Abstract
Tumors induce tolerance toward their antigens by producing the chemokine CCL21, leading to the formation of tertiary lymphoid organs (TLOs). Ins2-CCL21 transgenic, nonobese diabetic (NOD) mice express CCL21 in pancreatic β-cells and do not develop autoimmune diabetes. We investigated by which mechanisms CCL21 expression prevented diabetes. Ins2-CCL21 mice develop TLOs by 4 weeks of age, consisting of naive CD4+ T cells compartmentalized within networks of CD45−gp38+CD31− fibroblastic reticular cell (FRC)–like cells. Importantly, 12-week-old Ins2-CCL21 TLOs contained FRC-like cells with higher contractility, regulatory, and anti-inflammatory properties and enhanced expression of β-cell autoantigens compared with nontransgenic NOD TLOs found in inflamed islets. Consistently, transgenic mice harbored fewer autoreactive T cells and a higher proportion of regulatory T cells in the islets. Using adoptive transfer and islet transplantation models, we demonstrate that TLO formation in Ins2-CCL21 transgenic islets is critical for the regulation of autoimmunity, and although the effect is systemic, the induction is mediated locally likely by lymphocyte trafficking through TLOs. Overall, our findings suggest that CCL21 promotes TLOs that differ from inflammatory TLOs found in type 1 diabetic islets in that they resemble lymph nodes, contain FRC-like cells expressing β-cell autoantigens, and are able to induce systemic and antigen-specific tolerance leading to diabetes prevention.
Introduction
Type 1 diabetes (T1D) is an autoimmune disease characterized by the progressive destruction of insulin-producing β-cells in pancreatic islets, resulting in hyperglycemia and insulin dependency (1,2). Failure of central and peripheral immunological tolerance to islet cell autoantigens mediates T1D (3). Tumor cells are able to induce tolerance and promote their own survival (4). One mechanism used by tumor cells to induce tolerance is by secreting the secondary lymphoid chemokine CCL21 (5,6).
CCL21 is expressed by endothelial cells of high endothelial venules (HEVs), fibroblastic reticular cells (FRCs) in the lymph node (LN) paracortex, and lymphatic endothelial cells. CCL21 promotes interactions that are crucial to the adaptive T-cell immunity, by attracting various immune cell types expressing its receptor, CCR7, including dendritic cells, regulatory T cells (Tregs), and naive T cells (7–9). CCR7 signaling is critical for peripheral tolerance as it is required for Treg activation in the LN (10–12). Autologous secretion of CCL21 by melanoma cells is required for immune tolerance to melanoma antigens and is dependent on the induction of tolerogenic tertiary lymphoid organs (TLOs) (6).
TLO formation is reported in many organs during autoimmune diseases, chronic infections, and inflammation; in allogeneic transplantation (13–20); and in the fetal pancreas (21). However, the role of TLOs in modulating immunity and self-tolerance remains unclear. In the pancreas of nonobese diabetic (NOD) mice, islet infiltration is associated with TLO formation. Characterized by compartmentalization of T- and B-cell infiltrates as well as the appearance of HEVs, TLOs are considered sites of antigen presentation and activation of the immune response (22,23). In a C57BL/6 mouse model, Luther et al. (24) showed that TLO formation in the endocrine pancreas is induced by ectopic expression of CCL21 by the pancreatic islets without any signs of diabetes development. The presence and function of FRCs, which induce peripheral tolerance in LNs (25), remain to be elucidated in pancreatic TLOs in T1D.
Here, we investigated CCL21 as a novel regulator of immune tolerance to self-molecules implicated in the development of T1D. Local secretion of CCL21 in the pancreas of NOD mice was associated with the formation of TLOs containing β-cell autoantigen-expressing FRC-like cells, which induced systemic regulation of diabetogenic splenocytes.
Research Design and Methods
Mice
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Miami. Female NOD.Cg-Tg (Ins2-Ccl21b)2Cys/JbsJ (herein referred to as Ins2-CCL21) mice, NOD.CB17-Prkdcscid/J (NOD-scid), NOD/ShiLtJ, BALB/cJ, and C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME).
Histological Evaluation, Immunofluorescence, and Insulitis Grading
Sections from formalin-fixed paraffin-embedded or OCT frozen blocks were stained and imaged as previously reported (26). Islet size, Treg, and FRC density within pancreatic islets and islet size were quantified with ImageJ (National Institutes of Health [NIH]). Insulitis was graded depending on the percent of lymphocyte infiltration in the islet: 0% = grade 1, 1–10% = grade 2, 10–25% = grade 3, 25–50% = grade 4, and >50% = grade 5.
Islet Isolation, Culture, In Vitro Functionality, and CCL21 Assays
Isolation of murine pancreatic islets was performed at the Diabetes Research Institute (DRI) Preclinical Cell Processing and Translational Models Core as previously described (27). Glucose-stimulated insulin release was performed as previously described (27). CCL21 levels were measured by ELISA (R&D Systems, Minneapolis, MN).
Diabetes Induction, Blood Glucose Monitoring, Islet and Skin Transplantation, Adoptive Transfer of Splenocytes
Diabetes was chemically induced with a single intravenous injection of 200 mg/kg streptozotocin (STZ) (27) or with 50 mg/kg of STZ on five consecutive days. Diabetes was determined by three consecutive readings of nonfasting glycemic values >250 mg/dL. Transplants in the renal subcapsular space (KD) were performed as previously described (26–28). Islet transplant experiments and donor age/sex can be found in Table 1 and Supplementary Table 2. Skin grafting was performed as previously described (29). Adoptive transfer (AT) experiments by intravenous (IV) injection are summarized in Table 2.
Table 1.
Experimental details of experiments involving islet transplantation in mice
| Donors | Dose | Recipients | Site | n | Figure |
|---|---|---|---|---|---|
| Islets from Ins2-CCL21 NOD mice | 1,000 IEQ | Spontaneously diabetic NOD mice | EFP | 4 | Fig. 5B (red) |
| Islets from nontransgenic NOD mice | 1,000 IEQ | Spontaneously diabetic NOD mice | EFP | 1 | Fig. 5B (black) |
| Islets from CCL21 transgenic (CCL21+) C57BL/6 mice | 1,000 IEQ | Chemically diabetic BALB/c mice | EFP | 13 | Supplementary Fig. 2D (red) |
| Islets from CCL21− control C57BL/6 mice | 1,000 IEQ | Chemically diabetic BALB/c mice | EFP | 4 | Supplementary Fig. 2D (black) |
| Islets from Ins2-CCL21 NOD mice | 500 IEQ | 7-week-old NOD-scid mice | Kidney capsule (KD) | 7 | Fig. 5C (red) |
| Islets from nontransgenic NOD mice | 500 IEQ | 7-week-old NOD-scid mice | Kidney capsule (KD) | 6 | Fig. 5C (black) |
EFP, epididymal fat pad.
Table 2.
Experimental details of experiments involving AT of splenocytes in mice
| Donors | Dose | Recipients | Site | n | Figure |
|---|---|---|---|---|---|
| Splenocytes from 20- to 25-week-old Ins2-CCL21 NOD mice | 10 million | 4- to 6-week-old female NOD-scid mice | IV | 12 | Fig. 3A (red) |
| Splenocytes from 20- to 25-week-old diabetic NOD mice | 10 million | 4- to 6-week-old female NOD-scid mice | IV | 3 | Fig. 3A (black) |
| Splenocytes from 20- to 25-week-old diabetic NOD mice | 10 million | 10-week-old female Ins2-CCL21 NOD mice | IV | 6 | Fig. 5A (red) |
| Splenocytes from 20- to 25-week-old diabetic NOD mice | 10 million | 10-week-old female NOD mice | IV | 5 | Fig. 5A (black) |
| Splenocytes from 20- to 25-week-old diabetic NOD mice | 10 million | 11-week-old NOD-scid mice (4 weeks after NOD islet transplantation in KD) | IV | 6 | Fig. 5C (black) |
| Splenocytes from 20- to 25-week-old diabetic NOD mice | 10 million | 11-week-old NOD-scid mice (4 weeks after Ins2-CCL21 islet transplantation in KD) | IV | 7 | Fig. 5C (red) |
Immune Cell Isolation From Pancreas, Spleens, LNs, and Blood
LNs and spleens were processed by manual disruption. Collagenase D (Sigma-Aldrich) was used for the pancreatic distention and cell isolation. Single cell suspensions were stained for live/dead (Invitrogen) using the anti-mouse antibodies CD3, CD8, CD44, CD62L, CD25, CD127, Ki-67, B220 (BD Bioscience), CD4, FoxP3 (eBioscience), and CD45 (Biolegend) and acquired on a CytoFLEX or a BD LSRII. Tetramer staining for insulin and IGRP (NIH Tetramer Core) is detailed in Supplementary Table 1.
