Metaplasia in the stomach is associated with tissue atrophy, and loss of acid-secreting parietal cells is thought to trigger the development of a metaplastic mucous cell lineage that expresses high levels of TFF2 (spasmolytic polypeptide). Spasmolytic polypeptide-expressing metaplasia (SPEM) is linked to intestinal-type gastric adenocarcinoma and is also observed in numerous mouse models of parietal cell atrophy. Because SPEM may be the precursor to intestinal metaplasia and cancer, there has been intense interest in understanding how it arises. Genetic mouse models have been used to identify the cell-of-origin by lineage tracing, with starkly different conclusions as to whether SPEM arises from a mature, differentiated cell or a stem/progenitor cell, even when the same genetic strains were studied1, 2 (Figure 1). The Mills laboratory has addressed this controversy in a recent report by using a different experimental approach to provide compelling evidence that SPEM arises from zymogenic chief cell transdifferentiation and not from a proliferative progenitor (Radyk et al., Gastroenterology in press).
Figure 1.
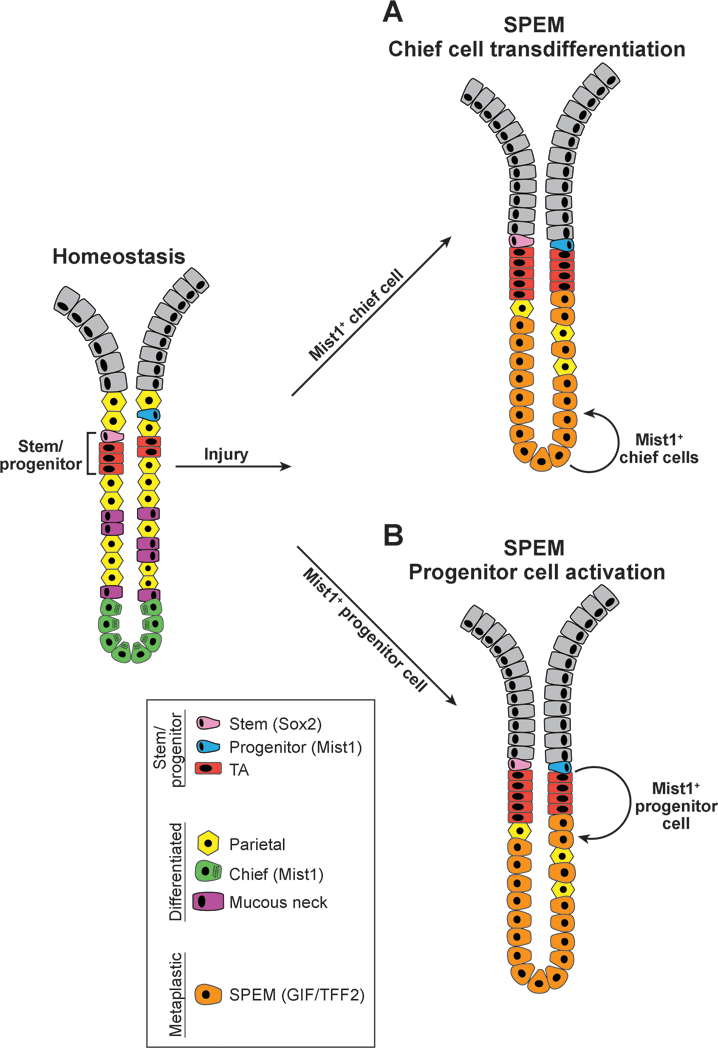
Competing theories for the cellular origin of Spasmolytic Polypeptide-Expressing Metaplasia (SPEM) in the stomach. Schematic representation of gastric chief cell-derived (A) vs. progenitor cell-derived (B) SPEM. Under homeostatic conditions (left), the gastric corpus is maintained by actively cycling stem and progenitor cells that proliferate and differentiate into mature epithelial cell types. After injury that results in parietal cell loss and gastric atrophy, such as observed with Helicobacter pylori infection or tamoxifen treatment, corpus glands undergo cellular remodeling with loss of chief cells and rapid development of the metaplastic cell lineage termed SPEM. The cellular source of SPEM has been controversial due to differing interpretations of genetic lineage tracing data from analysis of Mist1-CreERT2 mice. In model A, SPEM is derived from transdifferentiation of mature MIST1+ chief cells. In model B, SPEM is derived from a slowly cycling MIST1+ progenitor cell that expands in response to injury. The Mills laboratory used a non-lineage tracing method to support Model A that SPEM arises from differentiated chief cells rather than a progenitor cell.
