Abstract
OBJECTIVE
Guidelines for hypertension treatment in patients with diabetes diverge regarding the systolic blood pressure (SBP) threshold at which treatment should be initiated and treatment goal. We examined associations of early SBP treatment with atherosclerotic cardiovascular disease (ASCVD) events in U.S. adults with diabetes.
RESEARCH DESIGN AND METHODS
We studied 43,986 patients with diabetes who newly initiated antihypertensive therapy between 2002 and 2007. Patients were classified into categories based on SBP at treatment initiation (130–139 or ≥140 mmHg) and after 2 years of treatment (100–119, 120–129, 130–139, 140–159, and ≥160 mmHg). The primary outcome was composite ASCVD events (fatal and nonfatal myocardial infarction and stroke), estimated using inverse probability of treatment-weighted Poisson regression and multivariable Cox proportional hazards regression.
RESULTS
Relative to individuals who initiated treatment when SBP was 130–139 mmHg, those with pretreatment SBP ≥140 mmHg had higher ASCVD risk (hazard ratio 1.10 [95% CI 1.02, 1.19]). Relative to those with pretreatment SBP of 130–139 mmHg and on-treatment SBP of 120–129 mmHg (reference group), ASCVD incidence was higher in those with pretreatment SBP ≥140 mmHg and on-treatment SBP 120–129 mmHg (adjusted incidence rate difference [IRD] 1.0 [−0.2 to 2.1] events/1,000 person-years) and in those who achieved on-treatment SBP 130–139 mmHg (IRD 1.9 [0.6, 3.2] and 1.1 [0.04, 2.2] events/1,000 person-years for those with pretreatment SBP 130–139 mmHg and ≥140 mmHg, respectively).
CONCLUSIONS
In this observational study, patients with diabetes initiating antihypertensive therapy when SBP was 130–139 mmHg and those achieving on-treatment SBP <130 mmHg had better outcomes than those with higher SBP levels when initiating or after 2 years on treatment.
Introduction
Blood pressure (BP) control is a recommended component of cardiovascular disease risk reduction in individuals with diabetes (1,2). However, the optimal approach to BP management in hypertensive adults with diabetes remains unclear. Professional society guidelines from the American College of Cardiology/American Heart Association (ACC/AHA) and the American Diabetes Association (ADA) diverge on the BP threshold at which to initiate treatment and the BP therapeutic goal (1,3). The 2017 ACC/AHA guidelines classify all patients with diabetes as high risk for cardiovascular disease and recommend initiating therapy when systolic BP (SBP) is >130 mmHg and targeting a goal SBP of <130 mmHg (3). In contrast, the ADA recommends initiating hypertension treatment at a threshold of 140 mmHg with a goal of on-treatment SBP of <140 mmHg for most patients with diabetes (1).
The discordant professional society guidelines reflect divergent randomized trial results. The Systolic Blood Pressure Intervention Trial (SPRINT) found that randomization to a goal SBP of <120 mmHg in adults without diabetes but with hypertension was associated with lower mortality and cardiovascular events compared with a goal SBP of <140 mmHg (4). In contrast, the Action to Control Cardiovascular Risk in Diabetes–Blood Pressure (ACCORD-BP) trial reported no significant difference in cardiovascular events or mortality in patients with diabetes with hypertension who were randomized to a goal SBP <120 mmHg compared with those with a goal SBP <140 mmHg (5). Neither trial, however, directly addressed the threshold for antihypertensive medication initiation in individuals with diabetes, and there are few observational studies that specifically examine associations between early BP treatment and clinical outcomes. Thus, a critical gap remains regarding the clinical benefits of specific SBP thresholds for treatment initiation and treatment goals in individuals with diabetes and hypertension.
To address this gap, we performed a retrospective study in a national cohort of U.S. adults with diabetes and hypertension receiving primary care in the Veterans Affairs health care system (VA), the largest integrated health care system in the U.S. We assessed associations between both the threshold at which antihypertensive treatment was initiated and the SBP achieved in the first 2 years on treatment with long-term atherosclerotic cardiovascular disease (ASCVD) events and mortality among individuals with diabetes who were newly treated for hypertension.
Research Design and Methods
Study Population
We conducted a retrospective cohort study of individuals with diabetes receiving routine primary care in the VA. Diabetes status was defined as two or more uses of ICD-9-Clinical Modification diagnosis codes 250.xx or one or more use of 250.xx in conjunction with a primary care provider visit and use of an outpatient diabetes medication during 2002–2003. Among those with diabetes, we included those who had elevated BP at baseline (2003–2007) and who were newly started on an outpatient antihypertensive medication between 2003 and 2007. Elevated BP at baseline was defined as at least two measurements of SBP >140 mmHg before antihypertensive drug initiation, although the last SBP measurement before starting an antihypertensive medication did not have to be >140 mmHg. The date of the initial antihypertensive medication prescription was considered the date of treatment initiation. To identify those individuals newly started on BP medications, we excluded individuals with any antihypertensive prescriptions before the index prescription through the VA or outside the VA based on non-VA medication records and Medicare/Medicaid data. Medications considered antihypertensive drugs included the following classes: calcium channel blockers (CCB), diuretics, β-blockers (BB), ACE inhibitors (ACEi), and angiotensin receptor blockers (ARB). In addition, participants had to have age, sex, race/ethnicity, non-HDL cholesterol, SBP, smoking status, BMI, and hemoglobin A1c (HbA1c) at baseline. The Emory University and VA Boston Healthcare System Institutional Review Boards approved this study.
Exposures
In preliminary analyses, we evaluated two exposures: 1) last SBP before antihypertensive medication initiation, categorized as 130–139 and ≥140 mmHg; and 2) mean SBP in the 2nd year on treatment, categorized as 100–119, 120–129, 130–139, 140–159, and ≥160 mmHg. We used outpatient BP measurements recorded in the electronic health record (EHR) during routine clinical care to assign individuals to SBP categories. Because pretreatment and on-treatment SBP were both associated with the outcomes of interest, the exposure for the primary analysis was a combination of the last SBP before antihypertensive medication initiation and the mean SBP in the 2nd year on treatment. The last SBP before antihypertensive medication initiation was classified as 130–139 or ≥140 mmHg to discriminate between a discrepancy between the ACC/AHA guidelines and the ADA guidelines. The on-treatment SBP achieved was defined as the mean SBP across all measurements in the 2nd year (days 366–730) after antihypertensive medication initiation. We evaluated on-treatment SBP in the 2nd year after treatment initiation because the SBP levels plateaued in the 2nd year on treatment for most individuals in our cohort. For the primary analysis, we specified on-treatment SBP as a four-category variable: 100–119, 120–129, 130–139, and 140–159 mmHg. We did not include SBP ≥160 mmHg because individuals who had an SBP elevated to that level despite treatment diverged substantially from the other study participants across most baseline characteristics (Supplementary Table 1). To minimize potential reverse causality in which low on-treatment SBP was associated with higher mortality due to a common cause of low SBP and mortality, we excluded individuals with on-treatment SBP <100 mmHg.
