Abstract
Axon visualization techniques are important in assessing the efficacy of interventional approaches to stimulate neural regeneration. Whereas the labeling of descending tracts in the spinal cord has been well established using the intracortical injection of biotin dextran amine (BDA), the labeling of ascending sensory fibers of the dorsal funiculus is more problematic. Fluoro-Ruby (FR; dextran tetramethylrhodamine; MW 10,000) is a bidirectional permanent tracer, but the retrograde tracing of fibers is particularly prominent, and FR is a highly sensitive tracer that can be applied in discrete injection sites. In the present report, we used FR to efficiently label ascending fibers in the dorsal columns of the rat spinal cord. After transplantation of olfactory ensheathing cells into the transected dorsal funiculus, the application of FR was able to detect regenerating ascending fibers in the spinal cord. Regenerated fibers crossing the injury site were labeled and easily identified. It is likely that the tracer was taken up by damaged fibers. As additional advantages, the labeling is resistant to photobleaching and no additional tissue processing is necessary for visualization. It can be used for in vivo as well as in vitro injections. The findings indicate that FR can be used as a reliable fluorescent marker to study ascending regenerated fibers in the spinal cord axonal regeneration.
Keywords: axonal regeneration, cell transplantation, olfactory ensheathing cells, spinal cord injury
Introduction
Reliable and efficient anatomical methods to trace axons are important in assessing interventional approaches designed to enhance axonal regeneration. Labeling techniques to study descending cortical fibers of the spinal cord are well established using intracortical injections of biotin dextran amine (BDA). Veenman et al. reported the superior properties of BDA as an anterograde neuroanatomical tracer [1]. However, the labeling of ascending axons such as proprioceptive fibers in the dorsal columns is still problematic. Although BDA can be injected into a relatively discrete site in cortex where it is taken up by pyramidale neurons and anterogradely transported in corticospinal tract axons, this approach is not practical for ascending sensory axons that originate in numerous dorsal root ganglia [1], [2], [3]. Shi et al. reported that the injection of dextran amines can be applied to cut spinal cord tracts and the anterograde labeling of the axons can be observed [4]. Because the BDA tracer appears equally homogeneously distributed along the entire trajectories of fibers, a precise mapping of fiber tracts and the study of terminal projection patterns is possible. Furthermore, a quantitative estimation of the numbers and densities of labeled axon terminals can be attempted [5]. Because the combination of tracing and immunohistochemistry in a single experiment has become routine, Morecraft et al. could successfully demonstrate the injection of up to five anterograde tracers in the motor cortex of rhesus monkeys [6]. For the intracellular injection of presumed target neurons, two anterograde tracers can be combined, which allows the determination of multiple innervations of individual neurons [7].
The most problematic aspect of axonal marking techniques in regenerating axons is the establishment of primary axonal tracing methodologies. In this study, we are using a methodology to assess primary axonal regeneration in the nervous system.
Given these caveats, one must develop axonal tracing techniques to assess the reliable and consistent regenerative capacity of the nervous system [4], [5].
We describe a technique that may allow a quantitative and objective assessment of nerve regeneration. Given the difficulties of axonal regeneration, Fluoro-Ruby (FR) tracing may provide an important marking technique to establish the regeneration of neuronal circuits. Unlike conventional axonal tracers that have limited uptake capacity along the axonal trajectory, FR is taken up by the axon and provides an important tracer of the nervous system [8], [9]. This study aims to confirm the utility of FR as a useful axonal tracing element in regenerating nerve fibers.
Methods
Olfactory ensheathing cell (OEC) harvesting and isolation
Experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and the U.S. Veterans Affairs Connecticut Healthcare System Institutional Animal Care and Use Committee (IACUC) approved all animal protocols. Adult Wistar rats (250–400 g) were anesthetized and decapitated and the olfactory bulbs (OB) were removed. The meninges were carefully removed and the olfactory fiber and glomerular layers (ONGL) were dissected under microscopic control (Zeiss, OpMi-I) from the rest of the bulb. Obtained ONGL were enzymatically dissociated with collagenase A and D followed by mechanical dissociation. Cells were seeded on a poly-L-lysine (Sigma; average MW 25,000; 50 μg/mL in 15 mM sodium borate buffer, pH 8.4) pretreated flask at a density of 6.5×106 cells per flask. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (100 U/mL penicillin and 100 μg/mL streptomycin) and the culture medium was changed every 3 days.
