Abstract
Many patients with colorectal cancer are overweight. Even then, nutritional status is a frequently underestimated risk factor for perioperative complications. Enhanced Recovery after Surgery is the goal for perioperative management, and preoperative nutritional risk screening should be a standard. In case of nutritional risk, perioperative nutrition therapy should be started without delay and should follow recent guideline recommendations. The preservation of the microbiome has an emerging role in preventing postoperative anastomotic leakage and septic complications. The time window for recovery after neoadjuvant treatment for rectal cancer may be used for conditioning appropriate-risk patients in a “prehabilitation” program. In order to assess metabolic recovery and the prognosis for long-term survival, C-reactive protein/albumin ratio may be a promising parameter, which has to be validated in the future. This narrative review summarizes recent strategies and guideline recommendations.
Keywords: colorectal cancer, conditioning, Enhanced Recovery after Surgery (ERAS), immunonutrition, microbiome, nutrition therapy, prehabilitation
Introduction
For the development of colorectal cancer, obesity has been identified as a highly significant risk factor in a very recent “umbrella” review of systematic reviews and meta-analyses [1]. Although the precise mechanisms have not been elucidated yet, an interaction between diet, microbiota, mucosal immunity, and inflammation is considered to be critical for the promotion of colorectal cancer. The inflammatory potential of the diet may have an influence in compromising the integrity of the intestinal epithelial barrier [2]. Obesity with the intake of a high-fat, low-fiber diet may induce microbiota changes with a shift in the bacterial diversity and act as a possible initiator for carcinogenesis [3]. In comparison with matched controls, strong microbe-metabolite correlations were found in colorectal cancer cases, predominated by Proteobacteria and Actinobacteria [4].
In colorectal cancer patients, considerable weight loss is less common than in patients with upper gastrointestinal or pancreatic malignancy. More patients with colorectal cancer undergoing surgery are overweight or obese than undernourished [1], [5]. A higher body mass index (BMI) is associated with worse postoperative outcome in laparoscopic colorectal surgery [6]. A history of weight loss and clinical signs of malnutrition at the time of diagnosis may always be an indicator for advanced and metastasized cancer. However, during cancer treatment, nutritional deficiencies may stepwise develop with special regard to those patients undergoing neoadjuvant therapy. Therefore, a nutritional goal is the prevention of deterioration of nutritional status.
In order to achieve Enhanced Recovery after Surgery (ERAS), multimodality programs including perioperative care pathways have proven clinical effectiveness [7], [8]. Early oral food intake is favored, and perioperative nutrition therapy seems to be very “traditional” or even redundant. However, ERAS, which was developed for colorectal surgery, is a metabolic concept with a bundle of treatment modalities including nutrition as well. The program also has proven benefits in special-risk groups, such as the elderly [9], [10].
This narrative review focuses on nutritional status and the microbiome, and their impact on postoperative outcome in patients with colorectal cancer. A search strategy for literature in PubMed included recent trials, meta-analysis, and reviews using the key words colorectal cancer and enhanced recovery after surgery (ERAS), nutrition therapy, conditioning, microbiome, bowel preparation, prehabilitation, and immunonutrition.
Impact of nutritional status
From a metabolic point of view, restricted functional capacity and impaired nutritional status bear a special risk for postoperative complications. Functional limitations and care dependency have been proven to be significantly associated with postoperative morbidity and mortality [11]. Next to age-related comorbidity, functional status is determined by muscle mass, which is the key component of nutritional status.
In colorectal cancer, nutritional deficiencies are more frequently related to functional dependency than to the cancer itself, and in the latter associated with advanced tumor stage. Even in overweight and obese patients, impaired body composition may occur. Deficiency in muscle mass – so called sarcopenia – has a considerable impact on postoperative recovery regarding early mobilization and respiratory function. This has been impressively shown for patients with gastric cancer [12].
Recognizing and conditioning the surgical patient at risk is a classical and timeless issue in surgery with special regard to the increasing number of elderly patients with functional dependence. While it is a great challenge to offer cancer resection intended for cure in the elderly with considerable comorbidity, for 40 years, the high risk to neglect nutritional status has not changed [13].
To avoid postoperative complications and achieve the goal of long-term survival with a good quality of life, the perioperative period may be “exploited” for the improvement of functional and nutritional status [14]. Nevertheless, regarding short- and long-term mortality, there is still limited evidence for nutrition therapy in a recent Cochrane analysis [15].
Recent guidelines for clinical nutrition
The recent evidence has been updated in 2017 in the guidelines of the European Society for Clinical Nutrition and Metabolism (ESPEN) for Surgery and Oncology (available under www.espen.org and published in Ref. [16], copyright held by ESPEN).
Principles of nutritional care
From a metabolic and nutritional point of view, the key aspects of perioperative care include the following (Ref. [16], with kind permission from ESPEN):
Integration of nutrition into the overall management of the patient;
Avoidance of long periods of preoperative fasting;
Re-establishment of oral feeding as early as possible after surgery;
Start of nutritional therapy early, as soon as a nutritional risk becomes apparent;
Metabolic control, e.g. of blood glucose;
Reduction of factors that exacerbate stress-related catabolism or impair gastrointestinal function;
Minimization of the time on paralytic agents for ventilator management in the postoperative period;
Early mobilization to facilitate protein synthesis and muscle function.
Screening and assessment of nutritional status
Nutritional screening should be standard on hospital admission for all surgical patients. It has also been emphasized in the ERAS guidelines for colorectal surgery [7], [8]. “Patients should be screened for nutritional status, and if deemed to be at risk of undernutrition, given active nutritional support.”
The Nutritional Risk Score (NRS) according to Kondrup et al. [17] has been validated for hospitalized surgical patients with colorectal cancer [18], and has also been recommended for complex geriatric assessment of the elderly [19].
Malnutrition screening (e.g. nutritional risk screening – NRS) on admission or first contact;
Observation and documentation of oral intake;
Regular follow-up of weight and BMI;
Nutritional counseling.
The basic screening includes the following criteria:
BMI <20.5 kg/m2;
Weight loss >5% within 3 months;
Diminished food intake;
Severity of the disease.
Each cancer patient undergoing surgery should be screened differentiating weight loss and diminished food intake. Patients >70 years will score an additional point. A score of >3 points indicates metabolic risk, while 5 points correspond with a highly relevant risk related to clinical outcome.
It could be shown that in the case of appropriate coding, increased consumption of resources including nutritional therapy will be reimbursed by the German Diagnosis Related Groups system [20]. In case of positive screening, nutritional status should be assessed properly. Bioelectrical impedance analysis (BIA) is a non-invasive validated tool for the analysis and follow-up of body composition assessment differentiating more than the simple measurement of body weight [21]. A BIA-derived parameter is the phase angle, which indicates the amount of body cell mass. A low phase angle has been shown to be prognostic for postoperative complications of cancer patients [5], [22].
Definition of malnutrition and metabolic risk
A severe metabolic risk can be defined in case of at least one of the following criteria:
Weight loss >10–15% within 6 months;
BMI <18.5 kg/m2;
Subjective Global Assessment C or NRS ≥
Preoperative serum albumin <30 g/L (with no evidence of hepatic or renal dysfunction).