Outcomes
The primary outcome was a first ASCVD event after antihypertensive medication initiation, defined as fatal or nonfatal stroke or myocardial infarction. ASCVD events were identified using validated phenotyping algorithms that used diagnosis codes, notes, and other features in the EHR (6,7). We focused on first ASCVD events rather than cumulative incidence, including recurrent events, for two reasons: 1) to maintain comparability with recent clinical trials, and 2) to avoid changes in care after a nonfatal ASCVD event (e.g., medication intensification) that could add unmeasured confounding to the analysis of associations of BP levels in the first 2 years of treatment with subsequent events. Secondary outcomes were cardiovascular mortality and all-cause mortality. Causes of death were based on the primary cause of death in the National Death Index (8) through 2014, classified into 10 cause-of-death categories: cardiovascular disease, cancer, chronic obstructive pulmonary disease, diabetes, chronic kidney disease, infection, mental illness, abnormal/accident, other chronic diseases, and all other causes (Supplementary Table 2).
Statistical Analysis
We compared participant characteristics across levels of baseline SBP using χ2 tests for categorical data and two-sample t tests for continuous or ordinal data. In preliminary analyses evaluating the associations between pretreatment SBP and on-treatment SBP independently, we used multivariable Cox proportional hazards regression (to estimate hazard rates) and multivariable Poisson regression (to estimate incidence rates) using stabilized inverse probability of treatment weighting (IPTW), a method of balancing covariates between exposure categories by weighting individuals based on propensity for each BP category (9).
All multivariable analyses included demographic variables (baseline age, sex, race, and ethnicity), baseline cardiovascular disease risk factors (non-HDL cholesterol, BMI, and smoking status), diabetes-related variables (outpatient diabetes medications categorized as none, drug regimens without insulin, and drug regimens with insulin; and baseline HbA1c), renal function expressed as the estimated glomerular filtration rate (eGFR) calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (10), and Elixhauser comorbidity index score (11). We opted for the Elixhauser comorbidity index based on prior evidence that it performed comparably to alternative indices for predicting mortality in the VA and better than alternatives for predicting VA health care utilization (12,13). All comorbidities were based on ICD-9 diagnosis codes in the VA EHR (Supplementary Table 3).
In preliminary analyses evaluating on-treatment SBP as the exposure, we included additional covariates: the number of BP drug classes used at 2 years after antihypertensive medication initiation and whether an individual was or was not prescribed a CCB, BB, ACEi/ARB, or diuretic. Smoking status was determined using an algorithm developed for VA EHR that classified individuals as ever or never smokers (14).
For the primary analysis of the association of the combination of the SBP at antihypertensive medication initiation and SBP in the 2nd year of treatment, we used an IPTW approach to balance covariates. We estimated the propensity for both treatment initiation after an SBP of 130–139 or ≥140 mmHg and achievement of on-treatment SBP of 100–119, 120–129, 130–139, or 140–159 mmHg. Thus, the propensity model estimated probability of inclusion in one of eight categories defined by two possible treatment initiation thresholds and four possible levels of SBP achieved on treatment, capturing the differing thresholds for initiation and on-treatment goals highlighted by the ACC/AHA and ADA guidelines for hypertension management (1,3).
To account for time-varying covariates between treatment initiation and 2 years on treatment, we included baseline covariates available at the time of treatment initiation and also updated comorbidities, HbA1c, and renal function at 2 years on treatment in the propensity model. We then used stabilized IPTW Poisson regression and Cox proportional hazards models to compare outcomes across the eight categories defined by threshold of treatment initiation and SBP achieved on treatment with robust sandwich estimators of variance to calculate valid 95% CIs (15).
Because the primary exposure was based on BP measurements over the first 2 years of treatment, participants who died during the first 2 years after antihypertensive medication initiation were censored. Individuals who had an ASCVD event during the first 2 years of antihypertensive therapy were excluded from analyses of the ASCVD outcome but were included in analysis of mortality. As a sensitivity analysis, we limited the analyses to individuals aged 55–65 years at baseline to reduce the impact of age on the association between BP and ASCVD events.
For all Cox proportional hazards models with cardiovascular mortality as the outcome, we used cumulative incidence function methods to account for competing risk from mortality due to noncardiovascular causes (16). We used a significance threshold of P < 0.05 for all association tests. All analyses were conducted in SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC) or R 3.3 software (R Foundation for Statistical Computing, Vienna, Austria). Statistical code is available upon request.
Results
Study Participant Characteristics
The study included 43,986 participants (81% white and 16% black) in the study. Mean age was 62 years, mean BMI was 31.8 kg/m2, and mean follow-up time was 9.3 years. The pretreatment SBP was 130–139 in 14,064 individuals (32%) and ≥140 mmHg in 29,922 (68%). Individuals who initiated antihypertensive therapy after an SBP of 130–139 mmHg were younger, were less likely to have baseline cardiovascular disease or chronic kidney disease, and were more likely to have cancer or mental health disease at baseline compared with those who started treatment when SBP was ≥140 mmHg (Table 1). Despite statistically significant differences, the two groups were clinically similar with regard to baseline BMI, HbA1c, diabetes medications, lipid levels, and lipid medications (Table 1). There were clinically minor but statistically significant differences between on-treatment SBP groups across most covariates measured (Supplementary Table 1). A small proportion (4.0%) of the study population had an SBP ≥160 mmHg after 2 years of treatment; this group differed from the other on-treatment SBP groups across a number of important covariates (e.g., baseline cardiovascular disease, HbA1c, Elixhauser score, and eGFR) (Supplementary Table 1), and they were excluded from the remainder of the analyses. In addition, 330 individuals (0.8%; 85 and 245 with pretreatment SBP of 130–139 and ≥140 mmHg, respectively) died within 2 years of treatment initiation and were also excluded from the analyses.