Hemitransection of spinal cord
Adult female Wistar rats (250–280 g) were anesthetized by intramuscular injection with ketamine (15 mg/kg) and glycopyrule (0.0015 mg/kg). Under sterile conditions, the spinal cord was exposed at T9 followed by a laminectomy. The dorsal funiculus was transected [10] using an ophthalmic scalpel (P-715; Feather Safety Co., Osaka, Japan). The overlying muscles and skin were resutured and the animal was allowed to recover on a 37°C heating pad. Pain relief with buprenorphine (0.05 mg/kg/day SC) for 48 h and ibuprofen (0.15 mg/mL=5 mg/kg/day PO) for 72 h was provided to all animals.
Cell transplantation procedure for enhancing the promotion of axonal regeneration
Adult Wistar rats were prepared for surgery under sterile conditions and anesthetized by intramuscular injection with ketamine (15 mg/kg) and glycopyrule (0.0015 mg/kg). An incision was made in the skin overlying T6 to T8 vertebral levels and a laminectomy was performed at T7 or an incision was made in the skin overlying T8–T10 levels and a laminectomy was performed at T9. Three small openings (about 100 μm) separated by 2 mm were made in the dura. A sterile micropipette with a tip diameter of about 40 μm was mounted to a micromanipulator that was fixed to a stable base. Cell transplantation was performed immediately after hemitransection of the spinal cord. OECs were transplanted by direct injection proximal and distal to the lesion site into the dorsal column via the drawn glass micropipette. Typically, 1 μL of the cell suspension was injected at each of three depths (3.0×104 cells/μL, total cells injected ~2.5–3.0×105). For control lesions, 1 μL of the medium alone was injected into the lesion site.
FR injection and tissue processing
A micropipette with a tip diameter between 30 and 40 μm was filled with FR (MW 10,000, 5% dissolved in saline). The best results for spinal cord injections were found after a slow injection of 0.5 μL at a depth of 0.5 mm caudal from the lesion site 1 day before the sacrifice of the animals. After postoperative survival intervals of 21 days, rats were perfused with 0.1 M phosphate-buffered saline (PBS) and 4% paraformaldehyde. The tissue was dissected and postfixed at 4°C for 12 h and then incubated in sucrose (30% in PBS) overnight. The spinal cords were cut in 0.5 cm pieces, frozen in embedding medium, and cut at 30 μm using a cryostat.
Immunohistochemistry of transplanted OECs in the spinal cord
The animals were prepared for histological preparation between 3 and 5 weeks after transplantation. After intracardial perfusion with saline followed by 4% paraformaldehyde, the tissue was postfixed overnight. To identify the transplanted OECs, immunohistochemistry for p75NGFR expression was performed on 6 μm frozen sections from the perfusion-fixed rat spinal cords. Negative controls were performed on lesioned rat spinal cords without OEC transplantation. The sections were preblocked in normal blocking serum before incubation with primary anti-human p75NGFR monoclonal antibody (MAB365 EMD Millipore, clone 192-IgG, Chemicon) followed by incubation with a fluorescein isothiocyanate (FITC)-conjugated IgG secondary antibody. Photographs were taken on a Spot RT Color CCD.
Results
Marking of axonal fibers by FR
Regenerated fibers were seen throughout the lesion site. FR is a bidirectional tracer, but especially in the tracing of anterograde fibers remarkable results were found. FR is a highly sensitive tracer that can be used in small injection sites and the tracer is taken up by damaged fibers. No cytotoxic effect or massive invasion of macrophages as a sign of inflammation was observed.