FRC Isolation From Islets and LNs
FRCs were isolated from skin-draining LNs (axillary, brachial, and inguinal) and pancreatic islets by adapting published protocols (30). In brief, harvested tissues were digested with Dispase II, DNAse I, and Collagenase P for 1 h maximum. Every 15 min, released cells were collected on ice, while fresh enzyme solution was added to the undigested tissue. Single cell suspensions were stained with the antibodies gp38-PE (eBioscience), CD31-APC (Biolegend), and CD45-PeCy7 (Tonbo Biosciences) and sorted using a Beckman Coulter MoFlo Astrios EQ. LN FRCs and islet-derived FRC-like cells were gated as CD45−CD31−gp38+ cells.
Characterization of FRC-Like Cells by RNA Sequencing
RNAseq Sample Preparation and Sequencing
Total RNA was extracted from FRCs freshly sorted from LNs or from pancreatic islets using TRIzol reagent (Invitrogen) and the RNeasy Micro Kit (Qiagen). Preparation and sequencing of RNA libraries was performed in the John P. Hussman Institute for Human Genomics Center for Genome Technology. At least 10 ng of total RNA verified by an Agilent Bioanalyzer was used as input for the KAPA RNA HyperPrep Kit with RiboErase (HMR), to create rRNA-depleted sequencing libraries, including sample indexing to allow for multiplexing. Cluster generation and sequencing was performed using the Illumina cBOT and HiSEq 3000, generating >32 million single-end 100 base reads per sample.
RNAseq Analysis
De-multiplexed FASTQ files were created with the Illumina-supplied scripts in the BCL2FASTQ software (version 2.17). The quality of the reads was determined with FASTQC software (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) for per base sequence quality, duplication rates, and overrepresented k-mers. Illumina adapters were trimmed using the Trim Galore! package (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) and aligned to the mouse reference genome (mm10) with the STAR aligner (version 2.5.0a) (31) with default alignment parameters. Gene count quantification for total RNA was performed using the GeneCounts function within STAR against the GENCODE vM19 transcript file.
Differential Expression Analysis
Gene count data were input into edgeR for differential expression analysis. In brief, gene counts were normalized using the trimmed mean of M values (32) method to account for the compositional difference between the libraries. Differential expression between groups was calculated with the exact test implemented in edgeR.
Statistical Analysis
Prism 6 was used for all data analysis. Data are presented as mean ± SD. ANOVA with Tukey post hoc test was performed on data sets with more than two groups. A confidence level of 95% was considered significant. Actuarial survival curves and log rank test were used to compare diabetes reversal and graft survival among experimental groups.
Data and Resource Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. The RNAseq data were deposited in Gene Expression Omnibus (GEO, accession no. GSE130979).
Results
CCL21 Local Secretion by β-Cells Prevents Autoimmune Diabetes in Ins2-CCL21 Transgenic NOD Mice
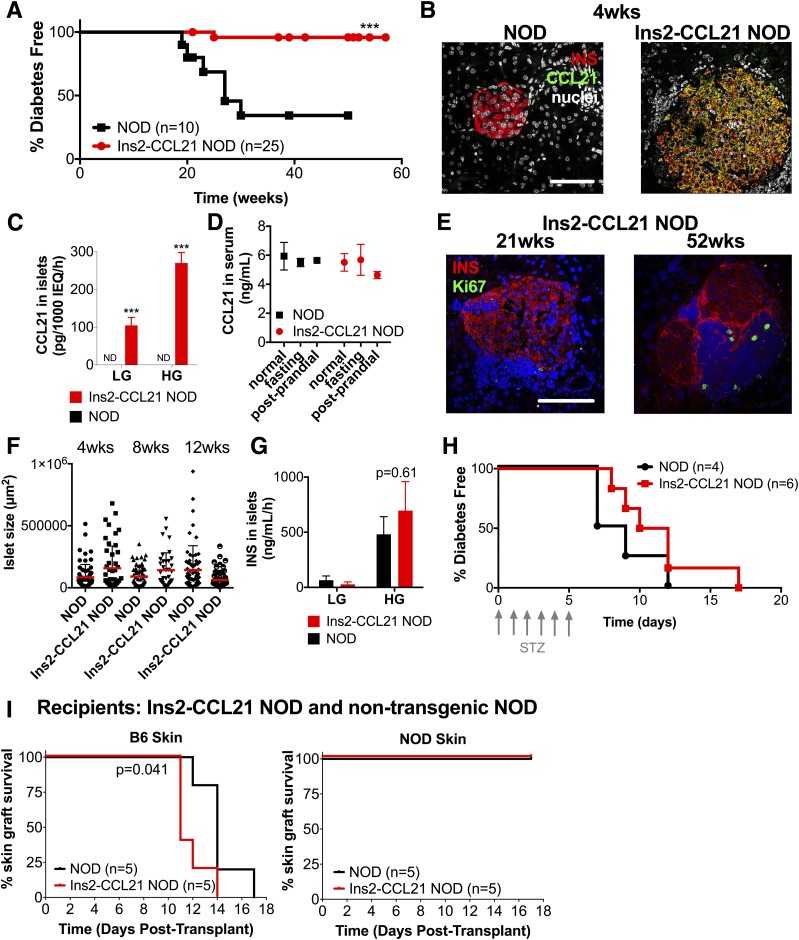
Ins2-CCL21 transgenic NOD mice do not develop diabetes (J. Bluestone, unpublished communication, https://www.jax.org/strain/006254). In our colony, 96% of female transgenic mice remained diabetes-free at 50 weeks of age compared with 40% littermate control female mice (P < 0.001) (Fig. 1A).
Figure 1.
CCL21 local secretion by β-cells prevents autoimmune diabetes in Ins2-CCL21 transgenic NOD mice. A: Percent of diabetes-free Ins2-CCL21 NOD (96%, red, n = 25) and nontransgenic littermate control (40%, black, n = 10) female mice. B: Confocal images of pancreatic sections of 4-week-old nontransgenic and Ins2-CCL21 NOD mice immunostained with insulin (red) and CCL21 (green) antibodies. Nuclei were counterstained with DAPI (gray). Scale bar, 50 μm. C: CCL21 concentrations in the supernatant of pancreatic islets isolated from 19- to 33-week-old nontransgenic (black) and Ins2-CCL21 NOD (red) mice stimulated with 2.2 mmol/L (LG) followed by 16.7 mmol/L (HG) glucose. ND, not detectable. ***P < 0.01. D: Normal (P = 0.83), fasting (P = 0.98), and postprandial (P = 0.23) secretion of CCL21 in the serum of 6- to 7-week-old nontransgenic (n = 3) compared with Ins2-CCL21 NOD (n = 3) mice. E: Confocal images of pancreatic sections of 21- and 52-week-old Ins2-CCL21 NOD mice stained with insulin (red) and Ki67 (green) antibodies. Nuclei were counterstained with DAPI (blue). Scale bar, 50 μm. F: Quantification of islet size from pancreatic sections of 4- (n = 6), 8- (n = 5), and 12-week-old (n = 5) nontransgenic littermate control and age-matched Ins2-CCL21 NOD mice. G: Insulin secretion after stimulation of islets from 19- to 33-week-old nontransgenic (black, n = 3) and Ins2-CCL21 NOD (red, n = 3) mice with 2.2 mmol/L (LG) followed by 16.7 mmol/L (HG) glucose. Islets were isolated from a minimum of five mice per experiment and plated in n = 3 wells per condition. H: Percent of diabetes-free 10-week-old Ins2-CCL21 NOD (red, n = 6) or nontransgenic (black, n = 4) NOD mice treated daily with five low doses (50 mg/kg) of STZ. I: Percent survival of C57BL/6 fully allogeneic (left) or syngeneic NOD (right) skin grafts into Ins2-CCL21 mice (red, n = 5, median survival rate: 14 days) or control nontransgenic littermate NOD mice (black, n = 5). wks, weeks.
