Abstract
Activated beige adipocytes have therapeutic potential due to their ability to improve glucose and lipid homeostasis. To date, the origin of beige adipocytes remains enigmatic. Whether beige cells arise through de novo differentiation from resident precursors or through reprogramming of mature white adipocytes has been a topic of intense discussion. Here, we offer our perspective on the natural origin of beige adipocytes in mice. In particular, we revisit recent lineage-tracing studies that shed light on this issue and offer new insight into how environmental housing temperatures early in life influence the mode of beige adipocyte biogenesis upon cold exposure later in life. We suggest a unified model in which beige adipocytes (UCP1+ multilocular cells) in rodents initially arise predominantly from progenitors (i.e., de novo beige adipogenesis) upon the first exposure to cold temperatures and then interconvert between “dormant beige” and “active beige” phenotypes (i.e., beige cell activation) upon subsequent changes in environmental temperature. Importantly, we highlight experimental considerations needed to visualize de novo adipogenesis versus beige cell activation in mice. A precise understanding of the cellular origins of beige adipocytes emanating in response to physiological and pharmacological stimuli may better inform therapeutic strategies to recruit beige adipocytes in vivo.
Introduction
The evolution of adipose tissue has provided mammals with an extraordinary ability to adapt to changes in energy demand and nutrient availability. White adipose tissue (WAT) serves as the principle site for safe energy storage in the form of triglyceride, whereas brown adipose tissue (BAT) functions to dissipate excess energy to produce heat (adaptive thermogenesis). In comparison with white adipocytes, brown adipocytes are rich in mitochondria and exhibit a multilocular, rather than unilocular, lipid droplet appearance. Historically, the defining feature of active thermogenic brown adipocytes has been the expression of the mitochondrial protein uncoupling protein 1 (UCP1) and multilocular lipid droplet appearance (1). For decades, it has been believed that UCP1 was the sole thermogenic engine driving energy expenditure in brown adipocytes. Today, it is becoming increasingly evident that additional thermogenic mechanisms are present and might be critical for BAT function. In fact, some placental mammals do not express UCP1 and thus rely on distinct thermogenic mechanisms (2). The relative contribution of such UCP1-independent mechanisms of adipocyte thermogenesis is still being defined; however, understanding these mechanisms may inform novel strategies to drive energy expenditure in a therapeutic manner. An excellent overview of these pathways has recently been described in detail (3).
Brown adipocytes likely evolved as a mechanism for small mammals to defend against stressful periods of cold environmental temperatures, beginning at the time of birth; however, WAT depots of cold-exposed rodents can undergo extensive remodeling and adopt a thermogenic phenotype, elicited by the emergence of UCP1+ energy-burning adipocytes (4). This “browning” of WAT exemplifies the remarkable capacity of adipose tissue to adapt to its environment. Recruited UCP1+ cells residing within WAT depots were long considered “brown” adipocytes; these cells express UCP1, are abundant in mitochondria, and exhibit a multilocular lipid droplet appearance. As such, these cells within WAT depots are often referred to as “BRITE” cells (brown in white adipose tissue) (5). Today, it has become increasingly apparent that these thermogenic adipocytes residing within WAT depots are developmentally, molecularly, and functionally distinct from classical brown adipocytes. Studies from Kozak and colleagues indicated that UCP1+ cells within white and brown fat depots were regulated by distinct genetic control mechanisms (6,7). Subsequent genetic lineage-tracing studies, gene expression profiling, biochemical analysis, and functional analyses have cumulatively provided evidence that classical interscapular brown adipocytes, but not most thermogenic adipocytes within inguinal WAT, share a common lineage with skeletal muscle cells (8–12). Wu et al. (13) determined that UCP1+ adipocytes differentiated from inguinal subcutaneous WAT-derived clonal precursor cell lines exhibit properties of both brown and white adipocytes. These adipocytes resemble white adipocytes in having low basal expression of UCP1; however, like classical brown adipocytes, they respond to cyclic AMP stimulation with high UCP1 expression and respiration rates. Importantly, the global gene expression profile of the UCP1+ fat cells appears distinct from white and brown adipocytes. As such, Wu et al. (13) adopted the term “beige adipocytes” to describe these cells. Additionally, these studies provided the first suggestion that beige adipocytes may arise from their own committed preadipocytes residing within the adipose tissue stromal vascular fraction.
Over the past 10 years, beige adipocytes have been the subject of intense focus in the field of energy metabolism. Importantly, adult humans harbor functional BAT, which likely consists of both classical brown adipocytes and beige adipocytes (14–16). As described in several recent reviews, rodent studies highlight the significant contribution of beige adipocytes to energy balance and nutrient homeostasis (17,18). It has been observed repeatedly that strains of mice that are able to increase these UCP1-positive cells are often also relatively resistant to diet-induced obesity (6,19,20). The ability to induce “browning” of WAT in rodents is protective against obesity and can trigger weight loss when activated in obese mice (21,22). Interestingly, many of the beneficial effects of beige adipocytes on glucose and lipid homeostasis can be observed prior to changes in body weight. Some of these effects might be mediated by functions independent of thermogenesis per se (e.g., secreted proteins) (18).
Tremendous effort is being placed on developing strategies to stimulate functional beige adipocyte biogenesis. The abundance of brown and beige adipocytes is heavily regulated in adult animals. Activation of β-adrenergic receptor signaling following cold exposure appears to be the most robust and powerful pathway leading to the formation and activation of thermogenic adipocytes; however, recent studies from Kajimura and colleagues reveal that functionally unique beige adipocytes (glycolytic beige adipocytes) can arise in the absence of β-adrenergic receptor signaling (23). There is a growing list of pharmacological and physiological stimuli that can potently trigger beige adipocyte accumulation in rodents beyond cold exposure, including cancer cachexia, gastric bypass, and exercise (24–26). Moreover, great progress has been made in elucidating the transcriptional machinery driving beige adipocyte differentiation (27). Despite the progress made on several key fronts, the natural origin of beige adipocytes has remained unclear and a topic of considerable discussion. Below, we discuss several recent lineage-tracing studies, including ones from our own groups, that aim to address the origins of beige adipocytes and their cellular fate. We attempt to reconcile seemingly disparate findings in this literature with a unified model in which beige adipocytes initially arise predominantly from progenitors upon the first exposure to cold temperatures and then interconvert between “dormant beige” and “active beige” phenotypes upon subsequent changes in environmental temperature.
