Abstract
Background
Elevated depressive symptoms among survivors of acute coronary syndromes (ACS) confer recurrent cardiovascular events and mortality, worse quality of life, and higher healthcare costs. While multiple scientific groups advise routine depression screening for ACS survivors, no randomized trials exist to inform this screening recommendation. We aimed to assess the effect of screening for depression on change in quality of life over 18 months among ACS patients.
Methods
The Comparison of Depression Identification after Acute Coronary Syndrome on Quality of Life and Cost Outcomes (CODIACS-QoL) trial is a pragmatic, 3-arm trial that randomized ACS patients to 1) systematic depression screening using the 8-item Patient Health Questionnaire (PHQ-8) and if positive screen (PHQ-8≥10), notification of primary care providers (PCPs) and invitation to participate in centralized, patient-preference, stepped depression care (Screen, Notify, and Treat, N=499); 2) systematic depression screening and PCP notification only (Screen and Notify, N=501); and 3) usual care (No Screen, N=500). Adults hospitalized for ACS in the previous 2–12 months without prior history of depression were eligible for participation. Key outcomes will be quality-adjusted life years (primary), cost of health care utilization, and depression-free days across 18 months.
Results
A total of 1,500 patients were randomized in the CODIACS-QOL trial (28.3% women; 16.3% Hispanic; mean age 65.9 (11.5) years). Only 7% of ACS survivors had elevated depressive symptoms.
Conclusions
Using a novel randomization schema and pragmatic design principles, the CODIACS-QoL trial achieved its enrollment target. Eventual results of this trial will inform future depression screening recommendations in cardiac patients.
Trial registration
Keywords: depression, screening, protocol
Background
Depression is three times more common in patients following acute coronary syndromes (ACS) than in the general population, with a prevalence of approximately 15% to 20%.1 With over one million ACS cases occurring annually,2 almost 200,000 individuals will have clinically significant depression. ACS survivors with depression have increased healthcare costs and utilization,3 reduced quality of life and functional status,4 as well as higher risk of myocardial infarction (MI) recurrence and all-cause mortality.5,6,7,8
Given this compelling observational data, the American Heart Association (AHA) issued a science advisory in 2014 recommending routine post-ACS depression screening using validated tools and referral for comprehensive depression evaluation by a mental health specialist.9,10 There are now similar recommendations from the American Academy of Family Practitioners,11 European professional cardiology societies,12 and the National Heart Foundation of Australia.13
These recommendations for systematic depression screening in post-ACS patients have not been without controversy. Some experts argue that, while well-intentioned, they are not supported by evidence from randomized clinical trials (RCTs); in fact, two systematic reviews have concluded that no existing clinical trials exist assessing whether screening for depression improves depressive symptoms and cardiac outcomes in patients with cardiovascular disease.14–16 Further, despite the robust epidemiologic association between depression and adverse outcomes, there are few trials confirming that depression treatment improves cardiovascular prognosis.15 Experts have also raised concerns about low positive predictive values (below 50%) of depression screening in the post-ACS population,17 potentially resulting in misallocated resources. Furthermore, the potential for unintended harms from depression screening include adverse medication effects and stigma arising from labeling patients as depressed. Some have also argued that a true depression screening trial should exclude patients already previously diagnosed with depression.18,19
Two critical gaps must be addressed to determine if public health will be improved by depression screening after ACS: 1) Does depression screening, with or without direct referral to a mental health specialist for treatment, improve quality-adjusted life years, and 2) Is the cost of screening within acceptable amounts (e.g., ≤ $174,000/QALY)? To answer these questions, trials evaluating depression screening must randomize patients prior to screening. To our knowledge, there exists one cluster randomized screening trial of 45 English general practices testing an electronic point-of-care prompt for anxiety, depression and pain intensity screening among patients aged ≥45 years old with osteoarthritis compared to usual care (pain prompts only); researchers found no difference in depressive symptoms and higher pain symptoms in the enhanced screening group, calling into question the benefits of screening.20 Thus far, post-ACS depression screening recommendations were not based on a systematic review of the evidence. Even primary care guidelines, like the United States Preventative Services Task Force, which recommend systematic depression screening when depression care support is in place, are largely based on trials combining depression screening with adequate treatment support, thus confounding our understanding of the effect of screening alone. 19 Meanwhile, Canadian Task Force on Preventative Health Care21 and United Kingdom National Screening Committee recommend against systematic depression screening.22
The Comparison of Depression Identification after Acute Coronary Syndrome: Quality of Life and Cost Outcomes (CODIACS-QoL) RCT aims to address gaps in the understanding of the effectiveness of depression screening in cardiac patients by comparing the effectiveness of (1) screening, provider notification and centralized treatment, (2) screening and provider notification only and (3) no screening on health-related quality of life (primary outcome), depression-free days (measured at 6-month intervals), and cost of health care utilization and ultimately inform AHA’s post-ACS depression screening advisory.
Design Overview
The CODIACS-QoL trial is an investigator-initiated, multicenter, randomized prospective 3-arm trial randomizing post-ACS patients to 1) depression screening with notification of treating providers and linkage to enhanced depression treatment; 2) depression screening and notification, alone; or 3) no depression screening. The timetable of key study procedures and assessments is shown in Table 1. The Center for Behavioral Cardiovascular Health at Columbia University Medical Center served as the centralized coordinating center for the trial. To maximize the applicability of trial results to clinical practice, the trial design was pragmatic,23 an innovative approach with the potential to inform other real world trials. Pragmatic characteristics included: eligibility criteria that were inclusive of the broad group of post-ACS patients eligible for depression screening; case identification using automated electronic medical record (EMR) searches; enrollment of a socioeconomically diverse patient population through recruitment at geographically-diverse health systems; remote (via telephone or internet) study assessments; availability of telephone-delivered psychotherapy for positive screens who were referred for treatment; and no withdrawals for noncompliance or unresponsiveness following randomization (i.e., intent-to-screen analyses). The study was approved by the Institutional Review Board of Columbia University Medical Center (New York, NY), as well as the three other recruitment sites. The trial was registered at ClinicalTrials.gov () prior to enrolling patients. A Data Safety and Monitoring Board (DSMB) comprised of experts in biostatistics, psychiatry, and cardiology ensured the safety of patients and the rigor of the conduct of the trial. The study was funded by NHLBI (R01 HL114924).
Table 1.
