Abstract
Objective
Tofacitinib is an oral JAK inhibitor for the treatment of rheumatoid arthritis (RA). This study was undertaken to evaluate the risk of major adverse cardiovascular events (MACE) in patients with RA receiving tofacitinib.
Methods
Data were pooled from patients with moderately to severely active RA receiving ≥1 tofacitinib dose in 6 phase III and 2 long‐term extension studies over 7 years. MACE (myocardial infarction, stroke, cardiovascular death) were independently adjudicated. Cox regression models were used to evaluate associations between baseline variables and time to first MACE. Following 24 weeks of tofacitinib, changes in variables and time to future MACE were evaluated after adjusment for age, baseline values, and time‐varying tofacitinib dose. Hazard ratios and 95% confidence intervals were calculated.
Results
Fifty‐two MACE occurred in 4,076 patients over 12,873 patient‐years of exposure (incidence rate 0.4 patients with events per 100 patient‐years). In univariable analyses of baseline variables, traditional cardiovascular risk factors and glucocorticoid and statin use were associated with MACE risk; disease activity and inflammation measures were not. In subsequent multivariable analyses, baseline age, hypertension, and the total cholesterol to high‐density lipoprotein (HDL) cholesterol ratio remained significantly associated with risk of MACE. After 24 weeks of treatment, an increase in HDL cholesterol and a decrease in the total to HDL cholesterol were associated with decreased MACE risk; changes in total cholesterol, low‐density lipoprotein (LDL) cholesterol, and disease activity measures were not. Increased erythrocyte sedimentation rates trended with increased future MACE risk.
Conclusion
In this post hoc analysis, after 24 weeks of tofacitinib treatment, increased HDL cholesterol, but not increased LDL cholesterol or total cholesterol, appeared to be associated with lower future MACE risk. Further data are needed to test the cardiovascular safety of tofacitinib.
Introduction
Cardiovascular disease (CVD) is one of the most common comorbidities in patients with rheumatoid arthritis (RA), with a prevalence of 9.3% for any CV event 1. Compared with the general population, patients with RA have an increased risk of CVD 2 and higher rates of CVD‐induced mortality 3. As a consequence, CVD is the leading cause of death in patients with RA, accounting for almost 31% of mortality 4.
Traditional CV risk factors, such as hypertension, smoking, and type 2 diabetes mellitus, contribute to the increased risk of CVD among patients with RA as they do in the general population 5, 6; however, after adjustment for traditional risk factors, a proportion of the increased CV risk in patients with RA remains unexplained 7. A key driver of increased CV risk in RA appears to be the high systemic inflammatory burden. There is an apparent inverse relationship between inflammation and lipid levels in patients with RA, such that increased inflammation is associated with reduced lipid levels and also with changes in the composition of lipid profiles 8.
The relationship between lipid levels and CV risk in patients with RA is complex. Within the general population, an increased risk of CVD is associated with high serum total cholesterol, high serum low‐density lipoprotein (LDL) cholesterol, and low serum high‐density lipoprotein (HDL) cholesterol levels 9, 10. Individuals with an elevated total cholesterol to HDL cholesterol ratio have an increased risk of developing CVD, and the total cholesterol to HDL cholesterol ratio has been shown to be a reliable predictor of CVD risk 11. In contrast, in patients with active RA, increased risk of CVD has been associated with relatively lower levels of serum total cholesterol and LDL cholesterol, as well as HDL cholesterol. These lower lipid levels may be driven by inflammation associated with RA 12, 13. In RA, changes are also seen in the composition and function of HDL particles; previous studies have shown that active disease is associated with impaired antioxidative function of HDL, decreased HDL‐mediated cholesterol efflux, and alterations in the levels and function of several HDL‐associated proteins 14, 15, 16.
Overall CV risk in patients with RA is affected by disease activity and likely by the resultant systemic inflammation. In a previous study by Maradit‐Kremers et al, high clinical disease activity, as measured by 3 erythrocyte sedimentation rates (ESRs) of ≥60 mm/hour, correlated with a 2‐fold increased risk of death from CVD in an inception cohort of 603 patients 17. In the same cohort of 172 patients who developed congestive heart failure (CHF), the proportion of patients with an ESR of ≥40 mm/hour was highest during the 6‐month period immediately preceding CHF onset 18. Other studies have demonstrated associations of markers of inflammation with subclinical atherosclerosis 19, 20. Finally, in a post hoc analysis of data from patients with RA treated with the interleukin‐6 (IL‐6) receptor inhibitor tocilizumab, increases in disease activity measures, such as the Disease Activity Score in 28 joints (DAS28) score 22 and joint counts, were associated with risk of future major adverse CV events (MACE), while changes in lipid levels were not 22.