Table 1.
Study participant characteristics
| All participants |
SBP at treatment initiation |
P value | ||
|---|---|---|---|---|
| 130–139 mmHg | ≥140 mmHg | |||
| (N = 43,986) | (n = 14,064) | (n = 29,922) | ||
| Age (years) | 61.5 ± 10.4 | 59.5 ± 10.6 | 62.4 ± 10.2 | <0.001 |
| Male | 42,906 (97.5) | 13,638 (97) | 29,268 (97.8) | <0.001 |
| Race | <0.001 | |||
| Black or African American | 7,161 (16.3) | 2,526 (18) | 4,635 (15.5) | |
| White | 35,401 (80.5) | 11,066 (78.7) | 24,335 (81.3) | |
| Other | 1,424 (3.2) | 472 (3.4) | 952 (3.2) | |
| Ethnicity | <0.001 | |||
| Hispanic or Latino | 3,183 (7.2) | 1,220 (8.7) | 1,963 (6.6) | |
| Not Hispanic or unknown | 40,803 (92.8) | 12,844 (91.3) | 27,959 (93.4) | |
| CVD at baseline | 9,797 (22.3) | 2,962 (21.1) | 6,835 (22.8) | <0.001 |
| Cancer at baseline | 10,001 (22.7) | 3,866 (27.5) | 6,135 (20.5) | <0.001 |
| Mental health disease at baseline | 6,827 (15.5) | 3,031 (21.6) | 3,796 (12.7) | <0.001 |
| Kidney disease at baseline | 339 (0.8) | 52 (0.4) | 287 (1) | <0.001 |
| Smoking status | <0.001 | |||
| Current | 8,621 (18.3) | 3,088 (22) | 4,903 (16.4) | |
| Former | 29,174 (61.8) | 8,146 (57.9) | 19,015 (63.5) | |
| Never | 9,426 (20) | 2,830 (20.1) | 6,004 (20.1) | |
| BMI (kg/m2) | 31.8 ± 6 | 31.6 ± 5.8 | 31.9 ± 6.1 | <0.001 |
| HbA1c (%) | 7.5 ± 1.7 | 7.5 ± 1.8 | 7.6 ± 1.7 | <0.001 |
| HbA1c (mmol/mol) | 58 ± 13 | 58 ± 14 | 60 ± 13 | |
| Glucose (mg/dL) | 158.2 ± 65.2 | 155.9 ± 65.3 | 159.3 ± 65.2 | <0.001 |
| Diabetes medications | 0.01 | |||
| None | 8,224 (17.4) | 2,404 (17.1) | 5,219 (17.4) | |
| Regimens with insulin | 9,767 (20.7) | 2,991 (21.3) | 6,000 (20.1) | |
| Regimens without insulin | 29,230 (61.9) | 8,669 (61.6) | 18,703 (62.5) | |
| Total cholesterol (mg/dL) | 186.6 ± 44 | 183.5 ± 42.9 | 188.1 ± 44.4 | <0.001 |
| HDL cholesterol (mg/dL) | 41.5 ± 11.6 | 41.1 ± 11.5 | 41.7 ± 11.6 | <0.001 |
| LDL cholesterol (mg/dL) | 109.9 ± 35.7 | 107.3 ± 34.5 | 111.1 ± 36.1 | <0.001 |
| Triglyceride (mg/dL) | 192.4 ± 177.3 | 191.7 ± 187.9 | 192.8 ± 172.1 | 0.53 |
| Lipid medications | 30,847 (70.1) | 9,890 (70.3) | 20,957 (70) | 0.55 |
| eGFR (mL/min/1.73 m2) | 81.3 ± 18.8 | 83.9 ± 18.2 | 80 ± 19 | <0.001 |
| Elixhauser score | −2.8 ± 5.9 | −3.4 ± 6.6 | −2.5 ± 5.5 | <0.001 |
| SBP (mmHg) | 146.4 ± 12 | 135 ± 2.8 | 151.7 ± 10.9 | <0.001 |
| DBP (mmHg) | 80.6 ± 8.5 | 77.6 ± 6.9 | 82 ± 8.8 | <0.001 |
| BP medications, n | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | <0.001 |
| Antihypertensive class | ||||
| ACEi/ARB | 27,126 (61.7) | 8,338 (59.3) | 18,788 (62.8) | <0.001 |
| CCB | 6,668 (15.2) | 970 (6.9) | 5,698 (19) | <0.001 |
| BB | 10,261 (23.3) | 2,463 (17.5) | 7,798 (26.1) | <0.001 |
| Diuretic | 11,013 (25) | 2,191 (15.6) | 8,822 (29.5) | <0.001 |
| Follow-up time (years) | 9.3 ± 1.9 | 9.2 ± 1.8 | 9.3 ± 2.0 | <0.001 |
Data are presented as n (%), mean ± SD, or median (interquartile range). CVD, cardiovascular disease; DBP, diastolic blood pressure.
Associations With ASCVD Events
In preliminary analyses, we found that pretreatment SBP and SBP achieved after 2 years of treatment were both associated with composite ASCVD events (Supplementary Tables 4–6). The adjusted hazard ratio (HR) of ASCVD events was 1.10 (95% CI 1.02, 1.19) for individuals who initiated therapy when SBP was ≥140 mmHg compared with those who initiated therapy when SBP was 130–139 mmHg (Supplementary Table 5). The adjusted HRs (95% CIs) of ASCVD events were 0.90 (0.76, 1.06), 1.03 (0.93, 1.14), 1.24 (1.13, 1.37), and 1.57 (1.34, 1.85) for individual who achieved on-treatment SBP of 100–119, 130–139, 140–159, and ≥160 mmHg, respectively, relative to those with on-treatment SBP of 120–129 mmHg (Supplementary Table 6).