Preoperative serum albumin is a prognostic factor for complications after surgery [23], [24], [25]. While primarily reflecting inflammation and fluid balance, serum albumin level is also associated with impaired nutritional status. Therefore, albumin may also be considered to define surgical patients with metabolic risk.
A persistently low, even decreasing or increasing serum albumin concentration is a good parameter of whether recovery is successful or not [24], [25], [26]. In a study including 627 patients after colorectal surgery, the Kaplan-Meier analysis and log rank test demonstrated a significant difference in the overall survival curves between patients with low C-reactive protein/albumin ratio (CAR) (≤0.038) and those with high CAR (>0.038; p<0.001) [24]. The magnitude of the postoperative systemic inflammatory response shown in the C-reactive protein level may be even significantly associated with long-term outcome after colorectal surgery independent of postoperative complications or disease stage [26].
Routine CT for the assessment of body composition
With special regard to those undergoing neoadjuvant treatment, CT scans will be performed several times in colorectal cancer patients. Quantitative analysis of muscle in abdominal cross sections on the level of L3 has shown good correlation with muscle and fat mass of the whole body. The prognostic impact of sarcopenia from diminished muscle cross section has been shown in oncological patients [27], [28], [29]. Two software tools are available: ImageJ from the National Institutes of Health (Bethesda, MD, USA) and Siliceomatic from TomoVision (Montreal, Quebec, Canada).
Strategies of nutritional therapy
Unrelated to the nutritional risk, a dietitian should be involved early after the diagnosis of colorectal cancer in order to optimize oral food intake. Special care should be provided during neoadjuvant treatment [30]. In the hospital, the quantity of oral intake should be observed and documented. Body weight and BMI should be measured and respectively calculated.
Nutritional therapy means first-line dietary counseling and, if necessary, fortification of the diet. Oral nutritional supplements may be offered for supplementing total intake and have shown, in a recent meta-analysis, significant impact on decreasing postoperative complications and length of hospital stay [31]. After surgery, early oral/enteral or even combined enteral/parenteral nutrition may be indicated.
For patients with normal nutritional status, potential restriction of adequate oral food intake for a longer period during cancer treatment should be taken into account. In case of anticipated inability for oral feeding for 5 days or intake <50% of the caloric requirements for 7 days, the recent guidelines recommend commencement of nutritional therapy. The oral/enteral route is the first choice. Patients with malnutrition risk (NRS >3) and those with obvious malnutrition should receive nutritional therapy immediately [16].
ERAS and plan B
ERAS is a multimodality program for the reduction of perioperative stress and catabolism, aiming at a faster recovery and functional rehabilitation. The program includes a series of different components with good but also lesser evidence [15], [16], [32]. These include preoperative preparation and medication, fluid balance, anesthesia and postoperative analgesia, preoperative and postoperative nutrition, and mobilization. Preoperative fasting should be minimized, and patients should be encouraged to take normal food as soon as possible after surgery.
While ERAS was originally developed for open surgery, a recent meta-analysis confirmed reduction of major morbidity and hospital stay by a combination of laparoscopic surgery and ERAS [33].
It has to be emphasized that strong evidence is available for early oral food intake in patients undergoing colorectal surgery without any increase in the risk of anastomotic leakage [34], [35]. The amount of initial oral intake should be adapted to the state of gastrointestinal function and to individual tolerance.
In comparison with conventional open surgery, early oral intake is tolerated even better after laparoscopic colonic resection, due to earlier return of peristalsis and bowel function [36]. However, in combination with ERAS, no differences were found between laparoscopic and conventional open colonic surgery when the full ERAS protocol was employed. In a multicentric randomized clinical trial, the postoperative hospital length of stay was significantly shorter in the ERAS group undergoing laparoscopic surgery [37].
High adherence to ERAS protocols may be associated with improved 5-year cancer-specific survival after major colorectal surgery [38]. Of course, adherence is also associated with high compliance to other treatment modules affecting outcome. The implementation of ERAS is not easy and may be optimized in many institutions dedicated to colorectal surgery, as had been shown in a recent study from several European countries [39]. Support is available and provided with an implementation program from the ERAS Society (www.erassociety.com).
Prehabilitation
Prehabilitation is a more recent approach aiming ERAS toward patients with compromised functional and nutritional status [40]. In colorectal cancer patients, prehabilitation has to be considered with special regard to patients with cardiopulmonary comorbidity and those with advanced tumors undergoing pelvic exenteration or cytoreduction with hyperthermic intraperitoneal chemotherapy.
After neoadjuvant therapy, the time period for recovery before surgery is about 4–6 weeks. Thus far, structured preparation for surgery for several weeks is very uncommon. This period may be “exploited” much more and used for conditioning in a prehabilitation program [40]. Prehabilitation first includes physical exercise and endurance training in order to improve cardiopulmonary resistance, but may also be accompanied by nutritional and psychological therapy or other measures, whenever appropriate. First results have shown significant improvement of cardiopulmonary parameters like the 6-min walking test and diminished oxygen consumption. In colorectal cancer patients and those undergoing liver resection, no significant difference in postoperative complications and outcome has been observed [41]. Long-term results are missing. Most likely, selected high-risk patients will benefit the most. In a recent randomized blinded controlled trial in high-risk patients undergoing elective major abdominal surgery prehabilitation enhanced postoperative outcome by a reduction of the number of patients with complications, as well as the rate of complications [42]. In Germany, appropriate outpatient modalities in the framework of interprofessional cooperation reimbursed by health-care insurance systems are pending.
Preservation of the microbiome
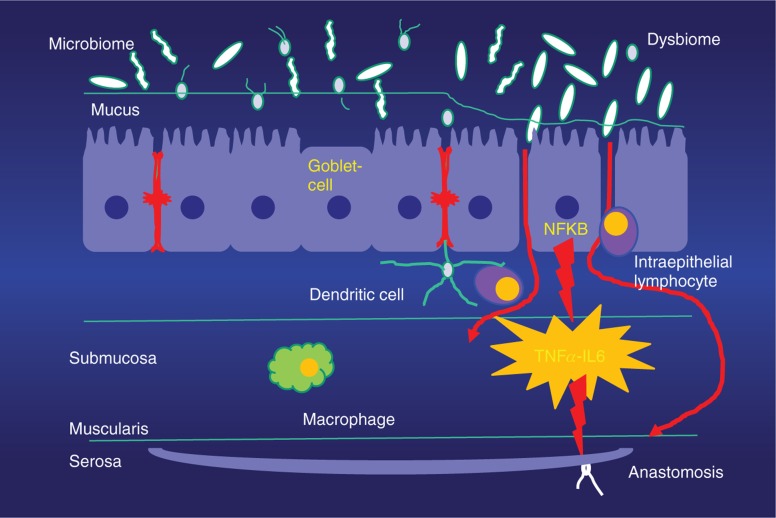
A new dimension for our understanding of septic complications after colorectal surgery is the emerging role of the colonizing physiological intestinal microbiome and its interaction with the intestinal immune cells [43], [44]. Under healthy conditions, the commensal microbiome has a colonizing and symbiotic relationship with the host, maintaining gut homeostasis. An intact microbiome in crosstalk with the host may also govern the immune response after injury. Short-chain fatty acids are produced by microbes and are considered a key mediator. However, stress and catabolism, as well as prolonged administration of antibiotics, lead to a change of bacterial colonization and metabolism, while decreased host resilience and cytokines as well as signals from the surrounding bacterial microenvironment (“sensing”) induce a selected increase of virulence in special bacteria. This shift of the physiological microbiome to a pathobiome is accompanied by a loss of bacterial diversification. Virulent bacteria may interact with a loss of function of the intestinal barrier with subsequent maladaptation of the immune response. These mechanisms can be considered a starter for local intestinal disturbance, like surgical site infection by impaired healing of a bowel anastomosis, but also for systemic septic complications and organ dysfunction (Figure 1). It has also been shown that gut microbes such as Lactobacillus spp. and Akkermansia muciniphila are involved in the process of wound healing by mechanisms dependent on reactive oxygen species and activated specific formyl peptide receptors [45].
