INTRODUCTION
Multiplex panels for cancer susceptibility including BRCA1 and BRCA2 became available in 2013. Since that time, panel testing has become the dominant means by which individuals undergo genetic risk evaluation. This approach is more efficient and less costly than Sanger sequencing. Because multiplex panels are now used widely for individuals presenting with many types of cancer, it is important for oncologists to understand the risks associated with mutations in various genes. Here, we focus on epithelial ovarian cancer (OC) and endometrial cancer (EC).
When considering germline pathogenic variants (mutations) and their association with cancer, genes are clustered by their penetrance (lifetime risk) for the development of the disease. The terms “high,” “moderate,” and “lower” penetrance provide an assessment of associated cancer risks. Genes in which mutations are associated with a relative risk (RR) of cancer of greater than 4 are considered high penetrance, with RRs of 2 to 4 and less than 2 considered moderate and lower penetrance genes, respectively.
Clinical validity for genetic susceptibility to cancer refers to how robustly a genetic variant is linked to an increased risk of disease as well as the degree of confidence in the understanding of the magnitude of that risk. Different study designs are used to assess risk. Case-only studies can identify mutations in candidate genes but cannot assess the contribution of those mutations to risk without controls. Case-control studies can be confounded by case selection (ascertainment bias) and by use of controls from populations other than the case population. Segregation analyses examine the correlation of the presence of mutation with disease in individuals in families and can allow the calculation of penetrance through kin-cohort modeling or other techniques. These studies can also be confounded by ascertainment bias if only a portion of the family is tested. Prospective cohorts in which individuals with specific gene mutations and without cancer are followed for development of disease provide the strongest level of evidence but are expensive, may be prohibitively large and/or take many years, and can be affected by differential follow-up and interventions.
For the genes associated with gynecologic cancer, the strength of evidence for clinical validity is variable. For some genes, there are multiple studies confirming risk with some precision, and for others the data are more limited or conflicting. In some situations, such as for BRCA1/2 and Lynch syndrome genes, lifetime risk estimates are derived from prospective cohorts. Case-control data are available for other genes, with lifetime risk estimates extrapolated from the relative risks observed. In several case-control studies, cases are represented by clinical samples sent to commercial testing laboratories (with an intrinsic ascertainment bias because these women met clinical criteria for genetic testing). The prevalence of mutations in these samples is then compared with the prevalence in “public controls,” which are publicly available data sets of sequences derived from individuals undergoing sequencing in other projects and aggregated by groups such as the Exome Aggregation Consortium (ExAC) or the Genome Aggregation Database. These controls are not derived from the same population as the cases (although analyses do attempt to correct for genetic background), which is relevant because polygenic background influences in the clinical case population may lead to higher case prevalence and risk estimates than would be observed in a true population-based ascertainment. In addition, older control data sets may not have undergone up-to-date sequencing and variant annotation, which could lead to a falsely lower apparent prevalence of mutations, again inflating risk estimates. For these reasons, the clinical validity of several more recently described associations is uncertain, and the magnitude of associated risks even more so.
EPITHELIAL OC
The lifetime risk of OC is 1.3% by the age of 80 years in the United States.1 Although OC is a heterogenous disease with multiple histologic subtypes, more than 90% of cases of OC are epithelial in origin. More than 70% of epithelial OC is diagnosed at stage III and IV, with a subsequent poor prognosis. OC has a significant heritable component, providing opportunities for identification of women at risk and subsequent surgical risk reduction. Family history of OC is a significant factor with a RR of approximately 3.2 Of the heritable component of OC risk, 25% can be explained by BRCA1/2 mutations,3 10% by mutations in other single genes, and approximately 6% by polygenic risk represented by single nucleotide polymorphisms (SNPs) identified in genome-wide association studies (GWAS). In considering a risk threshold for intervention, it is worth noting that guidelines do not recommend routine risk-reducing salpingo-oophorectomy (RRSO) for individuals with a first-degree relative with OC and no known genetic susceptibility; such risk is estimated at approximately 2% to 3%73. Cost-effectiveness analyses have suggested that RRSO is cost-effective when a lifetime risk of OC exceeds 4%.4 However, consideration needs to be given to timing of RRSO even in the setting of an elevated risk, given the harms of premature menopause, in terms of quality of life and long-term health.
