Abstract
While diabetes is characterized by hyperglycemia, nutrient metabolic pathways like amino acid and tricarboxylic acid (TCA) cycle are also profoundly perturbed. As glycemic control alone does not prevent complications, we hypothesized that these metabolic disruptions are responsible for the development and progression of diabetic cardiovascular autonomic neuropathy (CAN). We performed standardized cardiovascular autonomic reflex tests and targeted fasting plasma metabolomic analysis of amino acids and TCA cycle intermediates in subjects with type 1 diabetes and healthy control subjects followed for 3 years. Forty-seven participants with type 1 diabetes (60% female and mean ± SD age 35 ± 13 years, diabetes duration 13 ± 7 years, and HbA1c 7.9 ± 1.2%) had lower fumarate levels and higher threonine, serine, proline, asparagine, aspartic acid, phenylalanine, tyrosine, and histidine levels compared with 10 age-matched healthy control subjects. Higher baseline fumarate levels and lower baseline amino acid levels—asparagine and glutamine—correlate with CAN (lower baseline SD of normal R-R interval [SDNN]). Baseline glutamine and ornithine levels also associated with the progression of CAN (lower SDNN at 3 years) and change in SDNN, respectively, after adjustment for baseline HbA1c, blood glucose, BMI, cholesterol, urine microalbumin-to- creatinine ratio, estimated glomerular filtration rate, and years of diabetes. Therefore, significant changes in the anaplerotic flux into the TCA cycle could be the critical defect underlying CAN progression.
Introduction
Cardiovascular autonomic neuropathy (CAN) is a widely prevalent chronic diabetes complication that is characterized by impaired autonomic control of the cardiovascular system (1). Although the initial prevalence of CAN in patients newly diagnosed with type 1 diabetes is low, later prevalence after 15 years of diabetes increases to 35% in patients with type 1 diabetes and 60% in patients with type 2 diabetes (1–4). CAN is an independent predictor of chronic kidney disease progression and of cardiovascular disease morbidity and mortality in patients with diabetes (5–8). CAN is also associated with an increased risk of cardiac arrhythmias, silent myocardial ischemia, myocardial dysfunction, and sudden death (1,2,4,5,8). The earliest clinical manifestations of CAN are insidious and include reduced heart rate variability (HRV) at rest and during several challenges such as standing, deep breathing, and the Valsalva maneuver. Currently, objective measures of HRV using recommended standard cardiovascular autonomic testing for research studies or clinical care remain the gold standard diagnostic (1). Given the critical prognostic consequences of CAN, its targeted and timely diagnosis is paramount. However, the current cardiovascular autonomic testing and other tests such as the baroreflex or imaging studies remain both cumbersome and expensive.
Several risk factors play essential roles in the development of CAN including chronic hyperglycemia, diabetes duration, hypertension, hyperlipidemia, chronic inflammation, oxidative stress, and, more recently, glucose variability (1,2,4,9). While CAN progression is prevented with tight glucose control in type 1 diabetes (2,9) and possibly with combined multifactorial interventions in type 2 diabetes (10), to date there are no specific disease-modifying therapies for CAN. Thus, a deeper understanding of the mechanisms that modulate CAN development and progression is crucial for both risk assessment and therapeutic interventions.
In addition to the changes in carbohydrate metabolism, diabetes is characterized by profound alterations in amino acid and lipid metabolism. In fact, elevated levels of branched-chain amino acids (BCAAs) are a well-characterized risk predictor of future type 2 diabetes risk and insulin resistance (11–13). While glycemic control as documented by hemoglobin A1c (HbA1c) has been the primary goal for diabetes management, a broader understanding of nutrient metabolism can offer a potential mechanistic risk modifier in the care of patients with diabetes. Indeed, microvascular complications like neuropathy, diabetic kidney disease, and retinopathy are also associated with increased oxidative stress and alterations in the levels of intermediary metabolites (14). There are tissue-specific patterns of altered metabolic flux through the glycolytic and tricarboxylic cycles in diabetic mouse models (15,16). Importantly, diabetic neuropathy and retinopathy show a distinctly different metabolic signature compared with diabetic kidney disease in animal models (14,16,17). Recent advancement in mass spectrometry techniques—both targeted and untargeted approaches—have made simultaneous large-scale assessments of various metabolic pathways possible. Many studies have demonstrated that these intermediary metabolites can predict the progression and severity of disease in type 1 and type 2 diabetic kidney disease (16,18–20) and diabetic retinopathy (21). These studies have clarified the pathophysiological mechanisms, highlighted biomarkers, and identified potential therapeutic targets. However, such targeted metabolomic profiling has not been used to characterize the metabolic perturbations associated with CAN in diabetes. Therefore, the primary objective of this study was to evaluate the association between perturbations in metabolic intermediates (tricarboxylic acid [TCA] cycle metabolites and amino acids) and measures of CAN in subjects with type 1 diabetes. The discovery of a distinct metabolomic biomarker signature associated with CAN may provide insight into the pathogenic pathways that are currently unknown and may allow for the clinical stratification of these patients early in the course of the disease so that interventions can be targeted to specific vulnerable subjects.
Research Design and Methods
Subjects
Forty-seven subjects with type 1 diabetes and 10 age-matched healthy control subjects were enrolled in a 3-year longitudinal observational study. Main inclusion criteria for subjects with diabetes were age 18–65 years; presence of type 1 diabetes, with a minimum of 5 years’ diabetes duration; and no signs of microvascular complications or uncontrolled hypertension at baseline. All subjects had normal resting electrocardiogram and normal exercise treadmill test results before enrolling in the study. Patients with a history of cardiovascular disease were excluded from the study. Healthy control subjects were age matched with normal weight, normal glucose tolerance, and normal blood pressure (BP). Forty subjects with type 1 diabetes completed the study. Demographic and anthropometric measures were collected through questionnaires and physical examination; fasting blood and urine samples were obtained for the measurement of various metabolic parameters including HbA1c, lipid panel, and renal function tests. The University of Michigan Institutional Review Board approved the study, and written informed consent was obtained from all subjects.