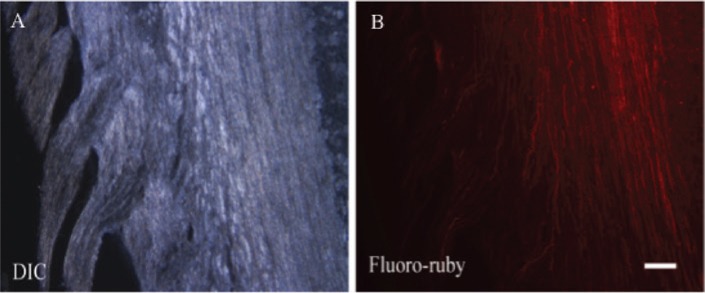
FR was used for in vivo as well as in vitro injections. The injection of FR in regenerated fibers allowed easy nerve identification and stained damaged and regenerated axons. It was possible to inject FR caudally from the injection site because it was taken up by the regenerated fibers and transported through the lesion site. To obtain information of marked axons, no additional processing of the tissue was necessary. Directly after removal of the spinal cord, the cord could be cut in frozen sections and observed under the microscope. After injection, single nerve fibers could be easily identified (Figure 1A and B).
Figure 1:
Image of longitudinal spinal cord section (A) with corresponding image demonstrating the labeling of axons after in vivo FR injection (B).
Scale bar in B=and pertains to A and B.
Absence of toxicity of FR and impact on tissue damage; stability of marking, absence of fading, and consistency of marking
There were no signs of toxicity and the injection of FR via microinjection with a Hamilton syringe did not result in additional damage of the tissue (Figure 2). The dye was transported through fast axonal transport and no additional lesion was induced.
Figure 2:
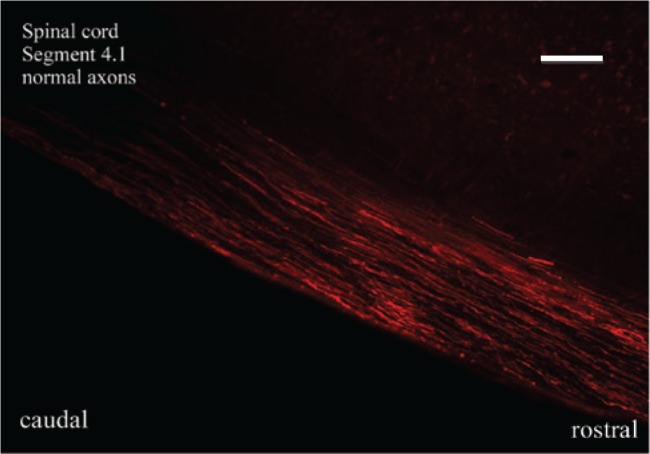
Distribution of FR after injection and labeling of ascending and descending axonal fibers with consistency of marking and without signs of toxicity or tissue damage.
Scale bar=40 μm.
On the microscopic level, no signs of macrophage invasion were observed. The injection of FR was performed for in vivo experiments 1 day before the sacrifice of the rat. On the following day, the animal was sacrificed and the spinal cord was removed. The animals showed no changes in their habits or any signs of intoxication. The injected FR was transported retrogradely and anterogradely. Twenty-four hours after in vivo injection, FR was fully absorbed by nerve fibers and no spreading or washout effects were observed.
In some animals, FR was injected after the removal of the semitransected spinal cord. The injection of FR was performed caudally from the transection site and the spinal cord was then kept alive in Hank’s balanced salt solution (HBSS) for 12 h. There was no difference in the quality of the marking of the regenerated fibers to the in vivo injections.
In case of FR injections in very low concentrations, the dye was only observed at the direct injection site caudally from the transection. The regenerated fibers were not reached by FR. In that case, the transection was not labeled. Following high concentrations of FR or large volumes, no fibers could be distinguished and FR was observed everywhere.
The marking of axons with FR was resistant to light and only minimal fading was observed. Strong axon signaling was observed on the slides; even after months being kept at –20°C, the obtained marked axons showed strong fluorescence.