BRCA1 and BRCA2
Mutations in BRCA1 and BRCA2 are found in approximately 15% to 20% of unselected patients with OC, are associated with improved outcome,5-7 and are the strongest single risk factor for the development of disease. OCs in women with BRCA1 and BRCA2 mutations are more likely to be high-grade serous adenocarcinomas as opposed to mucinous tumors. Mutations in BRCA1 are associated with an OC risk in prospective studies as great as 44% by age 80 years,8-10 along with a risk of breast cancer of approximately 70%. Mutations in BRCA2 are associated with increased risks of breast (lifetime risk of up to 70%) and OC (lifetime risk of 10% to 20%), along with pancreatic cancer and high-grade prostate cancer. Knowledge of the presence of a BRCA1/2 mutation has significant implications for women. RRSO is associated with improved mortality risk,11,12 and thus the National Comprehensive Cancer Network and other guidelines recommend RRSO once childbearing is complete and by age 35 to 40 years for BRCA1 and 40 to 45 years for BRCA2.13 The development of targeted therapy for BRCA1/2 mutation-associated OC makes the identifications of carriers critically important for clinical treatment decisions. Multiple poly(ADP)ribose polymerase inhibitors have been approved by the Food and Drug Administration for the treatment of BRCA-mutated, relapsed OC (olaparib and rucaparib at the time of this writing), as maintenance therapy for either BRCA-mutated or wild-type, platinum-sensitive relapsed disease (olaparib, niraparib, and rucaparib), or as maintenance therapy (olaparib) after first-line therapy for patients with BRCA1/2 mutations.14-18 More detailed information regarding the role of poly(ADP)ribose polymerase inhibitors in the management of OC will be covered elsewhere in this Special Series issue. Because of opportunities for prevention in unaffected carriers and the availability of specific therapies, all women with epithelial OC should undergo genetic testing for BRCA1 and BRCA2.
Lynch Syndrome
Lynch syndrome, also known as hereditary non-polyposis colorectal cancer, is characterized by autosomal dominant inheritance of susceptibility to colon, endometrial, ovarian, pancreatic, small-bowel, ureteral, and other cancers and is caused by germline mutations in the mismatch repair (MMR) genes. Mutations in MLH1 and MSH2 are the most common cause of Lynch syndrome, followed by MSH6, PMS2, and EPCAM. Less than 1% of patients with OC have germline mutations in MMR genes.19 Cancer risks in Lynch syndrome appear to be gene dependent, with prospective studies estimating the lifetime risk of OC by age 70 years at between 11% and 20% for MLH1 and 15% and 24% for MSH2 mutations.20,21 Data regarding MSH6 mutations have been limited in prospective studies; however, multiple case-control studies have demonstrated risk ranging from an odds ratio (OR) of 1.92 (95% CI, 1.19 to 3.10) to 5.04 (95% CI, 3.70 to 6.70).21-25 Data regarding PMS2 mutations and OC are more limited and available information does not demonstrate a statistically significant increased risk.26 Risks for women with EPCAM mutations are likely similar to those with MSH2 mutations.27 EC (discussed later in this article) is strongly associated with Lynch syndrome and thus discussion regarding risk-reducing hysterectomy and salpingo-oophorectomy is recommended for all women with Lynch syndrome.13 Hysterectomy and oophorectomy significantly decrease the risk of development of gynecologic cancers in women with Lynch syndrome,28 and intensive screening for colon and other cancers is recommended.29 In addition to the opportunity for prevention, individuals with advanced cancer and Lynch syndrome are candidates for immune therapy with pembrolizumab regardless of tumor of origin.30
ATM mutations.
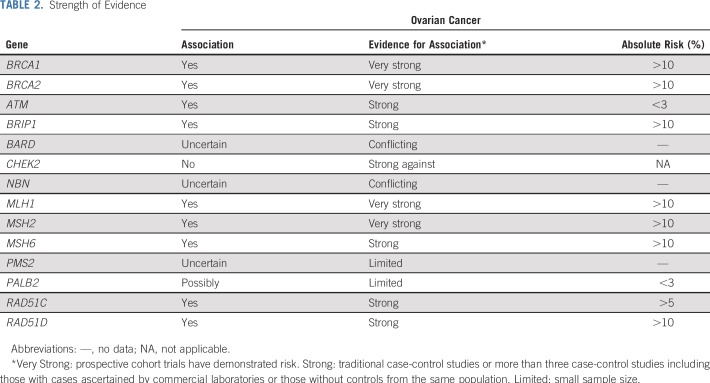
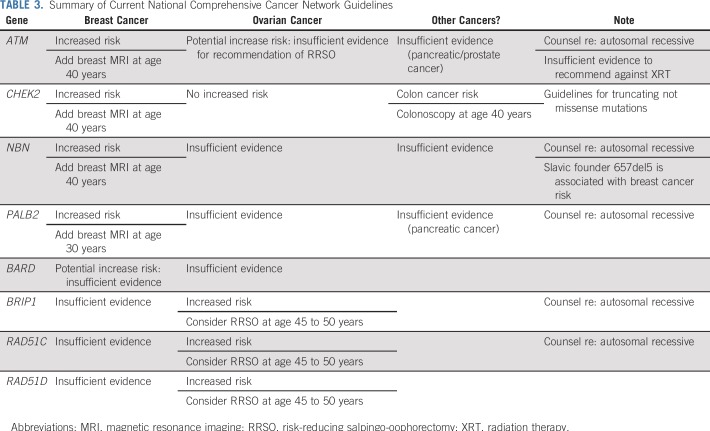
Several case-control studies have suggested a modestly increased risk of OC associated with ATM mutations (Table 1). In the first, Lilyquist et al25 examined the prevalence of mutations in 7,768 OC cases tested at a commercial laboratory compared with mutation frequencies in ExAC. ATM mutations were associated with a standardized risk ratio (SRR) of OC of 2.25 (95% CI, 1.7 to 2.9). Limitations of the study include the ascertainment bias of those tested in a commercial laboratory and the use of ExAC for controls, both of which could lead to inflation of the estimated risk. A subsequent study from the same laboratory (with likely substantial overlap of the patient population) with more extensive sequencing of 625 genes confirmed this association.31 In a different study of OC cases ascertained among patients participating in Gynecologic Oncology Group (GOG) trials or at the University of Washington, Norquist et al19 compared ATM mutations detected in cases to ExAC and Exome Sequencing Project controls. An increased risk (OR) was seen in comparison with ExAC controls (OR, 2.4; 95% CI, 1.2 to 4.4) but not in comparison with Exome Sequencing Project controls (OR, 2.5; 95% CI, 1.0 to 6.2), although the ORs were numerically similar. Kurian et al24 used a different approach to examine the association of specific genes to OC risk. They used multivariable logistic regression models adjusted for family history of breast and OC and applied to more than 95,000 individuals undergoing clinical testing at a different commercial laboratory. This approach also demonstrated an association, albeit more modest, between ATM mutations and OC with a OR of 1.69 (95% CI, 1.19 to 2.40). Therefore, despite limitations of the study designs, three large data sets have demonstrated an elevated risk of OC in ATM mutation carriers. Additional studies have come to similar conclusions. 74,75 However, the observed risk is modest and the lifetime risk of OC is estimated to be approximately 2.5%, below the threshold for which routine RRSO is recommended (Table 2).
