAR Treatment
This animal study protocol was approved by the animal care and use committee of the University of Washington (animal study protocol #4184-05) and was compliant with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. This study was performed in two experiments with half of the animals in each treatment group per round. Female Lewis rats 6 to 8 weeks of age were purchased (Envigo, Somerset, NJ) and maintained with standard chow and water ad libitum under specific pathogen-free conditions. EAU was generated with subcutaneous injection of 60 μg interphotoreceptor retinoid binding protein peptide R16 (ADGSSWEGVGVVPDV; Peptide 2.0, Inc., Chantilly, VA) in complete Freund's adjuvant (2.5 mg/mL H37Ra in incomplete Freund's Adjuvant) in two divided doses to each hip.39 Animals were weighed for health monitoring on days 0, 7, 10, 12, 13, and 14. The VNAR-mouse IgG2a constant domain (Fc) constructs are approximately 78 kDa fusion molecules with the VNAR domain N-terminally fused to the hinge region of a mouse IgG2a Fc region via a short (Gly4Ser)2 flexible linker. The Fc portion of the protein is derived from the wild type mouse IgG2a immunoglobulin molecule. S17-Fc and A5-Fc were expressed transiently in human embryonic kidney 283 cells. VNAR-Fc proteins were purified using Protein-A affinity chromatography and protein functionality was confirmed by target specific binding and neutralization assays.40–42 VNARs S17-Fc (20 mg/kg), A5-Fc (20 mg/kg), or PBS were administered by intraperitoneal (IP) injection on days 8, 10, and 12. Dexamethasone 0.2 mg/kg (Fresenius Kabi USA, LLC, Lake Zurich, IL) was administered by IP injection on days 10, 12, and 13. Previous unpublished experience had identified this steroid regimen as the minimum sufficient to prevent disease.
Clinical Scoring, Optical Coherence Tomography (OCT) System, Image Acquisition, and Analysis
Clinical scores and OCT images were obtained on day 0, 7, 10, 12, 13, and 14. Unmasked clinical scores were performed by a single unmasked grader (LW) using an external penlight exam and an established scale.39 OCT images were acquired using the Bioptigen Envisu R2300. Anterior segment volume scans centered on the corneal apex covering an area of 5 × 5 mm (1000Ascan/ Bscan × 200 B-scans) were captured using a Bioptigen 18 mm telecentric lens (product #90-BORE-G3-18; Bioptigen, Inc. Morrisville, NC). During imaging, animals were anesthetized with intraperitoneal ketamine/xylazine at a dose of 68.2 mg/kg (Ketamine: Ketaset 100 mg/mL; Zoeitis, Inc. Kalamazoo, MI; Xylazine: AnaSed 20 mg/mL; Lloyd Laboratories, Shenandoh, IA), and placed in the prone position in the Bioptigen rat-imaging cassette (Bioptigen, Inc.). Topical tetracaine (0.5%, Bausch and Lomb, Inc., Tampa, FL) was applied and eyes were dilated with phenylephrine (2.5%; Akorn, Inc., Lake Forest, IL) and corneal protection provided by Genteal gel (Alcon Laboratories, Inc., Fort Worth, TX). Two graders, masked to treatment and experimental day, scored the degree of inflammation on individual images using an adaptation of an established and validated OCT imaging score sytem.43 Disagreement in score between the two graders was arbitrated by a third masked grader. Briefly, cells on each image were counted and then assigned a semiquantitative score roughly paralleling the standardization of uveitis nomenclature (SUN) system:44 0 = no cells/image, 0.5+ = 1 to 5 cells/image, 1+ = 5 to 15 cells/image, 2+ = 16 to 24 cell/image, 3+ = 25+ cells/image and no hypopyon, 4+ = 25+ cells/image plus a hypopyon or pupillary membrane.
Histology and Aqueous Humor Analysis
Post mortem aqueous humor (right eyes) and whole eyes for histology (left eyes) were collected on day 14. Aqueous humor was collected in an ethylenediaminetetraacetic acid (EDTA)–containing capillary tube (Sarstedt, Nümbrecht, Germany) after corneal paracentesis with a 30-gauge needle (Becton Dickinson, Franklin Lakes, NJ). Then, 10 to 15 μL of aqueous was collected from each eye, and stored at −80°C in combination with 1 to 1.5 μL ×1 protease inhibitor (Sigma-Aldrich Corp., St. Louis, MO) until assayed. Whole eyes were fixed in 10% neutral buffered formalin (Sigma-Aldrich Corp.) for at least 24 hours. Paraffin block sections (4 μm) were stained with hematoxylin and eosin (H&E) and scored by a single grader (KP), masked to treatment using an established grading system.39
Aqueous protein was quantified using Pierce 660 nm Protein Assay Reagent (Thermo Fisher Scientific, Madison, WI) for colorimetric detection on the Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific). Then, 1 μL aqueous was used for total protein concentration determination. The remaining aqueous (9–14 μL) was diluted in an equal volume of radioimmunoprecipitation assay (RIPA) buffer containing phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor cocktail according to the manufacturer's protocol, and divided equally for testing in triplicate. Aqueous cytokine concentrations were determined using the MilliplexMAP rat cytokine/chemokine premixed 27 plex immunology multiplex assay (EMD Millipore Corp., Billerica, MA). The cytokines measured were granulocyte-colony stimulating factor (G-CSF), eotaxin, granulocyte monocyte-colony stimulating factor (GM-CSF), interleukin (IL)-1α, macrophage inflammatory protein-1α (MIP-1α), IL-2, epidermal growth factor (EGF), IL-13, IL-12p70, IL-5, monocyte chemoattractant protein-1 (MCP-1), interferon (IFN)-γ–induced protein 10 (IP-10), fractalkine, lipopolysaccharide-induced CXC chemokine (LIX), MIP-2, leptin, IL-4, IL-6, IL-10, IFN-γ, IL-17A, IL-18, growth-related oncogene/keratinocyte chemokine (GRO/KC), vascular endothelial growth factor (VEGF), TNFα, and regulated on activation, normal T cell expressed and secreted (RANTES). Samples were analyzed using the MAGPIX system (Luminex, Austin, TX) with xPonent software version 4.2 (EMD Millipore). Data analysis was performed using Milliplex Analyst Standard Version 5.1 software (EMD Millipore). Statistical analysis and graphing was performed using Graphpad Prism 7.0 software (Graphpad Software, La Jolla, CA). Clinical and histologic scores and aqueous protein concentrations of the four treatment groups were compared on day 14 using the Kruskal-Wallis test. Multiple pairwise comparisons were performed using Dunn's test. Adjusted P values <0.05 were significant.