Mouse models have been useful tools to study gastric metaplasia, with both chronic (Helicobacter infection, autoimmune gastritis) and acute (DMP-777, L635, tamoxifen) methods of parietal cell loss promoting SPEM. Cre-lox-based lineage tracing has been used to study the cellular source for SPEM. Several Cre drivers have been shown to mark cells that trace into SPEM, including Mist1-CreERT23, Lgr5–2A-CreERT24, Troy-eGFP-ires-CreERT25, and eR1-CreERT26. Some of these Cre drivers have also been used to express oncogenic KrasG12D, which rapidly induces SPEM and subsequent intestinal-type gastric tumors, lending credence to the notion that SPEM can be a precursor to cancer4, 6–8.
The controversy over the cell-of-origin for SPEM emerged from different interpretations of lineage tracing experiments using Mist1-CreERT23, 7,8. MIST1 is a transcription factor expressed in mature chief cells as well as other cell types throughout the body whose main function is to secrete large amounts of protein.9 Thus, Mist1-CreERT2 is primarily expressed in zymogenic cells in the corpus gland base. The observation of rapid lineage tracing into SPEM with this Cre driver suggested that chief cells were the cell-of-origin for SPEM.3 A similar conclusion was reached in lineage tracing experiments using Lgr5–2A-CreERT2 and Troy-eGFP-ires-CreERT2 Cre drivers that are also expressed in subsets of zymogenic chief cells.4, 5 However, although it is indisputable that the primary site for Mist1 expression in the stomach is in differentiated chief cells, Mist1 mRNA has also been observed in rare, ill-defined cells in the progenitor region of the gastric corpus4, 8. Because very occasional long-lasting Mist1-CreERT2 lineage traces can be observed emerging from the isthmus region, another study considered whether a progenitor cell was the true cell-of-origin for SPEM.8 To test this, they used cell ablation techniques associated with lineage tracing to determine the effect of depletion of chief cells or progenitor cells, concluding that Mist1-expressing progenitors, and not chief cells, were the source of SPEM.8
To address this controversy, the Mills laboratory used a non-genetic approach to test whether a differentiated cell or a proliferating progenitor cell is the source of SPEM (Radyk et al., Gastroenterology 2018). The authors acutely induced SPEM with high-dose tamoxifen while effectively blocking proliferation with 5-fluorouracil and observed no effect on SPEM formation, suggesting that SPEM does not arise from a proliferating stem/progenitor cell. Further, careful morphometric analysis for specific differentiated cell types showed rapid SPEM formation from the gland base, with increased SPEM cell numbers tracking closely with decreases in chief cell numbers. They also identified hybrid cells showing features of both chief and SPEM cells, consistent with transitional cells. Additional analysis of cellular proliferation revealed increased numbers of proliferating cells after SPEM induction, including cells at the gland base. They concluded from this work that SPEM arises by transdifferentiation of mature chief cells. This group also examined human gastric adenocarcinoma samples and found that cells expressing SPEM markers primarily occurred at the base of glands and not in the isthmal stem cell zone, further suggesting that SPEM originates through zymogenic cell reprogramming and not via proliferation of a stem cell.