Schedule and Timing of Key Measurements and Procedures for CODIACS-QOL Trial
| Data Collected | Baseline | 6 month follow-up | 12 month follow-up | 18 month follow-up |
|---|---|---|---|---|
| Consent | X | |||
| Socio-demographics | X | |||
| Depressive symptoms (CESD-10) | X | X | X | X |
| Patient Health Questionnaire (PHQ-8)a | X | X | ||
| Depression Treatment Preferenceb | X | |||
| Self-reported health service use | X | X | X | X |
| Quality of life scale (SF-12) | X | X | X | X |
| Interim depression treatment/care, work days missed, unemployment | X | X | X | |
| Healthcare utilization (outpatient, ED, hospital) | X | X | X | |
| Symptom checklist | X | X | X | X |
Depression Screen, Notify and Treat and Depression Screen and Notify groups only
Depression Screen, Notify and Treat group only (in positive depressions screens)
Abbreviations: SF-12, 12-item Short Form Version 2™ survey; ED, emergency department; CESD-10, 10-item Center for Epidemiologic Studies Depression scale
Study Setting
Patients were recruited from 4 sites in geographically diverse regions of the United States: HealthPartners (Minneapolis, MN); Duke University Health System (Durham, NC); Kaiser Permanente Northwest (Portland, OR); and New York-Presbyterian Hospital/Columbia University Irving Medical Center (New York, NY). The selection of participating health care organizations was informed by each having an integrated healthcare system and an established and searchable EMR to facilitate depression screening and data collection for cost-effectiveness analyses.
Eligibility criteria
Patients were eligible for enrollment if they met the following inclusion criteria: (1) documented ACS within 2–12 months of enrollment based on standardized International Classification of Diseases, Ninth Edition (ICD-9) or ICD-10 discharge codes for acute MI and unstable angina;24 (2) age at randomization of ≥ 21 years; and (3) access to a telephone. Patients were excluded if they had any of the following criteria (self-reported or EMR-based): primary language was not English or Spanish (self-report only); current receipt of depression treatment, past history of depression (self-reported or EMR); terminal illness (life expectancy of less than 1 year); prior or current history of bipolar disorder, suicidal risk, or psychosis; current substance abuse; dementia; current pregnancy; severe arthritis/rheumatologic illness; advanced liver disease requiring frequent hospitalizations; advanced heart failure such as New York Heart Association class IV requiring heart transplant or mechanical device; advanced lung disease needing oxygen at home; advanced HIV infection or AIDS, or advanced cancer of any kind. Of note, the study had planned to include those with a history of depression so long as they were not currently receiving depression treatment. However, prior to the start of the trial, eligibility criteria were modified to exclude those with a history of depression based on concerns raised by our DSMB that depression screening should refer to patients whose depression has not already been recognized by health care providers.19
Although the AHA scientific advisory recommends depression screening in all patients with coronary heart disease (CHD), we focused on patients with recent ACS (MI or unstable angina with documentation of the presence of coronary artery disease (CAD)), as this is the population for which there is the strongest observational evidence of ACS recurrence and mortality risk associated with elevated depressive symptoms.25 Patients were not eligible until at least 2 months after the ACS event to exclude those with a transient increase in depressive symptoms, which often spontaneously remit after the ACS event and may carry a lower prognostic risk.26,27
Recruitment, enrollment, and informed consent
To further align with our pragmatic design, each site worked within their capabilities (e.g., resources, workflows, and EMRs) to identify patients based on the above eligibility criteria. Sites identified potentially eligible patients by screening either the EMR or, when applicable, the healthcare organization’s insurance claims. Sites searched for potentially eligible participants primarily by identifying those with a recent documented ACS and by excluding those with depression history and other ineligible conditions based on ICD codes. Three sites sent an email and/or a letter introducing the study to potentially eligible patients with a link to an on-line eligibility questionnaire delivered 2 to 12 months after the ACS event. Participants learned that the study was about understanding the benefits of screening for depressive symptoms, with the exact wording customized by site. Those who did not complete the initial on-line eligibility questionnaire were contacted by phone to complete the questionnaire. A fourth site (New York-Presbyterian Hospital) provided potentially eligible patients with a study information sheet while hospitalized for the index ACS, and then contacted them by phone 2–12 months later to complete the eligibility questionnaire.
Patients who expressed interest in the study and remained eligible after completing the eligibility questionnaire then provided verbal informed consent by phone to be randomized. The consent forms reiterated that the study aimed to understand the benefits of screening for elevated depressive symptoms in cardiac patients. At this time, they also completed a baseline questionnaire which included age, sex, race, ethnicity, country of birth, education, language, partner status, and health insurance coverage.
Randomization, allocation, and blinding
Patients were randomized in a 1:1:1 ratio to one of three groups: 1) systematic depression screening with notification of primary care providers (PCP) and invitation to participate in patient-preference, stepped care depression treatment (Screen, Notify, and Treat), 2) systematic depression screening with notification of PCPs for positive screens, only (Screen and Notify), and 3) usual care (No Screen). Randomization was stratified by site and was generated using a random number generator in SAS version 9.4. Randomization assignments were made in block sizes of 3, 6, or 9 patients. Randomization occurred after an unblinded research assistant (RA) logged onto a secure website and entered required information about the participant (e.g., eligibility status and study site). Concealment was ensured as the randomization assignment only became visible to the RA after all necessary information about the participant had been entered. The RA then completed the visit according to the group to which the patient was assigned (see Interventions section, below). Randomization allocation was otherwise only known to the unblinded RA. To mask outcomes assessments, all subsequent study visits are currently being conducted by a different RA blinded to randomization assignment.
Interventions
1. Depression Screen, Notify and Treat
Patients assigned to the Screen, Notify, and Treat group were screened for depression using the 8-item Patient Health Questionnaire (PHQ-8). Both the PHQ-9 and PHQ-8 have good sensitivity and specificity for detecting depressive disorders in patients with cardiovascular disease,28 but the PHQ-8 omits the item asking about suicidal ideation and is thus ideally suited for research studies in which assessments are administered remotely. Patients with clinically significant depressive symptoms (PHQ-8 score ≥10) had their PCP and/or treating cardiologist notified of their elevated depressive symptoms via letter.
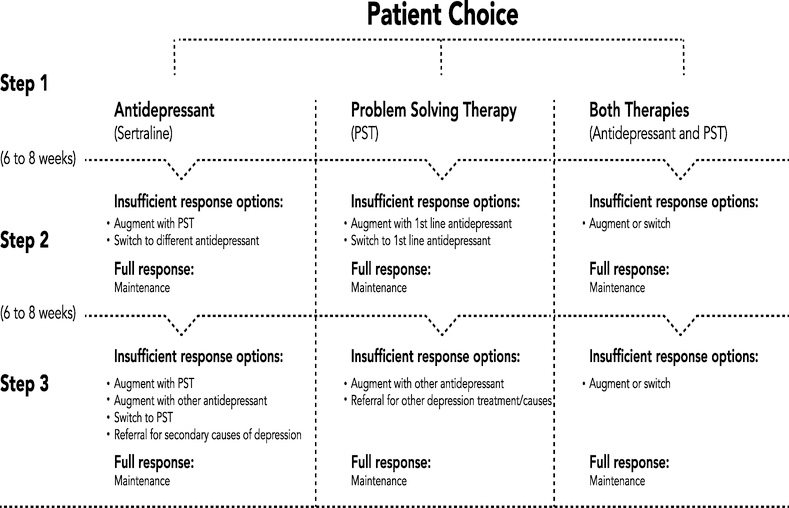
Patients with elevated depressive symptoms were provided with education regarding depression treatment options and offered a 6-month course of patient-preference, stepped care depression treatment regardless of insurance status following the format used in our previously published CODIACS trial.29 An unblinded RA provided patients with information about the following depression treatment options: telephone-delivered problem solving therapy (PST), antidepressant medication, both, or neither (Insert Figure 1). A team comprising the unblinded site coordinator, the central PST therapist (based at the Center for Behavioral Cardiovascular Health), and a site medication prescriber (psychiatrist, internist, or psychiatric nurse practitioner with prescribing privileges), then delivered the stepped care treatment based on patient preference. Patients notified that depression treatment was merited still had the option to seek depression treatment from their regular healthcare provider(s) rather than the study-provided treatment.