Results
Treatment With VNARs Decreases Inflammation in EAU
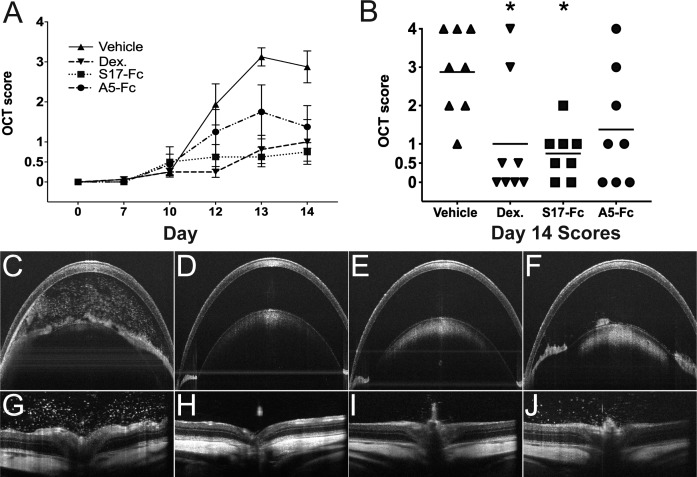
To test the efficacy of VNARs in the control of ocular inflammation, EAU was induced in 16 Lewis rats, and treatment with S17-Fc (Anti-TNFα) or A5-Fc (Anti-COSL) was compared to treatment with dexamethasone (positive control) and vehicle only (negative control). Control of inflammation was first evaluated using a masked OCT inflammation score (Fig. 1A). The mean score of eight eyes (both eyes of four animals) per treatment group was determined for each day, and then plotted longitudinally to reveal the course on inflammation over time. OCT score increased sharply between days 10 and 12 in vehicle-treated eyes and reached a mean score of 2.9 ± 1.1 on day 14. Treatment with S17-Fc, and dexamethasone decreased the daily inflammation score when compared to vehicle treatment starting on day 12 (Fig. 1A). On day 14, OCT score was significantly decreased with dexamethasone (mean = 1.0 ± 1.5, P < 0.02) and S17-Fc (mean = 0.75 ± 0.65, P < 0.03). Treatment with A5-Fc led to a decreased OCT score on day 14 (mean = 1.4 ± 1.5), but the difference from vehicle was not significant (P = 0.12; Fig. 1B). A large difference in score (≥ 2 step on day 14) between fellow eyes was noted in two animals; one vehicle-treated animal (right eye score = 3, left eye score = 1) and one A5-Fc–treated animal (right eye score = 0, left eye = 1). In seven of 16 (44%) animals, both eyes had the same score, and in the remaining seven of 16 (44%) there was a 1-step difference between eyes (Supplemental Fig. S1).
Figure 1.
Treatment decreases EAU inflammation score. (A) Longitudinal OCT score. Each point represents the mean score of eight eyes per treatment group. Error bars: SEM. (B) Dot plot of the scores for all eyes on day 14. Bar: Mean score. *P < 0.05. (C–F) Anterior chamber and retina (G–H) OCT image from each treatment group. (C, G) Vehicle. (D, H) Dexamethasone. (E, I) S17-Fc. (F, J) A5-Fc.
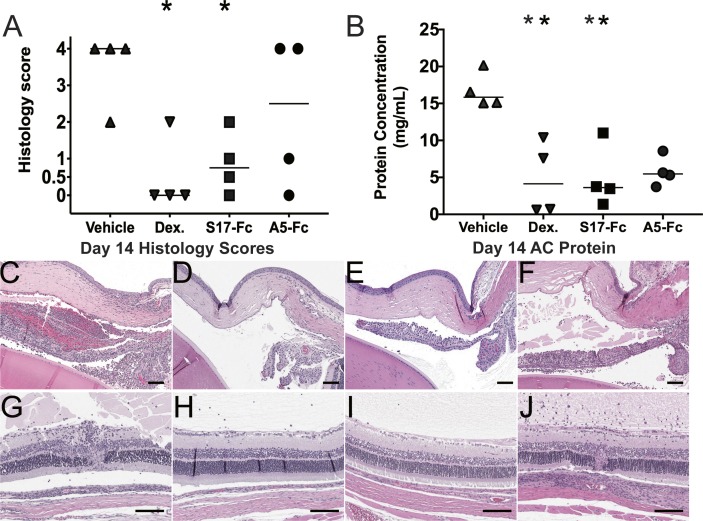
After OCT imaging on day 14, all animals were sacrificed. Left eyes were collected for histologic evaluation and scoring (Figs. 2A, 2C–F). From the right eye, aqueous was collected for total protein concentration determination (Fig. 2B) and inflammatory cytokine analysis (Table 1, Fig. 3). The comparisons of day 14 OCT to aqueous protein concentration (right eyes) or histology score (left eyes) for each treatment group are shown in Supplemental Figure S2. Histology of vehicle-treated eyes revealed extensive inflammation in the anterior and posterior chambers, including anterior chamber cells, pupillary membranes, retinal vasculitis, full thickness retinal lesions, and cellular choroidal infiltration (Fig. 2A). Median histologic score in vehicle-treated animals was 4 (interquartile ratio [IQR] = 2–4). Histologic score was significantly decreased by treatment with dexamethasone (median = 0, IQR = 0–1.5, P = 0.02) and S17-Fc (median = 0.5, IQR = 0–1.75, P = 0.03). Treatment with A5-Fc also decreased clinical score compared to vehicle, but this difference was not significant (median = 2.5, IQR = 0.25–4.0, P = 0.44). In the A5-Fc group, the range on histology score was large with two animals demonstrating almost complete control of inflammation (scores 0 and 1), but two animals demonstrated significant inflammation with a score of 4 (Fig. 2F).