It is unclear whether there are differential effects of RA therapeutics on CV outcomes. In previous studies, treatment with disease‐modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX) and tumor necrosis factor inhibitors (TNFi), has been shown to reduce the probability of CV events 23, 24. In the ENTRACTE study, which evaluated CV events in patients receiving tocilizumab versus etanercept, 83 MACE occurred over 4,900 patient‐years in the tocilizumab arm, compared with 78 MACE over 4,891 patient‐years in the etanercept arm (hazard ratio 1.05 [95% confidence interval 0.77–1.43]) 25. Taken together, these findings highlight the importance of investigating CV risk factors in patients with RA, as well as the effects of therapies for RA on the risk of MACE.
Tofacitinib is an oral JAK inhibitor for the treatment of RA. Formal comparison of the effect of tofacitinib on the risk of MACE is currently being investigated in a phase IIIb/IV prospective comparative study with TNFi (NCT02092467) 26. MACE is also being evaluated in patients receiving tofacitinib versus biologic DMARDs and conventional synthetic DMARDs in a real‐world setting using data from the Corrona registry and the following European registries: British Society for Rheumatology Biologics Register (BSRBR), Anti‐Rheumatic Therapy in Sweden (ARTIS), Rheumatoide Arthritis: Beobachtung der Biologika‐Therapie (RABBIT), and Base de Datos de Productos Biológicos de la Sociedad Española de Reumatología (BIOBADASER). However, during phase II studies, tofacitinib treatment was associated with increased LDL cholesterol and HDL cholesterol levels in patients with RA 27, 28, 29, 30, 31. Consequently, phase III and long‐term extension (LTE) studies included adjudication of potential CV events and deaths. In a pooled analysis of phase III data, tofacitinib treatment was associated ~10–20% of the increases in total cholesterol, HDL cholesterol, and LDL cholesterol levels from baseline to week 4, which were maintained to week 24. Changes in lipid levels stabilized after 12 weeks of tofacitinib treatment, and this was associated with a low incidence of CV events 32. The objective of the present post hoc analysis was to determine whether changes in lipids levels following administration of tofacitinib were associated with an increased risk of MACE in patients with RA enrolled in phase III and LTE studies.
Patients and methods
Design of phase III and LTE studies
Patients with RA participated in 1 of 6 randomized, double‐blind phase III studies and/or 2 open‐label LTE studies (for study names and details, see Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40911/abstract). Inclusion and exclusion criteria have been previously described 33, 34, 35, 36, 37, 38, 39, 40, 41. Briefly, patients were age ≥18 years, with active RA that fulfilled the American College of Rheumatology 1987 criteria (), and had active disease at screening and baseline.
All studies were conducted in compliance with the Declaration of Helsinki and the Good Clinical Practice Guidelines established by the International Conference for Harmonisation. The study protocols were approved by the institutional review board or independent ethics committee at each center. All patients provided written informed consent.
Two of the phase III studies evaluated tofacitinib 5 and 10 mg twice daily as monotherapy: ORAL Solo (NCT00814307), a 6‐month study of tofacitinib versus placebo in patients with an inadequate response to DMARDs 34 and ORAL Start (NCT01039688), a 24‐month study of tofacitinib versus MTX in MTX‐naive patients (37).
The remaining 4 phase III studies evaluated tofacitinib 5 and 10 mg twice daily in combination with conventional synthetic DMARDs versus placebo, as follows: ORAL Scan (NCT00847613), a 24‐month study of tofacitinib in patients with an inadequate response to MTX who were receiving background MTX 37; ORAL Standard (NCT00853385), a 12‐month study in patients with an inadequate response to MTX who were receiving background MTX 38; ORAL Sync (NCT00856544), a 12‐month study of tofacitinib in combination with conventional synthetic DMARDs in patients with an inadequate response to DMARDs 35; and ORAL Step (NCT00960440), a 6‐month study in patients with an inadequate response to TNFi who were receiving background MTX 33.
The 2 open‐label LTE studies (ORAL Sequel; NCT00413699 [database not locked at the time of analysis] and NCT00661661) enrolled patients who had completed phase I, phase II, or phase III index studies of tofacitinib. Regardless of treatment assignment in the qualifying index study, patients began treatment in the LTE studies with tofacitinib 5 or 10 mg twice daily (with the exception of Chinese and Japanese patients who received 5 mg twice daily per protocol), and were subsequently allowed to switch doses 39, 40, 41. In all studies, adverse events were recorded verbatim by the investigator and coded according to the Medical Dictionary for Regulatory Activities (MedDRA), version 13.0.
Post hoc analyses
The current post hoc analyses included all patients with RA who received at least 1 dose of tofacitinib 5 or 10 mg twice daily in the phase III and LTE studies and had exposure after week 24 (patients who had MACE before week 24, or who had withdrawn or completed the study before week 24 were excluded). As patients in the LTE studies were allowed to switch doses, patients were assigned into 5 or 10 mg twice daily treatment groups based on their average total daily dose (TDD; calculated by adding all doses received by each patient, and dividing by the number of days a dose was received). Patients were assigned to the 5 mg twice daily group if the TDD was <15 mg/day, and to the 10 mg twice daily group if it was ≥15 mg/day 43.