Figure 1.
Stepped Care Algorithm for CODIACS-QOL Trial Patients Assigned to the Screen, Notify, and Treat Group with Elevated Depression Scores
Problem Solving Therapy
PST is a relatively brief, goal-oriented, collaborative, and emotionally supportive form of treatment, that can be easily adapted to typical psychosocial problems and mild-to-moderate depression commonly observed in cardiac populations.30 Similar to protocols used in prior studies, patients who chose PST received sessions by telephone,31,32,33 PST is a protocol-driven and problem-focused form of cognitive-behavioral therapy that teaches patients how to systematically solve psychosocial problems that can perpetuate depression and that is tailored to specific problems faced by patients.34 PST was delivered centrally from the coordinating center at Columbia University by a licensed clinical social worker with expertise in PST. Sessions initially occurred weekly, with each session lasting up to 60 minutes. Treatment was augmented according to an algorithm (Figure 1). As depressive symptoms improved, session frequency was decreased to every 2 weeks, and if progress continued, the frequency was further reduced.
Antidepressant Medication
Patients who chose medication therapy are scheduled to meet with a site clinician, either a psychiatrist, internist, or nurse practitioner with expertise in mental health care, that could prescribe antidepressant medications. Antidepressant medication options included sertraline as first-line treatment and bupropion for treatment failure. Patients prescribed antidepressant medications were evaluated 1 week after initiating medication, and then every 1–2 weeks after dose titrations. The focus of these treatment contacts was to re-evaluate depressive symptoms and to detect adverse effects of medication. A more formal assessment of responsiveness to antidepressants occurred as part of the stepped care algorithm every 6–8 weeks.
Centralized Stepped Care Algorithm
Patients with elevated depressive symptoms who agreed to depression treatment by the study team subsequently underwent systematic tracking of depressive symptoms using the 10-item Center for Epidemiologic Studies Depression (CESD-10) scale every 6–8 weeks, with depression treatment adjusted according to a stepped-care algorithm with predefined decision rules (Figure 1).35 For those who started with both treatments, the augmentation procedures were determined by patient preference. A centralized psychiatrist was available to meet with both the PST specialist and medication prescriber bi-weekly to discuss active patients and participant issues as well as to jointly determine stepped-care decisions within the patient-chosen therapy. Successful treatment response was defined as CESD-10 ≤4 on two consecutive treatment sessions at which time patients review a relapse prevention plan and transition back to their PCPs.
2. Depression Screen and Notify
Similar to patients assigned to the Screen, Notify and Treat group, patients assigned to the Screen and Notify group were systematically screened for depression using the PHQ-8. Patients’ cardiologists and/or PCPs were notified in writing if a patient screened positive for depression (PHQ-8 score ≥10). Subsequent depression treatment decisions were made by the patient’s treating providers. Such patients were not eligible for centralized, patient-preference driven stepped depression care provided by study clinicians.
3. No Depression Screen (control group)
Patients assigned to the No Depression Screen group were not systematically screened for depression using the PHQ-8. Patients received usual care from their treating providers, and were eligible to seek mental health screening and/or treatments at their own discretion.
Primary Outcome
Quality-Adjusted Life Year (QALYs)
The primary outcome is change in QALYs from baseline through 18 months post-randomization. QALYs describe the duration of illness per years of survival, adjusted for quality of life experienced during that survival. One year in perfect health is equivalent to 1 QALY. All patients will complete a standardized measure of quality of life using the Short Form-12 Health Survey, Version 2™ (SF-12) at baseline, and again at 6-months, 12-months, and 18-months.36 QALYs will be estimated from the SF-12 using the Short Form 6 Duration (SF6D) which converts data from 7 items in the SF-12 assessing 6 domains (physical functioning, role limitations, social functioning, pain, mental health and vitality) to QALYs.37 Extensive validation, including construct validity, published normative data, and sensitivity to change have all been published for this scale.38–41 It has been used extensively in cost-effectiveness analyses of screening or treatment interventions and has successfully been used to assess the impact of depression interventions in other patient groups, allowing comparability with other data and settings.42 Change in QALYs were selected as the primary outcome to facilitate comparisons of the effect of depression screening with the effects of other preventive interventions. As QALYs do not directly assess depression, this outcome measure also minimized possible patient reporting bias as a result of lack of participant blinding or masking to condition. Accordingly, change in QALYs was the outcome we selected to determine whether the trial was a success, and was the sole outcome used to estimate our sample size. QALYs across 18 months will be calculated as the area-under-curve by interpolating linearly the scores at the four assessments.
Secondary Outcomes
Depression-free days will be calculated by using linear interpolation to estimate daily depression severity at each of the three follow-up assessment time points for the 3 study arms. To assess baseline and follow-up depressive symptoms across intervention arms, all patients will complete the 10-item Center for Epidemiologic Studies Depression (CESD-10) scale, a reliable and valid measurement for depressive symptoms, at baseline, 6-months, 12-months, and 18-months.35 Depression-free days have been validated in intervals as long as 6 months.43 The CESD-10 is a short version of the original 20-item scale. The scores range from 0 to 30, and a score of 10 or greater is considered significant. The CESD-10 will be used to estimate depressive symptoms throughout the trial. For calculations, we will convert a CESD score to a prorated depression day (Supplementary Material 2). Second, the prorated depression day will be used for interpolation to calculate depression days and then the depression free days will be calculated by subtracting the depression days from the total trial duration. Because the CESD-10 is considered an epidemiologic survey and not a diagnostic instrument, intervention among those with elevated scores in the No Depression Screen group is not mandated. Of note, the PHQ8 was chosen as the screening modality as it is more diagnostic and will be re-administered at 18-months for all 3 arms.
