Abstract
Liver surgery has become the standard treatment of primary liver cancer and liver metastases from colorectal cancer. Also, patients with non-colorectal liver metastases are increasingly offered surgery due to the low morbidity and excellent long-term results. The evolution of two-stage procedures helps to increase resectability. Also, laparoscopic and robotic liver surgery are constantly developed.
Keywords: cholangiocellular carcinoma, hepatocellular carcinoma, liver cancer, liver metastases, liver resection, liver surgery
Introduction
Liver surgery has become the standard treatment of primary and many secondary liver tumors over the past decades. Liver cancer is the most common primary liver tumor and the fifth most common human cancer worldwide [1]. Although most analyses do not differentiate between hepatocellular carcinoma (HCC) and cholangiocellular carcinoma (CCC), a very recent series from Sweden demonstrates that more than 80% of primary liver cancers are HCC and less than 20% are CCC [2]. As HCC is directly related to hepatitis B infection and liver cirrhosis, its incidence varies widely depending on the prevalence of these risk factors. For this reason, Asia accounts for about 70% of all HCC worldwide.
Metastatic colorectal cancer (CRC) is the third most common cancer death in men and women [3], and CRC liver metastases are the main indication for liver surgery in western countries. In particular for CRC metastases, a clear survival benefit for liver surgery has been demonstrated early [4]. Since then, liver resections have also been performed for metastases of other tumor entities, but the data are less strong. Currently, liver surgery for metastases can be performed with a low mortality (<3%) [5], [6], [7]. However, extended resections and resections in liver cirrhosis [e.g. for hepatocellular carcinoma (HCC)] harbor a higher mortality [7].
Due to the surgical progress of the recent 30 years, a large proportion of liver tumors are technically resectable, but the individual benefit for a patient has always to be balanced with the risk of the procedure. However, clear definitions of the technical resectability do not exist in the literature. In general, a tumor disease is technically resectable if a sufficient liver volume (>25%) with adequate arterial and portal-venous perfusion and venous drainage remains after surgery. However, patients with diseased livers, such as liver steatosis, cirrhosis, or long-term chemotherapy, require a larger volume of the remnant liver [8]. In the Celim study on the conversion of primarily unresectable to resectable CRC liver metastases, resectability varied widely even among these experts in liver surgery in a poststudy assessment: resectability varied significantly among surgeons (58% vs. 37%) as well as the definition of clear unresectability (20% vs. 49%) [9]. Similarly, the evaluation of resectability differs between general surgeons and liver surgeons, as crucial steps in extensive liver surgery often require particular expertise. In the absence of this expertise, a liver resection may be denied, although it would be offered by surgeons with the appropriate experience.
Increasing resectability by two-stage procedures
The liver has the unique potential to regenerate its volume after tissue loss. Similarly, the occlusion of a main branch of the portal vein induces a hypertrophy of the contralateral liver lobe. This phenomenon has been used increasingly over the past decades to increase resectability [10].
The evaluation of resectability should therefore include such two-step hepatectomy (TSH) procedures. In the absence of randomized trials, interventional portal vein embolization (PVE) and surgical portal vein ligation (PVL) as classical two-stage procedures appear equally effective in inducing hypertrophy of the contralateral lobe, although PVL was more effective in the mouse model [11], [12].
For multifocal tumor disease, the first step includes the atypical resection of tumors on one side (e.g. left lobe). Hypertrophy induction can be induced by PVL during step one or by PVE a few days later. The hypertrophy requires usually 3–4 weeks. However, the second step is often performed after 6 or more weeks, and this period is bridged by chemotherapy. During the second step, the lobe on the side of the occluded portal vein is resected by a hemihepatectomy. For unifocal disease with insufficient liver remnant, interventional PVE is performed first followed by surgery 4–6 weeks later (Figure 1). As this scenario mainly concerns primary liver cancer without effective systemic chemotherapy, the second step is often performed as early as a sufficient volume has been achieved radiologically.