CCL21 was expressed (Fig. 1B) and secreted (Fig. 1C) by pancreatic β-cells from transgenic mice. CCL21 secretion from cultured transgenic islets was influenced by glucose concentrations in vitro; the observation that serum levels of CCL21 did not differ between Ins2-CCL21 NOD mice and control littermates suggests that CCL21 is consumed locally (Fig. 1D).
CCL21 expression in β-cells did not induce either β-cell proliferation (Fig. 1E) or islet hypertrophy (Fig. 1F) or increased glucose-stimulated insulin release from isolated islets (Fig. 1G). Additionally, β-cells from transgenic NOD islets were equally sensitive to STZ as β-cells from NOD islets (Fig. 1H). Overall, these data show that CCL21 expression in β-cells protects from diabetes development independent of β-cell function, replication, and resistance to toxicity.
We also transplanted Ins2-CCL21 mice with fully allogeneic B6 skin grafts and syngeneic NOD grafts. Ins2-CCL21 NOD mice rejected B6 skin allografts at a similar rejection rate as NOD mice, whereas syngeneic skin grafts were accepted in both mouse strains (Fig. 1I). So, protection from diabetes development of CCL21-expressing β-cells is not caused by impaired immune function.
CCL21 Local Secretion by β-Cells Does Not Prevent Insulitis
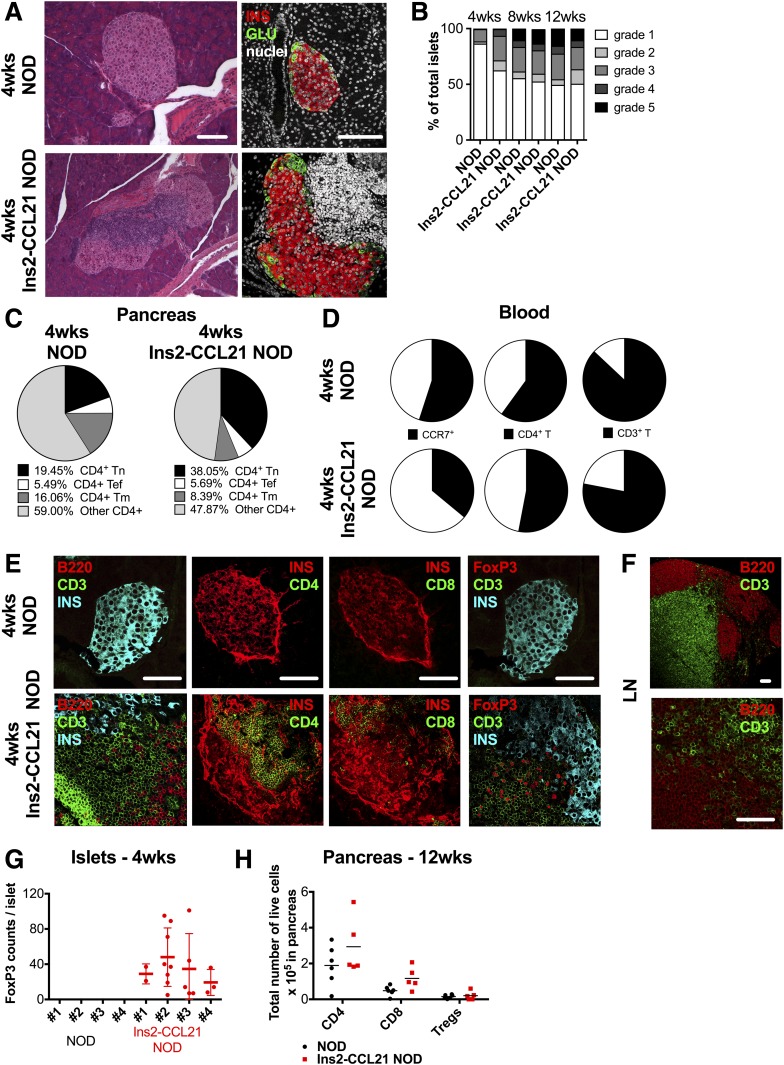
At 4 weeks of age, most (86%) female NOD mice exhibited no insulitis and only 14% showed grade 2–5 insulitis (Fig. 2A and B); in contrast, 83% of Ins2-CCL21 mice showed grade 2–5 insulitis in 38% of islets. These results suggest that CCL21 expression did not prevent islet infiltration, yet diabetes did not develop.
Figure 2.
CCL21 local secretion by β-cells does not prevent insulitis. A: Light and confocal microscope images of pancreatic sections of 4-week-old nontransgenic and Ins2-CCL21 NOD mice stained with hematoxylin and eosin (left) or with insulin (red) and glucagon (green) (right) antibodies. Nuclei were counterstained with DAPI (white). Scale bar, 50 μm. B: Insulitis quantification (grade 1–5) of pancreatic sections of 4- (n = 6), 8- (n = 5), and 12-week-old (n = 5) nontransgenic and age-matched Ins2-CCL21 NOD mice. Four-week-old nontransgenic NOD mice: 86% grade 1, 2% grade 2, 11% grade 3, 1% grade 4, and 1% grade 5. Four-week-old Ins2-CCL21 NOD mice: 62% grade 1, 9% grade 2, 22% grade 3, 6% grade 4, and 1% grade 5. C: Quantification of pancreatic infiltrates of 4-week-old nontransgenic (n = 5) and Ins2-CCL21 NOD (n = 5) mice by flow cytometry. Data are indicated as % CD4+ live cells. Tn: naive T cells (CD44−CD127−CD62L+) transgenic: 38.05 ± 0.08, nontransgenic: 19.45 ± 4.62, P < 0.001; Tef (effector T cells): CD44+CD127−; Tm (memory T cells): CD44+CD127+. D: Quantification of blood leukocytes of 4-week-old nontransgenic (n = 5) and Ins2-CCL21 NOD (n = 5) mice by flow cytometry. Data are indicated as % CD45+ live cells. CCR7+: transgenic, 13 ± 11; nontransgenic, 3 ± 3 (P < 0.05). E: Confocal images of pancreatic sections of 4-week-old nontransgenic and Ins2-CCL21 NOD mice stained with insulin (cyan), B cells (B220, red), and T cells (CD3, green) antibodies; insulin (red) and CD4 (green); insulin (red) and CD8 (green); insulin (cyan), T cells (CD3, green), and Tregs (CD3+FoxP3+, red) antibodies. Scale bar, 50 μm. F: Confocal images of skin-draining LN sections of 4-week-old Ins2-CCL21 NOD mice immunostained with B220 (red) and CD3 (green) at two different magnifications. Scale bar, 50 μm. G: Quantification of Treg density in islets from pancreatic sections of 4-week-old nontransgenic (n = 4) and age-matched Ins2-CCL21 (n = 4) NOD mice. H: Quantification of pancreatic infiltrates of 12-week-old nontransgenic littermate (n = 6) and Ins2-CCL21 NOD (n = 6) mice by flow cytometry. Data are indicated as total number of live cells. wks, weeks.
The relative proportions of naive CD4+ T cells (P < 0.001) (Fig. 2C) and of CCR7+CD62L+ leukocytes (P < 0.01) (Supplementary Fig. 1A) were increased in the pancreas of transgenic mice compared with NOD mice. Additionally, the relative proportions (P < 0.05) (Fig. 2D and Supplementary Fig. 1B) but not the total number of circulating CD3+, CD4+, and CD45+CCR7+ cells and the total number of splenic CD3+, CD4+, Treg, and naive CD4+ T cells (Supplementary Fig. 1C) were lower in transgenic compared with NOD mice (P < 0.001). These results suggest that CCL21 secretion by β-cells enriched the pancreas with naive T cells, likely via chemotactic recruitment through CCR7. Additionally, the relative proportion of macrophages and immature dendritic cells was decreased in the pancreas of transgenic compared with NOD mice (Supplementary Fig. 1D).