Figure 2.
Treatment decreases EAU histology score and aqueous protein concentration. (A) Histologic score of eyes collected on day 14. (B) Protein concentration of aqueous humor collected from the anterior chamber on day 14. *P < 0.05. (C–J) Histologic sections showing the (C–F) anterior chamber, including the cornea, iris, ciliary body, and anterior lens or (G–J) retina, vitreous, and choroid of an eye from each treatment group: (C, G) vehicle, (D, H) dexamethasone, (E, I) S17-Fc, (F, J) A5-Fc. Scale bar = 100 μm.
Table 1.
Changes in Aqueous Humor Inflammatory Cytokine Concentrations by Treatment Group
| Cytokine |
Median Concentration in Each Treatment Group (pg/mL) |
% of Vehicle |
Kruskal Wallis |
P Value Compared to Vehicle |
|||||||
| Vehicle |
Dex |
S17-Fc |
A5-Fc |
Dex |
S17-Fc |
A5-Fc |
Dex |
S17-Fc |
A5-Fc |
||
| VEGF | 3345 | 358 | 287 | 944 | 11 | 9 | 28 | 0.03 | 0.06 | 0.03 | 0.13 |
| RANTES | 816 | 102 | 139 | 179 | 13 | 17 | 22 | 0.02 | 0.03 | 0.05 | 0.07 |
| MIP-1α | 163 | 26 | 44 | 51 | 16 | 27 | 32 | 0.01 | 0.01 | 0.09 | 0.13 |
| IL-1β | 3498 | 797 | 945 | 1031 | 23 | 27 | 29 | 0.03 | 0.05 | 0.06 | 0.09 |
| LIX | 2446 | 698 | 1242 | 1303 | 29 | 51 | 53 | 0.03 | 0.02 | 0.41 | 0.11 |
| IP-10 | 5429 | 2451 | 2532 | 2408 | 45 | 47 | 44 | 0.38 | |||
| Leptin | 3437 | 1539 | 1893 | 2136 | 45 | 55 | 62 | 0.12 | |||
| IL-17A | 770 | 344 | 514 | 531 | 45 | 67 | 69 | 0.47 | |||
| MCP-1 | 3781 | 1789 | 2113 | 2951 | 47 | 56 | 78 | 0.01 | 0.01 | 0.11 | 0.90 |
| IL-18 | 4793 | 2592 | 2516 | 2736 | 54 | 52 | 57 | 0.05 | 0.11 | 0.11 | 0.053 |
| IL-6 | 6794 | 4430 | 15,857 | 8697 | 65 | 233 | 128 | 0.36 | |||
| Fractalkine | 99 | 72 | 88 | 112 | 73 | 89 | 113 | 0.37 | |||
| IL-10 | 528 | 392 | 505 | 613 | 74 | 96 | 116 | 0.05 | |||
| IL-4 | 226 | 173 | 204 | 244 | 76 | 90 | 108 | 0.18 | |||
| IFN-γ | 4467 | 3531 | 4218 | 4426 | 79 | 94 | 99 | 0.03 | 0.02 | >0.9999 | >0.9999 |
| G-CSF | 27 | 22 | 27 | 28 | 83 | 102 | 106 | 0.21 | |||
| IL-12p70 | 66 | 56 | 79 | 81 | 85 | 119 | 122 | 0.28 | |||
| IL-5 | 318 | 288 | 305 | 312 | 91 | 96 | 98 | 0.50 | |||
| IL-13 | 250 | 231 | 265 | 276 | 93 | 106 | 111 | 0.46 | |||
| IL-1α | 215 | 205 | 284 | 227 | 95 | 132 | 106 | 0.71 | |||
| IL-2 | 317 | 311 | 370 | 371 | 98 | 117 | 117 | 0.04 | >0.9999 | 0.19 | 0.36 |
| Eotaxin | 77 | 76 | 90 | 103 | 99 | 118 | 135 | 0.55 | |||
| TNFα | 14 | 14 | 14 | 14 | 100 | 100 | 100 | >0.9999 | |||
| EGF | 154 | 155 | 106 | 264 | 101 | 69 | 172 | 0.73 | |||
| MIP-2 | 596 | 770 | 851 | 879 | 129 | 143 | 147 | 0.16 | |||
| GM-CSF | 1412 | 1971 | 2168 | 2217 | 140 | 154 | 157 | 0.01 | 0.16 | 0.04 | 0.02 |
| GRO/KC | 1502 | 2254 | 2171 | 2361 | 150 | 145 | 157 | 0.02 | 0.08 | 0.053 | 0.03 |
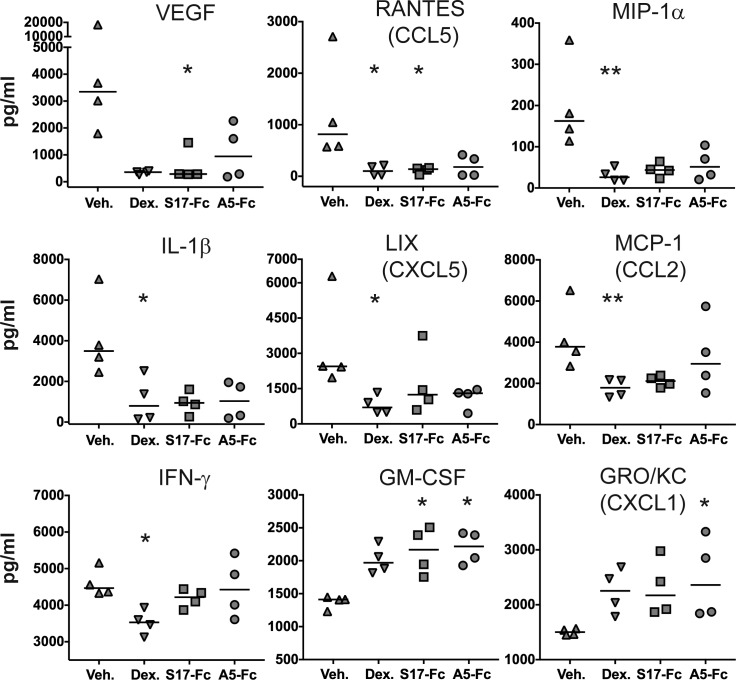
Figure 3.