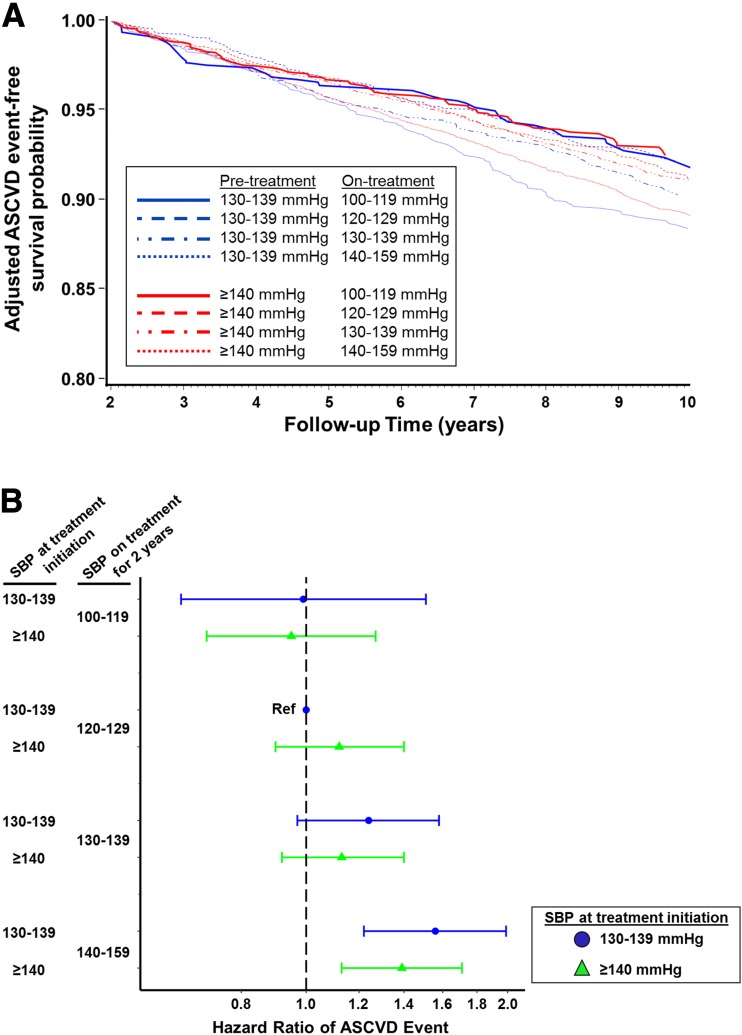
In the primary analysis of the combination of threshold for treatment initiation and on-treatment SBP, the lowest adjusted incidence rates of ASCVD events and highest ASCVD event-free survival were observed for individuals who achieved an on-treatment SBP level of 100–119 mmHg (adjusted incidence rate 7.8 [95% CI 5.4, 11.4] events/1,000-person-years and 7.6 [6.2, 9.4] events/1,000 person-years for those who initiated treatment when SBP was 130–139 and ≥140 mmHg, respectively) (Table 2 and Fig. 1A), and were similar in those who initiated treatment when SBP was 130–139 mmHg and achieved on-treatment SBP of 120–129 mmHg (referent; adjusted incidence rate 7.9 [6.5, 9.7] events/1,000 person-years). Relative to the reference group, the risk of ASCVD events was higher for individuals who initiated treatment when SBP was 130–139 mmHg and achieved on-treatment SBP of 130–139 mmHg (incidence rate difference [IRD] 1.9 [95% CI 0.6, 3.2] events/1,000 person-years) or 140–159 mmHg (IRD 4.5 [2.9, 6.1] events/1,000 person-years) and for those who initiated treatment when SBP was ≥140 mmHg and achieved an on-treatment SBP of 120–129 mmHg (IRD 1.0 [−0.2, 2.1] events/1,000 person-years), 130–139 mmHg (IRD 1.1 [0.04, 2.2] events/1,000 person-years), or 140–159 mmHg (IRD 3.2 [2.1, 4.3] events/1,000 person-years) (Table 2). Relative to achieving an SBP of 120–129 mmHg, achieving on-treatment SBP ≥130 mmHg was associated with higher incidence of ASCVD events in individuals who initiated treatment when SBP was 130–139 mmHg and when SBP was ≥140 mmHg (Table 2).
Table 2.
Association of early BP treatment pattern, ASCVD events, and cardiovascular and all-cause mortality
| SBP at treatment initiation (mmHg) | On-treatment SBP (mmHg) | Unadjusted incidence rate (95% CI)* | Adjusted incidence rate (95% CI)*† | Adjusted IRD (95% CI)*† |
|---|---|---|---|---|
| ASCVD | ||||
| 130–139 | 100–119 | 7.2 (5.2, 9.9) | 7.8 (5.4, 11.4) | −0.1 (−2.0, 2.0) |
| 120–129 | 7.2 (6.0, 8.6) | 7.9 (6.5, 9.7) | Reference | |
| 130–139 | 9.0 (7.9, 10.3) | 9.8 (8.6, 11.3) | 1.9 (0.6, 3.2) | |
| 140–159 | 12.4 (10.7, 14.4) | 12.4 (10.7, 14.3) | 4.5 (2.9, 6.1) | |
| ≥140 | 100–119 | 7.3 (5.9, 8.9) | 7.6 (6.2, 9.4) | −0.3 (−1.8, 1.1) |
| 120–129 | 8.6 (7.7, 9.5) | 8.9 (8.1, 9.9) | 1.0 (−0.2, 2.1) | |
| 130–139 | 8.9 (8.3, 9.5) | 9.1 (8.5, 9.8) | 1.1 (0.04, 2.2) | |
| 140–159 | 11.9 (11.2, 12.6) | 11.2 (10.5, 11.9) | 3.2 (2.1, 4.3) | |
| CVD mortality | ||||
| 130–139 | 100–119 | 3.5 (2.2, 5.5) | 4.2 (2.6, 6.7) | 1.4 (0.5, 2.4) |
| 120–129 | 2.3 (1.7, 3.1) | 2.8 (2.0, 3.9) | Reference | |
| 130–139 | 2.4 (1.8, 3.0) | 2.7 (2.1, 3.6) | −0.06 (−0.6, 0.5) | |
| 140–159 | 3.2 (2.4, 4.2) | 3.1 (2.3, 4.1) | 0.3 (−0.3, 0.9) | |
| ≥140 | 100–119 | 3.6 (2.8, 4.8) | 3.7 (2.8, 4.9) | 0.9 (0.2, 1.5) |
| 120–129 | 3.2 (2.7, 3.7) | 3.6 (3.0, 4.2) | 0.8 (0.3, 1.2) | |
| 130–139 | 3.6 (3.2, 4.0) | 3.7 (3.3, 4.1) | 0.9 (0.4, 1.3) | |
| 140–159 | 4.8 (4.3, 5.2) | 4.1 (3.7, 4.5) | 1.3 (0.8, 1.7) | |
| All-cause mortality | ||||
| 130–139 | 100–119 | 12.1 (9.5, 15.4) | 14.4 (11.2, 18.5) | 2.8 (0.08, 5.7) |
| 120–129 | 8.9 (7.7, 10.4) | 11.6 (9.9, 13.7) | Reference | |
| 130–139 | 9.0 (7.9, 10.2) | 10.5 (9.2, 12.0) | −1.1 (−2.7, 0.5) | |
| 140–159 | 11.9 (10.3, 13.7) | 11.7 (10.1, 13.5) | 0.1 (−1.7, 1.9) | |
| ≥140 | 100–119 | 15.4 (13.5, 17.6) | 15.1 (13.2, 17.3) | 3.5 (1.5, 5.5) |
| 120–129 | 12.8 (11.8, 13.8) | 14.1 (13.0, 15.3) | 2.5 (1.0, 4.0) | |
| 130–139 | 12.0 (11.3, 12.7) | 12.3 (11.6, 13.1) | 0.7 (−0.7, 2.0) | |
| 140–159 | 14.9 (14.2, 15.7) | 13.1 (12.4, 13.8) | 1.5 (0.05, 2.8) |
CVD, cardiovascular disease.