MACE, defined as any myocardial infarction (MI), cerebrovascular event (stroke), or CV death (defined as death caused by coronary, cerebrovascular, or cardiac events), were identified during the assessment of safety end points during the phase III and LTE studies. Patients were evaluated until withdrawal from the study, completion of the study, or the initial occurrence of MACE, whichever occurred first. In the event of multiple occurrences of MACE, only the first was counted.
Only adjudicated events were included in the analysis. Adjudication of MACE started in October 2009 with the chartering of the CV Safety Event Adjudication Committee (CVSEAC). Events reported prior to October 2009 were not adjudicated and are therefore not included in this analysis. The CVSEAC was retired in November 2013 and a new committee, the CV Event Adjudication Committee, was established. Both committees comprised independent, external experts in the fields of CV and/or neurovascular disease.
Statistical analysis
Baseline demographic and disease characteristics were summarized descriptively for patients with and without MACE. Cox regression models were used to evaluate the associations between baseline (pre‐tofacitinib) covariate values and time to first MACE, in univariable analyses (each covariate assessed singly) (Figure 1) and multivariable analysies (several covariates included together in 1 model) (Figures 2 and 3). The covariates included in the multivariable analysis were age, history of hypertension, total cholesterol to HDL cholesterol ratio, baseline body mass index (BMI), and time‐varying tofacitinib dosage (Figure 2). A second multivariable analysis was carried out using the same variables, but included history of diabetes mellitus instead of baseline BMI. A final model was selected via backward elimination with stay criteria at 15%. Associations were expressed as hazard ratios and 95% confidence intervals.
Figure 1.
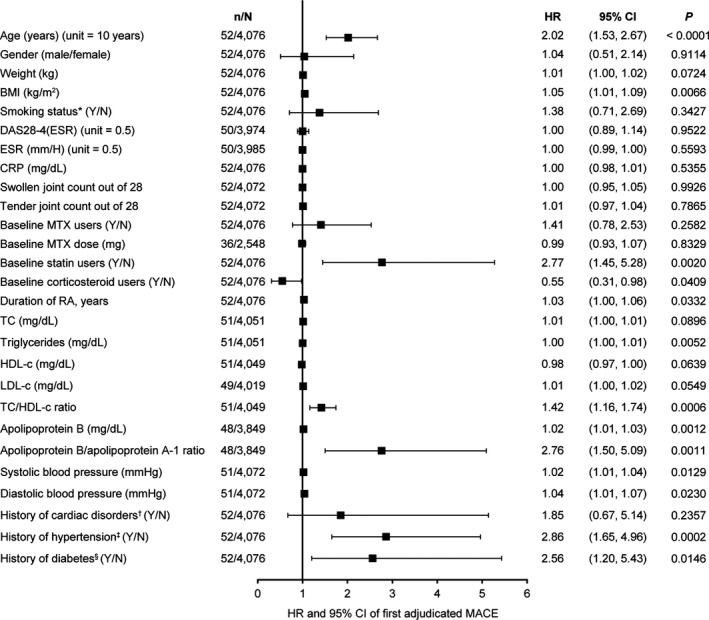
Univariable analyses of associations between baseline variables and the occurrence of major adverse cardiovascular events (MACE) during tofacitinib treatment. For all continuous variables, unit = 1 unless specified otherwise; in unit = x, “x” is the change in the continuous variable corresponding to which the change in hazards is observed. * = smoking status as recorded at baseline; † = including any of the Medical Dictionary for Regulatory Activities–coded terms: angina pectoris, arrhythmia, atrial flutter, atrial fibrillation, first‐degree atrioventricular block, left bundle branch block, cardiac failure, cardiac failure congestive, cardiac valve disease, cardiomegaly, cardiomyopathy, coronary artery disease, hypertensive cardiomyopathy, left ventricular hypertrophy, mitral valve incompetence, mitral valve prolapse, valve prolapse, myocardial infarction, palpitations, sinus bradycardia, sinus tachycardia, tachycardia, tachycardia paroxysmal, tricuspid valve incompetence, and ventricular extrasystole; ‡ = based on prior medical history; § = based on both use of diabetes medication and medical history. n = number of patients with future MACE for each baseline variable; N = number of patients included in the analysis for each baseline variable; HR = hazard ratio; 95% CI = 95% confidence interval; BMI = body mass index; DAS28‐4 (ESR) = 4‐variable Disease Activity Score in 28 joints using the erythrocyte sedimentation rate; CRP = C‐reactive protein; MTX = methotrexate; RA = rheumatoid arthritis; TC = total cholesterol; HDL‐c = high‐density lipoprotein cholesterol; LDL‐c = low‐density lipoprotein cholesterol.