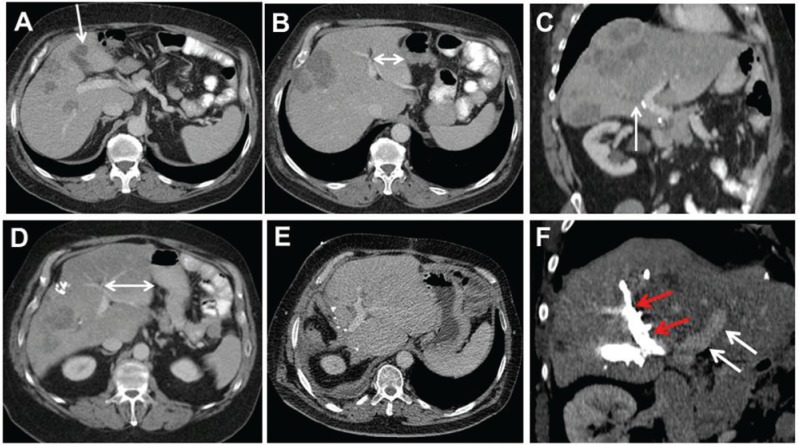
Figure 1:
A 61-year-old male patient presented with extensive metastatic disease to the liver from rectal cancer.
After 3 months of FOLFOX/bevazicumab treatment and sufficient downsizing of liver metastases, the patient underwent explorative laparotomy. Due to steatosis and preoperative chemotherapy, right hemihepatectomy with resection of segment 4b was considered too dangerous, and atypical resection of several metastases in the left liver (A, arrow) and ligation of the right portal vein was performed. Postoperatively, chemotherapy was continued. CT scan 5 months later demonstrates the hypertrophy of the left lateral sector (B and D, double arrows), and the TSH was completed by a formal right hemihepatectomy. Postoperative CT control 3 months later shows a tumor-free left liver. (E) Portal venous embolization (F) in another patient with CRC liver metastases achieves a similar amount of hypertrophy (white arrows, left portal vein; red arrows, embolized right branches).
The fastest hypertrophy of the future remnant liver results from the Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS). During the first step of surgery, the portal vein branches are divided (e.g. right portal vein and segment 4 branches) and the parenchymal dissection is completed. The hepatic artery and venous drainage remain patent. Seven to 10 days later (in selected cases also more), the resection is completed [13] (Figure 2). Both surgical approaches (PVL and ALPPS) offer the advantage of resecting additional metastases in the future remnant liver during the first step in contrast to PVE, which is particularly helpful in large or solitary tumors.
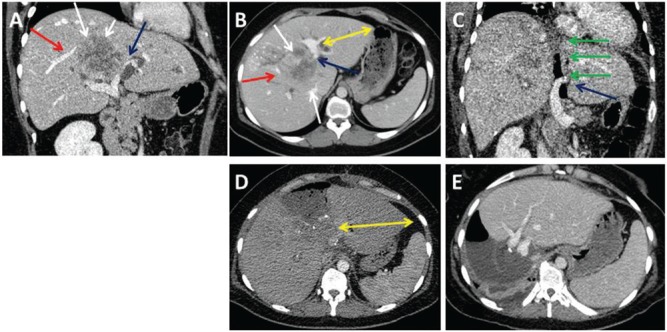
Figure 2:
A 32-year-old female patient with intrahepatic CCC.
The tumor (A and B, white arrows) had contact with a major branch of the right hepatic (A and B, red arrows) and left portal vein (A and B, blue arrows) and caused bilateral cholestasis. The ALPPS procedure was performed for curative resection. CT on POD1 after the first step demonstrates the patent left portal vein (C, blue arrow) and the dissection line along the falciforme ligament (C, green arrows). Due to the infiltration of the hilar bifurcation, the left bile duct was resected and a hepaticojejunostomy was performed for reconstruction. CT control on POD7 after the first step reveals sufficient hypertrophy of the left lateral sector (D, yellow arrows). The completion of ALPPS was done on POD7. CT 7 days after ALPPS completion displays hypertrophy of the left lateral lobe (E). Final histology confirmed a T3, N0 (0/3), G2, R0 intrahepatic CCC (∅7.5 cm).
The major disadvantage of TSH is the potential effect of liver regeneration on the tumor growth in the future liver remnant [14]. In the classical TSH concepts, about 25% of the patients do not undergo the second step due to tumor progression [15]. Those who complete both steps of the staged hepatectomy, however, have a median survival comparable to patients with primarily resectable liver metastases [16]. In contrast, nearly all patients undergo both steps of the ALPPS procedure, but a significant proportion develops early tumor recurrence [17]. Due to these observations, the indications for the ALPPS are controversial in the literature, and patients need to be carefully selected for two-stage concepts.