Very few B and T cells were detected in the islets of NOD mice at 4 weeks of age (Fig. 2E). However, B and T cells were observed in transgenic islets (Fig. 2E) with an organization that resembled the LN paracortex (Fig. 2F). Therefore, transgenic CCL21 expression in β-cells induced the formation of TLOs in the pancreas by 4 weeks of age.
By immunofluorescence, Tregs were readily detected in 4-week-old transgenic islets but not in age-matched NOD islets (Fig. 2E and G). Because the total number and the relative abundance (Supplementary Fig. 1A) of Tregs in the pancreas were unaffected, we conclude that CCL21 expression in β-cells leads to the redistribution of pancreatic Tregs and their enrichment in the islets, which may contribute to explain the enhanced regulation.
At 12 weeks of age, the total number of pancreatic lymphocyte populations were comparable in both strains (Fig. 2H). Thus, CCL21 production from β-cells leads to alterations in islet-infiltrating immune cells at early time points, but not at later time points.
Splenocytes From Transgenic Mice Are Not Diabetogenic
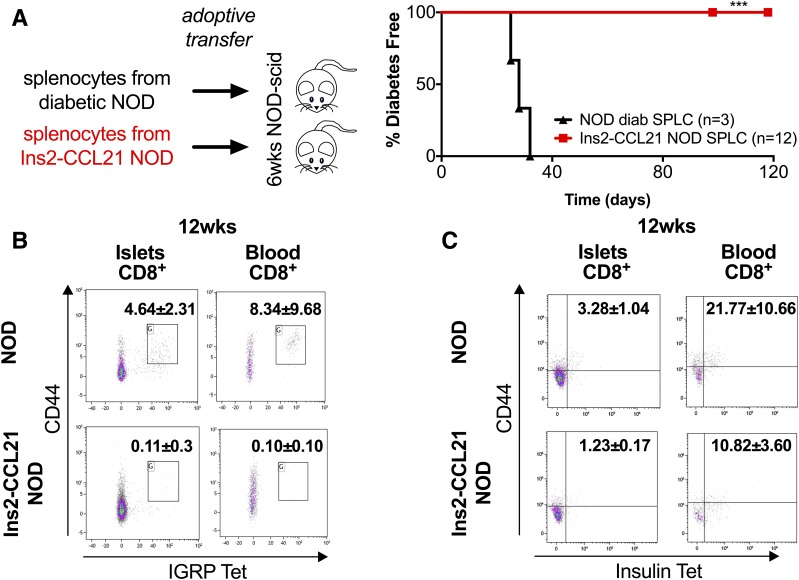
Whereas splenocytes from diabetic NOD mice transferred diabetes to 100% of NOD-scid recipients, splenocytes from transgenic mice (Table 2) failed to transfer diabetes (Fig. 3A), suggesting that they are nondiabetogenic and maintain this phenotype upon transfer to mice that do not express CCL21 in the islets.
Figure 3.
Splenocytes from transgenic mice are not diabetogenic. A: ATs of splenocytes from 20- to 25-week-old Ins2-CCL21 NOD mice (100%, red, n = 12) or from age-matched diabetic nontransgenic mice (0%, black, n = 3, median transfer time 28 days) into 6-week-old NOD-scid mice. At least three donors per group, two recipients per donor, and 10 million cells per AT were used. ***P < 0.01. Experimental details are reported in Table 2. B: Quantification of CD44+ IGRP-reactive CTLs in pancreatic islets (P < 0.05) and in blood (P < 0.05) of 12-week-old Ins2-CCL21 NOD mice (n = 5) compared with nontransgenic littermate mice (n = 5). Pancreatic islets: Ins2-CCL21 NOD, 0.11 ± 0.3%; nontransgenic NOD, 4.64 ± 2.31%, P < 0.05. Blood: Ins2-CCL21 NOD, 0.10 ± 0.10%; nontransgenic NOD, 8.34 ± 9.68%, P < 0.05. C: Quantification of CD44+ INS-reactive CTLs in pancreatic islets (P < 0.05) and blood (P = 0.08) of 12-week-old Ins2-CCL21 NOD mice (n = 5) compared with nontransgenic littermate NOD mice (n = 5). Pancreatic islets: Ins2-CCL21 NOD, 1.23 ± 0.17%; nontransgenic NOD, 3.28 ± 1.04%, P = 0.01. Blood: Ins2-CCL21 NOD, 21.77 ± 10.66%; nontransgenic NOD, 10.82 ± 3.60%, P = 0.08. Data are indicated as % CD8+ cells. wks, weeks.
Through tetramer staining (Supplementary Table 1), we found that the numbers of CD44+ IGRP-reactive CD8+ T cells (CTLs) (Fig. 3B) and of CD44+ insulin-reactive CTLs (Fig. 3C) in pancreatic islets and in blood of 12-week-old Ins2-CCL21 NOD mice were significantly decreased compared with nontransgenic mice. These results suggest that CCL21 local expression by β-cells decreased the number of autoreactive CTLs, which contributes to explain the lack of diabetogenic potential.
CCL21 Local Secretion by β-Cells Induces Formation of TLOs Containing FRC-Like Stromal Cells
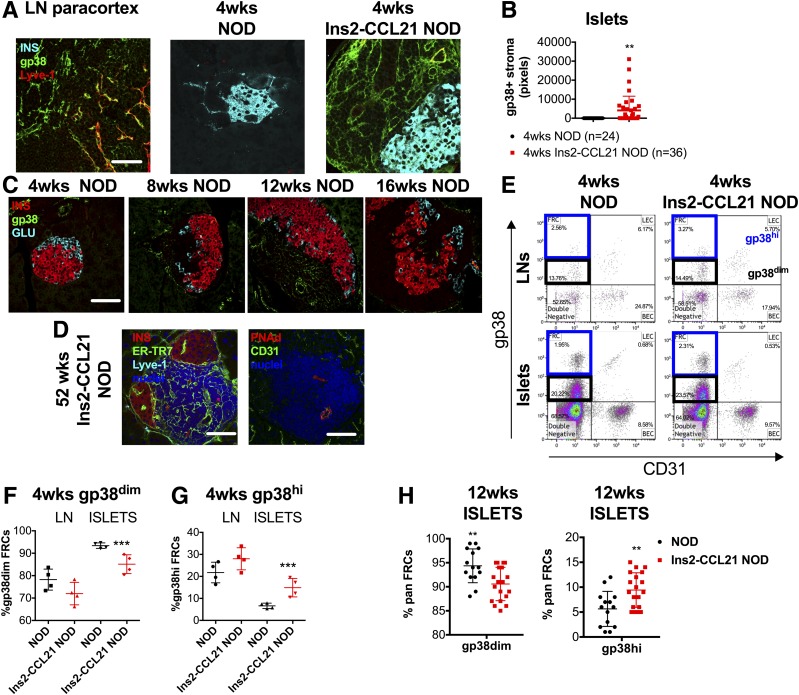
We hypothesized that protection from diabetes was associated with tolerogenic stromal cells within TLOs, which may impact autoreactive T-cells. Indeed, the pancreatic islets of 4-week-old transgenic NOD mice displayed a highly organized network of gp38+Lyve-1− FRC-like cells (Fig. 4A and B), closely resembling FRC reticular networks in LNs. This was not observed in nontransgenic littermate NOD mice until 8 weeks of age (Fig. 4C). Thus, the transgenic expression of CCL21 leads to the early appearance of highly organized TLOs in the pancreas.
Figure 4.