TABLE 1.
Association of Ovarian Cancer Risk With Mutations in Selected Genes
TABLE 2.
Strength of Evidence
BRIP1 mutations.
Germline mutations in BRIP1 are the most common finding in OC after BRCA1 and BRCA2 and have been shown in multiple studies to be associated with OC risk. BRIP1 mutations were initially detected case studies.32,33 Ramus et al34 then examined 3,374 epithelial OC cases and 3,487 controls (generally ascertained in the same populations as the cases) and found an increased frequency of BRIP1 mutations in patients with OC (0.9%) compared with controls (0.09%), with a RR of 11.22 (95% CI, 3.22 to 34.10). A segregation analysis was also performed in families with known BRIP1 mutations and demonstrated an RR of 3.41 (95% CI, 2.12 to 5.54). On the basis of these data, the authors estimated lifetime risk estimates of OC associated with BRIP1 mutations of 5.8% (95% CI, 3.6% to 9.1%).
Since that time, studies using data from testing laboratories and the GOG have provided validation of the association of BRIP1 mutations with OC. In one commercial laboratory study, BRIP1 mutations were associated with an SRR of 4.99 (95% CI, 3.79 to 6.45) in OC cases compared with ExAC controls.25 From the GOG/University of Washington study, BRIP1 mutations in cases compared with ExAC controls resulted in an OR of 6.4 (95% CI, 3.8 to 10.6).19 Finally, in the logistic regression analysis from a third study, mutations were associated with an OR of 2.62 (95% CI, 1.72 to 3.98). Thus, there are consistent data demonstrating an increased risk of OC with estimated lifetime risks from 5% to 12%. As such, RRSO is recommended between ages 45 and 50 years.13,35 Of note, the association between mutations and BRIP1 with breast cancer is much less clear, and most studies have not demonstrated a significant association.36,37
RAD51C/RAD51D mutations.
Initial studies suggested RAD51C33,38,39 and RAD51D 33,39 as potential epithelial OC susceptibility genes but were limited by the small number of mutation carriers or inadequate controls. Subsequent to these studies, Song et al40 examined 3,429 patients with invasive epithelial OC and 2,772 controls and found an increased prevalence of RAD51C/D mutations in OC with ORs of 5.2 (95% CI, 1.1 to 24.0) and 12 (95% CI, 1.5 to 90.0), respectively. The lifetime risks of OC risk by age 70 years were estimated at 5.2% (95% CI, 1.1% to 22.0%) for RAD51C and 12% (95% CI, 1.5% to 60.0%) for RAD51D. Additional studies have provided validation of the association of RAD51C mutations with OC risk (Table 1) and calculated ORs of 4.98 (95% CI, 3.09 to 8.04)24 and 3.4 (95% CI, 1.5 to 7.6),19 and SRR of 5.12 (95% CI, 3.72 to 6.88).25 In these same studies, RAD51D mutations were also associated with OC, with ORs of 4.78 (95% CI, 2.13 to 10.70)24 and 10.9 (95% CI, 4.6 to 26.0),19 and SRR of 6.34 (95% CI, 3.16 to 11.34).25
Despite the labile estimates resulting from small numbers of cases, the available data suggest that the lifetime risk of developing OC in the setting of a RAD51C or RAD51D mutation is greater than 5%. Guidelines recommend a discussion of RRSO between age 45 and 50 years (Table 3).13,35 Data regarding breast cancer risk are limited and conflicting at this time, although there may be an association specifically with triple-negative breast cancer.41,42
TABLE 3.
Summary of Current National Comprehensive Cancer Network Guidelines
PALB2 mutations.