CAN Assessments
Standardized CAN evaluations were performed on all subjects after an overnight fast. Subjects were asked to avoid caffeine and tobacco products for 8 h before testing and to hold any medication (except for basal insulin) until CAN testing was completed. Subjects who experienced a hypoglycemic episode after midnight (blood glucose ≤50 mg/dL [2.77 mmol/L]) before the testing were rescheduled. The electrocardiogram recordings were obtained in the supine position using a physiologic monitor (Nightingale PPM2; Zoe Medical), and data were collected during a resting study (5 min) and during several standardized cardiovascular autonomic reflex tests obtained under paced breathing (R-R response to deep breathing, Valsalva maneuver, and postural changes). Indices of CAN were derived using the ANX 3.1 (ANSAR Medical Technologies) as previously described (22). All CAN variables were assessed for the entire cohort at baseline and for 40 subjects with type 1 diabetes (out of 47) who completed the study at 3 years of follow-up.
CAN Outcome Measures
The following measures of CAN were predefined as outcomes of interests and analyzed: SD of normal R-R interval (SDNN), root-mean square differences of successive R-R intervals (RMSSD), expiration-to-inspiration (E:I) ratio during deep breathing, Valsalva ratio (average of two measures), 30:15 ratio, low-frequency (LF) power (0.04–0.15 Hz), high-frequency (HF) power (0.15–0.4 Hz), and LF/HF at rest and during cardiovascular autonomic reflex tests.
Metabolite Measurements
Amino acids were measured after purification and derivatization of 100-μL samples of plasma via gas chromatography–mass spectrometry (Agilent 6890N Gas Chromatograph coupled to 5973 MSD Mass Spectrometer) using a modified EZ:faast kit (Phenomenex); norvaline was used as an internal standard (16,20). TCA metabolites were extracted from 100 μL plasma with a mixture of methanol, chloroform, and water (8:1:1) containing 13C isotope–labeled internal standards for citrate, succinate, fumarate, malate, α-ketoglutarate, lactate, and pyruvate. Liquid chromatography–mass spectrometry analysis was performed on an Agilent system consisting of a 1260 ultraperformance liquid chromatography module coupled with a 6520 quadrupole time-of-flight mass spectrometer (Agilent Technologies, Santa Clara, CA). Data were processed using MassHunter Quantitative Analysis, version B.07.00. Metabolites were normalized to the nearest isotope-labeled internal standard and quantitated using a linear calibration curves (16).
Statistical Analysis
Data Integrity Check
Metabolomic variables were examined for evidence of problematic signal detection based on principal component analysis–based inspection of metabolite-level and subject-level outliers, batch effect, and detectability. All variables were checked for normality, and appropriate data transformation (natural log transformation or another scale) was performed to satisfy the assumption of normality for the various statistical techniques used. Optimal data normalization and scaling were performed to ensure the appropriate data format for the subsequent statistical analysis.
Analysis Plan
The differences in clinical characteristics, CAN measures, TCA cycle metabolites, and amino acid levels between subjects with type 1 diabetes and the healthy control subjects were analyzed by using Student t test or Fischer exact test for continuous variables and χ2 test for categorical variables. Bonferroni correction was applied to account for multiple comparisons. Pearson correlation coefficient was used to assess the relationship between the metabolic intermediates (TCA cycle metabolites and amino acids) and CAN parameters to detect strong trends in a biological context that were confirmed with subsequent regression models that account for the effects of clinical variables. Linear regression was used to predict the association with baseline metabolites and baseline CAN parameters and predict CAN measures at the 3-year follow up. Dimension reduction with principal component analysis was used to account for the high correlation between biologically related metabolites, and the principal component accounting for the most variance was used for further analysis. All statistical analysis was performed using the software SPSS (version 24; IBM Corp.).
Data and Resource Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. No applicable resources were generated or analyzed during the current study.
Results
Baseline Clinical Characteristics, Metabolite Measures, and CAN Measures in All Participants
Table 1 shows the baseline clinical characteristics of this cohort, with comparison of the 47 subjects with type 1 diabetes (61% female, 4% current smokers, and mean ± SD age 34 ± 13 years, diabetes duration 13 ± 6 years, and HbA1c 8 ± 1.2%) and the age- and sex-matched healthy control subjects. There were no significant differences in the baseline characteristics between patients with type 1 diabetes and healthy control subjects except for fasting blood glucose (153.7 ± 76 vs. 86.2 ± 14 mg/dL, respectively; P = 0.007) and HbA1c, which were the criteria used to define the group with type 1 diabetes. The subjects with type 1 diabetes were slightly heavier, with no evidence of microvascular or macrovascular complications at baseline (no retinopathy, normal serum creatinine, or microalbuminuria as per study design). While the healthy control subjects were not on medication, all subjects with type 1 diabetes were on insulin, and among these 24 (51%) were using continuous subcutaneous insulin infusion via a pump, 7 (15%) were on statins, 5 (11%) were on ACE inhibitors (despite no prior history of diabetic nephropathy), and none were on β-blockers. There were no differences in any CAN measures between the subjects with type 1 diabetes subjects and healthy control subjects at baseline (Table 1).
Table 1.