Surgery for primary liver cancer
The most frequent primary liver tumors are cholangiocellular carcinoma (CCC) and HCC. In contrast to CRC metastases, primary liver cancer often infiltrates major vessels or surrounding tissues and organs. Consequently, resection/reconstruction of the inferior vena cava or hepatic veins as well as portal vein resections may complicate the resection of such tumors. Whereas HCC is mostly associated with diseased livers, CCC predominantly occurs in healthy liver parenchyma. Surgery is the only treatment option with curative intent for both entities, as chemotherapy has only limited efficacy.
Cholangiocarcinoma
Cholangiocarcinoma can arise from all parts of the extrahepatic bile ducts or from intrahepatic cholangiocytes. At diagnosis, most intrahepatic CCC are large and diagnosed due to symptoms related to their size. As mentioned above, CCC tend to infiltrate surrounding organs (e.g. duodenum or diaphragm) and major vessels, and hilar lymph node metastases are frequent. Unfortunately, multifocal intrahepatic disease and metastases to peritoneum, lungs, or bone often preclude surgery due to the limited prognosis and little remnant liver volume.
Due to this aggressive biology, the resection of intrahepatic CCC often requires major or even extended liver and multivisceral resections to achieve complete tumor clearance. Due to the high incidence of hilar lymph node metastases, a hilar lymphadenectomy is standard during liver surgery for CCC (Figure 3).
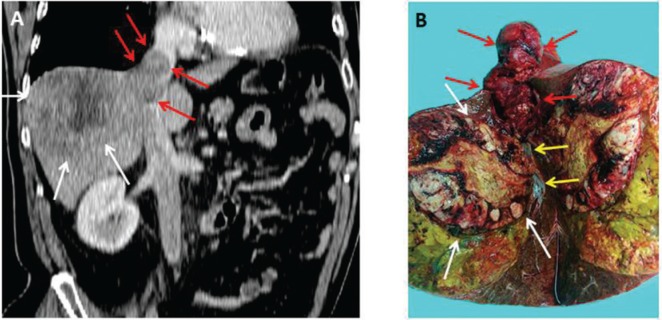
Figure 3:
A 75-year-old male patient with HCC in noncirrhotic liver presented with right upper abdominal pain.
CT scan depicted the large tumor in the right liver with infiltration of the right hepatic vein and consequent tumor thrombus extending into the right atrium of the heart (A). The resected specimen confirmed the tumor thrombus extending into the right hepatic vein (white arrows, tumor; red arrows, thrombus; yellow arrows, healthy right hepatic vein).
In the own experience of 102 liver resections for intrahepatic CCC, 50% of the patients required extended liver resections, 27.4% had additional vascular resections, and 34.3% required additional visceral extensions to achieve complete tumor clearance. By such aggressive surgical policy, more than 85% of the patients underwent R0 resections. The median survival was 22.9%. Moreover, we observed a 20% 5-year survival even after R1 resections, which would most likely not have been reached by palliative chemotherapy.
HCC
Due to the structured surveillance of patients with liver cirrhosis, HCC are usually diagnosed as small tumors but tend to be multifocal. However, more than 20% of HCC arise from healthy livers and then reach similar sizes to CCC and also infiltrate surrounding vasculature with the tendency to form tumor thrombi [18]. In contrast to CCC, lymph node metastases are infrequent in HCC. Consequently, a routine lymphadenectomy is not performed by most centers for the treatment of HCC.
Thus, surgery for HCC in noncirrhotic livers often requires extensive liver resections with venous reconstructions like for CCC (Figure 4). Due to the predominant association with liver cirrhosis, the treatment of HCC is, however, most frequently, complicated by the underlying liver disease, which limits the extent of liver surgery due to the limited liver function and increases perioperative morbidity and mortality.
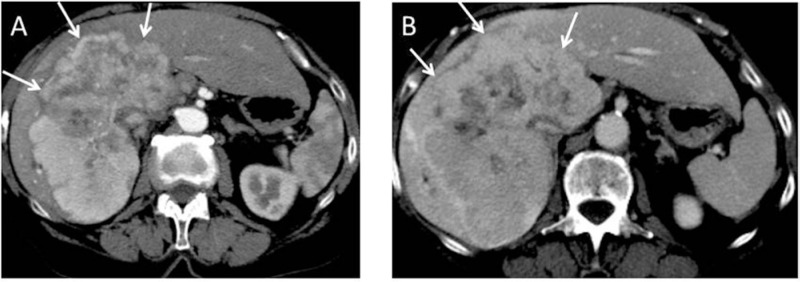
Figure 4:
A 79-year-old female patient with intrahepatic CCC.
An extended right hemihepatectomy was performed. Final histology revealed a T2b, N0 (0/5), M0, L0, V1, R0, G2 intrahepatic CCC (∅12 cm).