Beige Cells Can Arise From Differentiated Unilocular Adipocytes
Mature adipocytes are widely considered to be postmitotic. Thus, in principle, beige adipocytes can accumulate via de novo differentiation from resident precursors or via a conversion of mature white adipocytes into multilocular UCP1+ cells. The latter event is often referred to as “transdifferentiation” of white to beige fat cells. Cinti and colleagues have long proposed that beige cells arise through such a white to beige cell transdifferentiation (28). This hypothesis was initially raised on the basis of elegant electron microscopy studies of adipocytes following cold exposure. In recent years, genetic “pulse-chase” lineage-tracing studies have emerged to support the concept of adipocyte lineage plasticity. Lee et al. (29) utilized Adiponectin-CreERT2 mice to activate Cre-dependent reporter gene expression specifically in all mature adipocytes. Inducible and indelible labeling allows for mature adipocytes to be marked and their cell fate to be tracked over time. In such systems, labeled beige adipocytes emerging following the “browning” stimulus represent cells arising from mature white adipocytes. Unlabeled beige cells represent newly formed adipocytes, presumably arising from resident precursor populations. Using this system, Lee at al. report that nearly all UCP1+ cells retain label following cold exposure or stimulation with a β3-adrenergic receptor agonist. The authors concluded that inguinal beige adipocytes arise predominantly from adipoq-expressing unilocular adipocytes existing prior to cold exposure, in line with a potential “transdifferentiation” event.
Independent lineage-tracing studies from Wolfrum and colleagues shed considerable insight into the cellular fate of beige adipocytes once animals are reintroduced to regular housing conditions following cold exposure. Rosenwald et al. tracked the UCP1 lineage using UCP1-CreERT2 animals (30). With their model, the authors were able to map the fate of UCP1+ adipocytes as animals transitioned from cold temperatures back to room temperature housing conditions. They found that beige adipocytes have the capacity to revert to an apparent white adipocyte phenotype; the cells lose UCP1 expression and become unilocular. These same adipocytes can then revert back to UCP1+ cells upon repeated exposure to cold. All together, these data imply that white and beige adipocytes possess significant phenotypic plasticity, with the ability to interconvert between distinct cell states in response to physiological challenge.
A number of studies now shed insight into mechanisms facilitating the “whitening” of brown/beige adipocytes as thermogenic stimuli are withdrawn. Altshuler-Keylin et al. (31) demonstrated that the beige-to-white transition is linked to an autophagy-dependent clearance of mitochondria. In vitro, this occurs without passing through a definitive precursor stage. A more recent study by Roh et al. (32) explored the browning and whitening of subcutaneous adipose tissue with a focus on the changing epigenomic landscape within mature adipocytes. As beige cells transition between cold and warm temperatures, the global landscape of chromatin modifications changes in a pattern consistent with a white-to-beige cell fate switch. However, whitened beige adipocytes retain “poised” enhancers that allow thermogenic genes to reactivate upon repeated cold exposure. This epigenomic plasticity of brown adipocytes is not readily apparent, at least during the period examined. Brown adipocytes largely retain the epigenetic profile of “brown” adipocytes even as they undergo a morphological “whitening” at warm temperatures. These results provide unique insight into the cellular plasticity of white and beige adipocytes and further define differences between brown and beige fat cells.
Our own work on the transcription factor ZFP423 also sheds insight into plasticity of mature adipocytes (22,33). Zfp423 expression is enriched in white versus brown adipocytes and is suppressed in fat cells upon cold exposure or direct activation of β3-adrenergic signaling (22). Roh et al. (32) found that Zfp423 levels are activated in beige cells transitioning back to a white-like phenotype. Inducible genetic depletion of Zfp423 in mature adipocytes leads to a robust white-to-beige interconversion of nearly all differentiated adipocytes within the subcutaneous inguinal WAT depots and of numerous visceral adipocytes normally resistant to browning. Pulse-chase lineage tracing of Zfp423-deficient adipocytes clearly reveals that mature unilocular adipocytes are transitioning to a multilocular beige adipocyte phenotype when Zfp423 is removed. Taken all together, these aforementioned studies provide compelling evidence that beige adipocytes can indeed arise from mature unilocular adipocytes.
Beige Cells Can Arise Through De Novo Adipocyte Differentiation From Precursors
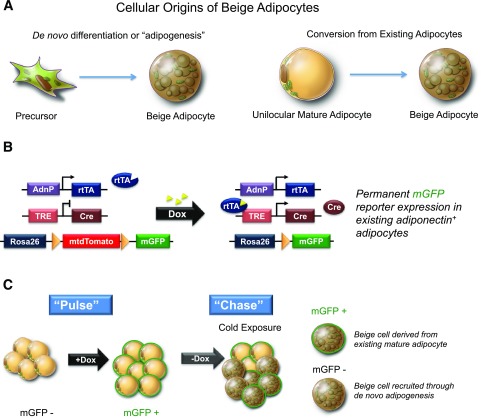
In multiple studies, our groups have independently employed the “AdipoChaser” model in a pulse-chase lineage-tracing experiment to investigate the origin of beige adipocytes induced by cold exposure/adrenergic signaling (22,34,35). The AdipoChaser model allows for tetracycline (doxycyline)-inducible Cre-mediated reporter gene activation in cells expressing the adiponectin gene. Similar to the tamoxifen-inducible lineage-tracing systems discussed above, this system can mark mature adipocytes and track their fate over time (Fig. 1). Using this system, we have observed that UCP1+ cells appear rapidly and arise through both de novo beige adipogenesis and from existing mature adipocytes upon stimulation with a β3-adrenergic receptor agonist or cold exposure (22,34,35). Our groups have independently and consistently made this observation; however, the exact degree of de novo adipogenesis occurring can vary, depending on the exact Rosa26 reporter employed (LacZ or mT/mG reporter).
Figure 1.