Treatment decreases intraocular proinflammatory cytokines. Significant differences in the aqueous concentration of nine cytokines were identified between vehicle-treated animals with EAU and animals treated with dexamethasone, S17-Fc, or A5-Fc. VEGF, RANTES, MIP-1α, IL-1β, LIX, MCP-1, and IFN-γ were all decreased by treatment. GM-CSF and GRO/KC were increased with treatment. *P ≤ 0.05, **P ≤ 0.01.
Anterior chamber protein is elevated in eyes with uveitis due to the breakdown of the blood ocular barrier during inflammation.45 In uninflamed rat eyes, aqueous protein is low. Valderrama et al.46 reported in normal eyes that protein concentration can fluctuate between 0.3 and 1.5 mg/mL. In rats with EAU treated with vehicle alone, median protein concentration of the aqueous was 15.85 mg/mL (IQR = 15.08–19.28 mg/mL). Median aqueous protein concentration was significantly lower in animals treated with dexamethasone (median = 4.13 mg/mL, IQR = 0.61–9.67 mg/mL, P = 0.028), and S17-Fc (median = 3.48 mg/mL, IQR = 1.89–9.19 mg/mL, P = 0.043). Treatment with A5-Fc also decreased aqueous protein concentration compared to vehicle-treated animals, but not significantly (median = 5.62 mg/mL, IQR = 4.12–7.83 mg/mL, P = 0.11).
Treatment Decreases Intraocular Proinflammatory Cytokines
The concentration of 27 proinflammatory cytokines was determined using a multiplex enzyme-linked immunosorbent (ELISA) assay (Table 1). In Table 1, the cytokines are ranked from top to bottom according to the ability of steroid treatment to decrease the aqueous humor concentration compared to vehicle treatment. Significantly decreased cytokines are located at the top of the table, while cytokines that were increased in treated eyes are located at the bottom. The presence of a significant difference between treatment groups was identified using Kruskal-Wallis analysis of variance testing. When a significant difference (P ≤ 0.05) within the group was identified, Dunn's multiple comparison test was performed to determine if a significant difference existed between the concentration of the cytokine in the vehicle control eyes and one of the treatment groups.
Eleven cytokines demonstrated a significant within group difference (Table 1). A significant difference between vehicle and dexamethasone treatment was identified in six of these groups: RANTES (P = 0.03), MIP-1α (P = 0.01), IL-1β (P = 0.05), LIX (P = 0.02), MCP-1 (P = 0.01), and IFN-γ (P = 0.02; Fig. 3). S17-Fc treatment significantly decreased VEGF (P = 0.04) and RANTES (P = 0.05), and significantly increased GM-CSF (P = 0.04) compared to vehicle. A5-Fc treatment did not significantly decrease any cytokine compared to vehicle, but did significantly increase GM-CSF (P = 0.02) and GRO/KC (P = 0.03). Local concentrations of TNFα were below the level of detection of the assay (<14.93 pg/mL) in three of the four vehicle control eyes. In one eye, TNFα concentration was detected at 18.7 pg/mL. In all eyes of the treated animals (dexamethasone, S17-Fc, and A5-Fc), TNFα concentrations were below the level of detection of the assay (<14.93 pg/mL).
For the individual eyes used to measure aqueous protein and cytokine concentrations, a significant correlation was found between day 14 OCT score and total aqueous protein concentration (Spearman's r = 0.82; 95% confidence interval [CI], 0.54–0.94; P < 0.001). Furthermore, eight cytokines were identified that demonstrated a significant correlation to aqueous protein concentration (Supplemental Table S1). Two demonstrated a negative correlation: GM-CSF (r = −0.77; 95% CI, −0.92 to −0.43; P < 0.001) and GRO/KC (r = −0.76; 95% CI, −0.92 to −0.42; P < 0.001). Six demonstrated a positive correlation to aqueous protein concentration: MIP-1α (r = 0.89; 95% CI, −0.69 to 0.96; P < 0.0001), IL-1β (r = 0.88; 95% CI, 0.67–0.96; P < 0.0001), VEGF (r = 0.84; 95% CI, 0.59–0.95; P < 0.0001), RANTES (r = 0.84; 95% CI, 0.59–0.95; P < 0.0001), and Leptin (r = 0.77; 95% CI, 0.44–0.92; P < 0.001).
The Effect of Treatment on Weight
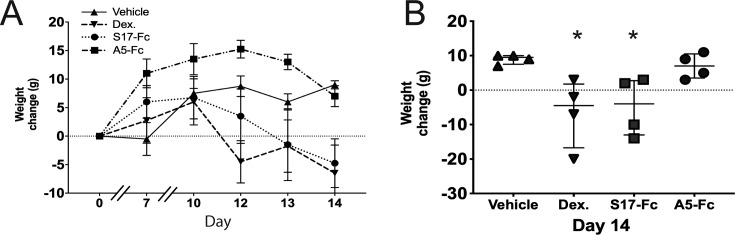
Systemic corticosteroid administration in rats leads to weight loss and decreased food intake.47–50 To monitor for weight loss with treatment, animals were weighed on days 0, 7, 10, 12, 13, and 14. The average change in weight from baseline per treatment group per day is shown in Figure 4A. Weight increased in all groups through day 10. On days 12 to 13, vehicle control animals maintained their weight, and on day 14 they demonstrated a median increase from baseline weight of 9.5 g (IQR = 7.5–10 g). In contrast, after initially gaining weight, dexamethasone- and S17-Fc–treated animals lost weight after day 10 for a final median weight loss of 4.5 g (IQR = −16.75 to +1.75 g) in the dexamethasone-treated animals and 4 g (IQR −13 to +2.75 g) in S17-Fc treated animals. A5-Fc–treated animals gained weight though day 12, but then lost weight through day 14. However, on day 14, A5-Fc animals still demonstrated a median weight gain of 7 g from baseline (IQR 3.5–10.5 g). The median change in weight in dexamethasone (−4.5 g, P = 0.03) and S17-Fc animals (−4.0 g, P = 0.03) was significantly different from the weight change in vehicle-treated animals (+9.5 g).
Figure 4.