Patient selection for liver surgery in liver cirrhosis is crucial. In general, liver surgery in decompensated liver cirrhosis is contraindicated. In addition, liver cirrhosis is often complicated by portal hypertension irrespective of liver function, which further increases morbidity and mortality. On the contrary, many patients in Child A stage may even tolerate a hemihepatectomy. Indocyanine green retention rate and Limax test may help selecting patients with impaired liver function for surgery, but reproducible cutoff levels for the indication for surgery in liver cirrhosis have not been validated, yet [19], [20], [21].
For HCC in cirrhotic livers, orthotopic liver transplantation (OLT) is the most attractive treatment alternative, as OLT is a curative treatment for the underlying liver disease, portal hypertension, and HCC. However, current regulations only provide the prioritization by standard exceptions for HCC within the Milan criteria (unifocal HCC up to 5 cm, maximum of three HCC up to 3 cm each) [22].
Liver surgery achieves a 5-year survival of about 50%. A recent analysis demonstrates that recurrence-free survival and overall survival are significantly superior after an uncomplicated perioperative course. Moreover, underlying liver disease, the extent of the tumor and portal hypertension are associated with surgical complications and long-term outcome [23].
The 5-year survival of patients undergoing OLT for HCC within the Milan criteria reaches 75% with a 5-year recurrence-free survival of 95%. Because patients outside these Milan criteria may achieve a 5-year survival of >50% despite the underlying liver disease, OLT should always be considered also for this cohort of patients. The major determinant of outcome after OLT appears to be a microvascular invasion of the HCC, which is, however, unassessable preoperatively [24].
Liver metastases
In contrast to primary liver cancer, liver metastases reflect a systemic disease, which should be considered in every patient before liver surgery. Due to the advances in chemotherapy for most gastrointestinal and some extraintestinal cancers, local treatments become increasingly attractive either with curative intent or to spare systemic chemotherapy for a certain period. Traditionally, liver metastases are divided in three groups reflecting differences in biology and prognosis: colorectal liver metastases, metastases from endocrine tumors, and nonendocrine/noncolorectal metastases.
Colorectal liver metastases
CRC predominantly metastasizes to the liver and the lungs. Due to this pattern of metastasis, liver surgery has been used for the treatment of metastases from CRC for decades. Due to the early results of surgery, liver resection has become the standard treatment for CRC liver metastases.
The median cancer-specific survival after liver resection for colorectal liver metastases overall is 42.5 months [5].
Multivariate analyses have identified high carcinoembryonic antigen (CEA) levels, short interval between primary tumor and diagnosis, number and size of liver metastases, and N+ stage as negative predictors of survival after liver resection for CRC liver metastases. Scoring systems based on these clinical risk factors can stratify patients preoperatively in groups regarding prognosis [5], [25], [26]. These scores predict the survival of patients with CRC liver metastases well: whereas more than 50% of the patients with up to 5 points survive 5 years, less than 10% survive in a high-risk situation with more than 20 points [5].
Although incomplete (R1) resections are strong risk factors for tumor recurrence, a minimal safety margin has not been defined and the minimal required resection margin is under debate: whereas some centers demonstrated significantly better survival by achieving larger resection margins, others reported no difference in outcome if the resection margin is below 1 cm or even only a few millimeters [27]. In selected cases, even R1 resections may have a role in the surgical treatment of CRC metastases with comparable long-term outcome to R0 resections [28].
Although not proven by the literature, most centers will primarily treat patients with high-risk constellations using neoadjuvant chemotherapy and recommend liver resection in case of at least stable disease after 3–4 months even for primarily resectable metastases. Due to the efficacy of systemic chemotherapy for CRC, primarily unresectable metastases can be converted to resectable with comparable outcome, and several series demonstrate the benefit of liver resection for this group of patients.
Effective chemotherapy can also result in complete radiological response – a clinical dilemma – as 25%–40% of these diminished metastases still contain viable tumor cells [29], [30]. Consequently, most authors recommend the resection of these respective areas.
On the contrary, few patients do not respond to chemotherapy. In patients with unresectable liver metastases, this scenario is detrimental or requires local alternatives. Patients with technically resectable but unresponsive metastases also benefit from liver resection if complete tumor clearance is possible. However, progressive disease to chemotherapy indicates unfavorable long-term outcome [31]. Therefore, liver resection should be considered very carefully in progressive disease.