Evaluating the cellular origin of beige adipocytes through lineage tracing. A: Potential cellular origins of beige adipocytes. In principle, beige adipocytes (UCP1+ multilocular adipocytes) can arise through de novo differentiation of beige adipocyte precursors (i.e., adipogenesis) or through a cellular conversion in which existing mature adipocytes transform into beige cells. B: Genetic components of the AdipoChaser mouse model. AdipoChaser mice are a combination of three published transgenic lines: 1) transgenic mice expressing the gene encoding the “tet-on” transcription factor rtTA under the control of the adipoq gene promoter (“AdnP-rtTA”), 2) a tet-responsive CRE (TRE-Cre) line that can be activated by rtTA in the presence of doxycycline (Dox), and 3) Rosa26 reporter mice expressing membrane-bound GFP (mGFP) from the Rosa26 locus in a Cre-dependent manner (Rosa26-loxP-mtdTomato-loxP-mGFP). In the absence of doxycycline, all cells express membrane-bound tdTomato (mtdTomato). Upon treatment with doxycycline, rtTA activates the TRE promoter to induce Cre expression, and Cre protein will subsequently eliminate the floxed mtdTomato cassette and permanently turn on mGFP expression in every mature adiponectin-expressing adipocyte present during doxycycline exposure. C: Pulse-chase lineage tracing to reveal origins of beige adipocytes. Treatment of AdipoChaser mice with doxycycline (+ Dox) (via chow diet) for 10 days results in specific expression of mGFP in mature adipocytes (i.e., “pulse-labeling”). Upon removal of doxycycline (− Dox), animals are switched to cold temperatures or administered a thermogenic stimulus (e.g., β3-adrenergic receptor agonist) for a specified time period (i.e., “the Chase”). During this period there is no longer active Cre expression/adipocyte labeling. mGFP+ beige adipocytes appearing following the period of WAT “browning” represent beige adipocytes that arise from preexisting mature adipocytes. mGFP− beige adipocytes represent cells that arise de novo, presumably from resident adipocyte precursors.
The notion that beige adipocytes emerge from resident precursor cells is in line with the original observations by Wu et al. (13) and subsequent studies of beige adipocyte precursors. Wang et al. (36) observed that committed PDGFRα+ beige precursors reside within murine subcutaneous WAT and can be prospectively isolated on the basis of Ebf2-driven GFP reporter gene expression. Beige adipocyte precursors appear also to reside in adult humans. Kajimura and colleagues established beige adipocyte cell lines derived from the supraclavicular adipose tissue of adult individuals (16). A number of studies suggest that beige adipocyte precursors, like white adipocyte precursors, reside in the adipose tissue vasculature (9,34,37). Long et al. (9) revealed that beige adipocytes express a smooth muscle-like gene program. A lineage relationship between beige fat cells and vascular smooth muscle/mural cells is now supported by several lineage-tracing studies (9,37). Inguinal beige adipocytes rapidly emerging (within 7 days) upon cold exposure descend from cells expressing Acta2 (smooth muscle actin). Prolonged cold exposure (≥2 weeks) triggers de novo beige adipogenesis from cells expressing Myh11 (Myh11-CreERT2 lineage tracing) and/or Pdgfrb (Pdgfrb-rtTA; TRE-CRE). Functional studies further support this hypothesis. Stromal vascular cells deficient in myocardin-related transcription factor A (MRTFA) exhibit less of a smooth muscle–like phenotype and gain potential to undergo beige adipogenesis (38). Corvera and colleagues made the important observation that beige precursor cells emerge from capillary sprouts of explanted human subcutaneous adipose tissue (39). These precursor-derived beige adipocytes, upon transplantation, improve glucose homeostasis. Altogether, evidence supports the existence of beige adipocyte precursors within WAT and that beige adipocytes can indeed emerge from these precursors in response to cold exposure.
The Favored Mode of Beige Cell Recruitment Is Dependent on History of Prior Cold Exposure and Exact Thermogenic Stimulus
There has been considerable discussion about the predominant developmental mechanism leading to beige cell formation. A number of studies, including our own original study, have suggested beige adipocytes emerge predominantly through de novo adipocyte differentiation in response to cold exposure. The studies by Lee et al. (29) and Rosenwald et al. (30) suggest that a vast majority arise from existing mature adipocytes. Further complicating matters, Graff and colleagues made the interesting observation that the mode of beige cell recruitment may differ in response to physiological (cold) versus pharmacological (β3-adrenergic receptor agonist) stimulation (40). One possible explanation for the discrepancy in results may lie in the technical approach. As mentioned above, different Rosa26 reporter alleles are used in the various studies; differences in the methods used to visualize reporter expression may confound direct comparison between data sets. Moreover, genetic variance heavily influences the degree of beige accumulation in rodents (6,7,20). It is conceivable that even slight differences in mouse strains used by different laboratories may influence results. Importantly, a major difference between many of these studies is in the choice of lineage-tracing system and inducing agent. We previously demonstrated that tamoxifen lingers inside WAT for a prolonged period of time following injection. This has the potential to confound lineage-tracing results, as Cre remains active beyond the desired labeling period (41). Moreover, high doses of tamoxifen are toxic to adipose tissue and lead to an artificial wave of adipocyte differentiation associated with tissue recovery. Additional studies have suggested that tamoxifen itself can trigger beige cell recruitment (42). Even a low dose of tamoxifen can lead to widespread beiging of adipose tissue (43).
In most of the aforementioned lineage-tracing studies, animals are born and raised under “room temperature” housing conditions prior to cold challenge. A temperature of 22°C represents a mild cold stress for rodents, particularly in the early postnatal period. In fact, Xue et al. (7) previously described a transient surge in beige adipocytes in the retroperitoneal depot of approximately postnatal day 10 mice. These cells disappeared by weaning age and appear again in response to cold. As such, we reasoned that such housing conditions, particularly during early postnatal period, might influence the observed mode of beige cell recruitment and lineage-tracing results.