PALB2 mutations may be associated with a modest risk of OC, but data remain limited. Three studies have not shown a statistically significant association, although numbers were small and confidence intervals wide, such that a modest increase in risk was not excluded.24,34,43 Two other series have demonstrated a risk. In the first, Lilyquist et al25 demonstrated an SRR of 3.08 (95% CI, 1.93 to 4.67), which was not significant when adjusted for the presence of breast cancer. Norquist et al19 also demonstrated an association of PALB2 mutations with OC with an OR 4.4 (95% CI, 2.1 to 9.1). Given the conflicting studies and small numbers, more data are needed. However, even if a risk is confirmed, it is likely to be modest and will likely not meet the current threshold for routine RRSO in the absence of other risk factors. PALB2 mutations are strongly associated with breast cancer with reported RRs between 4.0 and 7.5.24,41,43
NBN mutations.
Data are conflicting regarding the role of NBN mutations in OC risk. Most studies have not detected evidence of a statistically significant association19,25,34; however, Kurian et al24 did find an RR of 1.85 (95% CI, 1.05 to 3.24). Studies of NBN in breast cancer have revealed the importance of founder mutations. The Slavic founder mutation (657del5) had been associated with breast cancer risk, whereas NBN mutations generally have not.37,44,45 The role of founder mutations relative to OC is not known at this time.
BARD1 mutations.
Similar to NBN, most studies have failed to demonstrate an association between BARD1 mutations and OC risk.24,25,34 Norquist et al19 did note an elevation of risk, with an OR of 4.2 (95% CI, 1.4 to 12.5); however two of the four BARD1 mutation carriers in this series also had BRCA1 mutations and, therefore, the contribution of the BARD1 mutations alone on risk is unknown. The number of BARD1 mutation carriers in each of these series was very small and larger samples are needed to further clarify any potential risk. However, any such risk with a BARD mutation is likely to be modest and unlikely to change clinical management. Data suggest that BARD1 mutations may be associated with an RR of approximately 2 for breast cancer, but additional data are needed.24,37
Genes with no evidence for an association with OC risk.
Mutations in CHEK219,24,25,46 MRE11,19,25 RAD50,19,25 and RAD51B40 have not been associated with OC risk. There are sufficient numbers of CHEK2 mutation carriers included in studies that it is highly unlikely there is a significantly increased risk of OC. Because mutations in CHEK2 are commonly found in multiplex panel testing for breast cancer susceptibility, it is important for providers to know that RRSO should not be recommended in the absence of other risk factors for OC or as part of treatment of breast cancer. (Tables 1 and 2)
SNPs and polygenic risk scores.
GWAS have found multiple common but low-penetrance susceptibility alleles associated with cancer. Several SNPs are each individually associated with small increased risks of OC (RR < 1.5)47,48. Although such SNPS may have little impact individually (unlike mutations in the genes discussed in detail in this section) cumulatively as part of a polygenic risk score (PRS), they may be able to identify women at significantly increased risk of ovarian compared with the general population. An analysis using the prospective UK Collaborative Trial of OC Screening49 evaluated a PRS on the basis of the combined effects of 15 SNPs associated with epithelial OC.50 In this nested case-control study (750 cases and 1,428 controls), women in the top fifth percentile of the PRS had a 3.4-fold increased risk for epithelial OC compared with women in the bottom 5%. The corresponding absolute risks of developing epithelial OC risk by age 80 years were 2.9% and 0.9%. The demonstration that the PRS can predict epithelial OC risk prospectively is important, although even at the highest level of polygenic risk, the impact on clinical management is unclear. It may be possible to use PRS to further refine risks in known mutation carriers of genes such as BRCA2,51 although more work is necessary to determine clinical utility.
ENDOMETRIAL CANCER
Increasing age, obesity, exposure to unopposed estrogen, tamoxifen use, complex atypical hyperplasia, pelvic radiation, and a family history of EC52 are all known risk factors for EC. In addition, certain germline mutations increase the risk of disease.53 In a series of 1,170 unselected ECs, 4.5% had a mutation in one of 12 cancer susceptibility genes.54 A study of individuals ascertained through clinical testing at a commercial laboratory demonstrated that 11.9% of 453 patients with EC had detectable mutations, 60% of which were in genes associated with Lynch syndrome.55 Recent data from another laboratory suggest that women with both breast and EC have higher rates of mutations in cancer susceptibility genes (14%) than women with either cancer alone.56 However, mutations were seen in some genes in both of these series at the same prevalence as in the general population. This illustrates that the presence of a germline mutation in a patient with a particular cancer does not prove that the mutation is causal.