Nonneuroendocrine/noncolorectal liver metastases
Due to the different patterns of metastasis, surgery has not been considered for noncolorectal liver metastases for a long time. However, local therapies and surgery are increasingly used also for this subgroup of liver metastases. In general, a comparable outcome can be achieved for nonendocrine/noncolorectal metastases as for CRC liver metastases by adequate patient selection.
The Adam et al. score is based on more than 1000 patients and stratifies for three prognostic groups, which are based on the tumor entity, patient age, interval from the diagnosis of the primary tumor, presence of extrahepatic metastases, necessity of a major hepatectomy, and probability of an R2 resection. In the multivariate analysis, the resection of breast cancer liver metastases was associated with the best survival, whereas the resection of melanoma and squamous cell cancer liver metastases revealed the worst survival. All other tumor entities, in particular from intestinal cancers, were associated with a favorable outcome. By appointing each risk factor 0–3 points, the individual benefit from liver surgery can be presumed preoperatively: the 5-year survival is more than 30% in patients with 0–3 risk points, 10%–30% in patients with 4–6 risk points, and less than 10% in patients with 7–10 risk points. This analysis demonstrates that a certain proportion of patients with noncolorectal benefits as much as patients with CRC metastases from liver surgery.
Consequently, oncological concepts for synchronous and metachronous liver metastases are constantly shifting during recent years, and patients with upper gastrointestinal or pancreatic cancer with limited liver metastases are increasingly offered treatment protocols based on liver surgery in selected cases [32], [33].
Laparoscopic liver surgery
Following the general trend to less invasiveness in visceral surgery, laparoscopic liver surgery has been developed over the past 15 years. In addition to the general advantages such as better cosmesis and less pain, specific advantages of laparoscopic liver surgery are shorter hospital stay and less blood loss. Moreover, several series suggest less hepatic decompensations after laparoscopic surgery than after open surgery in patients with liver cirrhosis [34], [35], [36]. Laparoscopic liver surgery is particularly feasible in superficial metastases in the anterior segments but is increasingly used also for major resections such as hemihepatectomy. Also, tumors in the dorsal segments can be resected in left lateral position.
In patients with colorectal liver metastases, systemic chemotherapy can be initiated earlier after laparoscopic surgery than after open liver surgery, which might also relate to better long-term outcome [36]. Since the establishment of laparoscopic colorectal surgery, laparoscopic liver surgery may also offer advantages in patients with synchronous liver metastases from CRC [37].
As pointed out above, laparoscopic surgery seems to be beneficial for liver function. Several case series suggest less hepatic decompensations after resection of HCC in liver cirrhosis. However, these data from laparoscopic liver surgery are generated from retrospective case series and require confirmation by larger cohorts and randomized trials.
Future developments
Recently, robotic surgery has been implemented in liver surgery. This technology provides the advantages of 3D imaging and 7 degrees of freedom of the human hand, which would be particularly beneficial in apical and dorsal segment resections. Valid data on this technology are missing, but the currently available literature suggests higher blood loss and longer operating time for robotic surgery. In addition, the cost of this technology still outrages those of laparoscopic surgery [38], [39], [40]. It is anticipated, however, that future developments in robotic surgery will overcome these shortcomings of robotic technology.
In particular for laparoscopic liver surgery, intraoperative 3D navigation is very attractive. This technology helps the intraoperative orientation, as the resection is simulated in the actual computed tomography (CT) images. The relation to major vessels can be anticipated online, and even diminished liver metastases can be safely resected with the parenchyma sparing technique [40].
Conclusion
Liver surgery is the standard treatment for primary liver tumors, as systemic treatment remains ineffective, and the resection of HCC and CCC results in excellent long-term survival. Due to the low morbidity and mortality, liver surgery has also become a cornerstone in the treatment of liver metastases from various primary tumors. As for most gastrointestinal primaries, laparoscopic liver surgery is increasingly used and will become the standard of care for primary and secondary liver tumors.
Supporting Information
Supplementary Material:
The article (DOI: iss-2017-0009) offers reviewer assessments as supplementary material.
Author Statement
Funding: Authors state no funding involved. Conflict of interest: Authors state no conflict of interest. Informed consent: Informed consent is not applicable. Ethical approval: The conducted research is not related to either human or animals use.
Publication Funding
The German Society of Surgery funded the article processing charges of this article.
Author Contributions
Hauke Lang: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – original draft. Stefan Heinrich: Conceptualization, Data curation, Formal analysis, Validation, Visualization, Writing – original draft, Writing – review and editing.