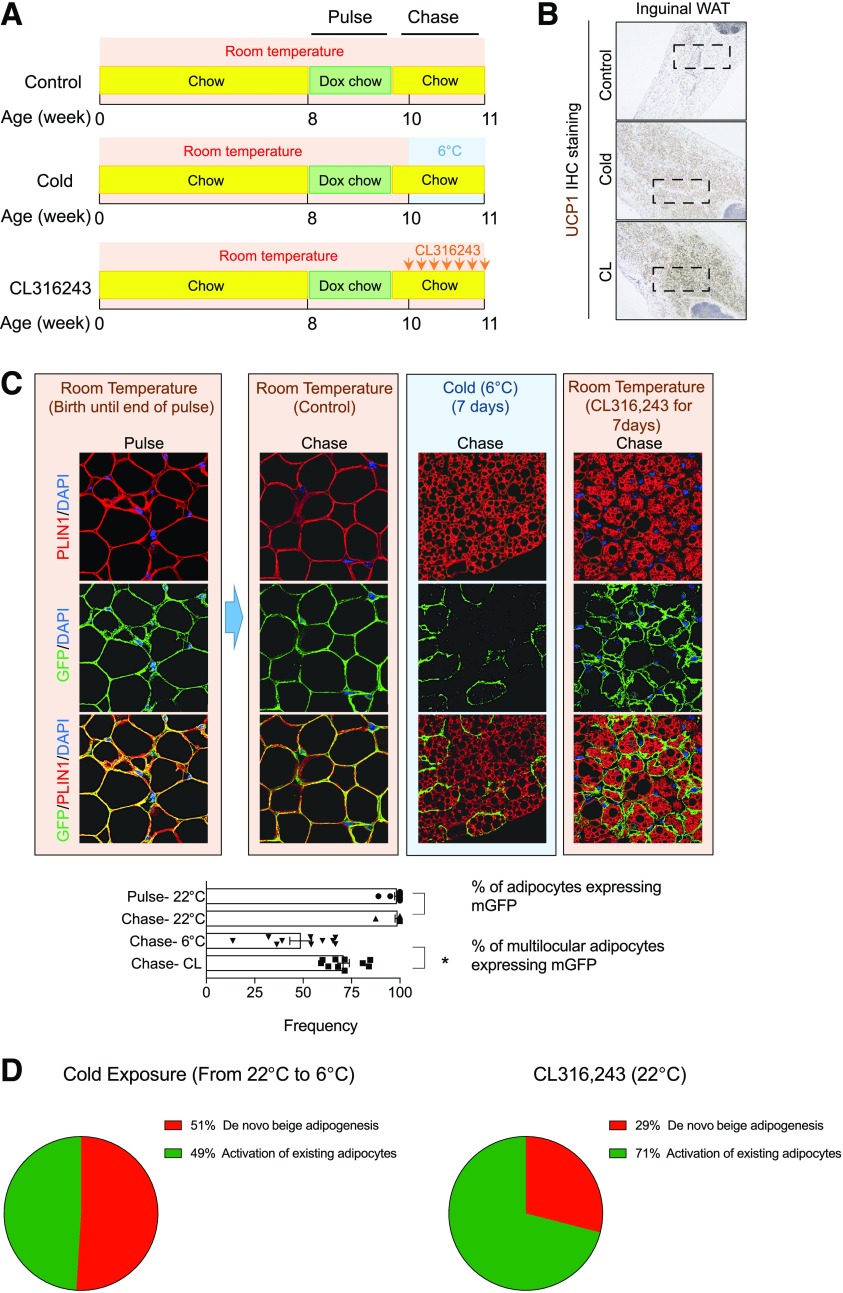
We have recently addressed this possibility by performing an additional series of quantitative lineage-tracing experiments, carefully taking into account animal housing temperature prior to cold exposure. Indeed, our studies reveal that housing conditions prior to cold exposure significantly impact the relative contribution of de novo beige adipogenesis to the emergence of beige adipocytes following cold exposure. As expected, 7 days of cold (6°C) exposure or administration of a β3-adrenergic receptor agonist (CL316,243) leads to widespread browning of the inguinal WAT depots of 10-week-old male C57BL/6 AdipoChaser mice that were born and raised at 22°C (room temperature) (Fig. 2A and B). Under these conditions, pulse-chase lineage tracing indicates that ∼50% of the multilocular PERILIPIN+ adipocytes are mGFP+ and thus represent beige adipocytes that emerged from mature adipocytes in response to cold exposure (Fig. 2C). The remaining 50% of multilocular adipocytes (unlabeled) presumably arise from resident precursors through de novo differentiation. Thus, as animals transition from 22°C to 6°C, both mechanisms of beige cell recruitment are readily apparent (Fig. 2D). Direct activation of the β3-adrenergic receptor yielded quantitatively distinct lineage-tracing results. Following 7 days of treatment with CL316,243, 71% of multilocular cells are GFP+ (Fig. 2C and D). This is consistent with prior tracing studies indicating that pharmacological activation of β3-adrenergic receptor signaling favors the formation of beige adipocytes from unilocular mature adipocytes. This can likely be explained by the fact that only mature adipocytes, rather than adipocyte precursor cells, express the β3-adrenergic receptor.
Figure 2.
Cellular origins of beige adipocytes in mice born and raised at 22°C (room temperature). A: AdipoChaser mice born and raised at room temperature were fed a standard chow diet until 8 weeks of age before being switched to doxycycline (Dox)-containing chow diet (600 mg/kg) for 10 days (“Pulse”). Following the pulse-labeling period, mice were switched back to the standard chow diet (no doxycycline) for 3 days before being subjected to cold exposure (6°C) or β3-adrenergic receptor agonist CL316,243 (CL) administration (10 mg/kg/day) for 7 days (“Chase”). B: Representative 4× brightfield image of UCP1 expression in inguinal WAT sections from mice following the Chase period. In this and all subsequent figures, boxed regions (black) highlight regions surveyed for quantitative analysis. C: Representative 63× image of inguinal WAT sections stained with anti-GFP (green) and anti-PERILIPIN (red) antibodies and counterstained with DAPI (blue [nuclei]) at indicated time points. Bar graphs/scatter plots (mean ± SD) depict the percentage of PERILIPIN+ cells expressing GFP at indicated time points. *P < 0.05 from Student t test. All immunohistochemistry (IHC) and indirect immunofluorescence assays in this figure and all other figures were performed as previously described (22,34). In this figure, and all other figures, each data point represents number of fields of cells counted within the boxed region shown in B. In each field (n = 10), >20 adipocytes were quantified. In total, >200 adipocytes were counted for each condition. D: Pie chart summarizing the relative contribution of de novo adipogenesis vs. adipocyte activation/interconversion to the total pool of beige adipocytes originating following exposure to cold temperatures or β3-adrenergic receptor agonist. Percentages indicated represent mean values from the data shown in C.