Nerve fiber regeneration by OECs, OEC harvesting, capacity of axonal regeneration, and transplantation
Direct transplantation of OECs into the transected spinal cord resulted in the anatomical regeneration of the transected fibers 21 days postlesional. The distribution of the transplanted cells was examined in sagittal frozen sections of the spinal cord. The cells survived and were dispersed extensively within the transection site and more limitedly beyond the lesion site. The outer margins of the lesion showed reduced fluorescence, but there was high fluorescence at the core of the lesion. At the lesion site, scar formation was examined. All control animals demonstrated limited scar formation similar to that reported previously (reviewed in [11]). All rats that had received OEC transplantations showed regrowth of axons through the lesion site (Figure 3). Axons occupying the lesion site were both large and small in diameter. Sporadically sprouting axons were observed.
Figure 3:
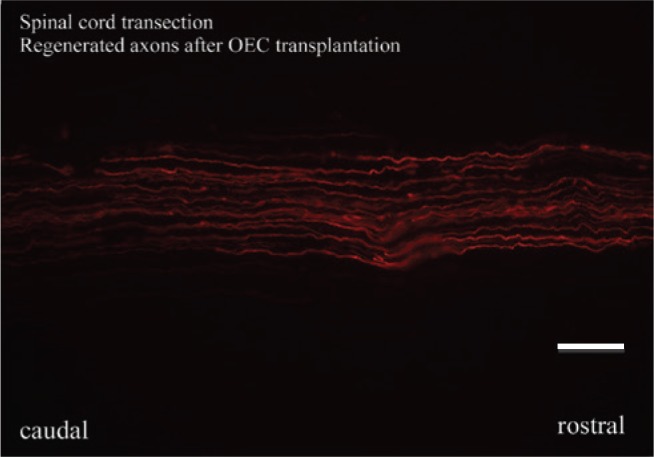
Visualization of individual regenerated fibers in the spinal cord after hemitransection and enhancement of axonal regeneration after OEC transplantation.
Scale bar=20 μm.
Discussion
In this study, dorsal column axons were visualized by a rhodamine-conjugated dextran amine (FR) that was applied to the dorsal columns. The spinal cord segments were removed and maintained in vitro in a slice chamber for 12 h. Ascending axons in the dorsal columns could be clearly visualized several millimeters from the injection site. In addition to the anterograde labeling of the ascending fibers, a retrograde labeling of dorsal root peripheral nerve fibers was observed. Moreover, after transection of the dorsal columns and transplantation of OECs, this technique allowed the identification of axons regenerating across the transection site. Thus, FR may provide an important tool to study the regenerating axons of the central and peripheral nervous systems. Given that this technique can be applied in vitro, it may provide a method to study axons derived from human tissue after biopsy.
A major problem in nerve surgery is the detection of regenerating axons during surgical intervention and the follow-up of regeneration. Thus far, no techniques are available to identify regenerating nerve fibers in vivo during surgical interventions. Histological staining methods (e.g. ChAT) require the surgical biopsy of the regenerating nerve leading to additional injury and causing minimal intraoperative time delay for detection [12], [13]. It was demonstrated that retrograde labeling with Fast Blue and FluoroGold produced strong labeling, but their visibility in the nuclei started with a delay of 2 days from injection and necessary adequate cell labeling was obtained 1 week after application. Labeling with Fast Blue persisted then for up to 8 weeks, whereas FluoroGold was not satisfactory at 8 weeks [14].
Intraoperative nerve conduction testing requires expensive technical equipment and expert technical assistance. Intraoperative muscle stimulation may not be detectable later than 6 weeks after injury. Thus, no further information may be gained by electrophysiological testing. An optimal tracing method for regenerating nerve fibers in peripheral nerves is easily available, easy to perform, fast, and nontoxic and does not cause additional injury to the nerve. FR staining has the potential to fulfill all these criteria. The presented technique with FR allows the immediate in vivo tracing of regenerating axons by simple injection into the peripheral nerve. Injection may be performed proximal or distal to the lesion, and the retrograde and anterograde transport of FR can be detected easily using fluorescent light. FR tracing across the lesion of a peripheral nerve shows the amount and location of regenerating nerve fibers.