References
- [1].Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed]; Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- [2].Torner A, Stokkeland K, Svensson Å, et al. The underreporting of hepatocellular carcinoma to the cancer register and a log-linear model to estimate a more correct incidence. Hepatology 2016;65:885–892. [DOI] [PubMed]; Torner A, Stokkeland K, Svensson Å. et al. The underreporting of hepatocellular carcinoma to the cancer register and a log-linear model to estimate a more correct incidence. Hepatology. 2016;65:885–892. doi: 10.1002/hep.28775. [DOI] [PubMed] [Google Scholar]
- [3].Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. CA Cancer J Clin 2012;62:10–29. [DOI] [PubMed]; Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- [4].Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet 1994;343:1405–1410. [DOI] [PubMed]; Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–1410. doi: 10.1016/s0140-6736(94)92529-1. [DOI] [PubMed] [Google Scholar]
- [5].Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125–135. [DOI] [PubMed]; Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- [6].Breitenstein S, DeOliveira ML, Raptis DA, et al. Novel and simple preoperative score predicting complications after liver resection in noncirrhotic patients. Ann Surg 2010;252:726–734. [DOI] [PubMed]; Breitenstein S, DeOliveira ML, Raptis DA. et al. Novel and simple preoperative score predicting complications after liver resection in noncirrhotic patients. Ann Surg. 2010;252:726–734. doi: 10.1097/SLA.0b013e3181fb8c1a. [DOI] [PubMed] [Google Scholar]
- [7].Aloia TA, Fahy BN, Fischer CP, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxf) 2009;11:510–515. [DOI] [PMC free article] [PubMed]; Aloia TA, Fahy BN, Fischer CP. et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxf) 2009;11:510–515. doi: 10.1111/j.1477-2574.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med 2007;356:1545–1559. [DOI] [PubMed]; Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- [9].Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 2010;11:38–47. [DOI] [PubMed]; Folprecht G, Gruenberger T, Bechstein WO. et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- [10].Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990;107:521–527. [PubMed]; Makuuchi M, Thai BL, Takayasu K. et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- [11].Furrer K, Tian Y, Pfammatter T, et al. Selective portal vein embolization and ligation trigger different regenerative responses in the rat liver. Hepatology 2008;47:1615–1623. [DOI] [PubMed]; Furrer K, Tian Y, Pfammatter T. et al. Selective portal vein embolization and ligation trigger different regenerative responses in the rat liver. Hepatology. 2008;47:1615–1623. doi: 10.1002/hep.22164. [DOI] [PubMed] [Google Scholar]
- [12].Aussilhou B, Lesurtel M, Sauvanet A, et al. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg 2008;12:297–303. [DOI] [PubMed]; Aussilhou B, Lesurtel M, Sauvanet A. et al. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg. 2008;12:297–303. doi: 10.1007/s11605-007-0410-x. [DOI] [PubMed] [Google Scholar]
- [13].Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405–414. [DOI] [PubMed]; Schnitzbauer AA, Lang SA, Goessmann H. et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- [14].Heinrich S, Jochum W, Graf R, Clavien PA. Portal vein ligation and partial hepatectomy differentially influence growth of intrahepatic metastasis and liver regeneration in mice. J Hepatol 2006;45:35–42. [DOI] [PubMed]; Heinrich S, Jochum W, Graf R, Clavien PA. Portal vein ligation and partial hepatectomy differentially influence growth of intrahepatic metastasis and liver regeneration in mice. J Hepatol. 2006;45:35–42. doi: 10.1016/j.jhep.2006.02.020. [DOI] [PubMed] [Google Scholar]
- [15].Brouquet A, Abdalla EK, Kopetz S, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 2011;29:1083–1090. [DOI] [PMC free article] [PubMed]; Brouquet A, Abdalla EK, Kopetz S. et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083–1090. doi: 10.1200/JCO.2010.32.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Heinrich S, Lang H. Liver metastases from colorectal cancer: technique of liver resection. J Surg Oncol 2013;107:579–584. [DOI] [PubMed]; Heinrich S, Lang H. Liver metastases from colorectal cancer: technique of liver resection. J Surg Oncol. 2013;107:579–584. doi: 10.1002/jso.23138. [DOI] [PubMed] [Google Scholar]