Lynch Syndrome
Lynch syndrome has the strongest association with EC risk. Germline mutations in MMR genes are found in less than 5% of individuals with EC, but when found, have significant implications for the patients and their relatives. Prospective data have estimated the lifetime risk of EC by age 70 years as approximately 46% to 54% for MLH1 mutations, 21% to 51% for MSH2 mutations, 16% to 49% for MSH6 mutations, and 13% to 24% for PMS2 mutations.20,21,26 Several clinical criteria have been proposed as indicators for germline testing for Lynch syndrome in patients with EC, including younger age at onset (younger than 50 years), a personal or family history of Lynch syndrome–associated cancers, and histopathologic features such as lymphocytic infiltration.57,58 Unfortunately, these features all seem to be relatively insensitive for the detection of germline mutations.5 This has led several groups to propose universal screening of all ECs with immunohistochemistry (IHC) for MMR protein loss and/or microsatellite instability analysis. When conducting universal screening, 15% to 25% of tumors will manifest loss of MLH1/PMS2, MSH2/MSH6, MSH6 alone, or PMS2 alone.59-64 MLH1/PMS2 loss is most common, and the great majority are the result of the hypermethylation of the promoter of MLH1. Germline testing identifies mutations in greater than 50% of the nonhypermethylated cases (approximately 2% to 6% of all cases entering universal screening), with many of those identified not meeting the traditional clinical criteria described previously. The practice of universal screening of patients younger than 70 years has been shown to be cost-effective in a study from the Netherlands,65 but logistical barriers remain.63
Cowden Syndrome
Germline mutations of PTEN cause Cowden syndrome, which is characterized by hamartomas, trichilemmomas, macrocephaly, and an increased risk of breast cancer, thyroid cancer, and EC, along with other features.66 The lifetime risk of EC in women with Cowden syndrome has been reported to be as high as 28%.67,68 However, whereas somatic PTEN mutations are common in EC, germline PTEN mutations are rare.69
BRCA1/2 mutations.
Several studies have examined the question of whether BRCA1 and BRCA2 mutations are associated with an increased risk of EC, and specifically with a risk of serous carcinoma. However, the studies to date do not seem to support the presence of a substantive risk of EC resulting from BRCA1/2 mutations. The total number of patients with EC in all of the series is small and study findings have been conflicting. Two prospective studies have demonstrated a small increased risk in EC; however, this risk may be limited to women who have taken tamoxifen. Segev et al70 identified 17 incident cases of EC in 4,456 women with a median follow-up of 5.7 years. The standardized incidence ratio was 4.14 (95% CI, 1.92 to 7.87) for women who received tamoxifen and 1.67 (95% CI, 0.81 to 3.07) for women who did not receive tamoxifen. This led to an estimated 10-year cumulative risk of EC in women who were treated with tamoxifen of 2.0%. In a study by Shu et al71 examining more than 1,000 women with BRCA1 and BRCA2 mutations, no increased risk in uterine cancers was observed overall. However, there were five serous/serous-like cancers (four in BRCA1 mutation carriers, and three of five cases had been exposed to tamoxifen). The authors estimated a risk of serous EC of approximately 2.6% by age 70 years. Finally, Long et al54 identified seven germline BRCA1/2 mutation carriers in 1,170 patients with uterine cancer, a prevalence similar to the general population. One BRCA1 mutation was seen in 135 uterine serous carcinomas. Additional studies are needed to clarify the robustness and magnitude of these associations.
Other Candidate Genes
Genes of potential interest regarding an association with EC risk include POLE and POLD1, among others,53 although data are too limited to provide any estimates of risk. Similar to the discussion regarding OC, SNPs associated with EC have been identified through GWAS with uncertain clinical utility at this time.72
Genetic Testing Strategies
In OC, the prevalence of clinically actionable gene mutations is high. The detection of a BRCA1, BRCA2, or Lynch syndrome gene mutation in a patient with cancer may have a direct impact, given Food and Drug Administration–approved therapies. In addition, the presence of RAD51C, RAD51D, and BRIP1 mutations in female relatives will lead to a discussion regarding RRSO. Thus at a minimum, all patients with epithelial OC should undergo testing for BRCA1, BRCA2, RAD51C, RAD51D, and BRIP1. Additional genes may be added to this list as evidence develops. Depending on the specifics of the patient’s cancer, family history, and preferences regarding information seeking and tolerance of uncertainty, larger panels can be considered, particularly including the Lynch genes. Optimal approaches to testing are evolving and under study. There are two potential approaches: simultaneous germline and somatic sequencing or somatic testing with reflex to germline testing if a pathogenic/likely pathogenic variant is found. For the latter approach, if a somatic mutation is found, it is extremely important that germline testing occur to understand future cancer risks for the patient and to provide information to family members. If a somatic mutation is not found, germline testing should still be considered for most patients because large genomic rearrangements can be missed with many of the current somatic sequencing platforms. Ten percent of BRCA1 mutations, for example, are large genomic rearrangements, which may be missed on somatic sequencing.