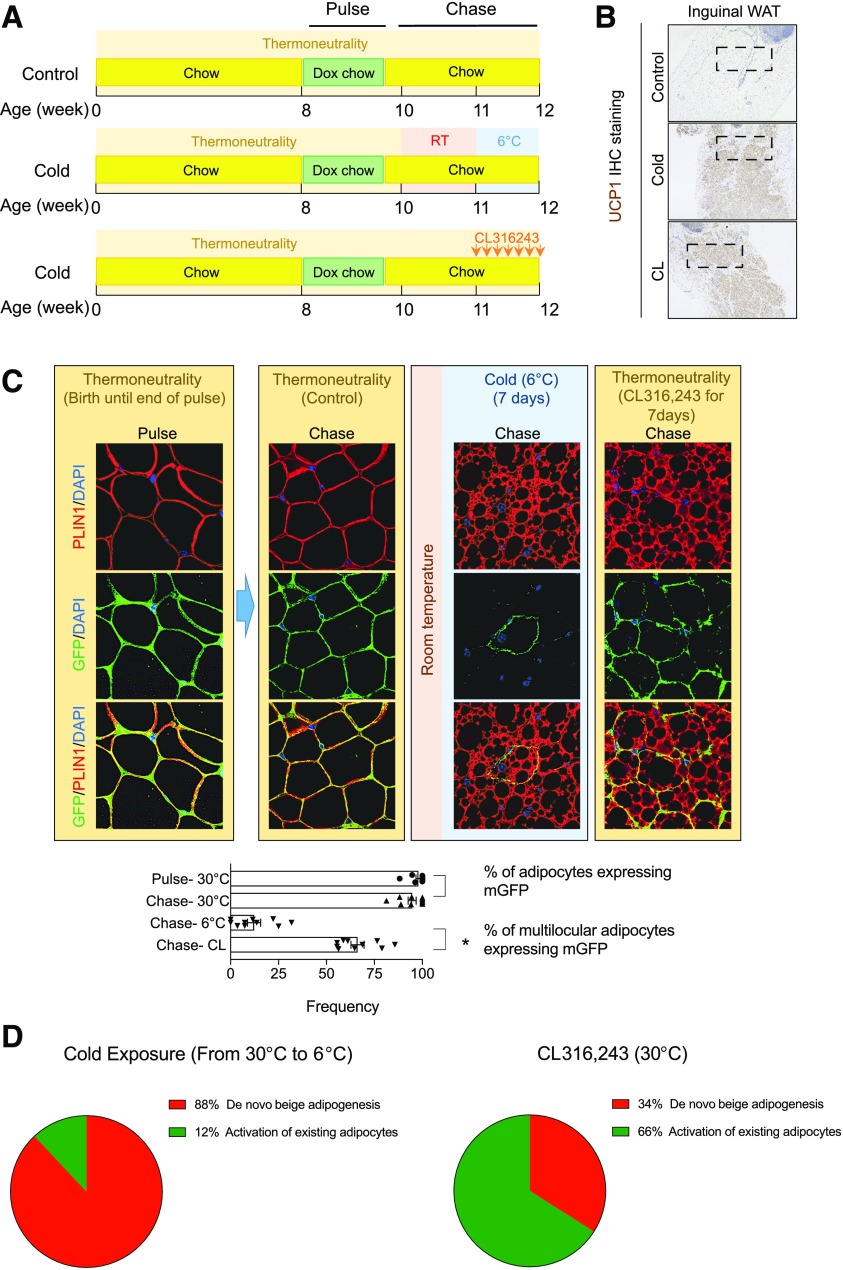
The results are strikingly different when animals are born and raised under thermoneutral housing conditions (30°C). Seven days of 6°C exposure treatment leads to widespread browning of the inguinal WAT depots of AdipoChaser mice that were born and raised at 30°C (Fig. 3A and B); however, after cold exposure, more than 80% of multilocular cells are unlabeled (GFP−) (Fig. 3C) and therefore represent beige adipocytes that emerge through de novo differentiation. These data suggest that upon the first significant cold stress experienced in life, beige adipocytes (UCP1+ multilocular cells) arise predominantly through de novo beige adipogenesis (Fig. 3D). Interestingly, housing conditions did not significantly impact the mode of beige cell recruitment activated by direct β3-adrenergic receptor agonist. Following 7 days of daily CL316,243 treatment at 30°C, widespread browning is also apparent; however, nearly 70% of multilocular cells are labeled (GFP+) (Fig. 3C). These data highlight potential differences in beige cell recruitment following physiological versus pharmacological stimuli.
Figure 3.
Cellular origins of beige adipocytes in mice born and raised at 30°C (thermoneutrality). A: AdipoChaser mice born and raised at thermoneutrality (30°C) were fed a standard chow diet until 8 weeks of age before being switched to doxycycline (Dox)-containing chow diet (600 mg/kg) for 10 days (“Pulse”). Following the pulse-labeling period, mice were switched back to the standard chow diet (no doxycycline) for 3 days before being subjected to cold exposure (6°C) or β3-adrenergic receptor agonist CL316,243 (CL) administration (10 mg/kg/day) for 7 days (“Chase”). RT, room temperature. B: Representative 4× brightfield image of UCP1 expression in inguinal WAT sections from mice following the Chase period. IHC, immunohistochemistry. C: Representative 63× image of inguinal WAT sections stained with anti-GFP (green) and anti-PERILIPIN (red) antibodies and counterstained with DAPI (blue [nuclei]) at indicated time points. Bar graphs/scatter plots (mean ± SD) depict the percentage of PERILIPIN+ cells expressing GFP at indicated time points. *P < 0.05 from Student t test. D: Pie chart summarizing the relative contribution of de novo adipogenesis vs. adipocyte activation/interconversion to the total pool of beige adipocytes originating following exposure to cold temperatures of β3-adrenergic receptor agonist. Percentages indicated represent mean values from the data shown in C.