Figure 1:
Intestinal wall and microbiome.
Intestinal wall with mucosa, intact mucus, and diversity of microbiome (A) and shift to a pathobiome with loss of diversity, increasing permeability of the mucosa, and initiation of inflammation (B).
Perioperative nutritional therapy should also focus on the preservation or refaunation of the microbiome. This means avoidance of stress and catabolism by prolonged periods of starving, and withholding early oral or enteral food intake after surgery. Appropriate measures of conditioning may also have an impact on the microbiome.
While the most appropriate strain is still a matter of debate, the use of probiotics/synbiotics is a promising nutritional intervention in order to influence the mucosa-associated microbiome. A modified microbiome has been shown in patients with colorectal cancer [46]. In patients undergoing elective surgery, a meta-analysis of 34 randomized controlled studies with 2634 patients revealed a lower risk for surgical site infection in the probiotics/synbiotics group [risk ratio, 0.65; 95% confidence interval (CI), 0.51–0.84; p=0.0007] [47]. In one more recent meta-analysis with nine trials and 1146 patients undergoing colorectal surgery, the combination of multistrain probiotics showed a significant reduction of total infections (odds ratio, 0.30; 95% CI, 0.15–0.61; p=0.0009), including surgical site and non-surgical site infections [48]. Most likely, the intervention should be started before surgery.
Bowel preparation
The role of bowel preparation before surgery remains to be elucidated in the framework of preservation of the microbiome, but may be an explanation for the conflicting results shown in recent meta-analyses. The preservation of the microbiome provides a strong argument to avoid any aggressive mechanical bowel preparation with special regard to hyperosmotic solutions. Despite some change in evidence during the past years, colorectal surgeons are still reluctant to operate on patients with an unprepared bowel due to chronic constipation, and this is the most common rationale to persist with systematic bowel preparation in these patients. Summarizing the recent evidence, the 2017 American Society for Enhanced Recovery guidelines recommend the use of a combined isoosmotic mechanic bowel preparation with oral antibiotics [49], [50]. Mechanical bowel preparation alone in the absence of oral antibiotics cannot be recommended. Regardless of the problems during surgery, the concept of the shift from microbiome to pathobiome is also an argument for a stepwise and very moderate bowel preparation, starting 7–10 days before surgery. Postoperative ileus in a patient with chronic constipation and an unprepared bowel may also be considered a stimulus for the shift to dysbiosis. Balancing the benefits of a clean bowel versus the potential harm by loss of microbiome diversification for the healing of anastomosis, the most appropriate bowel preparation will be an ongoing matter of debate and needs further controlled data.
Metabolic conditioning
Metabolic conditioning – so called “carbohydrate loading” – means a glucose drink focusing on avoiding perioperative hypoglycemia with special regard to the avoidance of postoperative insulin resistance and the reduction of perioperative discomfort. In the ESPEN guidelines, carbohydrate loading is recommended in the night before and 2 h before surgery [16]. A recent meta-analysis including 43 trials with 3110 patients showed a small reduction of hospital length of stay in comparison with fasting only. No benefits were observed in comparison with water and placebo effects. No reduction in postoperative complication rate was found [51]. It has to be argued that a considerable number of studies had included patients with minor surgery and very short hospital length of stay. Nevertheless, at present, this recommendation is based on expert opinion for colorectal surgery.
Immunonutrition
The enteral stimulation of immune defense by appropriate nutrition – so called “immunonutrition” – is a convincing concept with special regard to the conditioning of patients undergoing major cancer surgery [52]. Recently, stimulation of T-cell antitumoral activity has been shown for arginine [53]. For the combination of arginine, omega-3 fatty acids, and ribonucleotides, numerous prospective and randomized controlled studies and meta-analyses are available. In a meta-analysis, a sole administration of immunonutrition before surgery has shown significant benefits in comparison with a regular hospital diet, but not in comparison with a standard oral nutritional supplement [54].
In the recent ESPEN guidelines, the intake of oral nutritional supplements is recommended before major surgery, while immunomodulating substrates should be preferred for 5–7 days [16]. Aiming on the decrease of postoperative infection rate, the available data emphasize continuation of immunonutrition after surgery for 5–7 days. This may also be advantageous for cost-benefit analysis [55].
A new approach is the use of immunonutrition in an ERAS program. In a randomized controlled study, in 264 patients undergoing colorectal surgery, a diet enriched with immunonutrients was compared with a standard oral nutritional supplement and administered 7 days before surgery and continued for 5 days postoperatively. In the immunonutrition group, a significant decrease in the rate of infectious complications was found (23.8% vs. 10.7%, p=0.0007) [56]. Therefore, the integration of immunonutrition in an ERAS protocol may be recommended.
Indications for parenteral nutrition
Nowadays, exclusive total parenteral nutrition via a central venous line should be restricted to patients with severe long-term enteral intolerance, e.g. in advanced peritoneal carcinosis or short-bowel syndrome. The idea of partial supplemental parenteral nutrition is bridging a caloric gap in case of insufficient oral/enteral intake to avoid an accumulating caloric deficit. A special-risk group includes surgical intensive care patients with limited enteral tolerance after reoperation for septic complications. The ESPEN guideline recommends the combination of enteral and parenteral nutrition, anticipating that oral and enteral feeding together will remain <50% of the calculated caloric requirement for >7 days. Severely prolonged postoperative ileus may also require supplementing parenteral nutrition, e.g. administered via a peripheral vein, and should be started in high-risk patients and those with malnutrition not later than day 3 or 4 [14].
Parenteral nutrition before surgery may lead to considerable recovery of functional respiratory parameters, grip strength, and total body protein within 7 days. Further improvement may be expected within the second week [57]. This intervention has shown a significant decrease of postoperative complications in patients with severe metabolic risk, and is therefore strongly recommended in the guidelines for 10–14 days before surgery for those patients with severe metabolic risk and limited oral/enteral intake [14].
In the guidelines, commercially produced and standardized three-chamber bags containing glucose, lipids, and amino acids are also recommended for safety and economic reasons. An individual compounding is more complex and expensive, and should be reserved for special cases of long-term parenteral nutrition. For intensive care patients in the acute phase, recent guideline recommendations emphasize “hypocaloric high protein” – 80–90% of the required caloric intake with 1.2–2.5 g protein/kg body weight [58].
Supplementation of parenteral nutrition with glutamine and omega-3 fatty acids
In order to improve postoperative outcome by stimulating immune defense and anti-inflammation, supplementation with the conditional essential amino acid glutamine and polyunsaturated omega-3 fatty acids (“fish oil”) is a convincing concept and supported by plenty of data.