CCL21 local secretion by β-cells induces formation of TLOs containing FRC-like stromal cells. A: Confocal microscope images of pancreatic sections of 4-week-old nontransgenic and Ins2-CCL21 NOD mice and of skin-draining LN, stained with insulin (cyan), gp38 (stromal cell marker, green), Lyve-1 (lymphatic endothelial cell marker, red) antibodies, and nuclear counterstain (DAPI, white). FRC-like cells are identified by gp38+Lyve-1−. Scale bar, 50 μm. B: Quantification of FRC density in pancreatic islets of 4-week-old nontransgenic (n = 24 islets) and Ins2-CCL21 NOD (n = 36 islets) mice by image analysis of pancreatic sections. At least n = 3 mice per group were analyzed. C: Confocal microscope images of pancreatic sections of 4- to 16-week-old nontransgenic mice stained with insulin (red), glucagon (cyan), stromal cell (gp38, green) antibodies, and nuclear counterstain (DAPI, gray). Scale bar, 50 μm. D: Confocal microscope images of pancreatic sections of 52-week-old transgenic mice stained with insulin (red), Lyve-1 (cyan), FRC (ER-TR7, green) antibodies (left), and HEV antibodies (CD31: green; PNAd: red) (right); nuclear counterstain: DAPI (blue). Scale bar, 50 μm. Gating strategy (E) and relative quantification of CD45−CD31−gp38dim (F) and of CD45−CD31−gp38hi (G) FRCs from the skin-draining LNs and FRC-like cells from the pancreatic islets of 4-week-old nontransgenic (n = 4, black) and Ins2-CCL21 NOD mice (n = 4, red) by flow cytometry. H: Relative proportions of CD45−CD31−gp38dim and of CD45−CD31−gp38hi FRC-like cells from the pancreatic islets of 12-week-old nontransgenic (n = 5, black) and Ins2-CCL21 NOD mice (n = 5, red) by flow cytometry. **P ≤ 0.01, ***P ≤ 0.001. wks, weeks.
By 16 weeks of age, when nontransgenic NOD mice started developing diabetes, FRC networks started disappearing from the pancreas, as previously shown (14). In contrast, diabetes resistance in transgenic mice was associated with the persistence of both FRC-like networks (ERTR7+Lyve-1−) and HEVs (PNAd+CD31+) in the pancreas (Fig. 4D).
Further characterization of pancreatic and LN-derived stromal cells by flow cytometry (Supplementary Fig. 2A) revealed the presence of two populations of FRC-like cells: CD45−CD31−gp38hi and CD45−CD31−gp38dim (Fig. 4E). Transgenic islets were enriched in CD45−CD31−gp38hi stromal cells at 4 weeks (Fig. 4E–G) and 12 weeks (Fig. 4H) compared with nontransgenic islets. Proportions of FRCs in LNs were comparable between strains (Supplementary Fig. 2C and D).
Local CCL21 Expression by β-Cells Is Sufficient to Induce FRC-Like Cell–Containing TLOs and Mediate Systemic Protection From Diabetes
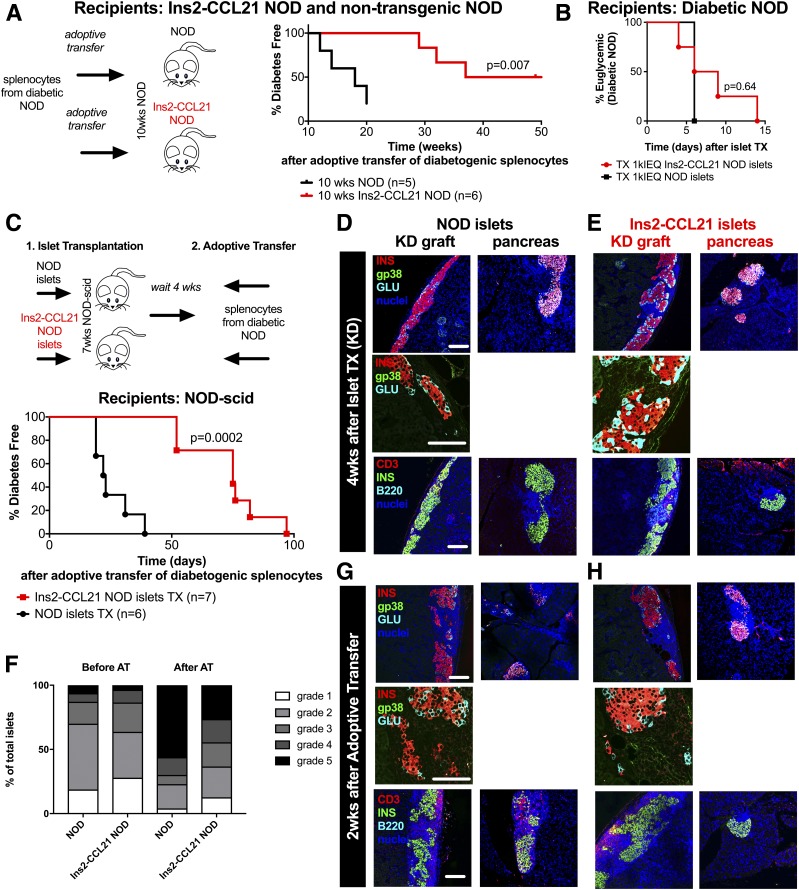
Next, we asked whether local formation of FRC-like cell–containing TLOs in CCL21-expressing islets was responsible for mediating systemic regulation of diabetogenic leukocytes. AT of diabetogenic splenocytes from recently diabetic NOD mice into 10-week-old transgenic NOD mice (Table 2) resulted in delayed diabetes development of 48 weeks compared with 18 weeks in NOD recipients (P = 0.007) (Fig. 5A), suggesting that formation of “regulatory” TLOs is crucial to prevent diabetes in NOD mice.
Figure 5.
Local CCL21 expression by β-cells is sufficient to induce FRC-like cell–containing TLOs and mediate systemic protection from diabetes. A: ATs of splenocytes from diabetic nontransgenic NOD mice into 10-week-old Ins2-CCL21 NOD mice (red, n = 6, median survival time 48 weeks, P = 0.007) or into age-matched nontransgenic mice (black, n = 5, median survival time 18 weeks). B: Percent of diabetes-free spontaneously diabetic NOD mice transplanted (epididymal fat pad) with 1,000 islet equivalents (IEQ) islets from Ins2-CCL21 NOD (red, n = 4) or nontransgenic (black, n = 1) NOD mice. Experimental details are reported in Table 2. C: AT of splenocytes from recently diabetic NOD mice into 7- to 11-week-old NOD-scid mice transplanted (TX) in the kidney capsule (KD) with a marginal dose (500 IEQ/mouse) of islets from either Ins2-CCL21 NOD (red, n = 7, median survival time 22.5 days, P = 0.0002) or nontransgenic (black, n = 6, median survival time 75 days) mice 4 weeks prior to injection. Confocal microscope images of KD islet graft sections (KD graft) (left) and of pancreatic sections (right) of 7-week-old NOD-scid mice that received either nontransgenic (D) or Ins2-CCL21 transgenic (E) NOD islets 4 weeks before. F: Insulitis quantification (grades 1–5) of pancreatic sections of NOD-scid mice before (left) and after (right) AT of diabetic splenocytes. Mice had received a transplant of either NOD (n = 3 for both timepoints) or Ins2-CCL21 NOD (n = 3 after transplant and n = 4 after AT) islets under the KD 4 weeks before the AT. G and H: Confocal microscope images of KD islet graft sections (KD graft) (left) and pancreatic sections (right) of NOD-scid mice that received either nontransgenic (G) or Ins2-CCL21 transgenic (H) NOD islets followed by AT of splenocytes from recently diabetic NOD mice. Sections were stained for insulin (red), stromal cells (gp38, green), and glucagon (cyan) (top panels) or for T cells (CD3, red), insulin (green), and B cells (B220, cyan) (bottom panels). Nuclei were counterstained with DAPI (blue). Scale bar, 100 µm. wks, weeks.
However, transgenic islets were not protected against recurrence of autoimmunity after transplantation in diabetic NOD mice (Fig. 5B) and from allorejection after transplantation into fully MHC-mismatched diabetic Balb/C mice (Supplementary Fig. 2E). To test whether lack of protection was due to insufficient time for the formation of “regulatory” TLOs at the graft site, we transplanted Ins2-CCL21 or nontransgenic NOD islets (Supplementary Table 2) under the kidney capsule of 7-week-old NOD-scid mice and waited 4 weeks before adoptively transferring splenocytes from diabetic NOD mice formation (Table 2), thus allowing time for TLO formation (Fig. 5C). Before AT, we could detect insulitis in recipient NOD-scid pancreas when either NOD or Ins2-CCL21 islets were transplanted (before AT) (Fig. 5D–F) where lymphocytes originated from the transplanted grafts as passenger leukocytes. Importantly, the formation of TLOs was observed in the Ins2-CCL21 graft (Fig. 5E) but not in nontransgenic islet grafts (Fig. 5D). Diabetes development after AT of diabetogenic splenocytes was delayed in recipients of Ins2-CCL21 islets compared with mice transplanted with NOD islets (P = 0.0002) (Fig. 5C). Importantly, mice transplanted with Ins2-CCL21 NOD islets displayed lower grades of insulitis in the pancreas (after AT; P = 0.0491) (Fig. 5F and H) than mice transplanted with NOD islets (Fig. 5G). Thus, CCL21 local expression in β-cell kidney grafts was sufficient to delay the onset of autoimmune diabetes and provide systemic protection in the pancreas, if time was allowed for the formation of regulatory TLOs at the graft site.