Treatment with dexamethasone and S17-Fc, but not A5-Fc leads to weight loss on day 14. (A) The four animals in each treatment arm were weighed at baseline and during the course of the experiment. The mean difference from weight at day 0 is plotted for each treatment group. (B) The change in weight from Days 0 to 14 is shown for each animal per treatment group. Mean and standard deviation are indicated. *P < 0.05. Dex., dexamethasone.
Discussion
We report on the use of two shark VNAR-Fc fusion proteins in the treatment of experimental uveitis in the Lewis rat. We find that the anti-TNFα agent, S17-Fc, was effective at reducing inflammation by clinical OCT score, postmortem histology, and aqueous humor protein concentration. Furthermore, the ability of S17-Fc to control inflammation by these measures was equivalent to the results of the positive control dexamethasone. Systemic treatment with the ICOSL inhibitor, A5-Fc VNAR, demonstrated an intermediate decrease in clinical score, OCT score, and histologic score, but showed a robust decrease in aqueous protein that was nearly equivalent to the effect of treatment with dexamethasone.
Not surprisingly, in this study we showed that systemic anti-TNFα therapy is effective in controlling experimental uveitis. Multiple prior studies have demonstrated the benefit of TNFα depletion in rat33–35 and mouse33 models of EAU. However, these studies used TNF receptor fusion proteins to bind TNFα rather than the commercially available anti-TNFα antibodies due to the inability of infliximab and adalimumab to bind the murine proteins.51–53 Our study is distinct from these prior animal model studies because the S17 anti-TNFα VNAR domain-Fc fusion protein can bind both mouse and rat TNFα. So for the first time, our data demonstrated in vivo efficacy of this anti-TNFα VNAR domain-Fc fusion protein in an important model of human uveitis, and establishes S17-Fc as new tool that can be used in a wide range of clinically relevant animal models of inflammatory disease.
The ability of anti-ICOSL VNAR A5-Fc was not consistent in demonstrating efficacy in the rat model of EAU. This is in contrast to a prior study using the A5-Fc VNAR in the mouse model of EAU.38 A potential reason for this difference is that in the mouse study, the A5-Fc was dosed daily starting day 1 through day 14 after EAU induction. Thus, the ICOSL co-stimulation was present during the “afferent” phase of uveitis induction rather than during the “efferent” phase of disease, and the dosing interval was relatively short. In the current study, A5-Fc was administered only three times during the efferent phase (days 8, 10, and 12). Despite this disadvantage, administration of A5-Fc still showed a trend towards preventing the full manifestations of EAU in many eyes of the treatment group. Furthermore, most of the intraocular cytokines that were decreased significantly by steroid treatment also were decreased, albeit nonsignificantly with A5-Fc administration. It is possible that further optimization of timing and dosing would clarify the potential benefits of ICOSL inhibition in the treatment of uveitis.
Cytokine analysis demonstrated that treatment with dexamethasone had superior ability to suppress inflammatory cytokine expression in the eye, but that both VNARs also led to a general trend toward decreased intraocular proinflammatory cytokine expression. Surprisingly, soluble TNFα was not identified in vehicle-treated eyes despite the presence of severe inflammation. This finding is in contradiction to a previous study that did identify TNFα (62 pg/mL) in rat eyes with EAU.54 However, the eyes of the rats in this study also had experienced ciliary body electroporation, which could have contributed to this difference. Establishing the importance of local versus systemic TNFα in the pathogenesis of uveitis has high clinical relevance. Local immune suppression with corticosteroids has a well-established role in the treatment of noninfectious uveitis.13,55,56 Since therapy with systemic anti-TNFα agents is effective, the next reasonable question is whether there is benefit from local anti-TNFα therapy.57,58 A few cases of intravitreal infliximab59 and adalimumab60,61 injection in humans have been reported. However, due to retinal toxicity associated with intravitreal infliximab in humans and a study in a rabbit model of uveitis that determined intravitreal injection of infliximab exacerbated inflammation while systemic administration ameliorated uveitis, local approaches have not been widely pursued.62,63 Taken together with our results of low to undetectable intraocular TNFα in all treatment conditions, the evidence suggested that systemic and not local TNFα has the key role in the pathogenesis of uveitis, possibly at the level of promoting Th1 polarization in regional lymph nodes.
IFNγ is a pivotal cytokine in rat EAU.64,65 We were surprised to find that intraocular IFNγ levels were not impacted by treatment with either S17-Fc or A5-Fc despite a decrease in clinical and histology score when compared to vehicle treatment. Furthermore, two proinflammatory cytokines, GM-CSF and GRO/KC, were elevated above vehicle in the VNAR and steroid treatment arms. GM-CSF is a key mediator of inflammation in the central nervous system, and sufficient to initiate experimental autoimmune encephalitis (EAE) when expressed in peripheral CD4+ T cells.66 GRO/KC, also known as chemokine (C-X-C motif) ligand 1 (CXCL1) is a neutrophil chemoattractant expressed by mast cells and macrophages during inflammation.67 How could these proinflammatory cytokines be elevated in treated eyes with lower clinical scores than those in the highly inflamed vehicle-treated eyes? We suspect that treatment delayed disease onset such that on day 14, eyes that received anti-inflammatory treatment (steroid and VNARs) were just beginning to express these early proinflammatory cytokines and chemokines, while the vehicle eyes were showing signs of a more mature inflammatory process with higher levels of a broad range of inflammatory cytokines. Future studies with a terminal time point on or after day 21 could be performed to test this hypothesis.
The animals in this study did not gain the weight typical for female rats in the 6- to 8-week age range. Animals in the vehicle and A5-Fc treatment arms did gain weight on average, but less than the expected range of 25 to 50 grams over 2 weeks. Animals in the steroid treatment and S17-Fc arms lost weight on average. Weight was not a study endpoint, so specific monitoring for important variables, such as food consumption and activity by each animal, was not part of the protocol. Additionally, no other signs of toxicity, such as diarrhea or general signs of distress or infection, were noted by study personnel. It is not clear what factors are responsible for these results. The design of future studies could include randomization to treatment arm by baseline weight and controls for variables known to contribute to poor weight gain to better establish the connection between weight loss and treatment suggested by our results.