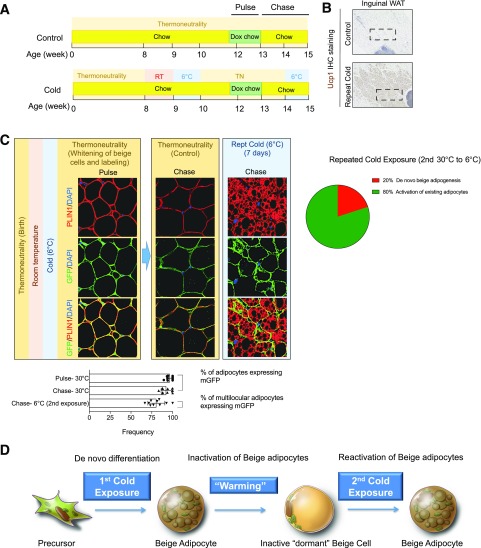
We returned a subset of these same cold-exposed animals to warmer temperature (30°C) for 4 weeks (Fig. 4A). After the 4 weeks of reintroduction to thermoneutrality, multilocular cells are rarely observed, and nearly all GFP-labeled adipocytes appear unilocular (Fig. 4B and C). This confirms prior studies demonstrating that beige adipocytes revert to a unilocular phenotype upon “warming” conditions and may represent “dormant” or “inactive” beige adipocytes (30,32). In a parallel study, we also quantitatively assessed the mode of beige cell recruitment in animals undergoing repeated cold exposure. Thermoneutral-raised AdipoChaser mice were cold exposed (first cold exposure) and then returned to 30°C for 4 weeks (as described above). Following the 4 weeks of reacclimation to 30°C, doxycycline was administered to label existing adipocytes with GFP expression. Then, mice were reintroduced to cold temperatures (6°C) for a second time (Fig. 5A). Following this repeated cold exposure, >75% of multilocular adipocytes retained GFP expression (Fig. 5B). Thus, the first cold exposure leads to beige cell recruitment through de novo adipogenesis, while the second exposure to cold temperatures favors a mechanism in which beige cells arise from differentiated unilocular adipocytes (Fig. 5C). The latter most likely reflects a reactivation of dormant beige adipocytes remaining following the first cold exposure. All together, these studies highlight the multiple cellular mechanisms leading to beige cell recruitment in vivo and important experimental considerations (i.e., history of the animals and housing conditions) needed to visualize them in vivo.
Figure 4.
Beige adipocytes acquire white adipocyte-like unilocular morphology after warm adaptation. A: AdipoChaser mice born and raised at thermoneutrality (TN) were fed a standard chow diet until 8 weeks of age before being switched to doxycycline (Dox)-containing chow diet (600 mg/kg) for 10 days (“Pulse”). After the pulse-labeling period, mice were switched back to the standard chow diet for 3 days. Mice were then transferred to room temperature (RT) adaptation for 7 days before 7 days of cold exposure (6°C). Following the cold exposure, mice were kept at thermoneutrality for another 4 weeks to induce the “whitening” of adipose tissue. B: Representative 4× brightfield image of UCP1 expression in inguinal WAT sections from mice following the final exposure to thermoneutrality. IHC, immunohistochemistry. C: Representative 63× image of inguinal WAT sections stained with anti-GFP (green) and anti-PERILIPIN (red) antibodies and counterstained with DAPI (blue [nuclei]) at indicated time points. D: Summary of results: beige adipocytes acquire white adipocyte-like unilocular morphology after warm adaptation.
Figure 5.
Repeated cold exposure influences the mode of beige adipocyte recruitment. A: AdipoChaser mice born and raised at thermoneutrality (TN) were fed a standard chow diet until 8 weeks of age before being transferred to room temperature (RT) adaptation for 7 days and then 6°C for 7 days. Following the cold exposure, the mice were returned to thermoneutrality for 4 weeks. During the 4 weeks of warm adaptation, the animals were administrated with doxycycline (Dox)-containing chow diet (600 mg/kg) for 10 days before being switched back to regular chow diet. Following the warm adaptation, the animals were subjected to repeated cold exposure (Rept Cold) (6°C) for 7 days (without Dox). For the single cold exposure cohort, age-matched mice housed at thermoneutrality were transiently fed with doxycycline-containing chow diet (600 mg/kg) for 10 days before being subjected to 7 days of room temperature adaptation and 7 days of cold exposure (6°C) (14–15 weeks of age). AdipoChaser mice born and raised at thermoneutrality and only transiently exposed to doxycycline-containing chow diet were used as control group. B: Representative 4× brightfield image of UCP1 expression in inguinal WAT sections from mice following the Chase periods. IHC, immunohistochemistry. C: Representative 63× image of inguinal WAT sections stained with anti-GFP (green) and anti-PERILIPIN (red) antibodies and counterstained with DAPI (blue [nuclei]) at indicated time points. Bar graphs (mean ± SD) depict the percentage of PERILIPIN+ cells expressing GFP at indicated time points. *P < 0.05 from Student t test. Pie chart summarizes the relative contribution of de novo adipogenesis vs. adipocyte activation/interconversion to the total pool of beige adipocytes following repeated cold exposure. D: Overall model: beige adipocytes initially arise predominantly from progenitors (i.e., de novo beige adipogenesis) upon the first exposure to cold temperatures and then interconvert between “dormant beige” and “active beige” phenotypes (i.e., beige cell activation) upon subsequent changes in environmental temperature.
Concluding Remarks and Future Directions
Adipose tissue gives mammals a remarkable capacity to adapt to the changing environment. As such, we should not be surprised to continuously find the lineage plasticity of adipocytes and their progenitors to be more complex than imagined. The initial observation from Seale et al. (10) that beige adipocytes emerge from a distinct cell lineage different from the precursors giving rise to brown adipocytes sparked tremendous efforts to unveil the natural origin of these cells. Based on the studies described here, we suggest a unified model that is in line with the initial observations made by Wu et al. (13) (Fig. 5D): beige adipocytes initially arise predominantly from progenitors (i.e., de novo beige adipogenesis) upon the first exposure to cold temperatures and then interconvert between “dormant beige” and “active beige” phenotypes (i.e., beige cell activation) upon subsequent changes in environmental temperature. Maintaining inactive, but transcriptionally “poised,” beige cells (UCP1− and unilocular) may be beneficial in order to rapidly defend against subsequent cold exposures. Although appearing inactive, such cells may also continue to play a role in other aspects of nutrient homeostasis and physiology.