Mechanisms of FRC-Like Cell–Mediated Immune Regulation
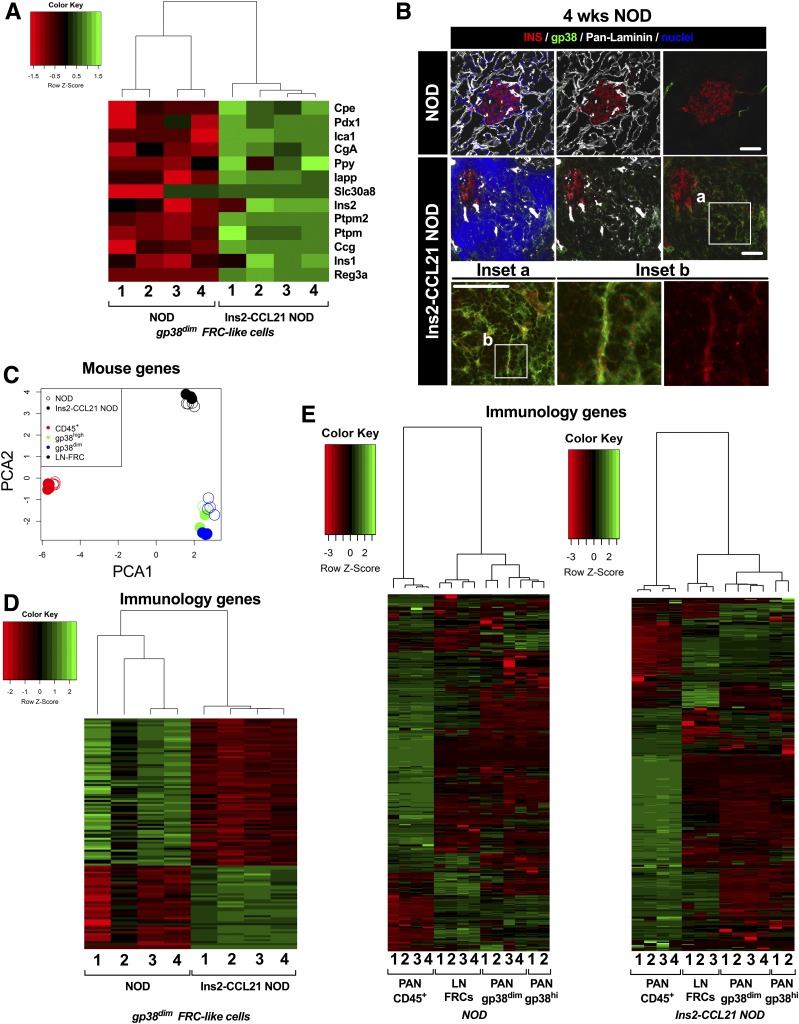
Since FRCs promote tolerance by expressing self-antigens (25), we assessed β-cell antigen expression in pancreatic FRC-like cells by RNAseq and immunostaining. RNAseq analysis revealed higher expression of β-cell antigens, including insulin (Ins1 and Ins2) autoantigen, in pancreatic gp38dimCD31− FRC-like stromal cells from 12-week-old Ins2-CCL21 NOD compared with nontransgenic NOD (Fig. 6A and Supplementary Table 3). We verified that pancreatic β-cells did not contaminate our FRC populations due to the following: 1) high sorting purity of pancreatic CD45−gp38+CD31− FRC-like cells (Ins2-CCL21 NOD: 97 ± 2%; nontransgenic NOD: 96 ± 3%) and 2) >3-log reduction in Ins2 mRNA levels between FRC-like cells and islets by quantitative RT-PCR (nontransgenic fold-change: 8,287; transgenic fold-change: 4,958; data not shown), which is consistent with what would be expected between Ins2 mRNA expression in β-cells and ectopic expression in LN. Insulin protein (Fig. 6B, inset b) was detected in conduits formed by islet-associated gp38+pan-laminin+ stromal cells of Ins2-CCL21 NOD mice at 4 weeks of age (Fig. 6B, inset a), reminiscent of FRC conduits found in LNs. These data suggest that pancreatic gp38+ stromal cells induced by CCL21 local expression in Ins2-CCL21 NOD mice express islet autoantigens, and this enhanced expression may be key for the lack of diabetes development in CCL21-transgenic mice.
Figure 6.
Mechanism of FRC-like cell–mediated immune regulation. A: Heat map of T1D autoantigen expression in gp38dim FRC-like cells freshly sorted from either Ins2-CCL21 NOD mice or nontransgenic NOD littermates analyzed byRNAseq. T1D autoantigens: carboxypeptidase E (Cpe), pancreatic and duodenal homeobox 1 (Pdx1), islet cell autoantigen 1 (Ica1), chromogranin A (Chga), pancreatic polypeptide (Ppy), islet amyloid polypeptide (Iapp), solute carrier family 30 member 8 (Slc30a8), insulin 2 (Ins2), protein tyrosine phosphatase, receptor type N (Ptprn), glucagon (Gcg), insulin (Ins1), and regenerating family member 3 α (Reg3a). B: Confocal microscope images of pancreatic sections of 4-week-old nontransgenic (top) and Ins2-CCL21 transgenic (bottom, different magnifications) NOD mice immunostained with insulin (red), pan-laminin (gray), stromal cell (gp38, green) antibodies, and nuclear counterstain (DAPI, blue). Scale bar, 50 μm. Bottom panels are higher magnifications of the areas indicated by the white boxes. C: Principal component analysis (PCA) of gene expression in LN-derived FRCs (n = 4 for nontransgenic NOD and n = 3 for Ins2-CCL21 NOD), islet-derived gp38dim FRC-like cells (n = 4 per mouse model), islet-derived gp38hi FRC-like cells (n = 2 per mouse model), and islet-derived CD45+ cells (n = 4 per mouse model) freshly sorted from 12-week-old Ins2-CCL21 NOD mice and from control NOD littermates. D: Heat map of significantly relevant (false discovery rate <0.05) inflammatory genes (list of genes taken from the Nanostring Immunology panel available on their website) in islet-derived gp38dim FRC-like cells from Ins2-CCL21 NOD mice compared with nontransgenic littermates. E: Heat map of inflammatory genes (Nanostring Immunology panel) expressed by LN-derived FRCs, islet-derived gp38dim FRC-like cells, islet-derived gp38hi FRC-like cells, and islet-derived CD45+ cells freshly sorted from 12-week-old Ins2-CCL21 NOD mice (right) or nontransgenic littermates (left). wks, weeks.
Next, we compared the RNAseq profiles of FRC-like cells from transgenic islets to FRC-like cells from nontransgenic islets, to LN-derived FRCs, and to islet-derived CD45+ leukocytes freshly sorted from both mouse strains. From principal component analysis, LN-FRCs clustered independently from islet-derived FRC-like cells (gp38hi and gp38dim), suggesting that they are distinct populations in both mouse strains (Fig. 6C). Similarly, forward scattering of islet-derived FRC-like cells was higher than LN FRCs (Supplementary Fig. 2F and G).
Differential expression of genes previously associated with LN stromal cells (33) (Supplementary Table 4) and of genes associated with cytoskeletal organization and cell contractility (34) (Supplementary Table 5) was found in CCL21-induced islet FRC-like cells compared with FRCs from nontransgenic mice. Cells from transgenic mice overexpressed genes that are implicated in cell shape regulation, rapid cytoskeletal reorganization, and cell contractility and downregulated genes associated with actin polymerization.