Regarding the clinical benefits of the decrease of infectious complications and hospital length of stay, there is an ongoing controversial discussion. Meta-analyses of the randomized controlled studies using glutamine with a standard dosage of 0.35 g/kg body weight emphasize the application in surgical patients [59], [60]. However, in a recent prospective randomized controlled multicenter trial in 150 surgical intensive care patients, no significant difference in the rate of infectious complications and 6-month survival was observed [61]. From these data, only an expert recommendation was given in the ESPEN guidelines for glutamine supplementation. Meta-analyses and more recent data recommend supplementation with omega-3 fatty acids in parenterally fed surgical patients [62], [63]. Taking into account the heterogeneity of the studies, the ESPEN guidelines provide a B recommendation [14].
Posthospital nutritional monitoring and therapy
All patients who underwent perioperative nutritional therapy should be monitored after discharge for nutritional intake and status. It is not surprising that the special-risk group comprises patients with a complicated course and considerable deterioration of nutritional status [64]. Follow-up data show the recovery of BMI within 4–6 months after surgery for colorectal cancer, while body cell mass remains under baseline before surgery and is compensated by an increase in extracellular mass with body water [65].
The significant impact of nutritional counseling and education about regular foods on the long-term outcome of colorectal cancer patients has been shown in the late follow-up of a randomized controlled trial comparing individualized nutritional counseling (group 1, n=34) versus oral nutritional supplements in addition to the usual diet (group 2, n=29) versus a usual diet of regular foods (group 3, n=26) [66]. The analyses were adjusted for tumor stage. While nutritional deterioration was higher in group 3 (p<0.001) and group 2 than in group 1, adequate nutritional status was maintained in 91% of group 1 and in 0% of group 3 (p<0.002). Food intake was within the prescribed recommendations; intake was significantly lower in groups 3 and 2. The median survival was 7.3 years in group 1 versus 6.5 years in group 2 and 4.9 years in group 3 (p<0.01). Late radiotherapy toxicity was significantly higher in groups 3 and 2 versus group 1. Radiotherapy toxicity, quality of life, and mortality were significantly associated with nutritional status and intake, while depleted intake and nutritional status as well as quality of life predicted toxicity and length of survival (hazard ratio, 8.25; 95% CI, 2.74–1.74; p<0.001). These data underline the effectiveness of personalized nutritional counseling, which outweighs the benefits of nutritional supplements alone. Therefore, the nutritional follow-up including nutritional counseling may not be underestimated and should be offered on a regular basis with special regard to those patients undergoing adjuvant therapy.
Conclusion
A severely compromised nutritional status in general and nutritional interventions have evidence-based impact on the outcome of patients undergoing surgery for colorectal cancer.
The key factors are as follows:
Nutritional screening and nutrition therapy in patients with metabolic risk;
Adherence to an ERAS protocol;
Perioperative administration of oral nutritional supplements;
Nutritional counseling and dietary education during postoperative radiotherapy.
In patients with colorectal cancer, the emerging role of the microbiome in carcinogenesis and the perioperative period has to be elucidated in order to develop more individualized strategies for modulation. Although colorectal cancer patients are usually not at a high metabolic risk at the time of diagnosis, malnutrition screenings should be mandatory before surgery, added by functional assessment in the elderly. Prevention of deterioration of the nutritional status should not be ignored and needs critical observation during neoadjuvant therapy. Within obvious deficits, “prehabilitation” offers a new concept of conditioning that is especially appropriate in the time interval after neoadjuvant treatment. Following guideline recommendations, perioperative nutrition therapy should be part of an ERAS program and focus on avoiding perioperative weight loss. In order to assess metabolic recovery for long-term survival, the CAR is a promising new prognostic parameter that has to be further validated in the future.
Supporting Information
Supplementary Material
The article (iss-2017-0039) offers reviewer assessments as supplementary material.
Author Statement
Research funding: Author received a research grant by Baxter, Danone. Conflict of interest: Author received speaker fees for Baxter Deutschland, Berlin Chemie, B. Braun Melsungen, Fresenius Kabi, Lilly, Medtronic, Nestlé, Nutricia. Informed consent: Informed consent is not applicable. Ethical approval: The conducted research is not related to either human or animal use.
Author Contributions
Arved Weimann: conceptualization; writing – original draft; writing – review and editing.
Publication Funding
The German Society of Surgery funded the article processing charges of this article.
References
- [1].Kyrgiou M, Kallala I, Markozannes G, Gunter MJ, Parakevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. Br Med J 2017;356:j477. [DOI] [PMC free article] [PubMed]; Kyrgiou M, Kallala I, Markozannes G, Gunter MJ, Parakevaidis E, Gabra H. et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. Br Med J. 2017;356:j477. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fan Y, Jin X, Man C, Gao Z, Wang X. Meta-analysis of the association between the inflammatory potential of diet and colorectal cancer risk. Oncotarget 2017;8:59592–600. [DOI] [PMC free article] [PubMed]; Fan Y, Jin X, Man C, Gao Z, Wang X. Meta-analysis of the association between the inflammatory potential of diet and colorectal cancer risk. Oncotarget. 2017;8:59592–600. doi: 10.18632/oncotarget.19233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Saetang J, Sangkhathat S. Diets link metabolic syndrome and colorectal cancer development. Oncol Rep 2017;37:1312–20. [DOI] [PubMed]; Saetang J, Sangkhathat S. Diets link metabolic syndrome and colorectal cancer development. Oncol Rep. 2017;37:1312–20. doi: 10.3892/or.2017.5385. [DOI] [PubMed] [Google Scholar]
- [4].Sinha R, Ahn J, Sampson JN, Shi JN, Shi J, Yu G, et al. Fecal microbiota, fecal metabolome, and colorectal cancer interrelations. PLoS One 2016;11:e0152128. [DOI] [PMC free article] [PubMed]; Sinha R, Ahn J, Sampson JN, Shi JN, Shi J, Yu G. et al. Fecal microbiota, fecal metabolome, and colorectal cancer interrelations. PLoS One. 2016;11:e0152128. doi: 10.1371/journal.pone.0152126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barao K, Vicente Cavagnari MA, Fucuta PS, Manoukian Forones N. Association between nutrition status and survival in elderly patients. Nutr Clin Pract 2017;32:658–63. [DOI] [PubMed]; Barao K, Vicente Cavagnari MA, Fucuta PS, Manoukian Forones N. Association between nutrition status and survival in elderly patients. Nutr Clin Pract. 2017;32:658–63. doi: 10.1177/0884533617706894. [DOI] [PubMed] [Google Scholar]