It is tempting to refer to the transition between dormant and active beige adipocytes as “transdifferentiation.” This term is classically used to describe the conversion of one differentiated cell type into another, without passing through an intermediate progenitor-like state (44). Such a phenomenon can be induced experimentally through genetic manipulation or tissue injury/insult; however, few, if any, examples of bona fide trans-differentiation events occurring under physiological conditions have been described (44). In our view, it is not clear that beige UCP1+ cells fully revert to a bona fide white adipocyte that is present prior to the first cold exposure. Evidence that beige or white adipocytes de-differentiate, at least to some degree, en route to assuming the alternate fate is lacking. In vitro studies suggest that de-differentiation/redifferentiation is likely not the case (31). Nevertheless, the possibility of this happening in vivo has yet to be formally excluded. In fact, our recent studies of the mammary gland demonstrate that mature adipocytes indeed undergo de-differentiation during lactation to a precursor-like state and then redifferentiate following the involution of the mammary ducts (45). Given these strict definitions, the term “transdifferentiation” should be used with caution. Future studies aimed at better identifying molecular markers of the “dormant beige adipocyte” will help address this question.
It now appears certain that adult WAT harbors precursor cells with the capacity to undergo beige adipocyte differentiation; however, the exact identity of these cells remains uncertain, and cell surface markers to selectively identify these cells have remained unidentified. Clonal analyses of cultured adipose-derived stromal cell lines suggest that committed beige precursors exist within adult WAT and are distinct from white adipocyte precursors (13,16,46). Whether functionally distinct beige and white adipocyte precursors are maintained in native adult WAT still remains unclear. Alternatively, native WAT may harbor bipotent precursors whose commitment to the beige adipocyte lineage is dictated by signals associated with cold exposure. Single-cell sequencing has recently shed insight into the molecular heterogeneity of WAT stromal cells (47–49). Additional single-cell sequencing analyses before/after cold exposure will likely clarify these issues.
To date, much of the work on beige adipocytes has focused on their emergence and activity within the subcutaneous inguinal WAT of rodents. Recently, our groups have derived an adipose tissue atlas of beige and brown fat depots in mice (50). Beige adipocytes emerge within multiple, but not all, WAT depots in response to cold exposure. Whether there are significant depot-specific differences in beige/brown adipocytes and their origin remains unclear. Moreover, a number of studies now suggest thermogenic adipocytes and/or their precursors may even be heterogeneous within single depots (23,51,52). Similar to white adipocytes, beige adipocytes may be quite heterogeneous with respect to their functional properties and developmental origin.
Over the past 10 years, positron emission tomography/computed tomography imaging studies have made it clear that adult humans have active brown fat that may influence various aspects of nutrient homeostasis (53,54). As a result, there has been tremendous excitement over the prospect that activating human brown fat may be an effective strategy to increase energy expenditure. One concern is that the absolute mass of existing brown fat in obese adults may be limiting. Lineage-tracing studies and various genetic rodent models highlight the remarkable functional plasticity of WAT. The ability of human WAT to undergo browning is also supported by studies of burn trauma victims and pheochromocytoma patients (55,56). Thus, the possibility exists that the pool of brown/beige adipose tissue can be expanded by “unlocking” the thermogenic potential of white adipose (57). Going forward, a better understanding of the distinct cellular origins of beige adipocytes arising in response to both physiological and pharmacological stimuli may better inform strategies to recruit beige adipocytes in vivo.
Article Information
Acknowledgments. The authors are grateful to members of the Touchstone Diabetes Center for useful discussion.
Funding. This study was supported by American Heart Association postdoctoral award 16POST26420136 and Career Development Award 19CDA34670007 from the American Heart Association and the Harry S. Moss Heart Trust to M.S.; National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, grants K01-DK107788 and R03-HD095414 to Q.A.W., DK-104789 to R.K.G., and R01-DK55758, R01-DK099110, P01-DK088761, and P01-AG051459 to P.E.S. Q.A.W. was also supported by City of Hope Shared Resources Pilot Award, Caltech–City of Hope Initiative Award, and American Diabetes Association Junior Faculty Development Award 1-19-JDF-023. P.E.S. was also supported by an unrestricted research grant from the Novo Nordisk Foundation. R.K.G. was also supported by American Heart Association Grant-in-Aid Award 16GRNT30790006.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.S., Q.A.W., P.E.S., and R.K.G. conceived the study and designed experiments. M.S., Q.A.W., A.S., L.V., and N.C.B. performed experiments. M.S., Q.A.W., A.S., L.V., N.C.B., P.E.S., and R.K.G. analyzed data. M.S., Q.A.W., P.E.S., and R.K.G. wrote the manuscript. P.E.S. and R.K.G. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data and Resource Availability. All primary data and animal models used in the study are available to investigators upon reasonable request.