*Incidence rates and IRDs in events/1,000 person-years.
†Adjusted models included age, sex, race, ethnicity, non-HDL cholesterol, BMI, smoking status, outpatient diabetes medications (categorized as none, drug regimens without insulin, and drug regimens with insulin), baseline HbA1c, renal function expressed as the eGFR calculated using the Chronic Kidney Disease Epidemiology Collaboration equation, Elixhauser comorbidity index score, and time-varying renal function, time-varying HbA1c, and time-varying Elixhauser comorbidity index score during the first 2 years on antihypertensive treatment.
Figure 1.
Association between early BP treatment pattern and ASCVD events in individuals with diabetes and hypertension. A: Adjusted event-free survival probability of ASCVD events during follow-up across eight BP treatment combinations based on two possible pretreatment SBP levels (130–139 mmHg in blue and ≥140 mmHg in red) and four possible on-treatment SBP levels (100–119, 120–129, 130–139, and 140–159 mmHg). B: Adjusted HRs for ASCVD events from multivariable Cox proportional hazards models across eight combinations of pretreatment and on-treatment SBP, with individuals initiating treatment after an SBP measurement of 130–139 mmHg and achieving on-treatment SBP of 120–129 mmHg as the reference group.
The pattern of results was similar in multivariable Cox proportional hazards models but not all of the contrasts achieved statistical significance (Fig. 1B). Relative to treatment initiation when SBP was 130–139 mmHg and on-treatment SBP of 120–129 mmHg, the hazard of ASCVD events was higher for individuals who initiated treatment when SBP was 130–139 mmHg or when SBP was ≥140 mmHg and achieved on-treatment SBP of 140–159 mmHg (Fig. 1B). The associations of pretreatment and on-treatment SBP with ASCVD events in analyses limited to individuals aged 55–65 years at baseline were similar to the full population, although the CIs were wide owing to reduced sample size (Supplementary Tables 7 and 8).
Associations With Mortality
Cardiovascular diseases and cancer were the two most common causes of death in the study population, irrespective of on-treatment SBP achieved (Supplementary Table 9). As with the ASCVD event outcome, we found in preliminary analyses that the pretreatment SBP and the SBP achieved after 2 years of treatment were both associated with cardiovascular and all-cause mortality (Supplementary Tables 5 and 6). The adjusted HR of cardiovascular mortality was 1.25 (95% CI 1.10, 1.43) and of all-cause mortality was 1.15 (95% CI 1.08, 1.24) for individuals who initiated therapy when SBP was ≥140 mmHg compared with those who initiated therapy when SBP was 130–139 mmHg (Supplementary Table 5).
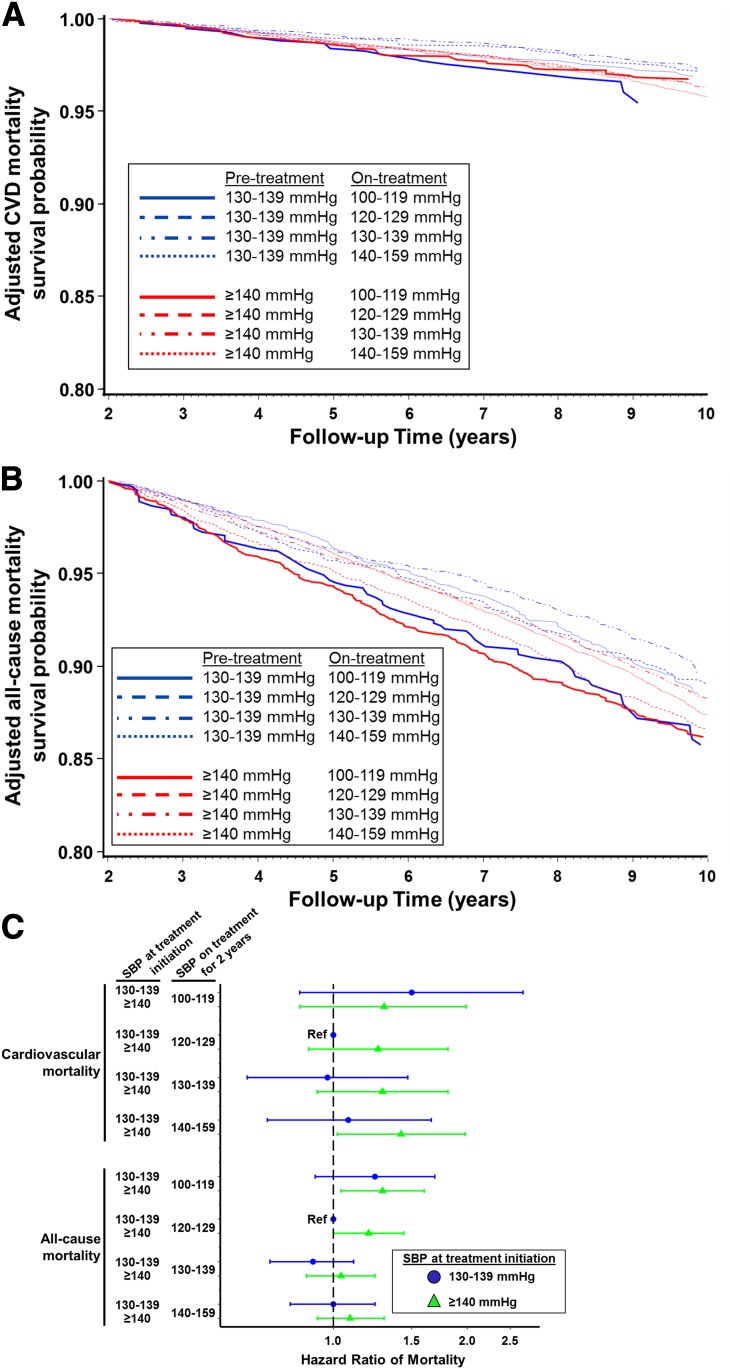
In the primary analysis of the combination of threshold for treatment initiation and on-treatment SBP, the lowest adjusted incidence rate and highest survival probability from cardiovascular mortality was observed for individuals who were started on antihypertensive treatment when SBP was 130–139 mmHg and achieved an on-treatment SBP of 130–139 mmHg, but cardiovascular mortality was nearly indistinguishable from those who initiated treatment when SBP was 130–139 mmHg and achieved an on-treatment SBP of 120–129 mmHg (referent) (Table 2 and Fig. 2A). Relative to the reference group, the risk of cardiovascular mortality was higher for individuals who initiated treatment when SBP was ≥140 mmHg, irrespective of the SBP level achieved on treatment (Table 2). For each level of on-treatment SBP ≥120 mmHg, the incidence rate and HR for cardiovascular mortality were higher among those who initiated treatment at SBP ≥140 mmHg than among those who initiated treatment at SBP 130–139 mmHg (Table 2 and Fig. 2C). However, the 95% CI for the HR for cardiovascular mortality failed to cross the null only among those who initiated treatment when SBP was ≥140 mmHg and achieved on-treatment SBP of 140–159 mmHg, relative to the reference group (HR of cardiovascular mortality 1.42 [95% CI 1.02, 1.98]) (Fig. 2C).