- [17].Adam R, Imai K, Castro Benitez C, et al. Outcome after associating liver partition and portal vein ligation for staged hepatectomy and conventional two-stage hepatectomy for colorectal liver metastases. Br J Surg 2016;103:1521–1529. [DOI] [PubMed]; Adam R, Imai K, Castro Benitez C. et al. Outcome after associating liver partition and portal vein ligation for staged hepatectomy and conventional two-stage hepatectomy for colorectal liver metastases. Br J Surg. 2016;103:1521–1529. doi: 10.1002/bjs.10256. [DOI] [PubMed] [Google Scholar]
- [18].Kaczynski J, Hansson G, Wallerstedt S. Diabetes: one of few remarkable differences in clinicopathologic features between cirrhotic and noncirrhotic Swedes with hepatocellular carcinoma. Dig Dis Sci 2006;51:796–802. [DOI] [PubMed]; Kaczynski J, Hansson G, Wallerstedt S. Diabetes: one of few remarkable differences in clinicopathologic features between cirrhotic and noncirrhotic Swedes with hepatocellular carcinoma. Dig Dis Sci. 2006;51:796–802. doi: 10.1007/s10620-006-3209-9. [DOI] [PubMed] [Google Scholar]
- [19].Jara M, Reese T, Malinowski M, et al. Reductions in post-hepatectomy liver failure and related mortality after implementation of the LiMAx algorithm in preoperative work-up: a single-centre analysis of 1170 hepatectomies of one or more segments. HPB (Oxf) 2015;17:651–658. [DOI] [PMC free article] [PubMed]; Jara M, Reese T, Malinowski M. et al. Reductions in post-hepatectomy liver failure and related mortality after implementation of the LiMAx algorithm in preoperative work-up: a single-centre analysis of 1170 hepatectomies of one or more segments. HPB (Oxf) 2015;17:651–658. doi: 10.1111/hpb.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Imamura H, Sano K, Sugawara Y, et al. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobil Pancreat Surg 2005;12:16–22. [DOI] [PubMed]; Imamura H, Sano K, Sugawara Y. et al. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobil Pancreat Surg. 2005;12:16–22. doi: 10.1007/s00534-004-0965-9. [DOI] [PubMed] [Google Scholar]
- [21].Malinowski M, Jara M, Lüttgert K, et al. Enzymatic liver function capacity correlates with disease severity of patients with liver cirrhosis: a study with the LiMAx test. Dig Dis Sci 2014;59:2983–2991. [DOI] [PubMed]; Malinowski M, Jara M, Lüttgert K. et al. Enzymatic liver function capacity correlates with disease severity of patients with liver cirrhosis: a study with the LiMAx test. Dig Dis Sci. 2014;59:2983–2991. doi: 10.1007/s10620-014-3250-z. [DOI] [PubMed] [Google Scholar]
- [22].Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–699. [DOI] [PubMed]; Mazzaferro V, Regalia E, Doci R. et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- [23].Doussot A, Lim C, Lahat E, et al. Complications after hepatectomy for hepatocellular carcinoma independently shorten survival: a western, single-center audit. Ann Surg Oncol 2017 [Epub ahead of print]. doi:10.1245/s10434-016-5746-6. [DOI] [PubMed]; Doussot A, Lim C, Lahat E. et al. Complications after hepatectomy for hepatocellular carcinoma independently shorten survival: a western, single-center audit. Ann Surg Oncol. 2017 doi: 10.1245/s10434-016-5746-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [24].Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35–43. [DOI] [PubMed]; Mazzaferro V, Llovet JM, Miceli R. et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- [25].Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309–318; discussion 318–321. [DOI] [PMC free article] [PubMed]; Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 1996;77:1254–1262. [PubMed]; Nordlinger B, Guiguet M, Vaillant JC. et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- [27].Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg 2011;253:656–665. [DOI] [PubMed]; Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg. 2011;253:656–665. doi: 10.1097/SLA.0b013e3181fc08ca. [DOI] [PubMed] [Google Scholar]
- [28].de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg 2008;248:626–637. [DOI] [PubMed]; de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248:626–637. doi: 10.1097/SLA.0b013e31818a07f1. [DOI] [PubMed] [Google Scholar]