References
- 1.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359 [DOI] [PubMed] [Google Scholar]
- 2.Gaudry MJ, Jastroch M, Treberg JR, et al. Inactivation of thermogenic UCP1 as a historical contingency in multiple placental mammal clades. Sci Adv 2017;3:e1602878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chouchani ET, Kazak L, Spiegelman BM. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab 2019;29:27–37 [DOI] [PubMed] [Google Scholar]
- 4.Loncar D. Convertible adipose tissue in mice. Cell Tissue Res 1991;266:149–161 [DOI] [PubMed] [Google Scholar]
- 5.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010;285:7153–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest 1998;102:412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res 2007;48:41–51 [DOI] [PubMed] [Google Scholar]
- 8.Forner F, Kumar C, Luber CA, Fromme T, Klingenspor M, Mann M. Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell Metab 2009;10:324–335 [DOI] [PubMed] [Google Scholar]
- 9.Long JZ, Svensson KJ, Tsai L, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab 2014;19:810–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008;454:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timmons JA, Wennmalm K, Larsson O, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A 2007;104:4401–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Seale P. Control of brown and beige fat development. Nat Rev Mol Cell Biol 2016;17:691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012;150:366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cypess AM, White AP, Vernochet C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 2013;19:635–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp LZ, Shinoda K, Ohno H, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 2012;7:e49452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinoda K, Luijten IH, Hasegawa Y, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med 2015;21:389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013;19:1252–1263 [DOI] [PubMed] [Google Scholar]
- 18.Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab 2015;22:546–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes 2004;53:3274–3285 [DOI] [PubMed] [Google Scholar]
- 20.Collins S, Daniel KW, Petro AE, Surwit RS. Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology 1997;138:405–413 [DOI] [PubMed] [Google Scholar]
- 21.Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011;121:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao M, Ishibashi J, Kusminski CM, et al. Zfp423 maintains white adipocyte identity through suppression of the beige cell thermogenic gene program. Cell Metab 2016;23:1167–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Ikeda K, Yoneshiro T, et al. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 2019;565:180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kir S, White JP, Kleiner S, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 2014;513:100–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neinast MD, Frank AP, Zechner JF, et al. Activation of natriuretic peptides and the sympathetic nervous system following Roux-en-Y gastric bypass is associated with gonadal adipose tissues browning. Mol Metab 2015;4:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes 2015;64:2361–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inagaki T, Sakai J, Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol 2017;18:527. [DOI] [PubMed] [Google Scholar]
- 28.Barbatelli G, Murano I, Madsen L, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 2010;298:E1244–E1253 [DOI] [PubMed] [Google Scholar]
- 29.Lee YH, Petkova AP, Konkar AA, Granneman JG. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J 2015;29:286–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 2013;15:659–667 [DOI] [PubMed] [Google Scholar]
- 31.Altshuler-Keylin S, Shinoda K, Hasegawa Y, et al. Beige adipocyte maintenance is regulated by autophagy-Induced mitochondrial clearance. Cell Metab 2016;24:402–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roh HC, Tsai LTY, Shao M, et al. Warming induces significant reprogramming of beige, but not brown, adipocyte cellular identity. Cell Metab 2018;27:1121–1137.e5 [DOI] [PMC free article] [PubMed]
- 33.Hepler C, Shao M, Xia JY, et al. Directing visceral white adipocyte precursors to a thermogenic adipocyte fate improves insulin sensitivity in obese mice. eLife 2017;6:e27669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vishvanath L, MacPherson KA, Hepler C, et al. Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab 2016;23:350–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013;19:1338–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Kissig M, Rajakumari S, et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci U S A 2014;111:14466–14471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berry DC, Jiang Y, Graff JM. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat Commun 2016;7:10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald ME, Li C, Bian H, Smith BD, Layne MD, Farmer SR. Myocardin-related transcription factor A regulates conversion of progenitors to beige adipocytes. Cell 2015;160:105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Min SY, Kady J, Nam M, et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med 2016;22:312–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Y, Berry DC, Graff JM. Distinct cellular and molecular mechanisms for β3 adrenergic receptor-induced beige adipocyte formation. eLife 2017;6:e30329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye R, Wang QA, Tao C, et al. Impact of tamoxifen on adipocyte lineage tracing: inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Mol Metab 2015;4:771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hesselbarth N, Pettinelli C, Gericke M, et al. Tamoxifen affects glucose and lipid metabolism parameters, causes browning of subcutaneous adipose tissue and transient body composition changes in C57BL/6NTac mice. Biochem Biophys Res Commun 2015;464:724–729 [DOI] [PubMed] [Google Scholar]
- 43.Zhao L, Wang B, Gomez NA, de Avila JM, Zhu MJ, Du M. Even a low dose of tamoxifen profoundly induces adipose tissue browning in female mice. Int J Obes. 31 January 2019 [Epub ahead of print]. DOI: 10.1038/s41366-019-0330-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol 2011;12:79–89 [DOI] [PubMed] [Google Scholar]
- 45.Wang QA, Song A, Chen W, et al. Reversible de-differentiation of mature white adipocytes into preadipocyte-like precursors during lactation. Cell Metab 2018;28:282–288. e3 [DOI] [PMC free article] [PubMed]
- 46.Xue R, Lynes MD, Dreyfuss JM, et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat Med 2015;21:760–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwalie PC, Dong H, Zachara M, et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 2018;559:103–108 [DOI] [PubMed] [Google Scholar]
- 48.Hepler C, Shan B, Zhang Q, et al. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife 2018;7:e39636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burl RB, Ramseyer VD, Rondini EA, Pique-Regi R, Lee YH, Granneman JG. Deconstructing adipogenesis induced by β3-adrenergic receptor activation with single-cell expression profiling. Cell Metab 2018;28:300–309.e4 [DOI] [PMC free article] [PubMed]
- 50.Zhang F, Hao G, Shao M, et al. An adipose tissue atlas: an image-guided identification of human-like BAT and beige depots in rodents. Cell Metab 2018;27:252–262.e3 [DOI] [PMC free article] [PubMed]
- 51.Lee YH, Kim SN, Kwon HJ, Granneman JG. Metabolic heterogeneity of activated beige/brite adipocytes in inguinal adipose tissue. Sci Rep 2017;7:39794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Liu L, Lin JZ, Aprahamian TR, Farmer SR. Browning of white adipose tissue with roscovitine induces a distinct population of UCP1+ adipocytes. Cell Metab 2016;24:835–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chondronikola M, Volpi E, Børsheim E, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014;63:4089–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chondronikola M, Volpi E, Børsheim E, et al. Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metab 2016;23:1200–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frontini A, Vitali A, Perugini J, et al. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim Biophys Acta 2013;1831:950–959 [DOI] [PubMed] [Google Scholar]
- 56.Sidossis LS, Porter C, Saraf MK, et al. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab 2015;22:219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shao M, Gupta RK. Transcriptional brakes on the road to adipocyte thermogenesis. Biochim Biophys Acta Mol Cell Biol Lipids 2019;1864:20–28 [DOI] [PubMed] [Google Scholar]