- [6].He Y, Wang J, Bian H, Deng X, Wang Z. BMI as a predictor for perioperative outcome of laparoscopic colorectal surgery: a pooled analysis of comparative studies. Dis Colon Rectum 2017;60:433–45. [DOI] [PubMed]; He Y, Wang J, Bian H, Deng X, Wang Z. BMI as a predictor for perioperative outcome of laparoscopic colorectal surgery: a pooled analysis of comparative studies. Dis Colon Rectum. 2017;60:433–45. doi: 10.1097/DCR.0000000000000760. [DOI] [PubMed] [Google Scholar]
- [7].Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Enhanced Recovery After Surgery Society Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012;31:783–800. [DOI] [PubMed]; Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N. et al. Enhanced Recovery After Surgery Society Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:783–800. doi: 10.1016/j.clnu.2012.08.013. [DOI] [PubMed] [Google Scholar]
- [8].Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN, et al. Enhanced Recovery after Surgery Society Guidelines for perioperative care in elective rectal/pelvic surgery: Enhance Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012;31:801–81. [DOI] [PubMed]; Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN. et al. Enhanced Recovery after Surgery Society Guidelines for perioperative care in elective rectal/pelvic surgery: Enhance Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:801–81. doi: 10.1016/j.clnu.2012.08.012. [DOI] [PubMed] [Google Scholar]
- [9].Braga M, Pecorelli N, Scatizzi M, Borghi F, Missana G, Radrizzani D, PeriOperative Italian Society. Enhance Recovery program in high-risk patients undergoing colorectal surgery: results from the PeriOperative Italian Society Registry. World J Surg 2017;41:860–7. [DOI] [PubMed]; Braga M, Pecorelli N, Scatizzi M, Borghi F, Missana G, Radrizzani D. PeriOperative Italian Society. Enhance Recovery program in high-risk patients undergoing colorectal surgery: results from the PeriOperative Italian Society Registry. World J Surg. 2017;41:860–7. doi: 10.1007/s00268-016-3766-9. [DOI] [PubMed] [Google Scholar]
- [10].Slieker J, Frauche P, Jurt J, Addor V, Blanc C, Demartines N, et al. Enhanced recovery ERAS for elderly: a safe and beneficial pathway in colorectal surgery. Int J Colorectal Dis 2017;32:215–21. [DOI] [PubMed]; Slieker J, Frauche P, Jurt J, Addor V, Blanc C, Demartines N. et al. Enhanced recovery ERAS for elderly: a safe and beneficial pathway in colorectal surgery. Int J Colorectal Dis. 2017;32:215–21. doi: 10.1007/s00384-016-2691-6. [DOI] [PubMed] [Google Scholar]
- [11].Scarborough JE, Bennett KM, Englum BR, Pappas TN, Lagoo-Deenadayalan SA. The impact of functional dependency on outcomes after complex general and vascular surgery. Ann Surg 2015;261:432–7. [DOI] [PMC free article] [PubMed]; Scarborough JE, Bennett KM, Englum BR, Pappas TN, Lagoo-Deenadayalan SA. The impact of functional dependency on outcomes after complex general and vascular surgery. Ann Surg. 2015;261:432–7. doi: 10.1097/SLA.0000000000000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine (Balt) 2016;95:e3164. [DOI] [PMC free article] [PubMed]; Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N. et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine (Balt) 2016;95:e3164. doi: 10.1097/MD.0000000000003164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hill GL, Blackett RL, Pickford I, Burkinshaw L, Young GA, Warren JV, et al. Malnutrition in surgical patients. Lancet 1977;1:689–92. [DOI] [PubMed]; Hill GL, Blackett RL, Pickford I, Burkinshaw L, Young GA, Warren JV. et al. Malnutrition in surgical patients. Lancet. 1977;1:689–92. doi: 10.1016/s0140-6736(77)92127-4. [DOI] [PubMed] [Google Scholar]
- [14].Horoitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol 2015;12:213–26. [DOI] [PMC free article] [PubMed]; Horoitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12:213–26. doi: 10.1038/nrclinonc.2014.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Feinberg J, Nielsen EE, Korang SK, Halberg Engell K, Nielsen MS, Zhang K, et al. Nutrition support in hospitalized adults at nutritional risk. Cochrane Database Syst Rev 2017;CD011598. [DOI] [PMC free article] [PubMed]; Feinberg J, Nielsen EE, Korang SK, Halberg Engell K, Nielsen MS, Zhang K. et al. Nutrition support in hospitalized adults at nutritional risk. Cochrane Database Syst Rev. 2017:CD011598. doi: 10.1002/14651858.CD011598.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. ESPEN Guideline: clinical nutrition in surgery. Clin Nutr 2017;36:623–50. [DOI] [PubMed]; Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S. et al. ESPEN Guideline: clinical nutrition in surgery. Clin Nutr. 2017;36:623–50. doi: 10.1016/j.clnu.2017.02.013. [DOI] [PubMed] [Google Scholar]
- [17].Kondrup J, Allison SP, Elia M, Vellas B, Plauth M, Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003;22:415–21. [DOI] [PubMed]; Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415–21. doi: 10.1016/s0261-5614(03)00098-0. [DOI] [PubMed] [Google Scholar]
- [18].Schwegler I, von Holzen A, Gutzwiller JP, Schlumpf R, Mühlebach S, Stanga Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg 2010;97:92–7. [DOI] [PubMed]; Schwegler I, von Holzen A, Gutzwiller JP, Schlumpf R, Mühlebach S, Stanga Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg. 2010;97:92–7. doi: 10.1002/bjs.6805. [DOI] [PubMed] [Google Scholar]
- [19].Cheema FN, Abraham NS, Berger DH, Albo D, Taffet GE, Naik AD. Novel approaches to perioperative assessment and intervention may improve long-term outcomes after colorectal cancer resection in older adults. Ann Surg 2011;253:867–74. [DOI] [PubMed]; Cheema FN, Abraham NS, Berger DH, Albo D, Taffet GE, Naik AD. Novel approaches to perioperative assessment and intervention may improve long-term outcomes after colorectal cancer resection in older adults. Ann Surg. 2011;253:867–74. doi: 10.1097/SLA.0b013e318208faf0. [DOI] [PubMed] [Google Scholar]
- [20].Aust J, Werner A, Grünewald G, Haberzettl D, Herbst A, Fedders M, et al. Results of “Screening for malnutrition” in a major community hospital. Aktuel Ernahrungsmed 2016;41:352–8.; Aust J, Werner A, Grünewald G, Haberzettl D, Herbst A, Fedders M. et al. Results of “Screening for malnutrition” in a major community hospital. Aktuel Ernahrungsmed. 2016;41:352–8. [Google Scholar]
- [21].Haverkort EB, Rejven PL, Binnekade JM, de van der Schueren MA, Earthman CP, Gouma DJ, et al. Bioelectrical impedance analysis to estimate body composition in surgical and oncological patients: a systematic review. Eur Clin Nutr 2015;69:3–13. [DOI] [PubMed]; Haverkort EB, Rejven PL, Binnekade JM, de van der Schueren MA, Earthman CP, Gouma DJ. et al. Bioelectrical impedance analysis to estimate body composition in surgical and oncological patients: a systematic review. Eur Clin Nutr. 2015;69:3–13. doi: 10.1038/ejcn.2014.203. [DOI] [PubMed] [Google Scholar]