Differential expression analysis of innate and adaptive immunity genes in CCL21-induced islet FRC-like cells compared with nontransgenic littermates (Fig. 6D and Supplementary Table 6) suggests a different immunological function. Genes implicated in the prevention of terminal differentiation of B cells into antibody-forming cells and in B cell receptor-induced B-cell apoptosis were upregulated in transgenic compared with nontransgenic NOD-derived FRC-like cells; in contrast, genes involved in protecting pro-B cells from programmed cell death, promoting pre–B-cell development, proliferation, and activation were downregulated in transgenic FRC-like cells. Costimulatory signals and proinflammatory molecule genes implicated in IL-1, TNF, IL-6, and IFNγ signaling, complement activation, Toll-like receptor signaling, and chemotaxis of inflammatory cells (e.g., activated T cells) were also downregulated in transgenic compared with nontransgenic NOD-derived FRC-like cells. Conversely, protolerogenic genes involved in IgM/IgA endocytosis, regulating T-cell activation and maturation, Th17 T-cell function, antagonizing IL-1 and TNF signaling, and promoting macrophage polarization were upregulated in transgenic FRC-like cells. Genes associated with lymphotoxin signaling were also upregulated in FRC-like cells from transgenic mice. Despite lower expression of β-cell antigens, FRC-like cells from nontransgenic islets expressed higher gene expression levels of proteins involved in antigen processing and presentation on MHC class I and II. Accordingly, we found that Ins2-CCL21 NOD islet FRC-like cells aligned their immunology gene expression pattern more with LN FRCs (Fig. 6E), which are well described to have tolerogenic properties (25), than with islet-derived CD45+ cells, although this has not been quantified.
We conclude that CCL21 expression in β-cells is associated with highly organized TLOs in the pancreas in which FRC-like cells express β-cell antigens and have enhanced contractility and anti-inflammatory function, resulting in an immunological profile that is more similar to tolerogenic LN-derived FRCs than proinflammatory FRC-like cells from the TLOs of inflamed islets of nontransgenic NOD mice.
Discussion
Understanding the mechanisms used in the immune-tolerant tumor microenvironment could advance approaches to re-establish self-tolerance in autoimmunity. Tumor tolerance can be mediated by tumor-derived secretion of the CCL21 chemokine and TLO formation (6). Here, we investigated whether local CCL21 secretion by β-cells could induce tolerogenic TLOs and whether these could play a role in preventing autoimmune diabetes in NOD mice. Protection from diabetes in the CCL21-transgenic NOD mice was associated with the formation of TLOs containing stromal cells that we identified as FRC-like cells based on either the gp38+Lyve-1− or CD45−gp38+CD31− phenotype. We identified properties of FRC-like populations that are unique to the CCL21 transgenic mice and changes in immune cell populations that are associated with tolerance and can explain lack of diabetes development.
Transgenic islets developed immune infiltrates containing TLOs with gp38+Lyve-1− FRC-like cells at earlier age (by 4 weeks) than nontransgenic mice, and unlike NOD mice in which TLOs are lost over time (14,35), these TLOs persisted in transgenic mice. We demonstrated the critical role of TLOs by using a transplant model, in which allowing time for the formation of TLOs promoted protection of both the CCL21-transgenic islet grafts and of the pancreas from diabetes transfer.
Local secretion of CCL21 by β-cells was predominantly associated with the recruitment of naive T cells to the pancreas, which are critical for the formation of TLOs (20,22,36–39). Accordingly, lymphotoxin pathways critical for TLO formation were upregulated in FRCs from transgenic islets. Since macrophages and immature dendritic cells are attracted by inflammation, their observed decrease in CCL21-transgenic islets suggests that the CCL21-induced TLOs are less inflammatory than TLOs found in inflamed islets of NOD mice. Additionally, we noted a redistribution of Tregs to CCL21-transgenic islets likely through expression of the CCL21 ligand CCR7, which could contribute to the regulatory phenotype we observe. We speculate that a different microenvironment created by local CCL21 secretion could impact the diabetogenic potential of autoreactive T cells. In agreement with this hypothesis, Ins2-CCL21 NOD mice had far fewer β-cell autoreactive CTLs in pancreatic islets and blood. Thus, CCL21 local expression by β-cells impacted autoreactive T cells at a systemic level, similarly to CCL21-secreting tumors (6). However, CCL21-induced TLOs did not cause a globally suppressive environment as allogenic skin grafts were rejected, suggesting that regulation was specific for β-cell antigen-specific T cells. We hypothesized that autoreactive CTLs could be impacted by tolerogenic expression of islet autoantigens by FRCs that results in CTL deletion or functional inactivation in LNs (25,40,41) while interactions with autoreactive CD4 T cells remain to be investigated.
Islet FRC-like cells from TLOs of CCL21 transgenic mice possessed higher expression of T1D-related autoantigen genes than pancreatic FRC-like cells from TLOs of inflamed islets of nontransgenic mice. Downregulation of autoantigens in LN FRCs of 12-week-old NOD mice was previously reported and is mediated by DEAF-1 downregulation (42). We did not observe differences in DEAF-1 expression, suggesting potentially different regulatory mechanisms.
Autoantigen downregulation in islet FRC-like cells from nontransgenic NOD mice was not associated with downregulation of genes associated with antigen processing and presentation, suggesting that reduced FRC antigen presentation was not a critical mechanism and emphasizing the importance of autoantigen levels. CCL21-induced pancreatic FRC-like cells expressed lower levels of proinflammatory genes than FRC-like cells found in TLOs of inflamed NOD islets. Because inflammation leads to MHC class I upregulation in the pancreas, it is likely that reduced inflammation in Ins2-CCL21 FRC-like cells might contribute to their observed downregulation of H2-K and H2-A genes. Genes associated with maturation, activation, and prosurvival of B cells were downregulated in CCL21-transgenic FRC-like cells compared with nontransgenic cells, suggesting that CCL21 may also regulate B lymphocytes, which also play a role in T1D development (13).
TLOs of transgenic mice harbored higher proportions of gp38hi FRCs. Podoplanin (gp38) regulates actomyosin contractility in FRCs and enables FRCs to reorganize their reticular network in LNs (43). Ablating podoplanin in FRCs reduced FRC contractility and enhanced immunity (43). Further, genes regulating contractility of islet gp38dim FRC-like cells were upregulated in transgenic compared with nontransgenic cells. Both increased proportions of gp38hi FRC-like cells, and the increased contractility of gp38dim FRC-like cells observed in CCL21-transgenic islets may enhance immune regulation early in the natural history of islet autoimmunity. Increased contractility of self-antigen–expressing FRCs may promote their interactions with autoreactive T cells and enhance regulatory properties of FRCs.
In conclusion, we characterized molecular events induced by CCL21 expression in pancreatic β-cells that lead to T1D prevention in NOD mice. We demonstrate that local CCL21 secretion is associated with the formation of tolerogenic TLOs in the pancreas. FRC-like cells in the pancreatic TLOs of CCL21 transgenic mice are phenotypically and functionally different than FRC-like cells from TLOs of inflamed NOD islets. Whereas inflammatory NOD TLOs stimulate polyclonal expansion of autoreactive T cells and autoantibody production (13), CCL21-induced TLOs promote systemic and antigen-specific regulation of islet autoimmunity, and this is mediated by FRC-like cells with enhanced expression of β-cell autoantigens, anti-inflammatory profiles, and higher contractility. Despite CCL21 local secretion having a systemic immunomodulatory effect, CCL21 was not detected systemically in CCL21-transgenic mice. This, together with the presence of HEVs, could imply that tolerance might be mediated locally by lymphocyte trafficking through TLOs. Overall, our findings suggest that CCL21 promotes TLOs that differ from inflammatory TLOs associated with inflamed islets in T1D in that they resemble LNs, contain FRC-like cells expressing β-cell autoantigens, and are able to induce systemic and antigen-specific tolerance leading to diabetes prevention. This provides a strong rationale for developing novel therapeutics based on inducing TLOs in confined sites to induce antigen-specific tolerance in autoimmune diseases by local CCL21 delivery through biomaterials. Induction of TLO formation at other tissue sites will also allow comparing the TLO characteristics in relation to their tissue localization and application of the approach to other autoimmune diseases.