Clinical characteristics of subjects with type 1 diabetes and healthy control subjects
| Subjects with type 1 diabetes (N = 47) | Healthy control subjects (N = 10) | P value | Type 1 diabetes (N = 40) |
P value | ||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | |||||
| Age, years | 34 ± 13 | 34 ± 12 | 0.85 | 35 ± 13 | 38 ± 13 | — |
| BMI, kg/m2 | 26 ± 5 | 23 ± 3 | 0.08 | 27 ± 5 | 27 ± 4 | 0.89 |
| Systolic BP, mmHg | 116 ± 11 | 115 ± 8 | 0.63 | 117 ± 11 | 117 ± 11 | 0.99 |
| Diastolic BP, mmHg | 72 ± 8 | 69 ± 8 | 0.29 | 73 ± 8 | 69 ± 11 | 0.08 |
| Heart rate, bpm | 67 ± 10 | 71 ± 8 | 0.16 | 67 ± 10 | 67 ± 11 | 0.97 |
| HbA1c, % | 8.0 ± 1.2 | 5.4 ± 0.3 | <0.0001 | 8 ± 1 | 8 ± 1 | 0.99 |
| Total cholesterol, mg/dL | 166 ± 28 | 162 ± 30 | 0.66 | 165 ± 29 | 173 ± 27 | 0.98 |
| LDL-c, mg/dL | 89 ± 23 | 86 ± 23 | 0.7 | 89 ± 24 | 90 ± 21 | 0.81 |
| HDL-c, mg/dL | 64 ± 19 | 58 ± 13 | 0.37 | 64 ± 20 | 67 ± 19 | 0.42 |
| Triglycerides, mg/dL | 70 ± 31 | 87 ± 33 | 0.12 | 67 ± 33 | 78 ± 41 | 0.24 |
| LF power | 2.96 ± 3.01 | 2.72 ± 2.59 | 0.81 | 3.23 ± 3.27 | 2.35 ± 4.05 | 0.07 |
| HF power | 2.95 ± 3.47 | 2.05 ± 2.02 | 0.44 | 2.85 ± 3.39 | 3.25 ± 7.38 | 0.68 |
| LF:HF ratio | 2.08 ± 1.89 | 1.43 ± 0.24 | 0.29 | 2.32 ± 2.03 | 2.65 ± 3.57 | 0.62 |
| Valsalva ratio | 1.36 ± 0.27 | 1.36 ± 0.20 | 0.99 | 1.35 ± 0.31 | 1.34 ± 0.33 | 0.81 |
| 30:15 ratio | 1.24 ± 0.15 | 1.23 ± 0.17 | 0.9 | 1.22 ± 0.15 | 1.21 ± 0.14 | 0.93 |
| E:I ratio | 1.23 ± 0.12 | 1.25 ± 0.13 | 0.71 | 1.23 ± 0.13 | 1.21 ± 0.14 | 0.29 |
| SDNN, ms | 53 ± 21 | 54 ± 31 | 0.87 | 51 ± 19 | 43.62 ± 22 | 0.02 |
| RMSSD, ms | 40 ± 27.6 | 39 ± 31 | 0.91 | 36 ± 24 | 34 ± 31 | 0.47 |
Data are means ± SD. HDL-c, HDL cholesterol; LDL-c, LDL cholesterol.
Forty subjects with type 1 diabetes returned at the end of 3 years for follow-up CAN and laboratory measures (Table 1). During the follow-up, there were no significant changes to glycemic control or changes to their BP, BMI, or lipid control. However, there was a significant decline in the SDNN, indicating worsening CAN (P < 0.05). As shown in Table 2, at baseline amino acids threonine, serine, proline, asparagine, aspartic acid, phenylalanine, tyrosine, and histidine were elevated in the subjects with type 1 diabetes as compared with their healthy counterparts. Among the TCA cycle metabolites, fumarate was lower in subjects with type 1 diabetes (Table 2). We stratified our cohorts based on the highest daily insulin requirements and found no differences in the levels of BCAA or CAN measures between these subgroups (Supplementary Table 1).
Table 2.
Baseline TCA cycle intermediates and amino acids in study participants
| Variable | Subjects with type 1 diabetes (n = 47) | Healthy control subjects (n = 10) | P value | q value |
|---|---|---|---|---|
| Amino acids | ||||
| Alanine | 433.0 ± 181.1 | 361.9 ± 94.2 | 0.48 | 0.58 |
| Glycine | 321.4 ± 124.3 | 266.4 ± 55.4 | 0.21 | 0.32 |
| Threonine | 186.1 ± 71.2 | 127.9 ± 34.8 | 0.01 | 0.05 |
| Serine | 197.6 ± 77.0 | 127.1 ± 20.6 | 0.0015 | 0.02 |
| α-Aminoisobutyric acid | 22.9 ± 9.6 | 22.6 ± 9.3 | 0.97 | 0.97 |
| Valine | 242.1 ± 76.1 | 204.4 ± 54.5 | 0.20 | 0.32 |
| Leucine | 103.2 ± 43.8 | 102.6 ± 32.4 | 0.80 | 0.84 |
| Isoleucine | 72.2 ± 22.8 | 62.5 ± 21.2 | 0.17 | 0.31 |
| Phenylalanine | 58.6 ± 17.1 | 40.5 ± 10.0 | 0.0007 | 0.02 |
| Tyrosine | 51.2 ± 21.4 | 35.8 ± 13.2 | 0.015 | 0.05 |
| Tryptophan | 49.5 ± 18.7 | 44.3 ± 14.2 | 0.49 | 0.58 |
| Asparagine | 75.5 ± 25.9 | 53.6 ± 11.9 | 0.01 | 0.05 |
| Aspartic acid | 6.2 ± 3.5 | 3.9 ± 1.0 | 0.11 | 0.22 |
| Glutamic acid | 55.4 ± 30.6 | 66.6 ± 28.4 | 0.18 | 0.31 |
| Glutamine | 1002.7 ± 537.0 | 597.9 ± 191.1 | 0.02 | 0.07 |
| Ornithine | 48.2 ± 24.7 | 54.1 ± 25.3 | 0.40 | 0.53 |
| Proline | 213.6 ± 69.8 | 162.8 ± 54.2 | 0.01 | 0.05 |
| 4-Hydroxyproline | 19.9 ± 12.2 | 14.3 ± 7.4 | 0.07 | 0.17 |
| Lysine | 224.3 ± 93.3 | 182.8 ± 51.2 | 0.30 | 0.42 |
| Histidine | 86.5 ± 25.6 | 67.5 ± 19.2 | 0.01 | 0.05 |
| TCA metabolites | ||||
| Citrate/isocitrate | 19.50 ± 3.72 | 21.09 ± 3.12 | 0.04 | 0.19 |
| α-Ketoglutarate | 1.80 ± 0.25 | 1.78 ± 0.14 | 0.57 | 0.88 |
| Succinate | 2.87 ± 0.66 | 2.84 ± 0.54 | 0.85 | 0.88 |
| Fumarate | 0.46 ± 0.04 | 0.54 ± 0.04 | <0.0001 | <0.0001 |
| Malate | 2.07 ± 0.62 | 2.02 ± 0.35 | 0.88 | 0.88 |
| Lactate | 180.17 ± 82.31 | 204.52 ± 57.17 | 0.61 | 0.88 |
| Pyruvate | 3.91 ± 1.58 | 4.43 ± 1.72 | 0.44 | 0.88 |
| Flavin adenine dinucleotide | 0.46 ± 0.001 | 0.46 ± 0.001 | 0.73 | 0.88 |
All data are presented as means ± SD and in μmol/L. Boldface type indicates P < 0.05 and q < 0.05.