- [22].Hekimian K, Haberzettl D, Fedders M, Weimann A. Parameters of nutritional risk in surgical patients – impact on hospital- and long-term mortality. Aktuel Ernahrungsmed 2011;36:103–7.; Hekimian K, Haberzettl D, Fedders M, Weimann A. Parameters of nutritional risk in surgical patients – impact on hospital- and long-term mortality. Aktuel Ernahrungsmed. 2011;36:103–7. [Google Scholar]
- [23].Aahlin EK, Tranø G, Johns N, Horn A, Søreide JA, Fearon KC, et al. Risk factors, complications and survival after upper abdominal surgery: a prospective cohort study. BMC Surg 2015;15:83. [DOI] [PMC free article] [PubMed]; Aahlin EK, Tranø G, Johns N, Horn A, Søreide JA, Fearon KC. et al. Risk factors, complications and survival after upper abdominal surgery: a prospective cohort study. BMC Surg. 2015;15:83. doi: 10.1186/s12893-015-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol 2016;23:900–7. [DOI] [PubMed]; Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol. 2016;23:900–7. doi: 10.1245/s10434-015-4948-7. [DOI] [PubMed] [Google Scholar]
- [25].Hübner M, Mantiziar S, Demartines N, Pralong F, Coti-Bertrand P, Schäfer M. Postoperative albumin drop is a marker for surgical stress and a predictor for clinical outcome. Gastroenterol Res Pract 2016;2016:8743187. [DOI] [PMC free article] [PubMed]; Hübner M, Mantiziar S, Demartines N, Pralong F, Coti-Bertrand P, Schäfer M. Postoperative albumin drop is a marker for surgical stress and a predictor for clinical outcome. Gastroenterol Res Pract. 2016;2016:8743187. doi: 10.1155/2016/8743187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McSorley ST, Watt DG, Horgan PG, McMillan DC. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol 2016;23:2832–40. [DOI] [PMC free article] [PubMed]; McSorley ST, Watt DG, Horgan PG, McMillan DC. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol. 2016;23:2832–40. doi: 10.1245/s10434-016-5204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Hunag D, et al. Impact of sarcopenia on outcomes on following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478–86. [DOI] [PMC free article] [PubMed]; Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Hunag D. et al. Impact of sarcopenia on outcomes on following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:1478–86. doi: 10.1007/s11605-012-1923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Carrara G, Pecorelli N, De Cobelli F, Christel G, Damascelli A, Beretta L, et al. Preoperative sarcopenia determinants in pancreatic cancer patients. Clin Nutr 2017;36:1649–53. [DOI] [PubMed]; Carrara G, Pecorelli N, De Cobelli F, Christel G, Damascelli A, Beretta L. et al. Preoperative sarcopenia determinants in pancreatic cancer patients. Clin Nutr. 2017;36:1649–53. doi: 10.1016/j.clnu.2016.10.014. [DOI] [PubMed] [Google Scholar]
- [29].Gomez-Perez SL, Haus JM, Shehan P, Patel B, Mar W, Chaudry V, et al. Measuring abdominal circumference and skeletal muscle from a single cross-sectional CT image: a step-by-step guide for clinicians using National Institutes of Health ImageJ. JPEN J Parenter Enteral Nutr 2016;40:308–18. [DOI] [PMC free article] [PubMed]; Gomez-Perez SL, Haus JM, Shehan P, Patel B, Mar W, Chaudry V. et al. Measuring abdominal circumference and skeletal muscle from a single cross-sectional CT image: a step-by-step guide for clinicians using National Institutes of Health ImageJ. JPEN J Parenter Enteral Nutr. 2016;40:308–18. doi: 10.1177/0148607115604149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ligthart-Melis GC, Weijs PJM, te Boveldt ND, Buskermolen S, Earthman CP, Verheul HMW, et al. Dietician-delivered intensive nutritional support is associated with a decrease in severe postoperative complications after surgery in patients with esophageal cancer. Dis Esophagus 2013;26:587–93. [DOI] [PubMed]; Ligthart-Melis GC, Weijs PJM, te Boveldt ND, Buskermolen S, Earthman CP, Verheul HMW. et al. Dietician-delivered intensive nutritional support is associated with a decrease in severe postoperative complications after surgery in patients with esophageal cancer. Dis Esophagus. 2013;26:587–93. doi: 10.1111/dote.12008. [DOI] [PubMed] [Google Scholar]
- [31].Elia M, Normand C, Norman K, Laviano A, Norman K. A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in the hospital setting. Clin Nutr 2016;35:370–80. [DOI] [PubMed]; Elia M, Normand C, Norman K, Laviano A, Norman K. A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in the hospital setting. Clin Nutr. 2016;35:370–80. doi: 10.1016/j.clnu.2015.05.010. [DOI] [PubMed] [Google Scholar]
- [32].Ljungqvist O. ERAS – Enhanced recovery after surgery: moving evidence-based perioperative care to practice. JPEN J Parenter Enteral Nutr 2014;38:559–66. [DOI] [PubMed]; Ljungqvist O. ERAS – Enhanced recovery after surgery: moving evidence-based perioperative care to practice. JPEN J Parenter Enteral Nutr. 2014;38:559–66. doi: 10.1177/0148607114523451. [DOI] [PubMed] [Google Scholar]
- [33].Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 2014;38: 1531–41. [DOI] [PubMed]; Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38:1531–41. doi: 10.1007/s00268-013-2416-8. [DOI] [PubMed] [Google Scholar]
- [34].Choi J, O’Connell TX. Safe and effective early postoperative feeding and hospital discharge after open colon resection. Am Surg 1996;62:853–6. [PubMed]; Choi J, O’Connell TX. Safe and effective early postoperative feeding and hospital discharge after open colon resection. Am Surg. 1996;62:853–6. [PubMed] [Google Scholar]
- [35].Osland E, Yunus RM, Khan S, Memon MA. Early versus traditional postoperative feeding in patients undergoing resectional gastrointestinal surgery: a meta-analysis. JPEN J Parenter Enteral Nutr 2011;35:473–87. [DOI] [PubMed]; Osland E, Yunus RM, Khan S, Memon MA. Early versus traditional postoperative feeding in patients undergoing resectional gastrointestinal surgery: a meta-analysis. JPEN J Parenter Enteral Nutr. 2011;35:473–87. doi: 10.1177/0148607110385698. [DOI] [PubMed] [Google Scholar]
- [36].Schwenk W, Bohm B, Haase O, Junghans T, Muller JM. Laparoscopic versus conventional colorectal resection: a prospective randomised study of postoperative ileus and early postoperative feeding. Langenbecks Arch Surg 1998;383:49–55. [DOI] [PubMed]; Schwenk W, Bohm B, Haase O, Junghans T, Muller JM. Laparoscopic versus conventional colorectal resection: a prospective randomised study of postoperative ileus and early postoperative feeding. Langenbecks Arch Surg. 1998;383:49–55. doi: 10.1007/s004230050091. [DOI] [PubMed] [Google Scholar]