Limitations of our study include the absence of a nonbinding VNAR-Fc isotype control and the small size of each treatment cohort. VNAR isotype control 2V has been extensively assessed in numerous in vitro cellular and non-cell based assays, and more importantly in in vivo pharmacokinetic and disease model studies across the mouse, rat, rabbit, and nonhuman primate model systems with no evidence to suggest any off-site target binding or pharmacologic effect.40–42,68 These data in conjunction with efficacy data from prior in vitro and animal studies using the S17-Fc and A5-Fc VNARs informed our decision to use the minimum number of animals that would be sufficient to identify a significant effect on the control of ocular inflammation in the EAU model. Further studies could be performed to replicate these data and confirm our findings in larger cohorts. Alternative treatment regimens, determination of serum and regional lymph node TNFα concentration, and later endpoints that have been used to determine efficacy in the rat model also could be explored.54,69–71
In summary, an anti-TNFα VNAR, S17-Fc, shows good efficacy in controlling inflammation in an animal model of uveitis. Targeting TNFα for the treatment of human uveitis with humanized monoclonal antibodies is effective, but has some limitations that can be overcome with the use of next generation biologic therapies. An anti-human TNFα VNARs recently has been produced and characterized, and shows high in vitro neutralizing ability.40 Thus, anti-TNFα VNARs are a promising new therapeutic option to explore for use in the future treatment of human uveitis.
Supplementary Material
Acknowledgments
Supported by an unrestricted departmental grant from Research to Prevent Blindness (New York, NY), NEI K08EY023998 (KLP), P30-EY001730 (RVG; Bethesda, MD), by a grant from Elasmogen Limited (RVG), and with support from the Mark J. Daily,MD Research Fund (RVG, KLP).
Disclosure: K.L. Pepple, None; L. Wilson, None; R.N. Van Gelder, Elasmogen (F); M. Kovaleva, Elasmogen (E); O.C. Ubah, Elasmogen (E); J. Steven, Elasmogen (E); C.J. Barelle, Elasmogen (E); A. Porter, Elasmogen (E)
References
- 1.Gritz DC, Wong I. G. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. discussion 500. [DOI] [PubMed] [Google Scholar]
- 2.Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PJ. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88:1159–1162. doi: 10.1136/bjo.2003.037226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80:844–848. doi: 10.1136/bjo.80.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kocur I, Resnikoff S. Visual impairment and blindness in Europe and their prevention. Br J Ophthalmol. 2002;86;:716–722. doi: 10.1136/bjo.86.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 6.Rothova A, Suttorp-van Schulten MS. Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332–336. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang DK, Chou YJ, Pu CY, Chou P. Epidemiology of uveitis among the Chinese population in Taiwan: a population-based study. Ophthalmology. 2012;119;:2371–2376. doi: 10.1016/j.ophtha.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Nakao K, Ohba N. [Prevalence of endogenous uveitis in Kagoshima Prefecture, Southwest Japan] Nippon Ganka Gakkai Zasshi. 1996;100:150–155. [PubMed] [Google Scholar]
- 9.Rathinam SR, Krishnadas R, Ramakrishnan R, et al. Population-based prevalence of uveitis in Southern India. Br J Ophthalmol. 2011;95:463–467. doi: 10.1136/bjo.2010.182311. [DOI] [PubMed] [Google Scholar]
- 10.Chang JH, Wakefield D. Uveitis: a global perspective. Ocul Immunol Inflamm. 2002;10:263–279. doi: 10.1076/ocii.10.4.263.15592. [DOI] [PubMed] [Google Scholar]
- 11.Miserocchi E, Fogliato G, Modorati G, Bandello F. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. 2013;23:705–717. doi: 10.5301/ejo.5000278. [DOI] [PubMed] [Google Scholar]
- 12.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 13.Kempen JH, Altaweel MM, Holbrook JT, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118:1916–1926. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suhler EB, Thorne JE, Mittal M, et al. Corticosteroid-related adverse events systematically increase with corticosteroid dose in noninfectious intermediate, posterior, or panuveitis: post hoc analyses from the VISUAL-1 and VISUAL-2 trials. Ophthalmology. 2017;124:1799–1807. doi: 10.1016/j.ophtha.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Kempen JH, Daniel E, Dunn JP, et al. Overall and cancer related mortality among patients with ocular inflammation treated with immunosuppressive drugs: retrospective cohort study. BMJ. 2009;339 doi: 10.1136/bmj.b2480. b2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kempen JH, Gangaputra S, Daniel E, et al. Long-term risk of malignancy among patients treated with immunosuppressive agents for ocular inflammation: a critical assessment of the evidence. Am J Ophthalmol. 2008;146:802–812. doi: 10.1016/j.ajo.2008.04.035. e801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen QD, Merrill PT, Jaffe GJ, et al. Adalimumab for prevention of uveitic flare in patients with inactive noninfectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet. 2016;388:1183–1192. doi: 10.1016/S0140-6736(16)31339-3. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe GJ, Dick AD, Brezin AP, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375:932–943. doi: 10.1056/NEJMoa1509852. [DOI] [PubMed] [Google Scholar]
- 19.Ramanan AV, Dick AD, Beresford MW. Adalimumab for Uveitis in juvenile idiopathic arthritis. N Engl J Med. 2017;377:789–790. doi: 10.1056/NEJMc1708646. [DOI] [PubMed] [Google Scholar]