Figure 2.
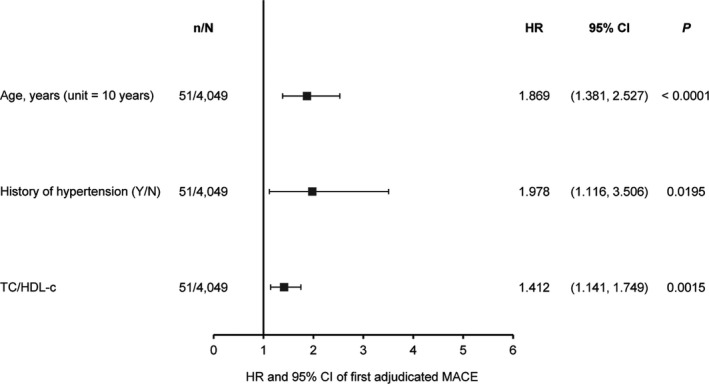
Multivariable analyses of associations between baseline variables and the occurrence of MACE during tofacitinib treatment. For all continuous variables, unit = 1 unless specified; in unit = x, “x” is the change in the continuous variable corresponding to which the change in hazards is observed. Only variables with significant associations with occurrence of MACE are shown; this analysis also included baseline body mass index and time‐varying tofacitinib dosage (both not significant). See Figure 1 for definitions.
Figure 3.
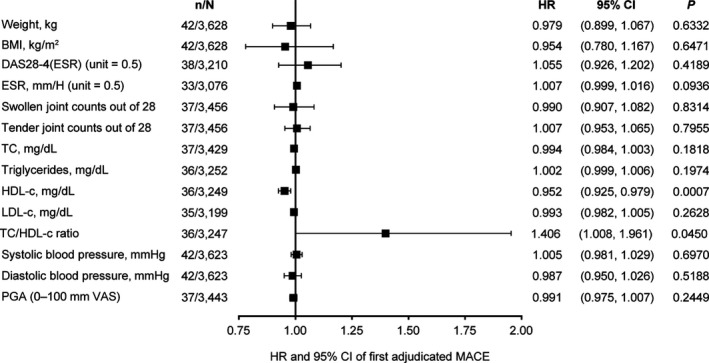
Multivariable analyses of associations between changes in variables after 24 weeks of tofacitinib treatment and the risk of future MACE. For each variable listed, a Cox regression model was fit, with change in the variable at week 24, the variable at baseline, age at baseline, and time‐varying dosage as predictors. In this model, only patients with exposure after week 24 were considered (i.e., patients who had MACE before week 24 or who had withdrawn or completed the study by week 24 were excluded). Patients with missing data for the week‐24 variable were excluded from the analysis of that variable (no imputation method). The HR corresponds to increased risk of MACE per 1‐unit increase in the parameter. n = number of patients with future MACE for each predictor; N = number of patients included in the analysis for each predictor; PGA = patient global assessment; VAS = visual analog scale (see Figure 1 for other definitions).
Cox regression models were also used to separately evaluate the associations between changes in the predictors of MACE from baseline to week 24 and the time to future development of MACE (defined as the first occurrence of MACE after 24 weeks), after adjustment for age, baseline values of covariates, and time‐varying tofacitinib dosage in a multivariable analysis (Figure 3). For each risk factor, the analysis included age, baseline value, and change from baseline to week 24; other risk factors were not included in the same model.
The time‐varying tofacitinib dosage was a time‐dependent covariate, and was determined by the time of first onset of MACE. If the first onset of MACE occurred during the index study, the randomized dosage in the index study was used. If the first onset of MACE occurred during the LTE study, then the average of the tofacitinib dosage (5 or 10 mg twice daily) was used.
For all analyses of covariates, unadjusted P values less than or equal to 0.05 were considered significant. No multiplicity adjustment was carried out, as this was a post hoc analysis for exploratory purposes.
Results
Tofacitinib exposure and patient disposition
The analysis population included 4,076 patients, representing a total of 12,932 patient‐years of tofacitinib exposure. In total, 52 patients had adjudicated MACE, resulting in a total of 12,873 patient‐years of exposure for the event and an incidence rate of 0.4 patients with events per 100 patient‐years of exposure (95% confidence interval 0.3–0.5), as of March 2015. There were 12 cases of CV death (cardiac death, n = 8; cerebrovascular death, n = 2; noncardiac/other vascular death, n = 2 [acute cardiac failure, n = 1; cerebral hemorrhage, n = 1]), 19 cases of nonfatal MI, and 23 cases of nonfatal stroke. Two patients had multiple categories of MACE (1 patient had a nonfatal stroke and a nonfatal MI, and 1 patient had a nonfatal stroke, followed by cerebrovascular death).