In case of macroscopic neuroma formation or scarring, this may guide the surgical treatment strategy. Once regeneration is observed, the resection of the neuroma and nerve grafting or nerve transfer may be avoided, and epifascicular or intrafascicular neurolysis may be performed instead. Potential intraoperative use includes the revision and neurolysis of brachial plexus injury and the revision of peripheral nerve injuries.
FR tracing presents a simple and valuable tool to detect regenerating nerve fibers after peripheral nerve injury. The intraoperative use gives important information to guide further surgical management strategy. Thus, therapy may be tailored more individually to the anatomical situation, and extensive and technically complex procedures may be shortened or completely avoided.
The combination of surgery, cell transplantation strategies, and sensitive marking techniques provide powerful future and promising tools in the hand of the peripheral nerve surgeon in the quest for best outcome after nerve injury.
In this study, ascending dorsal column axons were visualized by a rhodamine-conjugated dextran amine (FR) that was applied to the dorsal columns. The spinal cord segments were removed and maintained in vitro in a slice chamber for 12 h. Ascending axons in the dorsal columns could be clearly visualized several millimeters from the injection site. In addition to the anterograde labeling of the ascending fibers, a retrograde labeling of dorsal root peripheral nerve fibers was observed. Moreover, after transection of the dorsal columns and transplantation of OECs, this technique allowed the identification of axons regenerating across the transection site. Thus, FR may provide an important tool to study regenerating axons of the central and peripheral nervous systems. Given that this technique can be applied in vitro, it may provide a method to study axons derived from human tissue after biopsy.
Supporting Information
Supplementary Material:
The article (DOI: iss-2016-0019) offers reviewer assessments as supplementary material.
Author Statement
Funding: This work was supported by the German Research Foundation (FOR 1103) to C.R. and the Veterans Healthcare Administration. Conflict of interest: Authors state no conflict of interest. Informed consent: Informed consent has been obtained from all individuals included in this study. Ethical approval: The research related to human use complies with all the relevant national regulations and institutional policies, was performed in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Author Contributions
Christine Radtke: Writing of the manuscript; Design of Study. Jeffery Kocsis: Design of the study. Wolfgang Baumgärtner: Approval of the manuscript. Peter M. Vogt: Approval of the manuscript.
Publication Funding
The German Society of Surgery funded the article processing charges of this article.
References
- [1].Veenman CL, Reiner A, Honig MG. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J Neurosci Methods 1992;41:239–254. [DOI] [PubMed]; Veenman CL, Reiner A, Honig MG. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J Neurosci Methods. 1992;41:239–254. doi: 10.1016/0165-0270(92)90089-v. [DOI] [PubMed] [Google Scholar]
- [2].Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods 2000;103:23–37. [DOI] [PubMed]; Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods. 2000;103:23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- [3].Lazarov NE. Neuroanatomical tract-tracing using biotinylated dextran amine. Methods Mol Biol 2013;1018:323–334. [DOI] [PubMed]; Lazarov NE. Neuroanatomical tract-tracing using biotinylated dextran amine. Methods Mol Biol. 2013;1018:323–334. doi: 10.1007/978-1-62703-444-9_30. [DOI] [PubMed] [Google Scholar]