In EC, the most extensively evaluated strategy for identification of Lynch syndrome is universal IHC screening for loss of MMR proteins, with reflex analysis of cases with MLH1/PMS2 loss for MLH1 promoter hypermethylation preceding referral for germline testing. This strategy is cost-effective and identifies individuals who do not meet traditional clinical criteria.59,65 Several practical barriers impeded complete assessment in greater than 10% of cases,63 and the failure to identify germline mutations in patients with abnormal IHC (so-called Lynch-like syndrome) can create a clinical dilemma with respect to management recommendations. In addition, occasional patients with germline mutations will have tumors that do not manifest IHC loss, resulting in negative predictive values less than 100%.69
In conclusion, multigene panel testing for cancer susceptibility has led to the increasing identification of individuals with mutations in genes other than BRCA1 and BRCA2, and an expanding number of genes have been found to be associated with OC. The magnitude of OC risk is not the same across genes. As we move beyond testing for BRCA1 and BRCA2 for OC, it is crucial that patients and providers understand gene-specific cancer risks to recommend appropriate clinical management while avoiding unnecessary interventions (eg, RRSO in CHEK2-mutation carriers). All patients with epithelial OC should be offered germline genetic testing. Approaches to the genetic evaluation of EC (beyond assessment of family history) are evolving, but universal IHC testing for MMR genes has been proposed. For both OC and EC, the identification of germline findings can have both preventive and therapeutic implications. Prospective studies are ongoing and will be important to further clarify risks (eg, the Prospective Study of Multiplex Testing [ClinicalTrials.gov identifier: NCT02665195]; www.promptstudy.info). Gene-specific risks and management considerations are rapidly evolving.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Update on Genetic Testing in Gynecologic Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Susan M. Domchek
Honoraria: AstraZeneca, Clovis Oncology, Bristol-Myers Squibb
Research Funding: AstraZeneca (Inst), Clovis Oncology, Pharmamar (Inst)
Mark E. Robson
Honoraria: AstraZeneca
Consulting or Advisory Role: McKesson, AstraZeneca, Merck, Pfizer, Daiichi-Sanyko
Research Funding: AstraZeneca (Inst), Myriad Genetics (Inst), InVitae (Inst), AbbVie (Inst), Tesaro (Inst), Medivation (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stratton JF, Pharoah P, Smith SK, et al. A systematic review and meta-analysis of family history and risk of ovarian cancer. Br J Obstet Gynaecol. 1998;105:493–499. doi: 10.1111/j.1471-0528.1998.tb10148.x. [DOI] [PubMed] [Google Scholar]
- 3.Jervis S, Song H, Lee A, et al. A risk prediction algorithm for ovarian cancer incorporating BRCA1, BRCA2, common alleles and other familial effects. J Med Genet. 2015;52:465–475. doi: 10.1136/jmedgenet-2015-103077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manchanda R, Legood R, Antoniou AC, et al. Specifying the ovarian cancer risk threshold of ‘premenopausal risk-reducing salpingo-oophorectomy’ for ovarian cancer prevention: A cost-effectiveness analysis. J Med Genet. 2016;53:591–599. doi: 10.1136/jmedgenet-2016-103800. [DOI] [PubMed] [Google Scholar]
- 5.Harter P, Hauke J, Heitz F, et al. Prevalence of deleterious germline variants in risk genes including BRCA1/2 in consecutive ovarian cancer patients (AGO-TR-1) PLoS One. 2017;12:e0186043. doi: 10.1371/journal.pone.0186043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norquist BM, Brady MF, Harrell MI, et al. Mutations in homologous recombination genes and outcomes in ovarian carcinoma patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group Study. Clin Cancer Res. 2018;24:777–783. doi: 10.1158/1078-0432.CCR-17-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolton KL, Chenevix-Trench G, Goh C, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382–390. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 11.Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547–1553. doi: 10.1200/JCO.2013.53.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Comprehensive Cancer Network. Version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf.
- 14.Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 15.Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 16.Kristeleit R, Shapiro GI, Burris HA, et al. A phase I-II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2-mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23:4095–4106. doi: 10.1158/1078-0432.CCR-16-2796. [DOI] [PubMed] [Google Scholar]
- 17.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482–490. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–2310. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 21.Møller P, Seppälä TT, Bernstein I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: A report from the Prospective Lynch Syndrome Database. Gut. 2018;67:1306–1316. doi: 10.1136/gutjnl-2017-314057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan NAJ, Morris J, Green K, et al. Association of mismatch repair mutation with age at cancer onset in Lynch syndrome: Implications for stratified surveillance strategies. JAMA Oncol. 2017;3:1702–1706. doi: 10.1001/jamaoncol.2017.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engel C, Loeffler M, Steinke V, et al. Risks of less common cancers in proven mutation carriers with lynch syndrome. J Clin Oncol. 2012;30:4409–4415. doi: 10.1200/JCO.2012.43.2278. [DOI] [PubMed] [Google Scholar]