The baseline demographic characteristics of patients with and without adjudicated MACE are shown in Table 1. Overall, compared with patients without MACE, patients with MACE were older (mean age 52.7 years versus 60.2 years), had a higher mean BMI (27.0 versus 29.2 kg/m2), were more likely to have a history of diabetes mellitus (7.6% versus 15.4%) or hypertension (33.7% versus 57.7%), and were more likely to be receiving concomitant statins (10.4% versus 23.1%).
Table 1.
Baseline demographic characteristics of patients with and patients without MACEa
| Characteristic | No MACE (n = 4,024) | Adjudicated MACE (n = 52) |
|---|---|---|
| Age, mean ± SD years | 52.7 ± 11.9 | 60.2 ± 10.4 |
| Female, no. (%) | 3,334 (82.9) | 43 (82.7) |
| BMI, mean ± SD kg/m2 | 27.0 ± 6.4 | 29.2 ± 8.2 |
| History of CHD, no. (%) | 21 (0.5) | 0 (0.0) |
| History of cardiac disorders, no. (%)b | 199 (4.9) | 4 (7.7) |
| History of diabetes mellitus, no. (%)c | 307 (7.6) | 8 (15.4) |
| Abnormal BP, no. (%)d | 334 (8.3) | 4 (7.7) |
| History of hypertension, no. (%) | 1,358 (33.7) | 30 (57.7) |
| Smoking status, no. (%) | ||
| Never | 676 (16.8) | 14 (26.9) |
| Current | 678 (16.8) | 11 (21.2) |
| Ex‐smokere | 2,667 (66.3) | 27 (51.9) |
| Concomitant medications | ||
| Glucocorticoids, no. (%) | 1,909 (47.4) | 18 (34.6) |
| Statins, no. (%) | 420 (10.4) | 12 (23.1) |
| NSAIDs, no. (%) | 2,817 (70.0) | 34 (65.4) |
| MTX, no. (%) | 2,443 (60.7) | 36 (69.2) |
| MTX dose, mean ± SD mg | 15.0 ± 4.7 | 14.9 ± 4.0 |
MACE = major adverse cardiovascular event; BMI = body mass index; CHD = coronary heart disease; NSAIDs = nonsteroidal antiinflammatory drugs; MTX = methotrexate.
Including any of the Medical Dictionary for Regulatory Activities–coded terms: angina pectoris, arrhythmia, atrial flutter, atrial fibrillation, first‐degree atrioventricular block, left bundle branch block, cardiac failure, cardiac failure congestive, cardiac valve disease, cardiomegaly, cardiomyopathy, coronary artery disease, hypertensive cardiomyopathy, left ventricular hypertrophy, mitral valve incompetence, mitral valve prolapse, valve prolapse, myocardial infarction, palpitations, sinus bradycardia, sinus tachycardia, tachycardia, tachycardia paroxysmal, tricuspid valve incompetence, and ventricular extrasystole.
Based on both use of diabetes medication and medical history.
Defined as systolic blood pressure (BP) of >150 mm Hg or diastolic BP of >90 mm Hg.
Defined as those who had smoked previously but were not smokers at baseline.
The baseline disease characteristics of patients with and without adjudicated MACE are shown in Table 2. Compared with patients without MACE, patients with MACE had a longer mean disease duration (7.7 years versus 10.1 years), slightly higher mean total cholesterol levels (198.3 versus 208.2 mg/dl), LDL cholesterol levels (113.9 versus 123.3 mg/dl), total cholesterol to HDL cholesterol ratio (3.5 versus 4.0), and triglyceride levels (125.3 versus 152.1 mg/dl), and slightly lower HDL cholesterol levels (59.4 versus 55.3 mg/dl) at baseline.
Table 2.
Baseline disease characteristics in patients with and patients without MACEa
| Characteristic | No MACE (n = 4,024) | Adjudicated MACE (n = 52) |
|---|---|---|
| Duration of RA, years | 7.7 ± 7.9 | 10.1 ± 8.8 |
| 4‐variable DAS28‐ESR | 6.3 ± 1.1 | 6.3 ± 1.3 |
| Tender joint count | 14.1 ± 7.3 | 14.3 ± 7.5 |
| Swollen joint count | 10.4 ± 5.6 | 10.3 ± 5.9 |
| Total cholesterol, mg/dl | 198.3 ± 42.1 | 208.2 ± 48.9 |
| HDL cholesterol, mg/dl | 59.4 ± 16.9 | 55.3 ± 16.0 |
| LDL cholesterol, mg/dl | 113.9 ± 34.2 | 123.3 ± 43.2 |
| Total cholesterol to HDL cholesterol ratio | 3.5 ± 1.1 | 4.0 ± 1.5 |
| Triglycerides, mg/dl | 125.3 ± 72.6 | 152.1 ± 86.9 |
| Apolipoprotein A‐1, mg/dl | 153.6 ± 31.2 | 149.4 ± 27.8 |
| Apolipoprotein B, mg/dl | 94.4 ± 24.7 | 105.8 ± 29.4 |
| CRP, mg/dl | 17.1 ± 22.7 | 15.7 ± 16.9 |
| ESR, mm/hour | 50.4 ± 26.9 | 47.9 ± 23.8 |
Values are the mean ± SD. MACE = major adverse cardiovascular event; RA = rheumatoid arthritis; DAS28‐ESR = Disease Activity Score in 28 joints using the erythrocyte sedimentation rate; HDL = high‐density lipoprotein; LDL = low‐density lipoprotein; CRP = C‐reactive protein; ESR = erythrocyte sedimentation rate.