Correlation of Metabolites With Measures of CAN in Type 1 Diabetes at Baseline and 3-Year Follow-up
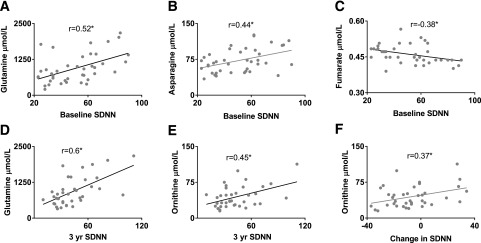
Table 3 and Fig. 1 show the Pearson correlations between measures of CAN and baseline metabolites levels in participants with type 1 diabetes. Figure 2 shows the Pearson correlations between the baseline metabolite levels and change in CAN measures at baseline and at 3-year follow-up of significant metabolites in a pathway-specific pattern. As observed, higher levels of baseline fumarate were associated with worsening baseline CAN parameters (SDNN, r = −0.46, P = 0.003; RMSSD, r = −0.40, P = 0.01; pNN50, r = −0.45, P = 0.003). Similarly, higher baseline citrate levels were associated with worse baseline CAN parameters (RMSSD, r = −0.51, P = 0.003) (Table 3). Meanwhile, lower levels of both amino acids asparagine (SDNN, r = 0.42, P = 0.007) and glutamine (SDNN, r = 0.51, P = 0.001) were positively correlated with baseline SDNN. The correlation of the entire metabolite panel and all baseline CAN measures are represented in Supplementary Table 2. Baseline estimated glomerular filtration rate (eGFR) and urine microalbumin-to-creatinine ratio have no relationship with baseline, year 3, or change in CAN measures.
Table 3.
Correlation between baseline and 3-year CAN parameters with baseline metabolites and principal components
| SDNN (r) |
RMSSD (r) |
|||||
|---|---|---|---|---|---|---|
| Baseline | 3-year | Difference | Baseline | 3-year | Difference | |
| Metabolites | ||||||
| Fumarate | −0.38 | −0.18 | 0.2 | −0.32 | −0.2 | 0.06 |
| Pyruvate | −0.34 | −0.19 | 0.19 | −0.31 | −0.19 | 0.08 |
| Citrate/isocitrate | −0.28 | −0.06 | 0.3 | −0.43 | −0.24 | 0.2 |
| α-Ketoglutarate | −0.26 | −0.09 | 0.21 | −0.14 | −0.04 | 0.09 |
| Asparagine | 0.44 | 0.3 | −0.04 | 0.21 | 0.19 | 0.1 |
| Glutamine | 0.52 | 0.6 | 0.16 | 0.26 | 0.24 | 0.1 |
| Ornithine | 0.11 | 0.45 | 0.37 | 0.24 | 0.44 | 0.47 |
| Principal components | ||||||
| Glutamine–asparagine–α-ketoglutarate | 0.44 | 0.62 | 0.02 | 0.32 | 0.4 | 0.08 |
| Ornithine-glutamine | 0.38 | 0.61 | 0.31 | 0.3 | 0.41 | 0.34 |
| α-Ketoglutarate–fumarate–citrate | −0.38 | −0.14 | 0.3 | −0.37 | −0.2 | 0.15 |
Values represent r values of Pearson correlation. Boldface type indicates P < 0.05. Italic type indicates P < 0.001.
Figure 1.
Correlation of baseline SDNN with baseline glutamine levels (A), asparagine levels (B), and fumarate levels (C). Correlation of 3-year SDNN with baseline glutamine levels (D) and baseline ornithine levels (E). Panel F demonstrates the correlation between the change in SDNN over 3-year follow-up with baseline ornithine levels. All metabolite levels in μmol/L and SDNN in seconds. Pearson correlation represented as r. yr, year. *P value <0.05.
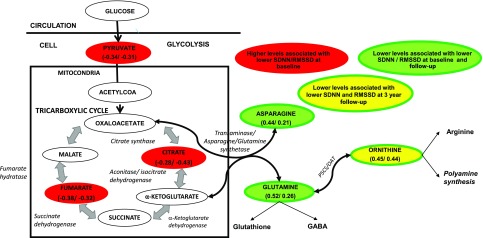
Figure 2.
A: Schematic diagram of the altered metabolites that associate with CAN parameters. Metabolites highlighted in red ovals are increased in subjects with type 1 diabetes and positively related to baseline SDNN and RMSSD, those in green-filled ovals are decreased in subjects with diabetes and positively related to SDNN and RMSSD at baseline and follow-up [r values by Pearson correlation to baseline CAN parameters, represented in parentheses as (SDNN/RMSSD)], and those in yellow-filled ovals are decreased in subjects with diabetes and positively related to SDNN and RMSSD at follow-up and the difference in SDNN and RMSSD from baseline and follow-up [r values by Pearson correlation to 3-year follow-up CAN parameters, represented in parentheses as (SDNN/RMSSD)]. OAT, ornithine amino transferase; P5CS, pyrroline 5-carboxylate synthetase.