- [29].Ferrero A, Langella S, Russolillo N, Vigano’ L, Lo Tesoriere R, Capussotti L. Intraoperative detection of disappearing colorectal liver metastases as a predictor of residual disease. J Gastrointest Surg 2012;16:806–814. [DOI] [PubMed]; Ferrero A, Langella S, Russolillo N, Vigano’ L, Lo Tesoriere R, Capussotti L. Intraoperative detection of disappearing colorectal liver metastases as a predictor of residual disease. J Gastrointest Surg. 2012;16:806–814. doi: 10.1007/s11605-011-1810-5. [DOI] [PubMed] [Google Scholar]
- [30].Bischof DA, Clary BM, Maithel SK, Pawlik TM. Surgical management of disappearing colorectal liver metastases. Br J Surg 2013;100:1414–1420. [DOI] [PubMed]; Bischof DA, Clary BM, Maithel SK, Pawlik TM. Surgical management of disappearing colorectal liver metastases. Br J Surg. 2013;100:1414–1420. doi: 10.1002/bjs.9213. [DOI] [PubMed] [Google Scholar]
- [31].Adam R, Pascal G, Castaing D, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg 2004;240:1052–1061; discussion 1061–1064. [DOI] [PMC free article] [PubMed]; Adam R, Pascal G, Castaing D. et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg 2004;240:1052–1061; discussion. :1061–1064. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Markar SR, Mikhail S, Malietzis G, et al. Influence of surgical resection of hepatic metastases from gastric adenocarcinoma on long-term survival: systematic review and pooled analysis. Ann Surg 2016;263:1092–1101. [DOI] [PubMed]; Markar SR, Mikhail S, Malietzis G. et al. Influence of surgical resection of hepatic metastases from gastric adenocarcinoma on long-term survival: systematic review and pooled analysis. Ann Surg. 2016;263:1092–1101. doi: 10.1097/SLA.0000000000001542. [DOI] [PubMed] [Google Scholar]
- [33].Crippa S, Bittoni A, Sebastiani E, et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur J Surg Oncol 2016;42:1533–1539. [DOI] [PubMed]; Crippa S, Bittoni A, Sebastiani E. et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur J Surg Oncol. 2016;42:1533–1539. doi: 10.1016/j.ejso.2016.06.398. [DOI] [PubMed] [Google Scholar]
- [34].Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831–841. [DOI] [PubMed]; Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- [35].Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol 2015;63:643–650. [DOI] [PubMed]; Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol. 2015;63:643–650. doi: 10.1016/j.jhep.2015.04.005. [DOI] [PubMed] [Google Scholar]
- [36].Tohme S, Goswami J, Han K, et al. Minimally invasive resection of colorectal cancer liver metastases leads to an earlier initiation of chemotherapy compared to open surgery. J Gastrointest Surg 2015;19:2199–2206. [DOI] [PMC free article] [PubMed]; Tohme S, Goswami J, Han K. et al. Minimally invasive resection of colorectal cancer liver metastases leads to an earlier initiation of chemotherapy compared to open surgery. J Gastrointest Surg. 2015;19:2199–2206. doi: 10.1007/s11605-015-2962-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lupinacci RM, Andraus W, De Paiva Haddad LB, Carneiro D’ Albuquerque LA, Herman P. Simultaneous laparoscopic resection of primary colorectal cancer and associated liver metastases: a systematic review. Tech Coloproctol 2014;18:129–135. [DOI] [PubMed]; Lupinacci RM, Andraus W, De Paiva Haddad LB, Carneiro D’ Albuquerque LA, Herman P. Simultaneous laparoscopic resection of primary colorectal cancer and associated liver metastases: a systematic review. Tech Coloproctol. 2014;18:129–135. doi: 10.1007/s10151-013-1072-1. [DOI] [PubMed] [Google Scholar]
- [38].Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 2014;259:549–555. [DOI] [PubMed]; Tsung A, Geller DA, Sukato DC. et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg. 2014;259:549–555. doi: 10.1097/SLA.0000000000000250. [DOI] [PubMed] [Google Scholar]
- [39].Dehlawi A, Memeo R, De Blasi V, et al. Robotic hepatectomies: advances and perspectives. Minerva Chir 2016;71:407–414. [PubMed]; Dehlawi A, Memeo R, De Blasi V. et al. Robotic hepatectomies: advances and perspectives. Minerva Chir. 2016;71:407–414. [PubMed] [Google Scholar]
- [40].Huber T, Baumgart J, Peterhans M, Weber S, Heinrich S, Lang H. Computer-assisted 3D-navigated laparoscopic resection of a vanished colorectal liver metastasis after chemotherapy. Z Gastroenterol 2016;54:40–43. [DOI] [PubMed]; Huber T, Baumgart J, Peterhans M, Weber S, Heinrich S, Lang H. Computer-assisted 3D-navigated laparoscopic resection of a vanished colorectal liver metastasis after chemotherapy. Z Gastroenterol. 2016;54:40–43. doi: 10.1055/s-0041-107542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.