- [4].Shi R, Borgens RB, Blight AR. Functional reconnection of severed mammalian spinal cord axons with polyethylene glycol. J Neurotrauma 1999;16:727–738. [DOI] [PubMed]; Shi R, Borgens RB, Blight AR. Functional reconnection of severed mammalian spinal cord axons with polyethylene glycol. J Neurotrauma. 1999;16:727–738. doi: 10.1089/neu.1999.16.727. [DOI] [PubMed] [Google Scholar]
- [5].Lanciego JL, Wouterlood FG. A half century of experimental neuroanatomical tracing. J Chem Neuroanat 2011;42:157–183. [DOI] [PubMed]; Lanciego JL, Wouterlood FG. A half century of experimental neuroanatomical tracing. J Chem Neuroanat. 2011;42:157–183. doi: 10.1016/j.jchemneu.2011.07.001. [DOI] [PubMed] [Google Scholar]
- [6].Morecraft RJ, Ugolini G, Lanciego JL, Wouterlood FG, Pandya DN. Classic and contemporary neural tract tracing techniques. In: Diffusion MRI. UK: Elsevier 2009:272–308.; Morecraft RJ, Ugolini G, Lanciego JL, Wouterlood FG, Pandya DN. Diffusion MRI. UK: Elsevier; 2009. Classic and contemporary neural tract tracing techniques; pp. 272–308. [Google Scholar]
- [7].Jorritsma-Byham B, Witter MP, Wouterlood FG. Combined anterograde tracing with biotinylated dextran-amine, retrograde tracing with Fast Blue and intracellular filling of neurones with Lucifer yellow: an electron microscopic method. J Neurosci Methods 1994;52:153–160. [DOI] [PubMed]; Jorritsma-Byham B, Witter MP, Wouterlood FG. Combined anterograde tracing with biotinylated dextran-amine, retrograde tracing with Fast Blue and intracellular filling of neurones with Lucifer yellow: an electron microscopic method. J Neurosci Methods. 1994;52:153–160. doi: 10.1016/0165-0270(94)90124-4. [DOI] [PubMed] [Google Scholar]
- [8].Schofield BR. Retrograde axonal tracing with fluorescent markers. Curr Protoc Neurosci. 2008; Chapter 1: Unit 1.17. [DOI] [PubMed]; Schofield BR. Retrograde axonal tracing with fluorescent markers. Curr Protoc Neurosci. 2008;(Chapter 1) doi: 10.1002/0471142301.ns0117s43. Unit 1.17. [DOI] [PubMed] [Google Scholar]
- [9].Lu J, Ashwell KW, Hayek R, Waite P. Fluororuby as a marker for detection of acute axonal injury in rat spinal cord. Brain Res 2001;915:118–123. [DOI] [PubMed]; Lu J, Ashwell KW, Hayek R, Waite P. Fluororuby as a marker for detection of acute axonal injury in rat spinal cord. Brain Res. 2001;915:118–123. doi: 10.1016/s0006-8993(01)02940-7. [DOI] [PubMed] [Google Scholar]
- [10].Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 1999;23:83–91. [DOI] [PubMed]; Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- [11].Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull 1999;49:377–391. [DOI] [PubMed]; Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- [12].Wainer BH, Rye DB. Retrograde horseradish peroxidase tracing combined with localization of choline acetyltransferase immunoreactivity. J Histochem Cytochem 1984;32:439–43. [DOI] [PubMed]; Wainer BH, Rye DB. Retrograde horseradish peroxidase tracing combined with localization of choline acetyltransferase immunoreactivity. J Histochem Cytochem. 1984;32:439–43. doi: 10.1177/32.4.6368680. [DOI] [PubMed] [Google Scholar]
- [13].Levey AI, Wainer BH, Mufson EJ, Mesulam MM. Co-localization of acetylcholinesterase and choline acetyltransferase in the rat cerebrum. Neuroscience 1983;9:9–22. [DOI] [PubMed]; Levey AI, Wainer BH, Mufson EJ, Mesulam MM. Co-localization of acetylcholinesterase and choline acetyltransferase in the rat cerebrum. Neuroscience. 1983;9:9–22. doi: 10.1016/0306-4522(83)90042-8. [DOI] [PubMed] [Google Scholar]
- [14].Choi D, Li D, Raisman G. Fluorescent retrograde neuronal tracers that label the rat facial nucleus: a comparison of Fast Blue, Fluoro-Ruby, Fluoro-Emerald, Fluoro-Gold and DiI. J Neurosci Methods 2002;117:167–172. [DOI] [PubMed]; Choi D, Li D, Raisman G. Fluorescent retrograde neuronal tracers that label the rat facial nucleus: a comparison of Fast Blue, Fluoro-Ruby, Fluoro-Emerald, Fluoro-Gold and DiI. J Neurosci Methods. 2002;117:167–172. doi: 10.1016/s0165-0270(02)00098-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.