Baseline predictors of MACE
In univariable analyses, risk of MACE was significantly associated with older age, higher BMI, statin use, or longer duration of RA. Other baseline predictors that were associated with a significantly increased risk of MACE were elevated levels of triglycerides, higher total cholesterol to HDL cholesterol ratio, elevated apolipoprotein B (Apo B) levels, higher Apo B to Apo A‐1 ratio, abnormal blood pressure, history of hypertension, and history of diabetes mellitus (Figure 1).
In contrast, glucocorticoid use was associated with a significantly lower risk of MACE (mean ± SD glucocorticoid dosage at baseline 3.5 ± 4.4 and 3.3 ± 6.4 mg/day for patients receiving tofacitinib 5 mg and 10 mg twice daily, respectively, and 3.9 ± 4.1 and 3.4 ± 3.9 mg/day for patients receiving placebo who advanced to tofacitinib 5 mg and 10 mg twice daily, respectively). Baseline disease activity and inflammation measures, including the 4‐variable DAS28 using the ESR 21, ESR and C‐reactive protein level, and swollen and tender joint counts, were not significantly associated with MACE risk (Figure 1).
In separate multivariable analyses, patient age, history of hypertension, and the total cholesterol to HDL cholesterol ratio continued to be significantly associated with an increased risk of MACE (Figure 2). The findings of the multivariable analyses were consistent across all selection methods used (backward, forward, and stepwise selection). Time‐varying tofacitinib dosage and baseline BMI were not found to be associated with risk of MACE in this analysis. Furthermore, in a second multivariable analysis that included history of diabetes mellitus in place of baseline BMI, history of diabetes mellitus was also not associated with MACE risk.
Changes in predictors of MACE between baseline and week 24
Previously, tofacitinib treatment was found to be associated with increases in total cholesterol, HDL cholesterol, and LDL cholesterol levels 32, and baseline and week 24 values for the other covariates included in the analysis are shown in Supplementary Table 2 (available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40911/abstract). The effects of 24 weeks of treatment with tofacitinib (and tofacitinib‐associated increases in lipid levels) on the risk of future MACE were assessed by multivariable analyses (Figure 3). After adjustment for age, time‐varying tofacitinib dosage, and the baseline value for each variable, increases in HDL cholesterol levels and decreases in the total cholesterol to HDL cholesterol ratio after 24 weeks of tofacitinib were associated with a reduced risk of MACE. In contrast, increases in total cholesterol levels and LDL cholesterol levels were not associated with increased risk of future MACE. A trend was observed between increases in ESR after 24 weeks of tofacitinib treatment and an increase in the future risk of MACE, although this did not reach statistical significance. Changes in disease activity measures, such as the 4‐variable DAS28 using the ESR, and swollen and tender joint counts, were not associated with increased risk of future MACE.
Discussion
In this post hoc analysis of data from phase III and LTE studies of tofacitinib, we assessed the associations between baseline variables and time to first MACE as well as the changes in variables after 24 weeks of tofacitinib treatment and time to future MACE.
In univariable analyses of baseline variables, an increased risk of MACE was associated with the presence of traditional risk factors at baseline (such as older age, higher BMI, abnormal blood pressure, and history of either hypertension or diabetes mellitus), elevated baseline triglyceride and Apo B levels, and higher baseline Apo B to Apo A‐1 ratio and total cholesterol to HDL cholesterol ratio. In subsequent multivariable analysis of baseline measures, age, history of hypertension, and the total cholesterol to HDL cholesterol ratio continued to be associated with an increased risk of MACE.
The analyses of baseline variables in this study are consistent with large population studies of CV outcomes, reinforcing the finding that traditional CV risk factors are important to CV risk in patients with RA 17. They also reinforce the findings from previous studies that have demonstrated the association between MACE risk and both traditional risk factors and Apo B to Apo A‐1 and total cholesterol to HDL cholesterol ratios in patients with RA 22, 44. In addition, the European League Against Rheumatism guidelines on management of CV risk in patients with RA suggest that the total cholesterol to HDL cholesterol ratio is a particularly important indicator of CV risk 45.