Figure 2.
Association between early BP treatment pattern and mortality in individuals with diabetes and hypertension. Adjusted survival probability of cardiovascular mortality (A) and all-cause mortality (B) during follow-up across eight BP treatment combinations based on two possible pretreatment SBP levels (130–139 mmHg in blue and ≥140 mmHg in red) and four possible on-treatment SBP levels (100–119, 120–129, 130–139, and 140–159 mmHg). CVD, cardiovascular disease. C: Adjusted HRs for mortality from multivariable Cox proportional hazards models across eight combinations of pretreatment and on-treatment SBP, with individuals initiating treatment after an SBP measurement of 130–139 mmHg and achieving on-treatment SBP of 120–129 mmHg as the reference group.
For all-cause mortality, the lowest adjusted incidence rate and highest survival probability during follow-up was observed for individuals who were started on antihypertensive treatment when SBP was 130–139 mmHg and achieved an on-treatment SBP of 130–139 mmHg (Table 2 and Fig. 2B). Relative to those who initiated treatment when SBP was 130–139 mmHg and achieved on-treatment SBP of 120–129 mmHg (referent), all-cause mortality was higher in individuals who initiated treatment when SBP was ≥140 mmHg and achieved on treatment SBP of 100–119 or 120–129 mmHg (HRs of all-cause mortality 1.29 [95% CI 1.04, 1.60] and 1.20 [1.00, 1.44] for achieving SBP 100–119 and 120–129 mmHg, respectively) (Table 2 and Fig. 2C). For each level of on-treatment SBP, the incidence rate and HR for all-cause mortality were higher among those who initiated treatment at SBP ≥140 mmHg than among those who initiated treatment at SBP 130–139 mmHg (Table 2 and Fig. 2C), but the 95% CIs did not always exclude the null.
Conclusions
In this retrospective cohort study set in the largest integrated health care system in the U.S., we found that patients with diabetes who were initiated on antihypertensive therapy when SBP was 130–139 mmHg had similar rates of ASCVD events but lower cardiovascular and all-cause mortality compared with individuals who began antihypertensive treatment when SBP was ≥140 mmHg. Furthermore, the patterns of early BP treatment observed in our data were associated with long-term clinical outcomes over a mean follow-up of 9 years—a much longer follow-up time than recent hypertension treatment trials. The on-treatment SBP at which we observed the lowest rate of outcomes depended on the threshold at which treatment was initiated and differed across outcomes. Lower ASCVD incidence, the primary outcome of this study, was observed in those who achieved on-treatment SBP of <130 mmHg than in those who achieved higher on-treatment SBP levels in individuals who began BP treatment when SBP was 130–139 mmHg and when SBP was ≥140 mmHg. In contrast, achieving on-treatment SBP of 130–139 was associated with the lowest all-cause mortality outcomes in individuals who initiated treatment when SBP was 130–139 mmHg and when SBP was ≥140 mmHg. In sum, earlier initiation of antihypertensive therapy, that is, at an SBP threshold of 130–139 mmHg, was associated with better outcomes in patients with diabetes receiving routine clinical care in a national U.S. health care system. However, the reduction in ASCVD events observed with intensive SBP lowering, that is, to <130 mmHg, may have to be balanced against increased mortality risk. Our results highlight the complexity of BP management for patients with diabetes faced by clinical providers.
By focusing on new initiators of BP treatment, our study was designed to address associations between the pattern of early hypertension care and clinical outcomes, an important dimension not evaluated by recent BP treatment trials. In the normotensive subgroup of the Appropriate Blood Pressure Control in Diabetes (ABCD) Trial, individuals randomized to intensive BP control (mean on-treatment SBP of 128 mmHg) had a lower risk of stroke (but not all ASCVD events) than those randomized to moderate BP control (mean on-treatment SBP of 137 mmHg) (17). In the hypertensive subgroup of the ABCD trial, individuals randomized to intensive BP control (mean on-treatment SBP of 132 mmHg) had lower all-cause mortality than those randomized to moderate BP control (mean on-treatment SBP of 138 mmHg) (18). In the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) randomized trial, patients with diabetes treated with perindopril-indapamide had mean reduction of SBP of 5.6 mmHg compared with those treated with placebo and had a lower rate of macro- and microvascular events, irrespective of baseline SBP (<140 vs. ≥140 mmHg) (19). In contrast, a secondary analysis of the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) randomized trial concluded that the relationship between SBP reduction and ASCVD outcomes was observed only in individuals with a baseline SBP of 143–155 mmHg (20). In the same study, the lowest incidence of myocardial infarction, cardiovascular mortality, or a composite ASCVD outcome occurred in individuals who achieved an SBP between 120 and 130 mmHg (20). However, none of the prior trials specifically addressed through randomization the threshold at which treatment is initiated.