- [37].Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 2011;254:868–75. [DOI] [PubMed]; Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF. et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study) Ann Surg. 2011;254:868–75. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- [38].Gustafsson UO, Oppelstrup H, Thorell A, Nygren J, Ljungqvist O. Adherence to the ERAS protocol is associated with 5-year survival after colorectal cancer surgery: a retrospective cohort study. World J Surg 2016;40:1741–7. [DOI] [PubMed]; Gustafsson UO, Oppelstrup H, Thorell A, Nygren J, Ljungqvist O. Adherence to the ERAS protocol is associated with 5-year survival after colorectal cancer surgery: a retrospective cohort study. World J Surg. 2016;40:1741–7. doi: 10.1007/s00268-016-3460-y. [DOI] [PubMed] [Google Scholar]
- [39].van Zelm R, Coeckelberghs E, Sermeus W, De Buck van Overstraeten A, Weimann A, Seys D, et al. Variation in care for surgical patients with colorectal cancer: protocol adherence in 12 European hospitals. Intern J Colorect Dis 2017;32:1471–8. [DOI] [PubMed]; van Zelm R, Coeckelberghs E, Sermeus W, De Buck van Overstraeten A, Weimann A, Seys D. et al. Variation in care for surgical patients with colorectal cancer: protocol adherence in 12 European hospitals. Intern J Colorect Dis. 2017;32:1471–8. doi: 10.1007/s00384-017-2863-z. [DOI] [PubMed] [Google Scholar]
- [40].Carli F, Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiology Clin 2015;33:17–33. [DOI] [PubMed]; Carli F, Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiology Clin. 2015;33:17–33. doi: 10.1016/j.anclin.2014.11.002. [DOI] [PubMed] [Google Scholar]
- [41].Looijaard SM, Slee-Valentijn MS, Otten RH, Maier AB. Physical and nutritional prehabilitation in older patients with colorectal carcinoma: a systematic review. J Geriatr Phys Ther 2017;Mar 1. doi: 10.1519/JPT.0000000000000125 [Epub ahead of print]. [DOI] [PubMed]; Looijaard SM, Slee-Valentijn MS, Otten RH, Maier AB. Physical and nutritional prehabilitation in older patients with colorectal carcinoma: a systematic review. J Geriatr Phys Ther. 2017:Mar 1. doi: 10.1519/JPT.0000000000000125. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [42].Barberan-Garcia A, Ubré M, Roca J, Lacy AM, Burgos F, Risco R, Momblán D, Balust J, Blanco I, Martinez-Palli G. Personalized prehabilitation in high-risk patients undergoing elective major abdominal sugrery: A randomized blinded controlled trial. Ann Surg 2017; May 9. doi 10.1097/SLA.0000000000002293. [Epub ahead of print]. [DOI] [PubMed]; Barberan-Garcia A, Ubré M, Roca J, Lacy AM, Burgos F, Risco R, Momblán D, Balust J, Blanco I, Martinez-Palli G. Personalized prehabilitation in high-risk patients undergoing elective major abdominal sugrery: A randomized blinded controlled trial. Ann Surg. 2017:May 9. doi: 10.1097/SLA.0000000000002293. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [43].Alverdy JC, Krezalek MA. Collapse of the microbiome, emergence of the pathobiome, and the immunopathology of sepsis. Crit Care Med 2017;45:337–47. [DOI] [PMC free article] [PubMed]; Alverdy JC, Krezalek MA. Collapse of the microbiome, emergence of the pathobiome, and the immunopathology of sepsis. Crit Care Med. 2017;45:337–47. doi: 10.1097/CCM.0000000000002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Alverdy JC, Luo JN. The influence of host stress on the mechanism of infection: lost microbiomes, emergent pathobiomes, and the role of interkingdom signaling. Front Microbiol 2017;8:322. [DOI] [PMC free article] [PubMed]; Alverdy JC, Luo JN. The influence of host stress on the mechanism of infection: lost microbiomes, emergent pathobiomes, and the role of interkingdom signaling. Front Microbiol. 2017;8:322. doi: 10.3389/fmicb.2017.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bachmann R, Leonard D, Delzenne N, Kartheuser A, Cani PD. Novel insight into the role of microbiota on colorectal surgery. Gut 2017;66:738–49. [DOI] [PubMed]; Bachmann R, Leonard D, Delzenne N, Kartheuser A, Cani PD. Novel insight into the role of microbiota on colorectal surgery. Gut. 2017;66:738–49. doi: 10.1136/gutjnl-2016-312569. [DOI] [PubMed] [Google Scholar]
- [46].Gao Z, Guo B, Gao R, Zhu Q, Wu W, Quin H. Probiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancer. Mol Med Rep 2015;12:6119–27. [DOI] [PubMed]; Gao Z, Guo B, Gao R, Zhu Q, Wu W, Quin H. Probiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancer. Mol Med Rep. 2015;12:6119–27. doi: 10.3892/mmr.2015.4124. [DOI] [PubMed] [Google Scholar]
- [47].Wu XD, Liu MM, Liang X, Hu N, Huang W. Effects of perioperative supplementation with pro-/synbiotics on clinical outcomes in surgical patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Clin Nutr 2016 Oct 26. pii: S0261-5614(16)31290-0. doi: 10.1016/j.clnu.2016.10.015 [Epub ahead of print]. [DOI] [PubMed]; Wu XD, Liu MM, Liang X, Hu N, Huang W. Effects of perioperative supplementation with pro-/synbiotics on clinical outcomes in surgical patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Clin Nutr. 2016;Oct 26 doi: 10.1016/j.clnu.2016.10.015. pii: S0261-5614(16)31290-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [48].Liu PC, Yan YK, Ma KJ, Wang XW, Gera J, Wang MC, et al. Probiotics reduce postoperative infections in patients undergoing colorectal surgery: a systematic review and meta-analysis. Gastroenterol Res Pract 2017;2017:6029075. [DOI] [PMC free article] [PubMed]; Liu PC, Yan YK, Ma KJ, Wang XW, Gera J, Wang MC. et al. Probiotics reduce postoperative infections in patients undergoing colorectal surgery: a systematic review and meta-analysis. Gastroenterol Res Pract. 2017;2017:6029075. doi: 10.1155/2017/6029075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kiran RP, Murray ACA, Chiuzcan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg 2015;262:416–25. [DOI] [PubMed]; Kiran RP, Murray ACA, Chiuzcan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg. 2015;262:416–25. doi: 10.1097/SLA.0000000000001416. [DOI] [PubMed] [Google Scholar]
- [50].Holubar SDF, Hedrick T, Gupta R, Kellum J, Hamilton M, Gan TJ, et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on prevention of postoperative infection within an enhanced recovery pathway for elective colorectal surgery. Perioper Med (Lond) 2017;6:4. [DOI] [PMC free article] [PubMed]; Holubar SDF, Hedrick T, Gupta R, Kellum J, Hamilton M, Gan TJ. et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on prevention of postoperative infection within an enhanced recovery pathway for elective colorectal surgery. Perioper Med (Lond) 2017;6:4. doi: 10.1186/s13741-017-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Amer MA, Smith MD, Herbison GP, Plank LD, McCall JL. Network meta-analysis of the effect of preoperative carbohydrate loading on recovery after elective surgery. Br J Surg 2017;104:187–97. [DOI] [PubMed]; Amer MA, Smith MD, Herbison GP, Plank LD, McCall JL. Network meta-analysis of the effect of preoperative carbohydrate loading on recovery after elective surgery. Br J Surg. 2017;104:187–97. doi: 10.1002/bjs.10408. [DOI] [PubMed] [Google Scholar]
- [52].Alazawi W, Pirmadid N, Lahiri R, Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann Surg 2016;64:73–80. [DOI] [PubMed]; Alazawi W, Pirmadid N, Lahiri R, Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann Surg. 2016;64:73–80. doi: 10.1097/SLA.0000000000001691. [DOI] [PubMed] [Google Scholar]