Health Care Costs and Lost Productivity
At baseline, 6-months, 12-months, and 18-months, patients will report measures of economic productivity, including employment status, occupation, hours spent at work, and time lost from work for health-related reasons.44 At 6-months, 12-months, and 18-months, patients will also report healthcare utilization since their last intake assessment, including emergency department (ED) visits, hospitalizations (location, admission and discharge dates), psychiatric medication use, name and dose, ambulatory care visits with mental health specialists, cardiologists, as well as PCPs and finally hospitalizations for cardiovascular events (Table 2). While we will not collect notification acknowledgement from the PCPs, we will ask patients about depression treatment at each follow up visit, which will further explain any potential improvements in these arms. Patient self-reports of healthcare utilization will be supplemented by review of the EMR and claims systems to collect data on healthcare utilization during the 18-month trial period. Average Medicare reimbursement rates according to diagnosis-related groups will be applied to inpatient visits to estimate hospitalization costs, and the Medicare physician fee schedule will be applied to outpatient and ED resource use according to current procedural terminology codes. Costs of study depression treatment will also be incorporated into estimates of healthcare utilization costs for those assigned to the Screen, Notify, and Treat group who agree to depression treatment by study personnel. To estimate economic costs from a societal perspective, changes in productivity and time spent traveling to appointments will also be accounted for. Costs will be standardized across years using the U.S. Consumer Price Index and presented in U.S. dollars.45 Of note, cost of health care utilization was erroneously listed as a co-primary outcome on the initial clinicaltrials.gov entry; this was corrected to being listed as a secondary outcome on clinicaltrials.gov prior to completion of data collection or any interim data analyses (change made on October 25, 2017). Further justifying cost-effectiveness as a secondary outcome, cost-effectiveness analyses were not guided by economic hypotheses involving statistical tests and sample size calculations for incremental cost-effectiveness ratios were never attempted.
Table 2.
Health Care Costs and Productivity Measures to be Collected for the CODIACS-QOL Trial
| From Electronic Medical Record | From Patient Report | From Trial Study Staff | |
|---|---|---|---|
| Total Depression Treatment Costs | |||
| Depression screening cost | X | ||
| Antidepressant cost, therapy cost, other mental health visits | X | X | X |
| Depression-free days | X | ||
| Time spent by patient in screening/treatment | X | ||
| Total Non-Depression Costs | |||
| Primary care, Emergency Department/urgent care, and other medical visits; diagnostic, inpatient, and other outpatient services | X | X | |
| Days able to work/impact on work and leisure productivity | X | ||
| Transit time | X | ||
Additional Measures
To further assess potential adverse consequences of depression screening and treatment, a symptom checklist assessing appetite, drowsiness/fatigue, insomnia, sexual side effects, gastrointestinal upset, and gastrointestinal bleeding will be administered at baseline, 6-months, 12-months, and 18-months. A summary of the timing of key measurements is provided in Table 1.
Statistical Analyses
Baseline characteristics were examined as means (standard deviation) or percentages by randomization assignment and site to assess for a balanced allocation. CESD-10 questionnaire with missing data were prorated if that participant answered more than 7 items. (There was only 28 (1.9%) participants whose CESD-10 score was prorated). All future analyses will use the principle of intention-to-screen.
Quality-adjusted life years
Change in QALYs from baseline through 18 months post-randomization will serve as the primary outcome for this trial. The goal of the primary analysis is to identify whether there is difference in QALYs among the three groups. We plan to perform a two-step gate-keeping test procedure: We will first perform an F-test using ANOVA, and then proceed to do all three pairwise comparisons using two-sided t-test at 5% nominal significance only if the F-test has a p-value less than 0.05. With three randomization groups and three pairwise comparisons, this two-step procedure can be shown to preserve the familywise error rate in the strong sense, that is, a false positive comparison will occur at most with 5% probability under all possible scenarios.46,47 This method is also generally more powerful than Bonferroni’s adjustments. We opted to use change in QALY as the primary endpoint as it’s a conventional way to summarize longitudinal quality of life data, and analyze the data using ANOVA without additional assumption on the variance-covariance structure of the data. All time points will be included (Supplementary Material 1.
In a secondary analysis, we will use a mixed model with a random intercept to assess the change in quality of life over time comparing individuals in the Screen, Notify, and Treat group vs. the other two randomization groups. Specifically, this model will include time (0-, 6-, 12-, and 18-month) and randomization group as main effects, and the time-by-group interaction. Under this model and randomization, we expect that there will be no main effect of randomization group. Also, a significant interaction will indicate difference in the trajectory of quality of life between groups. Additionally, under the mixed model regression framework, we will explore non-linear time trend by including a quadratic term for time, and we will explore adjusted analyses by including other baseline covariates should imbalances arise between groups (Supplementary Material 1).
Depression free days. We will first perform an omnibus F-test using ANOVA comparing the 3 arms to one another, and plan to do pairwise comparisons using two-sided t0test at 5% nominal significance only if the omnibus F-test had a p-value less than 0.05. Tis procedure would control the familywise type 1 error rate at 5%.
Cost-effectiveness
We will judge the cost-effectiveness of each of the groups on the basis of incremental cost-effectiveness ratios. The base case analysis will adopt a societal perspective with respect to costs and health benefits. Costs will include all healthcare costs and lost productivity as delineated above, and will be denominated in 2016 dollars and discounted at an annual rate of 3% according to recommendations from the Panel on Cost-Effectiveness in Health and Medicine.48 Incremental cost-effectiveness analyses for comparison of treatment groups will be calculated sequentially, using the ratio of the difference in average cost per participant divided by the difference in average QALY gained. We will first compare the strategy of Depression Screen, Notify, and Treat with Screen and Notify, then compare the strategy of Screen and Notify with No Screen. This allows us to assess whether either of the treatment strategies strongly dominates (i.e., lower costs but greater benefits). If no strategy strongly dominates, we will qualitatively compare the cost-effectiveness based on the World Health Organization’s recommendation of using 3 times per-capita GDP as an upper bound for cost-effectiveness to determine if the intervention arms provide benefit in QALYs at good value.49 The outcome variable for the cost-effectiveness analysis will be cost-effectiveness ratios in dollars per QALY. We will estimate confidence intervals for cost-effectiveness ratios using nonparametric bootstrapping with 1,000 random samples, and we will use the bias-corrected percentile method described by Efron and others.50–53 The proportion of dominated-cost-effectiveness ratios, which are unfavorable and occur then incremental costs are higher while incremental costs are higher while incremental QALYs are lower, will be estimated to further inform the uncertainty analysis. The 2016 US per-capita GDP of approximately $58,000 equates to a cutoff of $174,000 per QALY gained https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=US.
We will perform sensitivity analyses by adopting a range of ‘best and worst case’ scenarios for resource costs (including actual costs from the four health care systems, or from estimates obtained using charge-to cost ratios), a range of initial depression prevalence, and depression treatment benefit (including considerations of recall bias for self-reported measures). We will also recalculate the incremental cost-effectiveness ratios separately by health care system, so that a range by site can be presented. Nonparametric bootstrapping will be used to obtain 95% confidence intervals for estimates of cost-effectiveness ratios to determine the robustness of our results. In addition, we will repeat our baseline analysis and sensitivity analysis after restricting cost accounting to the payer perspective only.