Baseline glutamine and ornithine levels were also correlated with SDNN at the 3-year follow-up (glutamine, r = 0.60, P = 0.005; ornithine, r = 0.45, P = 0.005) (Table 3 and Fig. 1). The association of baseline glutamine levels with baseline SDNN and 3-year follow-up remained significant even after adjustment for years of diabetes, history of smoking, baseline HbA1c, blood glucose, BMI, total cholesterol, urine albumin-to-creatinine ratio, and eGFR (P = 0.014 and P = 0.005, respectively). In other words, increased citrate and fumarate levels and decreased glutamine and asparagine levels at baseline were associated with lower SDNN levels at baseline, indicating worsening CAN. The relationship with baseline glutamine and ornithine with SDNN was maintained at 3-year follow up. The correlation of the entire metabolite panel and 3-year CAN measures are represented in Supplementary Table 3.
As observed in Table 3, lower levels of baseline ornithine were associated with worsening of several CAN parameters from baseline to 3-year follow-up (SDNN, r = 0.37, P = 0.024; RMSSD, r = 0.466, P = 0.003; HF power, r = 0.342, P = 0.036; 30:15 ratio, r = 0.353, P = 0.03; E:I ratio, r = 0.365, P = 0.024). The association of baseline ornithine levels with change in RMSD, 30:15 ratio, and E:I ratio over 3 years remained significant even after adjustment for age, years of diabetes, history of smoking, BMI, baseline HbA1c, blood glucose, cholesterol, urine microalbumin-to-creatinine ratio, and eGFR). The correlation of the entire metabolite panel and change in CAN measures are represented in Supplementary Table 4.
To account for the close correlation between many of the metabolites, we reduced the dimensions of groups of similar metabolites based on biological context using principal component analysis. We found that the principal component representing glutamine, asparagine, and α-ketoglutarate (anaplerotic pathway/gluconeogenesis) and the principal component representing glutamine and ornithine (ornithine synthesis) both positively correlated with baseline SDNN and 3-year SDNN, indicating worsening CAN measures (Table 3). Similarly, the principal component representing the altered TCA metabolites citrate, α-ketoglutarate, and fumarate negatively correlated with baseline SDNN. Hence, the metabolite changes associated with CAN parameters are pathway specific and reveal systematic changes in metabolite patterns (Fig. 2).
Discussion
This is the first study to explore the association between the CAN and intermediates of central carbon metabolism (TCA cycle metabolites and amino acids) using a targeted metabolomics approach. In this study, we demonstrate that subjects with type 1 diabetes had baseline perturbations in levels of several amino acids and lower fumarate levels in the plasma compared with the healthy control subjects. Higher baseline levels of TCA cycle metabolites such as fumarate and citrate were also associated with lower SDNN measures in type 1 diabetes, indicating worsening CAN measures. Furthermore, lower baseline glutamine levels associated with lower SDNN measures at baseline and at 3-year follow-up, indicating worsening CAN measures after adjustment for other traditional risk factors. Similarly, baseline ornithine levels are associated with changes in SDNN and RMSDD measured at the 3-year follow-up after adjustment of clinical variables.
The metabolic profiles linked with diabetes risk and diabetes itself include metabolites beyond glucose metabolism. Type 1 diabetes risk is traditionally associated with autoimmunity, and lysophosphatidylcholine (18:0/0:0), BCAA, and glutamic acid are increased along with decreased glutamine and methionine levels before and after seroconversion with autoantibodies (23,24). Type 2 diabetes risk is associated with insulin resistance (25) and with elevated amino acids isoleucine, leucine, valine, tyrosine, and phenylalanine and decreased asparagine, glycine, and glutamine levels (25,26). Although this study only included participants with type 1 diabetes, given the contemporary changes in the phenotypes of patients with type 1 diabetes and that insulin resistance may be present in some participants, we stratified our cohorts based on highest daily insulin requirements and found no differences between these subgroups. In C-peptide–negative patients with type 1 diabetes, levels of leucine, isoleucine, valine, phenylalanine, and tyrosine are increased, whereas levels of glycine, glutamate, and threonine are decreased compared with both matched control subjects and insulin-treated patients (27); However, treatment with insulin with a euglycemic clamp removed these metabolic differences. Thus, the metabolome is very sensitive to the presence or absence of insulin in type 1 diabetes, and this could be the driving force for diabetes complications. In this study, subjects with type 1 diabetes demonstrated increased levels of threonine, serine, proline, histidine, asparagine, aspartic acid, phenylalanine, and tyrosine compared with healthy control subjects. Aromatic amino acids phenylalanine and tyrosine have previously been associated with insulin resistance, obesity, and future diabetes risk along with increased BCAA levels (25). The increase in these glucogenic amino acids compared with control subjects raises the possibility of altered metabolism of the metabolites in type 1 diabetes despite control of diabetes.
Our work on the db/db mouse model of diabetes demonstrated tissue-specific metabolic reprogramming and mitochondrial dysfunction that are associated with microvascular diabetes complications (16). Peripheral nerves are dependent on glycolysis independent of insulin action. However, diabetic db/db mice demonstrated decreased glucose metabolic flux and increased fatty acid oxidation in peripheral nerves (16,28). In a similar type 2 diabetes mouse model, decreased glycolytic and TCA intermediates in sural, sciatic, and dorsal root ganglion were observed (14). In contrast, other animal models of diabetic neuropathy reported a striking upregulation of mitochondrial oxidative phosphorylation and perturbation of lipid metabolism in the distal sciatic (29). While these studies indicate the perturbed metabolism in diabetic peripheral nerves in mouse models, there is no prior evidence linking changes in metabolomic profiles with the development or progression of CAN that involves deficits of the autonomic nervous system. Our data in circulating plasma levels in direct contradiction to the peripheral nerves in animal models of diabetes indicate elevated TCA metabolites and decreased glutamine, ornithine, and asparagine levels associated with worsening CAN measures. The data suggest anaplerotic flux into the TCA cycle in patients with worsening CAN, which needs to be confirmed with more definitive metabolic flux studies.