- [53].Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 2016;167:829–42.e13. [DOI] [PMC free article] [PubMed]; Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T. et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167:829–42.e13. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hegazi RA, Hustead DS, Evans DC. Preoperative standard oral nutrition supplements vs immunonutrition: results of a systematic review and meta-analysis. J Am Coll Surg 2014;219:1078–87. [DOI] [PubMed]; Hegazi RA, Hustead DS, Evans DC. Preoperative standard oral nutrition supplements vs immunonutrition: results of a systematic review and meta-analysis. J Am Coll Surg. 2014;219:1078–87. doi: 10.1016/j.jamcollsurg.2014.06.016. [DOI] [PubMed] [Google Scholar]
- [55].Chevrou-Séverac H, Pinget C, Cerantola Y, Demartines N, Wasserfallen JB, Schäfer M. Cost-effectiveness analysis of immune modulating nutritional support for gastrointestinal cancer patients. Clin Nutr 2014;33:649–54. [DOI] [PubMed]; Chevrou-Séverac H, Pinget C, Cerantola Y, Demartines N, Wasserfallen JB, Schäfer M. Cost-effectiveness analysis of immune modulating nutritional support for gastrointestinal cancer patients. Clin Nutr. 2014;33:649–54. doi: 10.1016/j.clnu.2013.09.001. [DOI] [PubMed] [Google Scholar]
- [56].Moya P, Soriano-Irigiaray L, Ramirez JM, Garcea A, Blasco O, Blanco F, et al. Perioperative standard oral nutrition supplements versus immunonutrition in patients undergoing colorectal resection in an enhanced recovery (ERAS) protocol: a multicenter randomized clinical trial (SONV) study. Medicine (Balt) 2016;95:e3704. [DOI] [PMC free article] [PubMed]; Moya P, Soriano-Irigiaray L, Ramirez JM, Garcea A, Blasco O, Blanco F. et al. Perioperative standard oral nutrition supplements versus immunonutrition in patients undergoing colorectal resection in an enhanced recovery (ERAS) protocol: a multicenter randomized clinical trial (SONV) study. Medicine (Balt) 2016;95:e3704. doi: 10.1097/MD.0000000000003704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hill GL. Impact of nutritional support on the clinical outcome of the surgical patient. Clin Nutr 1994;13:331–40. [DOI] [PubMed]; Hill GL. Impact of nutritional support on the clinical outcome of the surgical patient. Clin Nutr. 1994;13:331–40. doi: 10.1016/0261-5614(94)90021-3. [DOI] [PubMed] [Google Scholar]
- [58].Hurt RT, McClave SA, Martindale RG, Ochoa Gautier JB, Coss-Bu JA, Dickerson RN, et al. Summary points and consensus recommendations from the International Protein Summit. Nutr Clin Pract 2017;32(Suppl 1):142S–51S. [DOI] [PubMed]; Hurt RT, McClave SA, Martindale RG, Ochoa Gautier JB, Coss-Bu JA, Dickerson RN. et al. Summary points and consensus recommendations from the International Protein Summit. Nutr Clin Pract. 2017;32(Suppl 1):142S–51S. doi: 10.1177/0884533617693610. [DOI] [PubMed] [Google Scholar]
- [59].Bollhalder L, Pfeil AM, Tomonaga Y, Schwenkglenks M. A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin Nutr 2013;32:213–23. [DOI] [PubMed]; Bollhalder L, Pfeil AM, Tomonaga Y, Schwenkglenks M. A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin Nutr. 2013;32:213–23. doi: 10.1016/j.clnu.2012.11.003. [DOI] [PubMed] [Google Scholar]
- [60].Stehle P, Ellger B, Kojic D, Feuersenger A, Schneid C, Stover J, et al. Glutamine dipeptide-supplemented parenteral nutrition improves the clinical outcomes of critically ill patients: a systematic evaluation of randomised controlled trials. Clin Nutr ESPEN 2017;17:75–85. [DOI] [PubMed]; Stehle P, Ellger B, Kojic D, Feuersenger A, Schneid C, Stover J. et al. Glutamine dipeptide-supplemented parenteral nutrition improves the clinical outcomes of critically ill patients: a systematic evaluation of randomised controlled trials. Clin Nutr ESPEN. 2017;17:75–85. doi: 10.1016/j.clnesp.2016.09.007. [DOI] [PubMed] [Google Scholar]
- [61].Ziegler TR, May Ak, Hebbar G, Easley KA, Griffith DP, Dave N, et al. Efficacy and safety of glutamine-supplemented parenteral nutrition in surgical ICU patients: an American multicenter randomized controlled trial. Ann Surg 2016;263:646–65. [DOI] [PMC free article] [PubMed]; Ziegler TR, May Ak, Hebbar G, Easley KA, Griffith DP, Dave N. et al. Efficacy and safety of glutamine-supplemented parenteral nutrition in surgical ICU patients: an American multicenter randomized controlled trial. Ann Surg. 2016;263:646–65. doi: 10.1097/SLA.0000000000001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Pradelli L, Mayer K, Muscaritoli M, Heller AR. n-3 fatty acid-enriched parenteral nutrition regimens in elective surgical and ICU patients: a meta-analysis. Crit Care 2012;16:R184. [DOI] [PMC free article] [PubMed]; Pradelli L, Mayer K, Muscaritoli M, Heller AR. n-3 fatty acid-enriched parenteral nutrition regimens in elective surgical and ICU patients: a meta-analysis. Crit Care. 2012;16:R184. doi: 10.1186/cc11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gong Y, Liu Z, Liao Y, Mai C, Chen T, Tang H, et al. Effectiveness of ω-3 polyunsaturated fatty acids based lipid emulsions for treatment of patients after hepatectomy: a prospective clinical trial. Nutrients 2016;8:357. [DOI] [PMC free article] [PubMed]; Gong Y, Liu Z, Liao Y, Mai C, Chen T, Tang H. et al. Effectiveness of ω-3 polyunsaturated fatty acids based lipid emulsions for treatment of patients after hepatectomy: a prospective clinical trial. Nutrients. 2016;8:357. doi: 10.3390/nu8060357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Grass F, Benoit M, Coti Bertrand P, Sola J, Schäfer M, Demartines N, et al. Nutritional status deteriorates postoperatively despite preoperative nutritional support. Ann Nutr Metab 2016;68:291–7. [DOI] [PubMed]; Grass F, Benoit M, Coti Bertrand P, Sola J, Schäfer M, Demartines N. et al. Nutritional status deteriorates postoperatively despite preoperative nutritional support. Ann Nutr Metab. 2016;68:291–7. doi: 10.1159/000447368. [DOI] [PubMed] [Google Scholar]
- [65].Weimann A, Raab R, Selberg O, Bischoff S, Bornemann K, Müller MJ, et al. Perioperative changes in body composition and metabolism in patients with colorectal cancer according to tumor stage. Onkologie 1996;19:424–9.; Weimann A, Raab R, Selberg O, Bischoff S, Bornemann K, Müller MJ. et al. Perioperative changes in body composition and metabolism in patients with colorectal cancer according to tumor stage. Onkologie. 1996;19:424–9. [Google Scholar]
- [66].Ravasco P, Monteiro-Grillo I, Camillo M. Individualized nutrition intervention is of major benefit to colorectal cancer patients: long-term follow-up of a randomized controlled trial of a nutritional therapy. Am J Clin Nutr 2012;96:1346–53. [DOI] [PubMed]; Ravasco P, Monteiro-Grillo I, Camillo M. Individualized nutrition intervention is of major benefit to colorectal cancer patients: long-term follow-up of a randomized controlled trial of a nutritional therapy. Am J Clin Nutr. 2012;96:1346–53. doi: 10.3945/ajcn.111.018838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.