Sample size
The sample size of the trial was determined based on an assumed standard deviation for QALYs of 0.17.54 Additionally, based on a prior study of management of depression for patients with cancer, we assumed a net improvement in QALYs of 0.155 over 18 months of follow-up for depressed individuals who receive depression treatment in the Screen, Notify and Treat group.55 We assumed a 0.055 gain in QALYs for all patients not directly linked to depression treatment (i.e., those in the No Screen group, the Screen & Notify group and those without elevated depressive symptoms in the Screen, Notify, and Treat group). An important consideration for this trial is that only 20% of patients randomized to the Screen, Notify and Treat group were expected to meet criteria for elevated depressive symptoms and thus, to receive depression treatment. Therefore, assuming an increase in QALYs of 0.21 (0.055 background improvement + net improvement of 0.155) over the 18-month follow-up period for the 20% of patients diagnosed and treated for depression in the Screen, Notify, and Treat group and a 0.055 improvement in QALYs for the 80% of patients in this randomization group without depression, an overall gain in QALYs of 0.086 over the 18 month follow-up period was anticipated in this randomization group (0.21 * 0.2 + 0.055 * 0.8 = 0.086). Thus, we anticipated a difference in QALYs of0.031 (0.086 change in the Screen, Notify, and Treat group minus 0.055 in the No Screen group or Screen and Notify Group), leading to an expected effect size of 0.18 (= 0.031/0.17). With this effect size, we determined the sample size per group to be 475, which would yield 80% power for a two-sided t-test at 5% level. We chose to determine sample size based on a pairwise comparison at 80% power in the two-step procedure (described above), as a conservative approach relative to powering based on the F-test. Specifically, under the scenario where one group has higher QALY than the other two by an effect size 0.18, the F-test will yield 84% power. Adding in 5% loss to follow-up, we selected an overall sample size of n=500 in each randomization group for an overall sample size of n=1500.
Baseline Results
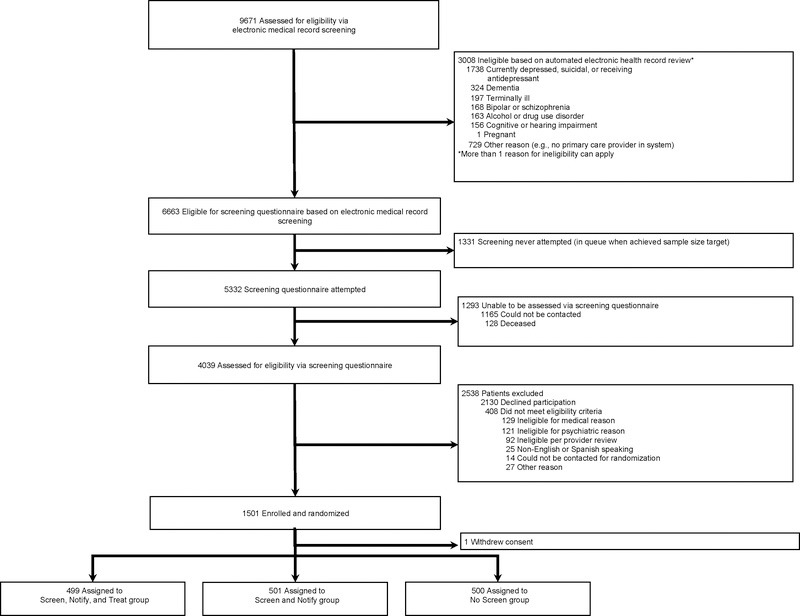
Recruitment took place from January 1, 2014 to April 30, 2017. In total 9,671 patients underwent EMR screening for eligibility, and 5332 were attempted to be contacted to complete a study eligibility screening questionnaire (Insert Figure 2). Of these, 4039 patients were reached and assessed for eligibility by completing an on-line or telephone screen, and 1,501 patients ultimately provided informed consent to be randomized into the RCT. One patient requested to have all data withdrawn (n=1), leaving 1,500 patients available to be analyzed in the trial (499, Screen, Notify, and Treat; 501, Screen and Notify; and 500, No Screen).
Figure 2.
Screening, Enrollment, and Randomization of Acute Coronary Syndrome Patients in CODIACS-QOL Trial
Mean age (standard deviation) of patients was 65.9 (11.5) years old; 71.7% were male; 16.3% were Hispanic; and 8.8% were black (Table 3). The average CESD-10 score at enrollment was 4.8 (4.8) and 14.1% had a CESD-10 score ≥10, indicative of elevated depressive symptoms. Of those in the depression screen arms, 38 (7.7%) in the screen, notify and treat group and 33 (6.6%) of screen and notify group screened positive for depression (PHQ-8 score ≥10); participants in the No Screen group did not complete the PHQ-8. There were no notable differences in baseline sociodemographic, medical, or psychiatric characteristics between the three groups.
Table 3.
CODIACS-QOL Trial Baseline Patient Characteristics Overall and by Randomization Group Assignmenta
| Variables | Overall (N=1,500) | Screen, Notify and Treat (n = 499)b | Screen and Notify (n = 501) | No Screen (n = 500) |
|---|---|---|---|---|
| Age in years, mean (SD) | 65.9 (11.5) | 66.2 (11.3) | 65.8 (11.7) | 65.8 (11.7) |
| Male | 1076 (71.7%) | 357 (71.5%) | 364 (72.7%) | 355 (71.0%) |
| Ethnicity | ||||
| Hispanic | 244 (16.3%) | 82 (16.4%) | 88 (17.6%) | 74 (14.8%) |
| Non-Hispanic | 1218 (81.2%) | 406 (81.4%) | 402 (80.2%) | 410 (82.0%) |
| Refused/Unknown | 38 (2.5%) | 11 (2.2%) | 11 (2.2%) | 16 (3.2%) |
| Education | ||||
| High school or lower | 536 (36.0%) | 191 (38.7%) | 178 (35.7%) | 167 (33.7%) |
| Some college | 336 (22.6%) | 105 (21.3%) | 119 (23.9%) | 112 (22.6%) |
| College and higher | 615 (41.4%) | 197 (40.0%) | 201 (40.4%) | 217 (43.8%) |
| Married | 966 (64.9%) | 318 (64.2%) | 336 (67.5%) | 312 (62.9%) |
| Covered by health insurance | 1432 (96.0%) | 475 (95.8%) | 482 (96.6%) | 475 (95.8%) |
| Race | ||||
| White | 1080 (73.5%) | 353 (71.7%) | 368 (75.6%) | 359 (73.3%) |
| Black | 130 (8.8%) | 47 (9.6%) | 37 (7.6%) | 46 (9.4%) |
| Other | 259 (17.6%) | 92 (18.7%) | 82 (16.8%) | 85 (17.3%) |
| PHQ-8 score ≥10 | N/A | 38 (7.7%) | 33 (6.6%) | N/A |
| CESD-10 score, mean (SD) | 4.8 (4.8) | 4.9 (5.1) | 4.8 (4.9) | 4.7 (4.5) |
| CESD-10 score ≥ 10 | 210 (14.1%) | 72 (14.6%) | 65 (13.0%) | 73 (14.8%) |
| SF-12 Mental score, mean (SD) | 54.0 (9.0) | 54.0 (9.6) | 53.8 (9.0) | 54.2 (8.4) |
| SF-12 Physical score, mean (SD) | 43.0 (11.8) | 42.5 (11.8) | 44.2 (11.5) | 42.2 (11.9) |
| Employed | 606 (40.7%) | 205 (41.7%) | 198 (39.6%) | 203 (40.9%) |
Data are presented as N (%) unless otherwise specified; N in denominator may vary due to missing data, but fewer than 5% missing for any category.