Diabetic retinopathy, a closely related microvascular complication, has elevated serum tryptophan metabolites: kynurenine, kynurenic acid, and 3-hydroxykynurenine (21). Diabetic kidney disease also demonstrates perturbed metabolic signatures (18–20). Serum leucine and phospholipids are altered in diabetic kidney disease compared with levels in subjects with diabetes and healthy control subjects (30). Elevated amino acid–derived acyl-carnitines, essential amino acids, and their derivatives are associated with progression to end-stage renal disease in patients with type 2 diabetes over many decades (20). Similarly, serum levels of seven modified amino acids (C-glycosyl tryptophan, pseudouridine, O-sulfo tyrosine, N-acetyl threonine, N-acetyl serine, N6-carbamoyl threonyl adenosine, and N6-acetyl lysine) were associated with renal function decline independent of the relevant clinical covariates in type 1 diabetes (19). In our study, lower levels of glutamine, asparagine, and ornithine associated with worsening CAN measures. In addition to amino acids, several TCA metabolites are increased in the urine of patients with type 2 diabetes, and urinary fumarate levels predicted chronic kidney disease progression in men (15). Mouse models also support the influence of higher fumarate levels in the kidney and urine as a result of NADPH oxidase 4 (NOX4) activity leading to decreasing renal function (31). In line with these changes in other diabetes complications, high circulating fumarate, citrate, and α-ketoglutarate levels were associated with low SDNN, indicating worsening CAN in our study.
Hyperglycemia is generally acknowledged as the driving factor for most of the diabetes complications. In our study, the metabolite associations with the CAN measures remained unaffected by the concurrent blood glucose in the same sample and long-term blood glucose control in the form of HbA1c. Increased glucose flux can result in the downstream production of advanced glycation end products, polyol, hexosamine, protein kinase C, and poly(ADP-ribose) polymerase pathways. Oxidative stress, apoptosis, and inflammation are also the consequences of the above-increased flux (32). Diabetic glucotoxicity along with these altered TCA metabolites can contribute to protein modifications that include glycation, carbonylation, nitration, cysteine S-nitrosylation, acetylation, sumoylation, ADP-ribosylation, O-GlcNAcylation, and succinylation (33). In addition to this, succinate and other TCA derivatives act on specific receptors and pathways to effect oxidative stress and inflammation (34,35). Hence, the preserved Krebs cycle intermediates that negatively associate with CAN measures might be driving altered posttranslational protein modifications, binding to receptors, oxidative stress, and inflammation in type 1 diabetes.
Glutamine is the most abundant amino acid in the circulation, and both glutamine and asparagine depending on conditions can feed into or be derived from the TCA cycle metabolites α-ketoglutarate and oxaloacetate (3). Certain cells rely solely on glutamine for critical cellular functions, and other metabolic inputs are unable to replace them. Glutamine itself is the source of many metabolites including glucose, α-ketoglutarate, glutamate, ornithine (and therefore arginine, urea, and nitric oxide production), and glutathione (3). Glutamine is responsible for gluconeogenesis and ammonia production in the kidney and liver, neurotransmitter synthesis in the brain (γ-amino butyrate [GABA]), NADPH, antioxidant defenses, and DNA and protein synthesis in cells of the immune system (3). In our study, lower levels of amino acids glutamine, asparagine, and ornithine were positively related to worsening CAN parameters SDNN and RMSD both at baseline and at follow-up. Glutamine metabolism is known to be perturbed in diabetes, and increased glutamine levels are associated with decreased risk of both type 2 diabetes and coronary artery disease; in addition, glutamine levels are also increased with rosiglitazone treatment in these patients (36,37). Glutamine supplementation was beneficial in preventing neuronal loss and development of experimental diabetic cardiomyopathy (38,39). Similarly, glutamine was shown to increase insulin sensitivity and cause overnight hypoglycemia postexercise in adolescents with type 1 diabetes by decreasing glucose production (40). Thus, glutamine and glutamine metabolism play crucial roles in the cardiovascular burden, insulin sensitivity, and microvascular complications in diabetes, and data from our study indicate that glutamine and its metabolism are central to CAN progression in type 1 diabetes. Glutamate product GABA acts on GABA receptors present in sympathetic ganglia, causing diminished ganglion blockade; thus, glutamate by-products could influence the autonomic nervous system (41). Similarly, ornithine, a product of glutamate, plays a central part of the urea cycle, polyamine synthesis, and collagen formation. Ornithine supplementation promotes weight loss in rats by increasing sympathetic nerve activity in white and brown adipose tissue and modulating lipid metabolism (42). Therefore, the relationship between decreased ornithine levels in our study and changes in CAN measures at 3-year follow up is possibly due to the modulation of autonomic nerve activity.
High baseline fumarate and citrate levels along with low glutamine levels are linked to worse baseline CAN measures indicating breaks in the citric acid cycle in CAN. In diabetic nephropathy, NADPH oxidase 4–induced decrease in kidney fumarate hydratase levels (the enzyme that converts fumarase to malate) caused increased urinary fumarate levels (31). Fumarate levels in the diabetic kidney stimulated endoplasmic reticulum stress, matrix gene expression, and expression of hypoxia-inducible factor-1α and transforming growth factor-β. So, oxidative stress could reduce fumarate hydratase levels, leading to fumarate accumulation (31). Inherited fumarate hydratase deficiency results in increased fumarate levels along with severe neurological deficits and failure to thrive. Therefore, it is possible that the increased oxidative stress associated with CAN could decrease fumarate hydratase levels and increase fumarate levels, leading to neurotoxicity. However, there is no published connection linking fumarate levels to neuropathy. Fumarate levels and glutamine levels are also linked via the urea cycle. Glutamate generates carbamoyl phosphate from ammonia as the first step of the urea cycle, while fumarate is a product of the urea cycle. Clearly, quantifying the entire plasma metabolome, including the metabolites of the urea cycle, will uncover the possible link between these metabolites.
Both SDNN and RMSSD are indices of heart rate variability over time. Though correlated, these indices provide information on different aspects of autonomic modulation. It is widely accepted that SDNN is a broad measure of both the sympathetic and parasympathetic modulation of HRV, while the RMSSD mainly characterizes the parasympathetic effect (43). In diabetic CAN, the interactions between the sympathetic/parasympathetic tone and function are complex and the changes in various indices may not be fully synchronized, with alterations in some measures preceding others. SDNN and RMSSD are very early indicators of CAN, and changes in these indicators usually precede manifest forms of CAN by many years. Given that the participants enrolled in this observational study had no evidence of complications at baseline (as per study design), and thus are in a very early, preclinical stage of CAN, it is conceivable to observe selectivity in the relationship between certain HRV indices and specific metabolites. Our findings could also suggest differentiated mechanisms contributing to the modulation of various aspects of the autonomic nervous system in early stages of disease that could be targeted.