Although not a randomized trial, our study provides observational data from routine clinical care in a national health system and demonstrates that individuals with diabetes who initiated antihypertensive therapy when SBP was 130–139 mmHg had similar rates of ASCVD events and lower mortality rates than those who began treatment when SBP was ≥140 mmHg. Prior analyses of trial data have suggested that the relationship between BP lowering and stroke may differ from other ASCVD events (5,20–23). Unfortunately, we did not have enough stroke events in our data to analyze with sufficient power whether our results were driven by a beneficial association between early initiation of BP treatment and stroke. Though prior randomized trials and meta-analyses in patients with diabetes have not found that reducing SBP to <130 mmHg is associated with statistically significant improvements in macrovascular outcomes when treatment was started when SBP was ≥140 (5,19,20,22,24), our results suggest fewer ASCVD events but higher cardiovascular mortality in individuals who achieved on-treatment SBP of 100–119 mmHg, though with wide CIs owing to the analytic approach and sample size.
Our observation of a higher incidence of cardiovascular and all-cause mortality in individuals who achieved an on-treatment SBP of 100–119 mmHg is similar to the findings of several prior observational studies (25–28) but not all (22,29). Our findings are also consistent with a series of studies using regional data from the U.S. that observed U-shaped associations between SBP and cardiovascular outcomes (coronary heart disease, stroke, and heart failure) among individuals with diabetes (30–32). While the excess mortality observed in individuals with an on-treatment SBP <120 mmHg did not consistently achieve statistical significance in our study, the consistent observation of this association across numerous other studies suggests that lower SBP than intended on treatment may be an indicator of poor prognosis.
Our study has several limitations. First, as an observational study, we cannot prove causality between hypertension treatment and clinical outcomes. Specifically, we cannot exclude unmeasured confounding related to provider and patient factors that determined both the BP at which antihypertensive therapy was initiated and the BP achieved within 2 years of treatment. Second, by censoring individuals who did not survive for 2 years on antihypertensive treatment, we may be inducing selection bias, but this represented <1% of the study population. Third, we limited the study population to individuals newly initiated on hypertension treatment to minimize confounding, so the interpretation of our results should be limited to treatment-naive individuals with hypertension and diabetes who are newly initiating antihypertensive therapy. Fourth, there were several limitations inherent to the data available: 1) we lacked information on adverse events related to treatment; 2) pretreatment SBP was based on a single clinical measurement, making that variable susceptible to misclassification; 3) BP measurements were based on routine clinic measurements rather than a standardized protocol, although a single-site VA study found minimal differences between measurement methods (33); 4) cause of death was based on the National Death Index and not adjudicated (8); and 5) there were too few stroke events in our data to analyze stroke as an outcome independently from the composite ASCVD outcome. Finally, care in the VA may not generalize to individuals receiving care outside the VA. A recent study found that VA facilities meet outpatient quality measures related to diabetes, hypertension, and cardiovascular disease care at a higher rate than non-VA facilities (34). Moreover, nearly the entire study population was male, limiting generalizability to women.
In conclusion, we report better clinical outcomes among individuals with diabetes started on antihypertensive therapy when SBP was 130–139 mmHg rather than ≥140 mmHg. While this result supports the ACC/AHA 2017 guidelines for antihypertensive treatment initiation in patients with diabetes, the benefits of lower ASCVD incidence associated with achieving on-treatment SBP <130 mmHg were mitigated by higher all-cause mortality, especially for individuals who initiate therapy only after SBP exceeds 140 mmHg. Given the concerns for adverse events related to hypertension overtreatment with advancing age (35–37), our results suggest that BP management early after hypertension diagnosis when treatments are better tolerated may have durable long-term effects on ASCVD. We conclude that our results provide further motivation for randomized clinical trials that explicitly address the threshold for hypertension treatment initiation, that consider more granular stratification of SBP targeted by treatment, and that use adaptive designs that acknowledge the heterogeneity of risks and benefits from BP treatment across the diverse population of patients with diabetes and hypertension.
Supplementary Material
Article Information
Funding. S.R. is supported by American Heart Association award 17MCPRP33670728. S.R. is supported by U.S. Department of Veterans Affairs VA Career Development Award IK2-CX001907-01 and J.L.V. by IK2-CX001262. Y.-L.H., D.R.G., K.C., P.W.F.W., and L.S.P. are supported by U.S. Department of Veterans Affairs VA Merit Grant I01-CX001025. V.K. is supported by American Heart Association award 18CDA34110037. L.S.P. is supported by U.S. Food and Drug Administration award RO1FD003527; U.S. Department of Veterans Affairs Health Services Research and Development Service award IIR 07-138; National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases awards R21-DK-099716, DK-066204, U01-DK-091958, U01-DK-098246, and P30-DK-111024; and Cystic Fibrosis Foundation award PHILLI12A0. Support for VA/Centers for Medicare & Medicaid Services data was provided by the Department of Veterans Affairs, VA Health Services Research and Development Service, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004). All authors are also supported in part by the Veterans Health Administration (VA).
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. This work is not intended to reflect the official opinion of the VA or the U.S. government.
Duality of Interest. L.S.P. has served on scientific advisory boards for Janssen and the Profil Institute for Clinical Research and has or had research support from Merck, Amylin, Eli Lilly, Novo Nordisk, Sanofi, PhaseBio, Roche, AbbVie, Vascular Pharmaceuticals, Janssen, GlaxoSmithKline, and Pfizer outside the present work. L.S.P. was a speaker for Novartis and Merck, but not for the last 5 years. L.S.P. is also a cofounder, officer, board member, and stockholder of DIASYST, which is developing software aimed to help improve diabetes management. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.R., Y.-L.H., V.K., M.K.R., J.L.V., D.R.G., K.C., P.W.F.W., and L.S.P. participated in interpreting results, manuscript writing, and critical revision. All authors approved of the manuscript submission in its current form. Study design was conceived by S.R., Y.-L.H., V.K., J.L.V., D.R.G., K.C., P.W.F.W., and L.S.P. Data collection and organization were performed by Y.-L.H., K.C., P.W.F.W., and L.S.P. Analyses were performed by Y.-L.H., D.R.G., and K.C. S.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0686/-/DC1.