1 participant withdrew and as such their data is not reported here.
As expected given the geographic diversity of our sites, there were substantial differences in key demographics across the four sites, including a higher proportion of Hispanics and lower educational attainment at the New York City site and a higher proportion of blacks enrolled from Duke University Health System in North Carolina (Table 4). In addition, patients recruited from HealthPartners in Minnesota had the lowest depressive symptom scores.
Table 4.
CODIACS-QOL Trial Baseline Patient Characteristics by Study Sitea
| Variables | Columbia-New York Presbyterian (n = 631) | Kaiser Permanente (n = 522) | Health Partners (n = 304) | Duke University Health System (n = 43) | P-value |
|---|---|---|---|---|---|
| Age in years, mean (SD) | 65.3 (11.9) | 68.7 (11.1) | 62.7 (10.8) | 65.6 (10.5) | <0.001 |
| Male | 460 (72.9%) | 356 (68.2%) | 231 (76.0%) | 29 (67.4%) | 0.08 |
| Hispanic | 228 (36.1%) | 13 (2.5%) | 3 (1.0%) | 0 (0%) | <0.001 |
| Education | <0.001 | ||||
| High school or lower | 272 (43.6%) | 147 (28.3%) | 105 (34.9%) | 12 (27.9%) | |
| Some college | 117 (18.8%) | 161 (31.0%) | 44 (14.6%) | 14 (32.6%) | |
| College and higher | 235 (37.7%) | 211 (40.7%) | 152 (50.5%) | 17 (39.5%) | |
| Married | 364 (58.1%) | 354 (68.7%) | 219 (72.0%) | 29 (67.4%) | <0.001 |
| Covered by health insurance | 594 (95.2%) | 502 (96.5%) | 296 (97.4%) | 40 (93.0%) | 0.27 |
| Race | |||||
| White | 311 (51.0%) | 464 (90.1%) | 279 (92.7%) | 26 (60.5%) | <0.001 |
| Black | 95 (15.6%) | 9 (1.7%) | 11 (3.7%) | 15 (34.9%) | |
| Other | 204 (33.4%) | 42 (8.2%) | 11 (3.7%) | 2 (4.7%) | |
| CESD-10 score, mean (SD) | 5.3 (5.2) | 5.3 (4.9) | 2.8 (3.0) | 5.9 (5.7) | <0.001 |
| CESD-10 score ≥ 10 | 107 (17.1%) | 82 (15.9%) | 12 (3.9%) | 9 (20.9%) | <0.001 |
Data are presented as N (%) unless otherwise specified; N in denominator may vary due to missing data, but fewer than 5% missing for any category.
Discussion
We have described the rationale and design of a multicenter randomized trial assessing the effectiveness of a widely recommended depression screening and treatment intervention in ACS survivors. We demonstrate successful recruitment of a diverse sample of ACS survivors, broadly representative of ACS-survivors across the United States. Strengths of our design include its pragmatic nature which increase the applicability of our results to health systems around the country. If effective at improving quality of life in a cost-effective manner, our centralized depression screening, notification, and treatment intervention may provide a way for health systems to broadly disseminate enhanced depression care strategies, even among health care systems without easy access to mental health resources for all patients. On the other hand, if our systematic depression screening intervention does not prove itself to be more effective than usual care, then it may suggest a need to reconsider depression screening recommendations.
Ours is among the first depression screening trials to assess the effect of direct depression screening in patients with no prior history of depression. Few if any rigorous depression screening RCTs using this criterion have ever been conducted, even among the more general patient population.18 Thus, our trial will have the opportunity to inform depression screening guidelines more broadly. Likely as a result of rendering ineligible those with a past history of depression or antidepressant use, we identified a lower than expected prevalence of elevated depressive symptoms (14% by CESD-10 and 7% by PHQ-8). Relatedly, we also identified differences in the prevalence of depression between sites. Site-specific differences in depression screening policies at primary care practices affiliated with our sites may have differentially impacted the ability of centralized depression screening approach to identify previously undetected cases of depression. Although cost-effectiveness analyses remain to be conducted, this low prevalence of positive depression screens among post-ACS individuals with no history of depression decreases the opportunity for systematic depression screening and linkage to depression treatment to be a cost-effective approach, if screening is to be for undetected and untreated patients as many experts believe.19 In addition, our baseline data reveals the large number of patients that need to be screened to identify positive depression cases, farther suggesting the limited opportunity for improvement with systematic screening given the low yield.
A few limitations will need to be considered when evaluating our data. As mentioned above, we excluded patients who reported a prior history of depression or antidepressant use. Patients with a history of depression still have potential to benefit from a systematic assessment of depressive symptoms after ACS as symptoms may worsen and/or may be sub-optimally treated, and this trial will not be able to comment on the effectiveness of systematically monitoring depressive symptoms in such patients.56 Further, the exclusion of those with a history of depression in the final study protocol may have contributed to our lower than expected prevalence of positive depression screens at baseline. This low case detection rate may have reduced the statistical power of our study. Nonetheless, even if we have lower than expected power to detect differences in QALYs between depression screening strategies, the assumptions used in our power calculation (i.e., standard deviation of QALYs equal = 0.17) will allow us to estimate a 95% confidence interval width of 0.02 QALY, which is quite narrow. Thus, even if the trial is negative, (i.e., no statistically significant difference in QALYs by depression screening strategy), we will still be able to confidently conclude that the effect of depression screening, if any, is small and of questionable clinical significance.