Lower baseline glutamine and higher baseline fumarate, citrate, and pyruvate levels are related to worse CAN measures at baseline and possibly related autonomic nerve dysfunction. Ornithine levels are associated with worsening CAN measures at follow-up, possibly indicating an early deficiency associated with causation. This distinct metabolite pattern may underlie unique aspects of the pathophysiology of the presence and development of CAN. Additionally, these metabolites could serve as a biomarker for autonomic dysfunction in patients with type 1 diabetes and as an outcome in exploratory trials of therapies for early CAN. Although these patterns are very informative, these findings need to be confirmed in a larger, adequately powered, independent cohort.
CAN is an independent predictor of progression of diabetic kidney disease, and patients with more advanced chronic kidney disease are also more likely to have CAN (5–7). Per study design, none of the patients had evidence of diabetic kidney disease as evidenced by normal creatinine and absence of microalbuminuria. Also, there were no relationships of the baseline eGFR or urine microalbumin-to-creatinine ratio with CAN measures at baseline or year 3 or with change in CAN during the follow-up period. Neither eGFR nor urine microalbumin-to-creatinine ratio diminished the relationship between baseline metabolites and CAN measures. Therefore, we can reasonably conclude that the relationship of these metabolites and CAN measures is independent of renal function.
In summary, this study elucidates specific patterns among TCA metabolites and amino acids that associate with baseline CAN and changes in CAN measures. Specifically, lower levels of α-ketoglutarate amino acids asparagine and glutamine, that feed into and/or can be synthesized from oxaloacetate and α-ketoglutarate of the TCA cycle, are associated with CAN measures (Fig. 2). This pattern might indicate a block in the flux of these metabolites out of the TCA cycle and/or increased flux in synthesizing other downstream derivatives. The data provided are derived from use of only static metabolomic analysis, and metabolic flux can only be inferred. For accurate flux analysis, isotopically labeled tracers documenting the flux of nutrients along various pathways need to be followed to estimate metabolic fluxes. Our cohort lacked information on diet and physical activity, but smoking status did not influence the relationship of the metabolites with the CAN measures in our models. The sample size was also limited, and we are unable to pinpoint the origin of the circulating markers without tissue-specific metabolomics; thus, the study is underpowered to detect many strong correlations. Our findings need to be followed up by larger, well-powered studies for confirmation. However, the strengths of this study include standardized, accurate, and repeated CAN measures and associated metabolomic profiling. For mitigation of the diurnal variation of the metabolites in relation to the circadian rhythm, mealtime, and physical activity (44,45), only fasting samples obtained in a standardized fashion were used in this study. Day-to-day metabolite variation in fasting samples is consistent with variation in plasma glucose in healthy subjects, and our analysis included adjustment for blood glucose at the time of the sample collection (46). In conclusion, we present a novel study profiling the metabolic perturbations associated with type 1 diabetes and progression of CAN measures. These findings, when expanded to a larger cohort, will test the biomarker potential of these altered metabolites and potentially open therapeutic avenues for the prevention of CAN. Furthermore understanding these alterations will shed light on the pathways involved in CAN development and progression.
Supplementary Material
Article Information
Funding. This work is supported in part by grants from the Center for Scientific Review, National Institutes of Health (NIH) (P30DK081943, DK089503, DK082841, DK097153, K08HL130944, and 1R01HL102334-01) and American Diabetes Association grant 1-14-MN-02. This work utilized Core Services supported by grant DK097153 of the NIH to the University of Michigan.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.V.M. conducted the metabolomics analyses and wrote the manuscript. M.J. coordinated additional data collection analysis, conducted the analysis, and cowrote the manuscript. L.A. recruited the subjects and reviewed the manuscript. G.M. analyzed the data and provided statistical guidance. S.P. designed the experiments, provided funding, and reviewed the manuscript. R.P.-B. designed the study, recruited the subjects, provided funding, and reviewed the manuscript. S.P. and R.P.-B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0145/-/DC1.