Risk of MACE was not associated with measures of disease activity or inflammation at baseline in this analysis. A previous study by Rao et al, which investigated risk factors for MACE in patients with RA during treatment with tocilizumab, also showed no association between risk of MACE and baseline measures of inflammation, but did demonstrate an association between the risk of MACE and baseline disease activity measures 22.
The present analyses also suggest an association between baseline statin use and risk of future MACE. However, this observation was potentially due to confounding by indication, as patients with RA who had the highest CV risk as judged by their physicians were more likely to be taking a statin at baseline.
In a previous pooled analysis, which assessed lipid concentrations in the same patient population as the current analysis, increased total, HDL, and LDL cholesterol levels were observed in patients receiving tofacitinib 32. We evaluated the effects of 24 weeks of tofacitinib treatment on risk of MACE in multivariable analyses and found that increases in HDL cholesterol levels and decreases in the total cholesterol to HDL cholesterol ratio were associated with reduced risk of future MACE in multivariable analyses, while increases in total cholesterol and LDL cholesterol levels were not. Rao et al also found no association of CV risk with total cholesterol and LDL cholesterol levels following 24 weeks of tocilizumab treatment. However, in contrast with our findings, increases in HDL cholesterol were not associated with risk of MACE following tocilizumab treatment in their study 22.
Higher HDL cholesterol levels have previously been associated with a decreased risk of MI in patients with active RA 46, 47. However, cholesteryl ester transfer protein inhibitors, which also increase HDL cholesterol levels, have failed to confer consistent reductions in CV events in clinical trials in the general population 48. Therefore, the association between modulation of HDL cholesterol level and risk of MACE remains unresolved. Consequently, additional consideration should be given to mechanisms independent of increases in HDL cholesterol levels, such as improved HDL particle function, which has been linked to CV outcomes in the general population 49. Previous studies have suggested that tofacitinib may improve the function of HDL particles via increases in the activity of the HDL‐associated enzyme paraoxonase 1 50. In addition, during the MEASURE study, “normalization” of HDL particle composition was observed in a detailed analysis of lipoprotein subfractions following tocilizumab treatment 51, and a small‐scale study of rituximab in patients with RA showed a reduction in proatherogenic HDL particle composition 52. Furthermore, cholesterol ester fractional catabolism, which is higher in patients with active RA than in the general population, was reduced following tofacitinib treatment, and this reduction was also associated with improvements in HDL functional markers and correlated significantly with increased HDL cholesterol levels 53. Therefore, the effects of tofacitinib treatment on CV risk are likely to be multifactorial and may include changes in lipoproteins that are independent of cholesterol levels, such as changes in HDL composition and/or cholesterol ester fractional catabolism 53.
The present study also showed a trend toward an association of elevated ESR following tofacitinib treatment with an increased risk of future MACE, and this is consistent with findings in a population‐based study of CV death, which suggested that patients with RA who have sustained elevation of ESR may have a higher risk of CV death 17. One possible explanation for this higher risk is that increased ESR may be a surrogate of failure to respond. In contrast to these findings, Rao et al (22) did not demonstrate changes in ESR to be associated with future risk of MACE following tocilizumab treatment; additionally, increases in DAS28 scores and swollen and tender joint counts were associated with higher CV risk in their study but not in ours. The disparities between our results and the findings of Rao et al may be due to differences in the pharmacokinetics/pharmacodynamics of tocilizumab and tofacitinib as well as the overall small number of CV events in both trial programs. In addition, while IL‐6–induced JAK/STAT signal transduction as a driver of CV events is well established in atherosclerosis 54, inflammatory processes are myriad and complex, with both multiple upstream activators and diverse downstream targets in different cell types. Therefore, tocilizumab inhibition of IL‐6 and tofacitinib inhibition of JAK/STAT may have different effects in the downstream inflammation process and may, therefore, have contrasting effects on lipid levels.
This study had a number of limitations. The data were obtained from a post hoc analysis of data pooled from 6 phase III and 2 LTE studies that were not designed to evaluate future MACE risk. Consequently, the number of patients and exposure times in this study were low, as were the number of adjudicated MACE. The adjudication process changed in November 2013 from consensus‐based to blinded independent review, and there were also changes in the individuals conducting adjudication as well as changes from committee‐created definitions of events to Food and Drug Administration−approved definitions 55. These procedural changes may have introduced variables into the adjudication outputs. Also, several covariates, including disease activity and measures of inflammation, as well as time‐varying confounders such as hypertension, statin use, and glucocorticoids, were only assessed at baseline in the multivariable analyses and this may not be sufficient to predict CV events with this sample size. Furthermore, we recognize that while the backward elimination method is the simplest to implement and most intuitive to explain, it may not always identify the best subset of variables or covariates for retention in the model. In this analysis, consistent results were also observed with forward and stepwise elimination methods. In addition, this study did not evaluate HDL and LDL particle size, or HDL particle function. As a consequence of these limitations, direct interpretation of the results may be confounded.