Cellular remodeling has been demonstrated in a variety of gastrointestinal tissues, often using inducible Cre drivers to mark non-proliferating cells to follow their fate after injury. In addition to gastric chief cells, facultative intestinal stem cells10, liver hepatocytes11 and pancreatic acinar cells12 have been demonstrated to serve as cellular sources to repair tissue damage. However, as highlighted above, there can be limitations to approaches that use Cre-lox-based labeling methods13. Cre drivers often exhibit inappropriate expression patterns, and lox-Stop-lox lineage markers have markedly different sensitivities to Cre recombination, which can confound the analysis. In addition, many Cre drivers are tamoxifen-regulated, which can result in tissue toxicity when the lineage trace is activated14. The current study (Radyk et al., Gastro in press) avoided these limitations by using a non-lineage-tracing method to address the cell-of-origin of SPEM. Their conclusions support the majority of the earlier Cre-lox-based lineage tracing studies, confirming that chief cells and not proliferating progenitors are the source of SPEM.
Cellular plasticity is an intriguing facet of gastrointestinal cells. In response to injury, mature cell types can reprogram to promote tissue repair and regeneration. The cellular remodeling is characterized by loss of differentiated cellular features and development of new lineages by transdifferentiation or de-differentiation, depending on the tissue context.15 The newly programmed cells have the potential for expansion and differentiation to repair the damage. Importantly, newly programmed cells can also convert into proliferating, regenerative cells. Understanding the molecular mechanisms regulating and driving cellular reprogramming in adult tissues in response to injury will be key to understanding the basic signaling pathways, epigenetic mechanisms, transcription factors and downstream effectors involved in tissue restitution. In turn, this information may identify novel therapeutic approaches for repair of gastrointestinal tissues.
Several future questions can be considered for further study of gastric tissue repair. What epigenetic mechanisms are used to reprogram differentiated cells to form metaplasia and allow cellular proliferation? What are the signals/cues initiating SPEM, and regulating persistence as a permanent cellular metaplasia vs. a transient cellular response to acute injury? Finally, do SPEM progenitors require inflammation or continued injury to induce proliferation and permanence?
Acknowledgments
Grant Support: E.S.H. is supported by an AACR-DDF Career Development Award and NIH K01-DK-111710. L.C.S has research support through NIH P01-DK062041 and NIH R01-DK096972.
Footnotes
The authors declare no conflicts of interest.
References:
- 1.Mills JC, Goldenring JR. Metaplasia in the Stomach Arises From Gastric Chief Cells. Cell Mol Gastroenterol Hepatol 2017;4:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayakawa Y, Fox JG, Wang TC. The Origins of Gastric Cancer From Gastric Stem Cells: Lessons From Mouse Models. Cell Mol Gastroenterol Hepatol 2017;3:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nam KT, Lee HJ, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 2010;139:2028–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leushacke M, Tan SH, Wong A, et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol 2017;19:774–786. [DOI] [PubMed] [Google Scholar]
- 5.Stange DE, Koo BK, Huch M, et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 2013;155:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuo J, Kimura S, Yamamura A, et al. Identification of Stem Cells in the Epithelium of the Stomach Corpus and Antrum of Mice. Gastroenterology 2017;152:218–231. [DOI] [PubMed] [Google Scholar]
- 7.Choi E, Hendley AM, Bailey JM, et al. Expression of Activated Ras in Gastric Chief Cells of Mice Leads to the Full Spectrum of Metaplastic Lineage Transitions. Gastroenterology 2016;150:918–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayakawa Y, Ariyama H, Stancikova J, et al. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell 2015;28:800–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo HG, Jin RU, Sibbel G, et al. A single transcription factor is sufficient to induce and maintain secretory cell architecture. Genes Dev 2017;31:154–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buczacki SJ, Zecchini HI, Nicholson AM, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 2013;495:65–69. [DOI] [PubMed] [Google Scholar]
- 11.Yanger K, Zong Y, Maggs LR, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev 2013;27:719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strobel O, Dor Y, Alsina J, et al. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology 2007;133:1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demitrack ES, Samuelson LC. Notch as a Driver of Gastric Epithelial Cell Proliferation. Cell Mol Gastroenterol Hepatol 2017;3:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huh WJ, Khurana SS, Geahlen JH, et al. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 2012;142:21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills JC, Sansom OJ. Reserve stem cells: Differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal 2015;8:re8. [DOI] [PMC free article] [PubMed] [Google Scholar]