- 24.Kurian AW, Hughes E, Handorf EA, et al. Breast and Ovarian Cancer Penetrance Estimates Derived From Germline Multiple-Gene Sequencing Results in Women. JCO Precis Oncol. 2017;1:1–12. doi: 10.1200/PO.16.00066. [DOI] [PubMed] [Google Scholar]
- 25.Lilyquist J, LaDuca H, Polley E, et al. Frequency of mutations in a large series of clinically ascertained ovarian cancer cases tested on multi-gene panels compared to reference controls. Gynecol Oncol. 2017;147:375–380. doi: 10.1016/j.ygyno.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ten Broeke SW, van der Klift HM, Tops CMJ, et al. Cancer risks for PMS2-associated Lynch syndrome. J Clin Oncol. 2018;36:2961–2968. doi: 10.1200/JCO.2018.78.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ligtenberg MJ, Kuiper RP, Geurts van Kessel A, et al. EPCAM deletion carriers constitute a unique subgroup of Lynch syndrome patients. Fam Cancer. 2013;12:169–174. doi: 10.1007/s10689-012-9591-x. [DOI] [PubMed] [Google Scholar]
- 28.Schmeler KM, Sun CC, Bodurka DC, et al. Prophylactic bilateral salpingo-oophorectomy compared with surveillance in women with BRCA mutations. Obstet Gynecol. 2006;108:515–520. doi: 10.1097/01.AOG.0000228959.30577.13. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins MA DJ, Dowty JG, Ait Ouakrim D, et al. Short-term risk of colorectal cancer in individuals with lynch syndrome: A meta-analysis. J Clin Oncol. 2015;33:326–331. doi: 10.1200/JCO.2014.55.8536. [DOI] [PubMed] [Google Scholar]
- 30.Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med. 2017;377:1409–1412. doi: 10.1056/NEJMp1709968. [DOI] [PubMed] [Google Scholar]
- 31.Lu HM, Li S, Black MH, et al. Association of breast and ovarian cancers with predisposition genes identified by large-scale sequencing. JAMA Oncol. doi: 10.1001/jamaoncol.2018.2956. [epub ahead of print on August 16, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rafnar T, Gudbjartsson DF, Sulem P, et al. Mutations in BRIP1 confer high risk of ovarian cancer. Nat Genet. 2011;43:1104–1107. doi: 10.1038/ng.955. [DOI] [PubMed] [Google Scholar]
- 33.Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108:18032–18037. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramus SJ, Song H, Dicks E, et al. Germline mutations in the BRIP1, BARD1, PALB2, and NBN genes in women with ovarian cancer. J Natl Cancer Inst. 2015;107:107. doi: 10.1093/jnci/djv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13:581–588. doi: 10.1038/nrclinonc.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Easton DF, Lesueur F, Decker B, et al. No evidence that protein truncating variants in BRIP1 are associated with breast cancer risk: Implications for gene panel testing. J Med Genet. 2016;53:298–309. doi: 10.1136/jmedgenet-2015-103529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190–1196. doi: 10.1001/jamaoncol.2017.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meindl A, Hellebrand H, Wiek C, et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet. 2010;42:410–414. doi: 10.1038/ng.569. [DOI] [PubMed] [Google Scholar]
- 39.Loveday C, Turnbull C, Ramsay E, et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;43:879–882. doi: 10.1038/ng.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song H, Dicks E, Ramus SJ, et al. Contribution of germline mutations in the RAD51B, RAD51C, and RAD51D genes to ovarian cancer in the population. J Clin Oncol. 2015;33:2901–2907. doi: 10.1200/JCO.2015.61.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372:2243–2257. doi: 10.1056/NEJMsr1501341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimelis H, LaDuca H, Hu C, et al. Triple-negative breast cancer risk genes identified by multigene hereditary cancer panel testing. J Natl Cancer Inst. 2018;110:855–862. doi: 10.1093/jnci/djy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antoniou AC, Casadei S, Heikkinen T, et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med. 2014;371:497–506. doi: 10.1056/NEJMoa1400382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson ER, Rowley SM, Li N, et al. Panel testing for familial breast cancer: Calibrating the tension between research and clinical care. J Clin Oncol. 2016;34:1455–1459. doi: 10.1200/JCO.2015.63.7454. [DOI] [PubMed] [Google Scholar]
- 45.Seemanová E, Jarolim P, Seeman P, et al. Cancer risk of heterozygotes with the NBN founder mutation. J Natl Cancer Inst. 2007;99:1875–1880. doi: 10.1093/jnci/djm251. [DOI] [PubMed] [Google Scholar]
- 46.Näslund-Koch C, Nordestgaard BG, Bojesen SE. Increased risk for other cancers in addition to breast cancer for CHEK2*1100delC heterozygotes estimated from the Copenhagen General Population Study. J Clin Oncol. 2016;34:1208–1216. doi: 10.1200/JCO.2015.63.3594. [DOI] [PubMed] [Google Scholar]
- 47.Kuchenbaecker KB, Ramus SJ, Tyrer J, et al. Identification of six new susceptibility loci for invasive epithelial ovarian cancer. Nat Genet. 2015;47:164–171. doi: 10.1038/ng.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phelan CM, Kuchenbaecker KB, Tyrer JP, et al. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat Genet. 2017;49:680–691. doi: 10.1038/ng.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet. 2016;387:945–956. doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang X, Leslie G, Gentry-Maharaj A, et al. Evaluation of polygenic risk scores for ovarian cancer risk prediction in a prospective cohort study. J Med Genet. 2018;55:546–554. doi: 10.1136/jmedgenet-2018-105313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuchenbaecker KB, McGuffog L, Barrowdale D, et al. Evaluation of polygenic risk scores for breast and ovarian cancer risk prediction in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2017;109:109. doi: 10.1093/jnci/djw302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnatty SE, Tan YY, Buchanan DD, et al. Family history of cancer predicts endometrial cancer risk independently of Lynch Syndrome: Implications for genetic counselling. Gynecol Oncol. 2017;147:381–387. doi: 10.1016/j.ygyno.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Spurdle AB, Bowman MA, Shamsani J, et al. Endometrial cancer gene panels: Clinical diagnostic vs research germline DNA testing. Mod Pathol. 2017;30:1048–1068. doi: 10.1038/modpathol.2017.20. [DOI] [PubMed] [Google Scholar]