Our reliance on a centralized approach to depression screening may have led to a lower uptake of depression screening (about 1 in 4 ACS patients declined to be screened) than if screening was implemented within the post-ACS clinical care setting, and we did not follow those who declined to assess the degree to which they differed from participants. That said, depression screening is often sub-optimally implemented in care settings with only a fraction of eligible patients screened, and a systematic, centralized process within integrated health care systems has the potential for greater reach than relying on front-line clinicians. The screening and consent process explained that the goal of the study was to assessed the benefit of screening for “elevated depressive symptoms” in cardiac patients, and this may have influenced participants’ willingness to participate. To avoid excessive contacts, we farther used 6-month intervals for the computation of depression free days, which may result in imprecise estimates. There is, however, precedent for intervals of this duration in prior literature.43 In recent years, depression screening has become more common in primary care-based settings, potentially decreasing opportunity to identify new positive depression screens through our centralized screening approach. In fact, the lower rates at HealthPartners may have been due to their better depression detection rates and EMR documentation, potentially resulting in fewer undiagnosed cases. The use of centralized, telephone-delivered psychotherapy as opposed to in-person psychotherapy in the intervention arm may also have influenced depression treatment acceptability, all potentially resulting in differing effect estimates than were used in our power calculations. Furthermore, we used the PHQ-8 to screen for depression in lieu of the full PHQ-9, partly in response to participant safety concerns raised by our DSMB. Nevertheless, prior research suggests that the omitted ninth item, which assesses suicidality, is rarely positive and may not be an accurate suicide screen in CAD patients, making the PHQ-8 a better option57 The PHQ-8 is also highly correlated with the PHQ-9,58 and has a high specificity (91%) for detecting probable depression case status compared with a psychiatric interview in ACS patients.59 Also, direct comparisons in regards to screen and treat arm will only apply to management and not screening parse, while comparisons between no screen and screen and treat arms will confound screening and better management options; we will not able to compare screening and depression management to the same management without screening, the ideal scenario. Nonetheless, if the screen and treat arm does not result in better health outcomes than the no screening arm, we will be able to conclude that screening is not a beneficial strategy. Furthermore, while recent trials suggest long-term benefits of depression treatment,60 18 months or shorter have been used widely in this literature in assessing quality of life.61,62 A final limitation is that our sample is limited to post-ACS patients. Patients with other cardiac diseases including stable CHD and heart failure are also susceptible to increased rates of depression and worse depression-associated outcomes. Our study will not provide direct evidence to inform depression screening guidelines in these patient populations.
These limitations notwithstanding, the CODIACS-QOL trial will be one of the largest depression screening trials conducted in any patient population. The results from this trial will provide a greater understanding of the costs and benefits of depression screening in ACS survivors. Accordingly, the results of this trial will bring the highest level of evidence to inform the next generation of depression screening guidelines in cardiac patients.
Supplementary Material
Acknolwedgements
Funding Sources: This work was supported by funds from the National Institutes of Health (R01 HL114924; 3 R01 HL114924-03S1; R01 HL141609). The sponsors had no role in the design and conduct of the study, including the collection, management, analysis, interpretation of the data, preparation, review or approval of the manuscript and decision to submit the manuscript for publication.
Funder: National Heart, Lung, and Blood Institute (R01 HL114924)
Footnotes
Conflicts of interest: none to report
Declarations: none to report
Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thombs BD, Bass EB, Ford DE, Stewart KJ, Tsilidis KK, Patel U, et al. Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med 2006;21:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 3.Frasure-Smith N, Lesperance F, Gravel G, Masson A, Juneau M, Talajic M, et al. Depression and health-care costs during the first year following myocardial infarction. J Psychosom Res 2000;48:471–8. [DOI] [PubMed] [Google Scholar]
- 4.Swenson JR, O’Connor CM, Barton D, Van Zyl LT, Swedberg K, Forman LM, et al. Influence of depression and effect of treatment with sertraline on quality of life after hospitalization for acute coronary syndrome. Am J Cardiol 2003;92:1271–6. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006;27:2763–74. [DOI] [PubMed] [Google Scholar]
- 6.van Melle JP, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004;66:814–22. [DOI] [PubMed] [Google Scholar]
- 7.Smolderen KG, Spertus JA, Gosch K, Dreyer RP, D’Onofrio G, Lichtman JH, et al. Depression Treatment and Health Status Outcomes in Young Patients With Acute Myocardial Infarction: Insights From the VIRGO Study (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients). Circulation 2017;135:1762–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May HT, Brunisholz K, Horne B, Muhlestein J, Bair T, Lappe D, et al. Can effective treatment of depression reduce future cardiovascular risk? J Am Coll Cardiol 2016;67:1921. [Google Scholar]
- 9.Lichtman JH, Bigger JT, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lespérance F, et al. Depression and Coronary Heart Disease: Recommendations for Screening, Referral, and Treatment: A Science Advisory From the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: Endorsed by the American Psychiatric Association. Circulation 2008;118:1768–75. [DOI] [PubMed] [Google Scholar]
- 10.Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350–69. [DOI] [PubMed] [Google Scholar]
- 11.Post-Myocardial Infarction Depression Clinical Practice Guideline Panel. AAFP Guideline for the Detection and Management of Post-Myocardial Infarction Depression. Ann Fam Med 2009;7:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J 2007;28:2375–414. [DOI] [PubMed] [Google Scholar]
- 13.Colquhoun DM, Bunker SJ, Clarke DM, Glozier N, Hare DL, Hickie IB, et al. Screening, referral and treatment for depression in patients with coronary heart disease. Med J Aust 2013;198:483–4. [DOI] [PubMed] [Google Scholar]
- 14.Whooley MA. To screen or not to screen? Depression in patients with cardiovascular disease. J Am Coll Cardiol 2009;54:891–3. [DOI] [PubMed] [Google Scholar]
- 15.Thombs BD, Roseman M, Coyne JC, de Jonge P, Delisle VC, Arthurs E, et al. Does evidence support the American Heart Association’s recommendation to screen patients for depression in cardiovascular care? An updated systematic review. PLoS One 2013;8:e52654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thombs BD, de Jonge P, Coyne JC, Whooley MA, Frasure-Smith N, Mitchell AJ, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA 2008;300:2161–71. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwsma JA, Williams JW Jr., Namdari N, Washam JB, Raitz G, Blumenthal JA, et al. Diagnostic Accuracy of Screening Tests and Treatment for Post-Acute Coronary Syndrome Depression: A Systematic Review. Ann Intern Med 2017;167:725–35. [DOI] [PubMed] [Google Scholar]
- 18.Thombs BD, Ziegelstein RC, Roseman M, Kloda LA, Ioannidis JP. There are no randomized controlled trials that support the United States Preventive Services Task Force Guideline on screening for depression in primary care: a systematic review. BMC Med 2014;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thombs BD, Coyne JC, Cuijpers P, de Jonge P, Gilbody S, Ioannidis JP, et al. Rethinking recommendations for screening for depression in primary care. CMAJ 2012;184:413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallen CD, Nicholl BI, Lewis M, Bartlam B, Green D, Jowett S, et al. The effects of implementing a point-of-care electronic template to prompt routine anxiety and depression screening in patients consulting for osteoarthritis (the Primary Care Osteoarthritis Trial): A cluster randomised trial in primary care. PLoS Med 2017;14:e1002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joffres M, Jaramillo A, Dickinson J, et al. Recommendations on screening for depression in adults. CMAJ. 2013;185(9):775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UK National Screening Committee. Current UKNSC Recommendations. https://legacyscreening.phe.org.uk/screening-recommendations.php. Accessed 5 August 2019.