References
- 1.Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40:136–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group . Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate--their central role in cell metabolism and function. Cell Biochem Funct 2003;21:1–9 [DOI] [PubMed] [Google Scholar]
- 4.Spallone V, Ziegler D, Freeman R, et al.; Toronto Consensus Panel on Diabetic Neuropathy . Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011;27:639–653 [DOI] [PubMed] [Google Scholar]
- 5.Orlov S, Cherney DZI, Pop-Busui R, et al. Cardiac autonomic neuropathy and early progressive renal decline in patients with nonmacroalbuminuric type 1 diabetes. Clin J Am Soc Nephrol 2015;10:1136–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra P, Sands RL, Gillespie BW, et al. Predictors of heart rate variability and its prognostic significance in chronic kidney disease. Nephrol Dial Transplant 2012;27:700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheelock KM, Jaiswal M, Martin CL, et al. Cardiovascular autonomic neuropathy associates with nephropathy lesions in American Indians with type 2 diabetes. J Diabetes Complications 2016;30:873–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pop-Busui R, Evans GW, Gerstein HC, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010;33:1578–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pop-Busui R, Low PA, Waberski BH, et al.; DCCT/EDIC Research Group . Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 2009;119:2886–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 [DOI] [PubMed] [Google Scholar]
- 11.Felig P, Marliss E, Cahill GF Jr. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 1969;281:811–816 [DOI] [PubMed] [Google Scholar]
- 12.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 2012;15:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinder LM, Vivekanandan-Giri A, McLean LL, Pennathur S, Feldman EL. Decreased glycolytic and tricarboxylic acid cycle intermediates coincide with peripheral nervous system oxidative stress in a murine model of type 2 diabetes. J Endocrinol 2013;216:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu JJ, Liu S, Gurung RL, et al. Urine tricarboxylic acid cycle metabolites predict progressive chronic kidney disease in type 2 diabetes. J Clin Endocrinol Metab 2018;103:4357–4364 [DOI] [PubMed] [Google Scholar]
- 16.Sas KM, Kayampilly P, Byun J, et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight 2016;1:e86976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sas KM, Lin J, Rajendiran TM, et al. Shared and distinct lipid-lipid interactions in plasma and affected tissues in a diabetic mouse model. J Lipid Res 2018;59:173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma K, Karl B, Mathew AV, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol 2013;24:1901–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niewczas MA, Mathew AV, Croall S, et al. Circulating modified metabolites and a risk of ESRD in patients with type 1 diabetes and chronic kidney disease. Diabetes Care 2017;40:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niewczas MA, Sirich TL, Mathew AV, et al. Uremic solutes and risk of end-stage renal disease in type 2 diabetes: metabolomic study. Kidney Int 2014;85:1214–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munipally PK, Agraharm SG, Valavala VK, Gundae S, Turlapati NR. Evaluation of indoleamine 2,3-dioxygenase expression and kynurenine pathway metabolites levels in serum samples of diabetic retinopathy patients. Arch Physiol Biochem 2011;117:254–258 [DOI] [PubMed] [Google Scholar]
- 22.Jaiswal M, McKeon K, Comment N, et al. Association between impaired cardiovascular autonomic function and hypoglycemia in patients with type 1 diabetes. Diabetes Care 2014;37:2616–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orešič M, Simell S, Sysi-Aho M, et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Exp Med 2008;205:2975–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pflueger M, Seppänen-Laakso T, Suortti T, et al. Age- and islet autoimmunity-associated differences in amino acid and lipid metabolites in children at risk for type 1 diabetes. Diabetes 2011;60:2740–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guasch-Ferré M, Hruby A, Toledo E, et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 2016;39:833–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urpi-Sarda M, Almanza-Aguilera E, Llorach R, et al. Non-targeted metabolomic biomarkers and metabotypes of type 2 diabetes: a cross-sectional study of PREDIMED trial participants. Diabetes Metab 2019;45:167–174 [DOI] [PubMed] [Google Scholar]
- 27.Lanza IR, Zhang S, Ward LE, Karakelides H, Raftery D, Nair KS. Quantitative metabolomics by H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS One 2010;5:e10538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greene DA, Winegrad AI. In vitro studies of the substrates for energy production and the effects of insulin on glucose utilization in the neural components of peripheral nerve. Diabetes 1979;28:878–887 [DOI] [PubMed] [Google Scholar]
- 29.Freeman OJ, Unwin RD, Dowsey AW, et al. Metabolic dysfunction is restricted to the sciatic nerve in experimental diabetic neuropathy. Diabetes 2016;65:228–238 [DOI] [PubMed] [Google Scholar]
- 30.Zhu C, Liang QL, Hu P, Wang YM, Luo GA. Phospholipidomic identification of potential plasma biomarkers associated with type 2 diabetes mellitus and diabetic nephropathy. Talanta 2011;85:1711–1720 [DOI] [PubMed] [Google Scholar]
- 31.You YH, Quach T, Saito R, Pham J, Sharma K. Metabolomics reveals a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. J Am Soc Nephrol 2016;27:466–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther 2008;120:1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng H, Wu J, Jin Z, Yan LJ. Protein modifications as manifestations of hyperglycemic glucotoxicity in diabetes and its complications. Biochem Insights 2016;9:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peti-Peterdi J, Kang JJ, Toma I. Activation of the renal renin-angiotensin system in diabetes--new concepts. Nephrol Dial Transplant 2008;23:3047–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lampropoulou V, Sergushichev A, Bambouskova M, et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab 2016;24:158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cha SA, Yun JS, Lim TS, et al. Diabetic cardiovascular autonomic neuropathy predicts recurrent cardiovascular diseases in patients with type 2 diabetes. PLoS One 2016;11:e0164807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ottosson F, Smith E, Melander O, Fernandez C. Altered asparagine and glutamate homeostasis precede coronary artery disease and type 2 diabetes. J Clin Endocrinol Metab 2018;103:3060–3069 [DOI] [PubMed] [Google Scholar]
- 38.Pereira RV, Tronchini EA, Tashima CM, Alves EP, Lima MM, Zanoni JN. L-glutamine supplementation prevents myenteric neuron loss and has gliatrophic effects in the ileum of diabetic rats. Dig Dis Sci 2011;56:3507–3516 [DOI] [PubMed] [Google Scholar]
- 39.Badole SL, Jangam GB, Chaudhari SM, Ghule AE, Zanwar AA. L-glutamine supplementation prevents the development of experimental diabetic cardiomyopathy in streptozotocin-nicotinamide induced diabetic rats. PLoS One 2014;9:e92697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mauras N, Xing D, Fox LA, Englert K, Darmaun D. Effects of glutamine on glycemic control during and after exercise in adolescents with type 1 diabetes: a pilot study. Diabetes Care 2010;33:1951–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vemulapalli S, Barletta M. The role of the sympathetic nervous system in the cardiovascular effects of systemically administered gamma-aminobutyric acid. Arch Int Pharmacodyn Ther 1984;267:46–58 [PubMed] [Google Scholar]
- 42.Konishi Y, Koosaka Y, Maruyama R, et al. L-ornithine intake affects sympathetic nerve outflows and reduces body weight and food intake in rats. Brain Res Bull 2015;111:48–52 [DOI] [PubMed] [Google Scholar]
- 43.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996;93:1043–1065 [PubMed] [Google Scholar]
- 44.Sato S, Parr EB, Devlin BL, Hawley JA, Sassone-Corsi P. Human metabolomics reveal daily variations under nutritional challenges specific to serum and skeletal muscle. Mol Metab 2018;16:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA. The human circadian metabolome. Proc Natl Acad Sci U S A 2012;109:2625–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim K, Mall C, Taylor SL, et al. Mealtime, temporal, and daily variability of the human urinary and plasma metabolomes in a tightly controlled environment. PLoS One 2014;9:e86223–e86223 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.