- 54.Long B, Lilyquist J, Weaver A, et al. Cancer susceptibility gene mutations in type I and II endometrial cancer. Gynecol Oncol. 2019;152:20–25. doi: 10.1016/j.ygyno.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. doi: 10.1038/gim.2015.166. Susswein LR, Marshall ML, Nusbaum R, et al: Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genet Med 18:823-832, 2016 [Erratum: Genet Med 18:531-532, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. doi: 10.1016/j.ygyno.2018.12.021. Fulk K, Milam MR, Li S, et al: Women with breast and uterine cancer are more likely to harbor germline mutations than women with breast or uterine cancer alone: A case for expanded gene testing. Gynecol Oncol 152:612-617, 2019. [DOI] [PubMed] [Google Scholar]
- 57.Lin DI, Hecht JL. Targeted screening with combined age- and morphology-based criteria enriches detection of Lynch syndrome in endometrial cancer. Int J Surg Pathol. 2016;24:297–305. doi: 10.1177/1066896916629782. [DOI] [PubMed] [Google Scholar]
- 58.Garg K, Leitao MM, Jr, Kauff ND, et al. Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am J Surg Pathol. 2009;33:925–933. doi: 10.1097/PAS.0b013e318197a046. [DOI] [PubMed] [Google Scholar]
- 59.Dillon JL, Gonzalez JL, DeMars L, et al. Universal screening for Lynch syndrome in endometrial cancers: Frequency of germline mutations and identification of patients with Lynch-like syndrome. Hum Pathol. 2017;70:121–128. doi: 10.1016/j.humpath.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 60.Leenen CH, van Lier MG, van Doorn HC, et al. Prospective evaluation of molecular screening for Lynch syndrome in patients with endometrial cancer ≤ 70 years. Gynecol Oncol. 2012;125:414–420. doi: 10.1016/j.ygyno.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 61.Watkins JC, Yang EJ, Muto MG, et al. Universal screening for mismatch-repair deficiency in endometrial cancers to identify patients with Lynch syndrome and Lynch-like syndrome. Int J Gynecol Pathol. 2017;36:115–127. doi: 10.1097/PGP.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 62.Adar T, Rodgers LH, Shannon KM, et al. Universal screening of both endometrial and colon cancers increases the detection of Lynch syndrome. Cancer. 2018;124:3145–3153. doi: 10.1002/cncr.31534. [DOI] [PubMed] [Google Scholar]
- 63.Bruegl AS, Ring KL, Daniels M, et al. Clinical challenges associated with universal screening for Lynch syndrome-associated endometrial cancer. Cancer Prev Res (Phila) 2017;10:108–115. doi: 10.1158/1940-6207.CAPR-16-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferguson SE, Aronson M, Pollett A, et al. Performance characteristics of screening strategies for Lynch syndrome in unselected women with newly diagnosed endometrial cancer who have undergone universal germline mutation testing. Cancer. 2014;120:3932–3939. doi: 10.1002/cncr.28933. [DOI] [PubMed] [Google Scholar]
- 65.Goverde A, Spaander MC, van Doorn HC, et al. Cost-effectiveness of routine screening for Lynch syndrome in endometrial cancer patients up to 70 years of age. Gynecol Oncol. 2016;143:453–459. doi: 10.1016/j.ygyno.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Ngeow J, Sesock K, Eng C. Clinical implications for germline PTEN spectrum disorders. Endocrinol Metab Clin North Am. 2017;46:503–517. doi: 10.1016/j.ecl.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 67.Bubien V, Bonnet F, Brouste V, et al. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet. 2013;50:255–263. doi: 10.1136/jmedgenet-2012-101339. [DOI] [PubMed] [Google Scholar]
- 68.Tan MH, Mester JL, Ngeow J, et al. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18:400–407. doi: 10.1158/1078-0432.CCR-11-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ring KL, Bruegl AS, Allen BA, et al. Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Mod Pathol. 2016;29:1381–1389. doi: 10.1038/modpathol.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segev Y, Iqbal J, Lubinski J, et al. The incidence of endometrial cancer in women with BRCA1 and BRCA2 mutations: An international prospective cohort study. Gynecol Oncol. 2013;130:127–131. doi: 10.1016/j.ygyno.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 71.Shu CA, Pike MC, Jotwani AR, et al. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. 2016;2:1434–1440. doi: 10.1001/jamaoncol.2016.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spurdle AB, Thompson DJ, Ahmed S, et al. Genome-wide association study identifies a common variant associated with risk of endometrial cancer. Nat Genet. 2011;43:451–454. doi: 10.1038/ng.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jervis S, Song H, Lee A, et al. Ovarian cancer familial relative risks by tumour subtypes and by known ovarian cancer genetic susceptibility variants. J Med Genet. 2014;51:108–113. doi: 10.1136/jmedgenet-2013-102015. [DOI] [PubMed] [Google Scholar]
- 74.Lee K, Seifert BA, Shimelis H, et al. Genet Med. 2018 [Google Scholar]
- 75.Castera L, Harter V, Muller E, et al. Genet Med. 2018;20:1677–1686. doi: 10.1038/s41436-018-0005-9. [DOI] [PubMed] [Google Scholar]