- 23.Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. CMAJ 2009;180:E47–E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis JC, Marra CA, Najafzadeh M, Liu-Ambrose T. The independent contribution of executive functions to health related quality of life in older women. BMC Geriatrics 2010;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strong V, Waters R, Hibberd C, Murray G, Wall L, Walker J, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet 2008;372:40–8. [DOI] [PubMed] [Google Scholar]
- 26.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003;108:2543–9. [DOI] [PubMed] [Google Scholar]
- 27.Zellweger MJ, Osterwalder RH, Langewitz W, Pfisterer ME. Coronary artery disease and depression. Eur Heart J 2004;25:3–9. [DOI] [PubMed] [Google Scholar]
- 28.Lane D, Carroll D, Ring C, Beevers DG, Lip GYH. The prevalence and persistence of depression and anxiety following myocardial infarction. Br J Health Psychol 2002;7:11–21. [DOI] [PubMed] [Google Scholar]
- 29.Kronish IM, Rieckmann N, Halm EA, Shimbo D, Vorchheimer D, Haas DC, et al. Persistent Depression Affects Adherence to Secondary Prevention Behaviors After Acute Coronary Syndromes. J Gen Intern Med 2006;21:1178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry 2010;32:345–59. [DOI] [PubMed] [Google Scholar]
- 31.Whang W, Burg MM, Carney RM, Freedland KE, Bigger JT, Catellier D, et al. Design and baseline data from the vanguard of the Comparison of Depression Interventions after Acute Coronary Syndrome (CODIACS) randomized controlled trial. Contemp Clin Trials 2012;33:1003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carney RM, Rich MW, Tevelde A, Saini J, Clark K, Jaffe AS. Major depressive disorder in coronary artery disease. Am J Cardiol 1987;60:1273–5. [DOI] [PubMed] [Google Scholar]
- 33.Whang W, Burg MM, Carney RM, Freedland KE, Bigger JT, Catellier D, et al. Design and Baseline Data from the Vanguard of the Comparison of Depression Interventions after Acute Coronary Syndrome (CODIACS) Randomized Controlled Trial. Contemp Clin Trials 2012;33:1003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106–16. [DOI] [PubMed] [Google Scholar]
- 35.Berkman L, Jaffe A, Carney R, Czajkowski S, Kaufman P, Blumenthal JA, et al. Enhancing recovery in coronary heart disease (ENRICHD) study intervention: Rationale and design. Psychosom Med 2001;63:747–55. [PubMed] [Google Scholar]
- 36.Ramanuj P, Ferenchick EK, Pincus HA. Depression in primary care: Part 2-management. BMJ. 2019;365:l835. [DOI] [PubMed] [Google Scholar]
- 37.Radloff LS. The CES-D ScaleA Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 38.Ware J, Kolinski M, Keller S. How to Score the SF-12 Physical and Mental Health Summaries: A User’s Manual. Boston, MA: The Health Institute, New England Medical Centre; 1995. [Google Scholar]
- 39.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851–9. [DOI] [PubMed] [Google Scholar]
- 40.De Smedt D, Clays E, Annemans L, De Bacquer D. EQ-5D versus SF-12 in coronary patients: are they interchangeable? Value Health 2014;17:84–9. [DOI] [PubMed] [Google Scholar]
- 41.Jenkinson C, Layte R, Jenkinson D, Lawrence K, Petersen S, Paice C, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med 1997;19:179–86. [DOI] [PubMed] [Google Scholar]
- 42.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Han Y, Zhao FL, Zhou J, Chen Z, Sun H. Validation and comparison of EuroQoL-5 dimension (EQ-5D) and Short Form-6 dimension (SF-6D) among stable angina patients. Health Qual Life Outcomes 2014;12:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grochtdreis T, Brettschneider C, Wegener A, Watzke B, Riedel-Heller S, Harter M, et al. Cost-effectiveness of collaborative care for the treatment of depressive disorders in primary care: a systematic review. PLoS One 2015;10:e0123078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vannoy SD, Arean P, Unutzer J. Advantages of Using Estimated Depression-Free Days for Evaluating Treatment Efficacy. Psychiatric services; (Washington, DC: ) 2010;61:160–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prasad M, Wahlqvist P, Shikiar R, Shih YC. A review of self-report instruments measuring health-related work productivity: a patient-reported outcomes perspective. Pharmacoeconomics 2004;22:225–44. [DOI] [PubMed] [Google Scholar]
- 47.Unutzer JHH, Schoenbau M., Druss B The collaborative care model: An approach for integrated physicial and mental health care in meidcad health homes. Health Home Information Resource Center Brief May 2013. https://www.chcs.org/resource/the-collaborative-care-model-an-approach-for-integrating-physical-and-mental-health-care-in-medicaid-health-homes/, Accessed October 17, 2018.
- 48.Cheung YK. Sequential Implementation of Stepwise Procedures for Identifying the Maximum Tolerated Dose. J Am Stat Assoc 2007;102:1448–61. [Google Scholar]
- 49.Hochberg Y, Tamhane AC. Multiple comparison procedures. New York: Wiley; 1987. [Google Scholar]
- 50.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996;276:1253–8. [PubMed] [Google Scholar]
- 51.Choosing interventions that are cost-effective [Internet]. Geneva: World Health Organization; 2014. Available from: http://www.who.int/choice/en/, Accessed October 17, 2018 [Google Scholar]
- 52.Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: A non-parametric approach to confidence interval estimation. Health Econ. 1997;6(4):327–340. [DOI] [PubMed] [Google Scholar]
- 53.Campbell MK, Torgerson DJ. Bootstrapping: estimating confidence intervals for cost-effectiveness ratios. QJM. 1999;92(3):177–182. [DOI] [PubMed] [Google Scholar]
- 54.Chaudhary MA, Stearns SC. Estimating confidence intervals for cost-effectiveness ratios: An example from a randomized trial. StatMed. 1996;15(13):1447–1458. [DOI] [PubMed] [Google Scholar]
- 55.Efron B Better Bootstrap Confidence-Intervals. J Am Stat Assoc. 1987;82(397):171–185. [Google Scholar]
- 56.Carney RM, Freedland KE, Jaffe AS. Depression screening in patients with heart disease. JAMA 2009;301:1337; author reply 8. [DOI] [PubMed] [Google Scholar]
- 57.Razykov I, Ziegelstein RC, Whooley MA, Thombs BD. The PHQ-9 versus the PHQ-8 —Is item 9 useful for assessing suicide risk in coronary artery disease patients? Data from the Heart and Soul Study. J Psychosom Res 2012;73:163–8. [DOI] [PubMed] [Google Scholar]
- 58.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–73. [DOI] [PubMed] [Google Scholar]
- 59.Thombs BD, Ziegelstein RC, Whooley MA. Optimizing Detection of Major Depression Among Patients with Coronary Artery Disease Using the Patient Health Questionnaire: Data from the Heart and Soul Study. J Gen Intern Med 2008;23:2014–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim JM, Stewart R, Lee YS, Lee HJ, Kim MC, Kim JW, et al. Effect of Escitalopram vs Placebo Treatment for Depression on Long-term Cardiac Outcomes in Patients With Acute Coronary Syndrome: A Randomized Clinical Trial. JAMA 2018;320:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Houck PR, Counihan PJ, et al. Telephone-delivered collaborative care for treating post-CABG depression: a randomized controlled trial. JAMA 2009;302:2095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tully PJ, Baumeister H. Collaborative care for comorbid depression and coronary heart disease: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2015;5:e009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.