Supplementary Material
Article Information
Acknowledgments. The authors are grateful to Dr. Vickie Zhang (Diabetes Research Institute, University of Miami) for technical assistance, the personnel of the DRI Preclinical Cell Processing and Translational Models Core for their help with islet isolation, transplantation, and management of diabetic mice, the DRI Imaging Core for providing expertise on confocal imaging, and the DRI Histology Core headed by Kevin Johnson for his help with histological processing of all samples. Additionally, the authors thank Dr. William Hulme and the Center for Genome Technology of the John P. Hussman Institute for Human Genomics (University of Miami Miller School of Medicine) for their assistance with RNA sequencing and data analysis.
Funding. Funding was provided by philanthropic funds from the Diabetes Research Institute Foundation, a grant from JDRF (2-SRA-2016-316-S-B), a grant from the Iacocca Family Foundation, a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-109929), A.A.T.’s 2017 provost’s research award, A.A.T.’s Biomedical Engineering start-up package, and the European Foundation for the Study of Diabetes (ZUW80166).
Duality of Interest. A.A.T. is a co-inventor of intellectual property used in this study and may gain royalties from future commercialization of the technology. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. F.E.G.B., F.Z.T., M.M.A., R.M., D.S., M.N., S.H.W., A.L.B., É.K., R.D.M., and A.A.T. generated data, reviewed and edited the manuscript, and contributed to discussion. F.E.G.B., M.M.A., A.P., and A.A.T. designed the research. A.A.T. wrote the manuscript. F.E.G.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0239/-/DC1.
References
- 1.Pugliese A. The multiple origins of type 1 diabetes. Diabet Med 2013;30:135–146 [DOI] [PubMed] [Google Scholar]
- 2.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang P, Lu Q. Genetic and epigenetic influences on the loss of tolerance in autoimmunity. Cell Mol Immunol 2018;15:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostrand-Rosenberg S. Tolerance and immune suppression in the tumor microenvironment. Cell Immunol 2016;299:23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 2007;11:526–538 [DOI] [PubMed] [Google Scholar]
- 6.Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 2010;328:749–752 [DOI] [PubMed] [Google Scholar]
- 7.Förster R, Schubel A, Breitfeld D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999;99:23–33 [DOI] [PubMed] [Google Scholar]
- 8.Gunn MD, Kyuwa S, Tam C, et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med 1999;189:451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen SC, Vassileva G, Kinsley D, et al. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J Immunol 2002;168:1001–1008 [DOI] [PubMed] [Google Scholar]
- 10.Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med 2007;204:735–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Förster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 2008;8:362–371 [DOI] [PubMed] [Google Scholar]
- 12.Comerford I, Harata-Lee Y, Bunting MD, Gregor C, Kara EE, McColl SR. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev 2013;24:269–283 [DOI] [PubMed] [Google Scholar]
- 13.Astorri E, Bombardieri M, Gabba S, Peakman M, Pozzilli P, Pitzalis C. Evolution of ectopic lymphoid neogenesis and in situ autoantibody production in autoimmune nonobese diabetic mice: cellular and molecular characterization of tertiary lymphoid structures in pancreatic islets. J Immunol 2010;185:3359–3368 [DOI] [PubMed] [Google Scholar]
- 14.Penaranda C, Tang Q, Ruddle NH, Bluestone JA. Prevention of diabetes by FTY720-mediated stabilization of peri-islet tertiary lymphoid organs. Diabetes 2010;59:1461–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown K, Sacks SH, Wong W. Tertiary lymphoid organs in renal allografts can be associated with donor-specific tolerance rather than rejection. Eur J Immunol 2011;41:89–96 [DOI] [PubMed] [Google Scholar]
- 16.Li W, Bribriesco AC, Nava RG, et al. Lung transplant acceptance is facilitated by early events in the graft and is associated with lymphoid neogenesis. Mucosal Immunol 2012;5:544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang N, Schröppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity 2009;30:458–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baddoura FK, Nasr IW, Wrobel B, Li Q, Ruddle NH, Lakkis FG. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am J Transplant 2005;5:510–516 [DOI] [PubMed] [Google Scholar]
- 19.Nasr IW, Reel M, Oberbarnscheidt MH, et al. Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection. Am J Transplant 2007;7:1071–1079 [DOI] [PubMed] [Google Scholar]
- 20.Ruddle NH. Lymphoid neo-organogenesis: lymphotoxin’s role in inflammation and development. Immunol Res 1999;19:119–125 [DOI] [PubMed] [Google Scholar]
- 21.Jansen A, Voorbij HA, Jeucken PH, Bruining GJ, Hooijkaas H, Drexhage HA. An immunohistochemical study on organized lymphoid cell infiltrates in fetal and neonatal pancreases. A comparison with similar infiltrates found in the pancreas of a diabetic infant. Autoimmunity 1993;15:31–38 [DOI] [PubMed] [Google Scholar]
- 22.Lee Y, Chin RK, Christiansen P, et al. Recruitment and activation of naive T cells in the islets by lymphotoxin beta receptor-dependent tertiary lymphoid structure. Immunity 2006;25:499–509 [DOI] [PubMed] [Google Scholar]
- 23.Kendall PL, Yu G, Woodward EJ, Thomas JW. Tertiary lymphoid structures in the pancreas promote selection of B lymphocytes in autoimmune diabetes. J Immunol 2007;178:5643–5651 [DOI] [PubMed] [Google Scholar]
- 24.Luther SA, Bidgol A, Hargreaves DC, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol 2002;169:424–433 [DOI] [PubMed] [Google Scholar]
- 25.Fletcher AL, Acton SE, Knoblich K. Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol 2015;15:350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manzoli V, Villa C, Bayer AL, et al. Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol. Am J Transplant 2018;18:590–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomei AA, Manzoli V, Fraker CA, et al. Device design and materials optimization of conformal coating for islets of Langerhans. Proc Natl Acad Sci U S A 2014;111:10514–10519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villa C, Manzoli V, Abreu MM, et al. Effects of composition of alginate-polyethylene glycol microcapsules and transplant site on encapsulated islet graft outcomes in mice. Transplantation 2017;101:1025–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cabello-Kindelan C, de la Barrera A, Malek TR, Bayer AL. In vivo environment necessary to support transplanted donor mouse T regulatory cells. Am J Transplant 2014;14:1032–1045 [DOI] [PubMed] [Google Scholar]
- 30.Fletcher AL, Malhotra D, Acton SE, et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front Immunol 2011;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 2010;11:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodda LB, Lu E, Bennett ML, et al. Single-cell RNA sequencing of lymph node stromal cells reveals niche-associated heterogeneity. Immunity 2018;48:1014–1028.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaidel-Bar R, Zhenhuan G, Luxenburg C. The contractome--a systems view of actomyosin contractility in non-muscle cells. J Cell Sci 2015;128:2209–2217 [DOI] [PubMed] [Google Scholar]
- 35.Korpos É, Kadri N, Kappelhoff R, et al. The peri-islet basement membrane, a barrier to infiltrating leukocytes in type 1 diabetes in mouse and human. Diabetes 2013;62:531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Randall TD, Carragher DM, Rangel-Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol 2008;26:627–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy DD, Summers-Deluca L, Vu F, Chiu S, Gao Y, Gommerman JL. The lymphotoxin pathway: beyond lymph node development. Immunol Res 2006;35:41–54 [DOI] [PubMed] [Google Scholar]
- 38.Wu Q, Salomon B, Chen M, et al. Reversal of spontaneous autoimmune insulitis in nonobese diabetic mice by soluble lymphotoxin receptor. J Exp Med 2001;193:1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol 1999;17:399–433 [DOI] [PubMed] [Google Scholar]
- 40.Yip L, Fuhlbrigge R, Taylor C, et al. Inflammation and hyperglycemia mediate Deaf1 splicing in the pancreatic lymph nodes via distinct pathways during type 1 diabetes. Diabetes 2015;64:604–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thayer TC, Pearson JA, De Leenheer E, et al. Peripheral proinsulin expression controls low-avidity proinsulin-reactive CD8 T cells in type 1 diabetes. Diabetes 2016;65:3429–3439 [DOI] [PubMed] [Google Scholar]
- 42.Yip L, Su L, Sheng D, et al. Deaf1 isoforms control the expression of genes encoding peripheral tissue antigens in the pancreatic lymph nodes during type 1 diabetes. Nat Immunol 2009;10:1026–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Astarita JL, Cremasco V, Fu J, et al. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat Immunol 2015;16:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.