References
- 1.American Diabetes Association 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S103–S123 [DOI] [PubMed] [Google Scholar]
- 2.de Boer IH, Bangalore S, Benetos A, et al. . Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017;40:1273–1284 [DOI] [PubMed] [Google Scholar]
- 3.Whelton PK, Carey RM, Aronow WS, et al. . 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in J Am Coll Cardiol 2018;71:2275–2279]. J Am Coll Cardiol 2018;71:e127–e248 [DOI] [PubMed] [Google Scholar]
- 4.Wright JT Jr., Williamson JD, Whelton PK, et al.; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control [published correction appears in N Engl J Med 2017;377:2506]. N Engl J Med 2015;373:2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cushman WC, Evans GW, Byington RP, et al.; ACCORD Study Group . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu S, Chakrabortty A, Liao KP, et al. . Surrogate-assisted feature extraction for high-throughput phenotyping. J Am Med Inform Assoc 2017;24:e143–e149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Cai T, Yu S, et al. . Methods for high-throughput phenotyping with electronic medical record data using a common semi-supervised approach (PheCAP). Nat Protoc. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Center for Excellence for Suicide Prevention; Joint Department of Veterans Affairs (VA) and Department of Defense (DoD) Suicide Data Repository. National Death Index (NDI) [Internet], 2018. Available from https://www.dspo.mil/About-Suicide/Suicide-Data-Repository/. Accessed 3 April 2019
- 9.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–560 [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27 [DOI] [PubMed] [Google Scholar]
- 12.Dominick KL, Dudley TK, Coffman CJ, Bosworth HB. Comparison of three comorbidity measures for predicting health service use in patients with osteoarthritis. Arthritis Rheum 2005;53:666–672 [DOI] [PubMed] [Google Scholar]
- 13.Radomski TR, Zhao X, Hanlon JT, et al. Use of a medication-based risk adjustment index to predict mortality among veterans dually-enrolled in VA and Medicare. Healthc (Amst). 25 April 2019 [Epub ahead of print]. DOI: 10.1016/j.hjdsi.2019.04.003 10.1016/j.hjdsi.2019.04.003 [DOI] [PMC free article] [PubMed]
- 14.Song RJH, Nguyen Y-L, Honerlaw X-MT, et al. . Development of an electronic health record-based algorithm for smoking status using the Million Veteran Program (MVP) Cohort survey response (Abstract). Circulation 2016;134(Suppl. 1):A18809 [Google Scholar]
- 15.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22 [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509 [Google Scholar]
- 17.Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002;61:1086–1097 [DOI] [PubMed] [Google Scholar]
- 18.Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000;23(Suppl. 2):B54–B64 [PubMed] [Google Scholar]
- 19.Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007;370:829–840 [DOI] [PubMed] [Google Scholar]
- 20.Redon J, Mancia G, Sleight P, et al.; ONTARGET Investigators . Safety and efficacy of low blood pressures among patients with diabetes: subgroup analyses from the ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial). J Am Coll Cardiol 2012;59:74–83 [DOI] [PubMed] [Google Scholar]
- 21.Cooper-DeHoff RM, Gong Y, Handberg EM, et al. . Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA 2010;304:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015;313:603–615 [DOI] [PubMed] [Google Scholar]
- 23.Mancia G, Kjeldsen SE, Zappe DH, et al. . Cardiovascular outcomes at different on-treatment blood pressures in the hypertensive patients of the VALUE trial. Eur Heart J 2016;37:955–964 [DOI] [PubMed] [Google Scholar]
- 24.Mancia G, Grassi G. Blood pressure targets in type 2 diabetes. Evidence against or in favour of an aggressive approach. Diabetologia 2018;61:517–525 [DOI] [PubMed] [Google Scholar]
- 25.Adamsson Eryd S, Gudbjörnsdottir S, Manhem K, et al. . Blood pressure and complications in individuals with type 2 diabetes and no previous cardiovascular disease: national population based cohort study. BMJ 2016;354:i4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kontopantelis E, Springate DA, Reeves D, et al. . Glucose, blood pressure and cholesterol levels and their relationships to clinical outcomes in type 2 diabetes: a retrospective cohort study [published correction appears in Diabetologia 2015;58:1142]. Diabetologia 2015;58:505–518 [DOI] [PubMed] [Google Scholar]
- 27.Vamos EP, Harris M, Millett C, et al. . Association of systolic and diastolic blood pressure and all cause mortality in people with newly diagnosed type 2 diabetes: retrospective cohort study. BMJ 2012;345:e5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan EYF, Yu EYT, Chin WY, et al. . Effect of achieved systolic blood pressure on cardiovascular outcomes in patients with type 2 diabetes: a population-based retrospective cohort study. Diabetes Care 2018;41:1134–1141 [DOI] [PubMed] [Google Scholar]
- 29.Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ 2016;352:i717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao W, Katzmarzyk PT, Horswell R, et al. . Blood pressure and heart failure risk among diabetic patients. Int J Cardiol 2014;176:125–132 [DOI] [PubMed] [Google Scholar]
- 31.Zhao W, Katzmarzyk PT, Horswell R, et al. . Blood pressure and stroke risk among diabetic patients. J Clin Endocrinol Metab 2013;98:3653–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao W, Katzmarzyk PT, Horswell R, et al. . Aggressive blood pressure control increases coronary heart disease risk among diabetic patients. Diabetes Care 2013;36:3287–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papademetriou V, Tsioufis C, Chung A, Geladari C, Andreadis EA. Unobserved automated office BP is similar to other clinic BP measurements: a prospective randomized study. J Clin Hypertens (Greenwich) 2018;20:1411–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing quality of care in Veterans Affairs and non-Veterans Affairs settings. J Gen Intern Med 2018;33:1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerr EA, Lucatorto MA, Holleman R, Hogan MM, Klamerus ML, Hofer TP; VA Diabetes Quality Enhancement Research Initiative (QUERI) Workgroup on Clinical Action Measures . Monitoring performance for blood pressure management among patients with diabetes mellitus: too much of a good thing? Arch Intern Med 2012;172:938–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mossello E, Pieraccioli M, Nesti N, et al. . Effects of low blood pressure in cognitively impaired elderly patients treated with antihypertensive drugs. JAMA Intern Med 2015;175:578–585 [DOI] [PubMed] [Google Scholar]
- 37.Sussman JB, Kerr EA, Saini SD, et al. . Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med 2015;175:1942–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.