- 20.Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthal mology. 2014;121:785–796. doi: 10.1016/j.ophtha.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 21.Cordero-Coma M, Salom D, Diaz-Llopis M, Lopez-Prats MJ, Calleja S. Golimumab for uveitis. Ophthalmology. 2011;118:1893–1894. doi: 10.1016/j.ophtha.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Cordero-Coma M, Sobrin L. Anti-tumor necrosis factor-alpha therapy in uveitis. Surv Ophthalmol. 2015;60:575–589. doi: 10.1016/j.survophthal.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Kruh JN, Yang P, Suelves AM, Foster CS. Infliximab for the treatment of refractory noninfectious uveitis: a study of 88 patients with long-term follow-up. Ophthalmology. 2014;121:358–364. doi: 10.1016/j.ophtha.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Kahn P, Weiss M, Imundo LF, Levy DM. Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmology. 2006;113:860–864. doi: 10.1016/j.ophtha.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Sukumaran S, Marzan K, Shaham B, Reiff A. High dose infliximab in the treatment of refractory uveitis: does dose matter? ISRN Rheumatol. 2012. 765380. [DOI] [PMC free article] [PubMed]
- 26.Greenberg AS, Avila D, Hughes M, Hughes A, McKinnev EC, Flajnik MF. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature. 1995;374:168–173. doi: 10.1038/374168a0. [DOI] [PubMed] [Google Scholar]
- 27.Kovaleva M, Ferguson L, Steven J, Porter A, Barelle C. Shark variable new antigen receptor biologics - a novel technology platform for therapeutic drug development. Exp Opin Biol There. 2014;14:1527–1539. doi: 10.1517/14712598.2014.937701. [DOI] [PubMed] [Google Scholar]
- 28.Barelle C, Gill DS, Charlton K. Shark novel antigen receptors--the next generation of biologic therapeutics? Adv Exp Med Biol. 2009;655:49–62. doi: 10.1007/978-1-4419-1132-2_6. [DOI] [PubMed] [Google Scholar]
- 29.Liu JL, Zabetakis D, Brown JC, Anderson GP, Goldman ER. Thermal stability and refolding capability of shark derived single domain antibodies. Mol Immunol. 2014;59:194–199. doi: 10.1016/j.molimm.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Ubah OC, Steven J, Porter AJ, Barelle CJ. An Anti-hTNF-alpha variable new antigen receptor format demonstrates superior in vivo preclinical efficacy to Humira(R) in a transgenic mouse autoimmune polyarthritis disease model. Front Immunol. 2019;10:526. doi: 10.3389/fimmu.2019.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caspi RR. Experimental autoimmune uveoretinitis in the rat and mouse. Curr Protoc Immunol. Chapter 15, Unit 15.6. [DOI] [PubMed]
- 32.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Medical. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busch M, Bauer D, Hennig M, et al. Effects of systemic and intravitreal TNF-alpha inhibition in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2013;54:39–46. doi: 10.1167/iovs.12-10138. [DOI] [PubMed] [Google Scholar]
- 34.Dick AD, McMenamin PJ, Korner H, et al. Inhibition of tumor necrosis factor activity minimizes target organ damage in experimental autoimmune uveoretinitis despite quantitatively normal activated T cell traffic to the retina. Eur J Immunol. 1996;26:1018–1025. doi: 10.1002/eji.1830260510. [DOI] [PubMed] [Google Scholar]
- 35.Dick AD, Duncan L, Hale G, Waldmann H, Isaacs J. Neutralizing TNF-alpha activity modulates T-cell phenotype and function in experimental autoimmune uveoretinitis. J Autoimmun. 1998;11:255–264. doi: 10.1006/jaut.1998.0197. [DOI] [PubMed] [Google Scholar]
- 36.Usui Y, Akiba H, Takeuchi M, et al. The role of the ICOS/B7RP-1 T cell costimulatory pathway in murine experimental autoimmune uveoretinitis. Eur J Immunol. 2006;(2006);36:3071–3081. doi: 10.1002/eji.200636138. [DOI] [PubMed] [Google Scholar]
- 37.Xing L, Yang P, Wu C, et al. Inducible co-stimulator (ICOS) is upregulated in experimental autoimmune uveoretinitis. Graefes Arch Clin Exp Ophthalmol. 2006;244:1650–1658. doi: 10.1007/s00417-005-0207-0. [DOI] [PubMed] [Google Scholar]
- 38.Kovaleva M, Johnson K, Steven J, Barelle CJ, Porter A. Therapeutic potential of shark Anti-ICOSL VNAR domains is exemplified in a murine model of autoimmune non-infectious uveitis. Front Immunol. 2017;8:1121. doi: 10.3389/fimmu.2017.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agarwal RK, Silver PB, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Biol. 2012;900:443–469. doi: 10.1007/978-1-60761-720-4_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ubah OC, Steven J, Kovaleva M, et al. Novel, anti-hTNF-alpha variable new antigen receptor formats with enhanced neutralizing potency and multifunctionality, generated for therapeutic development. Front Immunol. 2017;8:1780. doi: 10.3389/fimmu.2017.01780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steven J, Muller MR, Carvalo MF, et al. In vitro maturation of a humanized shark vnar domain to improve its biophysical properties to facilitate clinical development. Front Immunol. 2017;8:1361. doi: 10.3389/fimmu.2017.01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Dwyer R, Kovaleva M, Zhang J, et al. Anti-ICOSL new antigen receptor domains inhibit T cell proliferation and reduce the development of inflammation in the collagen-induced mouse model of rheumatoid arthritis. J Immunol Res. 2018;2018:4089459. doi: 10.1155/2018/4089459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pepple KL, Choi WJ, Wilson L, Van Gelder RN, Wang RK. Quantitative assessment of anterior segment inflammation in a rat model of uveitis using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 216. 57:3567–3575. doi: 10.1167/iovs.16-19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krause U, Raunio V. The proteins of the pathologic human aqueous humour. An in vivo investigation. Ophthalmologica. 1970;160:280–287. doi: 10.1159/000306003. [DOI] [PubMed] [Google Scholar]
- 46.Valderrama CM, Li R, Liu JH. Direct effect of light on 24-h variation of aqueous humor protein concentration in Sprague-Dawley rats. Exp Eye Res. 2008;87:487–491. doi: 10.1016/j.exer.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu XY, Shi JH, Du WH, et al. Glucocorticoids decrease body weight and food intake and inhibit appetite regulatory peptide expression in the hypothalamus of rats. Exp Ther Med. 2011;2:977–984. doi: 10.3892/etm.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zakrzewska KE, Cusin I, Stricker-Krongrad A, et al. Induction of obesity and hyperleptinemia by central glucocorticoid infusion in the rat. Diabetes. 1999;48:365–370. doi: 10.2337/diabetes.48.2.365. [DOI] [PubMed] [Google Scholar]
- 49.Konno J, Yoshida S, Ina A, et al. Upregulated expression of neuropeptide Y in hypothalamic-pituitary system of rats by chronic dexamethasone administration. Neurosci Res. 2008;60:259–265. doi: 10.1016/j.neures.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 50.De Vos P, Saladin R, Auwerx J, Staels B. Induction of ob gene expression by corticosteroids is accompanied by body weight loss and reduced food intake. J Biol Chem. 1995;270:15958–15961. doi: 10.1074/jbc.270.27.15958. [DOI] [PubMed] [Google Scholar]
- 51.Scallon BJ, Trinh H, Nedelman M, et al. Functional comparisons of different tumour necrosis factor receptor/IgG fusion proteins. Cytokine. 1995;7:759–770. doi: 10.1006/cyto.1995.0091. [DOI] [PubMed] [Google Scholar]
- 52.Assas BM, Levison SE, Little M, et al. Anti-inflammatory effects of infliximab in mice are independent of tumour necrosis factor alpha neutralization. Clin Exp Immunol. 2017;187:225–233. doi: 10.1111/cei.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knight DM, Trinh H, Le J, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993;30:1443–1453. doi: 10.1016/0161-5890(93)90106-l. [DOI] [PubMed] [Google Scholar]
- 54.Kowalczuk L, Touchard E, Camelo S, et al. Local ocular immunomodulation resulting from electrotransfer of plasmid encoding soluble TNF receptors in the ciliary muscle. Invest Ophthalmol Vis Sci. 2009;50:1761–1768. doi: 10.1167/iovs.08-3027. [DOI] [PubMed] [Google Scholar]
- 55.Jaffe GJ, Foster CS, Pavesio CE, Paggiarino DA, Riedel GE. Effect of an injectable fluocinolone acetonide insert on recurrence rates in chronic noninfectious uveitis affecting the posterior segment: twelve-month results. Ophthalmology. 2019;126:601–610. doi: 10.1016/j.ophtha.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 56.Thorne JE, Sugar EA, Holbrook JT, et al. Periocular triamcinolone vs. intravitreal triamcinolone vs. intravitreal dexamethasone implant for the treatment of uveitic macular edema: the periocular vs. intravitreal corticosteroids for uveitic macular edema (POINT) trial. Ophthalmology. 2019;126:283–295. doi: 10.1016/j.ophtha.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leal I, Rodrigues FB, Sousa DC, et al. Efficacy and safety of intravitreal anti-tumour necrosis factor drugs in adults with noninfectious uveitis - a systematic review. Acta Ophthalmol. 2018;96:e665–e675. doi: 10.1111/aos.13699. [DOI] [PubMed] [Google Scholar]
- 58.Pascual-Camps I, Hernandez-Martinez P, Monje-Fernandez L, et al. Update on intravitreal anti-tumor necrosis factor alpha therapies for ocular disorders. J Ophthalmic Inflamm Infect. 2014;4:26. doi: 10.1186/s12348-014-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farvardin M, Afarid M, Mehryar M, Hosseini H. Intravitreal infliximab for the treatment of sight-threatening chronic noninfectious uveitis. Retina. 2010;30:1530–1535. doi: 10.1097/IAE.0b013e3181d3758a. [DOI] [PubMed] [Google Scholar]
- 60.Hamam RN, Bankien AW, Antonios RS, et al. Intravitreal adalimumab in active noninfectious uveitis: a pilot study. Ocul Immunol Inflamm. 2016;24:319–326. doi: 10.3109/09273948.2014.990041. [DOI] [PubMed] [Google Scholar]
- 61.Kheir WJ, Mehanna CJ, Abdul Fattah M, et al. Intravitreal adalimumab for the control of breakthrough intraocular inflammation. Ocul Immunol Inflamm. 2018;26:1206–1211. doi: 10.1080/09273948.2017.1335756. [DOI] [PubMed] [Google Scholar]
- 62.Giganti M, Beer PM, Lemanski L, et al. Adverse events after intravitreal infliximab (Remicade) Retina. 2010;30:71–80. doi: 10.1097/IAE.0b013e3181bcef3b. [DOI] [PubMed] [Google Scholar]
- 63.Donmez O, Yaman A, Ozturk I, et al. The efficacy of systemic and intravitreal infliximab treatments in an endotoxin-induced uveitis model. Cutan Ocul Toxicol. 2019;2:1–10. doi: 10.1080/15569527.2019.1632883. [DOI] [PubMed] [Google Scholar]
- 64.Egwuagu CE, Sztein J, Mahdi RM, et al. IFN-gamma increases the severity and accelerates the onset of experimental autoimmune uveitis in transgenic rats. J Immunol. 1999;162:510–517. [PubMed] [Google Scholar]
- 65.Kaufmann U, Diedrichs-Mohring M, Wildner G. Dynamics of intraocular IFN-gamma, IL-17 and IL-10-producing cell populations during relapsing and monophasic rat experimental autoimmune uveitis. PLoS One. 2012;7:e49008. doi: 10.1371/journal.pone.0049008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spath S, Komukzki J, Hermann M, et al. Dysregulation of the cytokine GM-CSF induces spontaneous phagocyte invasion and immunopathology in the central nervous system. Immunity. 2017;46:245–260. doi: 10.1016/j.immuni.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 67.De Filippo K, Dudeck A. Hasenberg M, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 68.Muller MR, Saunders K, Grace C, et al. Improving the pharmacokinetic properties of biologics by fusion to an anti-HSA shark VNAR domain. MAbs. 2012;4:673–685. doi: 10.4161/mabs.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Smet MD, Bitar G, Roberge FG, Gery I, Nussenblatt RB. Human S-antigen: presence of multiple immunogenic and immunopathogenic sites in the Lewis rat. J Autoimmun. 1993;6:587–599. doi: 10.1006/jaut.1993.1048. [DOI] [PubMed] [Google Scholar]
- 70.Touchard E, Benard R, Bigot K, et al. Non-viral ocular gene therapy, pEYS606, for the treatment of non-infectious uveitis: preclinical evaluation of the medicinal product. J Control Release. 2018;285:244–251. doi: 10.1016/j.jconrel.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 71.Yun J, Xiao T, Zhou L, et al. Local S100A8 levels correlate with recurrence of experimental autoimmune uveitis and promote pathogenic T cell activity. Invest Ophthalmol Vis Sci. 2018;59:1332–1342. doi: 10.1167/iovs.17-23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.