In conclusion, in pooled analyses of tofacitinib‐treated patients, traditional CV risk factors at baseline appeared to be associated with an increased risk of future MACE, while no apparent association was observed between future risk of MACE and baseline disease activity or measures of inflammation. Following adjustment for age, baseline values, and time‐varying tofacitinib dosage, increases in HDL cholesterol and decreases in the total cholesterol to HDL cholesterol ratio were associated with reduced future MACE risk after 24 weeks of tofacitinib treatment, while increases in LDL cholesterol and total cholesterol were not associated with future MACE risk. Increases in ESR after 24 weeks of tofacitinib therapy may be associated with increased future MACE risk and, conversely, a decrease in inflammation as measured by ESR with tofacitinib may convey some CV protection. More data are needed to confirm these findings, which could be beneficial in future profiling of tofacitinib‐treated patients with RA who may be at greatest risk of MACE. The CV event safety of tofacitinib versus adalimumab or etanercept in patients with RA is currently being investigated in a phase IIIb/IV randomized open‐label study (A3921133; NCT02092467) 26.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Soma had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
DeMasi, Soma, Biswas.
Acquisition of data
Soma, Hwang, Biswas.
Analysis and interpretation of data
Charles‐Schoeman, DeMasi, Valdez, Soma, Hwang, Boy, Biswas, McInnes.
Role of the Study Sponsor
All authors interpreted the results, provided critical revision, approved the final draft, and had the final decision to submit the manuscript for publication. Pfizer Inc did not control the analysis or interpretation of the study results. Publication of this article was not contingent upon approval by Pfizer Inc. Medical writing support, under the guidance of the authors, was provided by Anthony G. McCluskey, PhD, at CMC Connect, a division of McCann Health Medical Communications Ltd and was funded by Pfizer Inc.
Supporting information
Supported by Pfizer Inc.
Dr. Charles‐Schoeman's work was supported by the NIH (National Heart, Lung, and Blood Institute grant HL‐123064).
Dr. Charles‐Schoeman has received consulting fees from Gilead, Pfizer Inc, and Regeneron‐Sanofi (more than $10,000 each) and has received research support from AbbVie, Bristol‐Myers Squibb, and Pfizer Inc. Drs. DeMasi, Valdez, Soma, Hwang, Boy, and Biswas own stock in Pfizer Inc. Dr. McInnes has received research support from AbbVie, Bristol‐Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer Inc, and Roche.
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de‐identified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices 1) for indications that have been approved in the US and/or EU, or 2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de‐identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data‐access agreement with Pfizer.
References
- 1. Naranjo A, Sokka T, Descalzo MA, Calvo‐Alén J, Hørslev‐Petersen K, Luukkainen RK, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST‐RA study. Arthritis Res Ther 2008;10:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avina‐Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta‐analysis of observational studies. Ann Rheum Dis 2012;71:1524–9. [DOI] [PubMed] [Google Scholar]
- 3. Aviña‐Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta‐analysis of observational studies. Arthritis Rheum 2008;59:1690–7. [DOI] [PubMed] [Google Scholar]
- 4. Young A, Koduri G, Batley M, Kulinskaya E, Gough A, Norton S, et al. Mortality in rheumatoid arthritis: increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology (Oxford) 2007;46:350–7. [DOI] [PubMed] [Google Scholar]
- 5. Dessein PH, Joffe BI, Veller MG, Stevens BA, Tobias M, Reddi K, et al. Traditional and nontraditional cardiovascular risk factors are associated with atherosclerosis in rheumatoid arthritis. J Rheumatol 2005;32:435–42. [PubMed] [Google Scholar]
- 6. Boyer JF, Gourraud PA, Cantagrel A, Davignon JL, Constantin A. Traditional cardiovascular risk factors in rheumatoid arthritis: a meta‐analysis. Joint Bone Spine 2011;78:179–83. [DOI] [PubMed] [Google Scholar]
- 7. Del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001;44:2737–45. [DOI] [PubMed] [Google Scholar]
- 8. Choy E, Ganeshalingam K, Semb AG, Szekanecz Z, Nurmohamed M. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology (Oxford) 2014;53:2143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile: a statement for health professionals. Circulation 1991;83:356–62. [DOI] [PubMed] [Google Scholar]
- 10. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2012;33:1635–701. [DOI] [PubMed] [Google Scholar]
- 11. Castelli WP. Cholesterol and lipids in the risk of coronary artery disease: the Framingham Heart Study. Can J Cardiol 1988;4 Suppl A:5A–10. [PubMed] [Google Scholar]
- 12. Myasoedova E, Crowson CS, Kremers HM, Fitz‐Gibbon PD, Therneau TM, Gabriel SE. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis 2010;69:1310–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robertson J, Peters MJ, McInnes IB, Sattar N. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat Rev Rheumatol 2013;9:513–23. [DOI] [PubMed] [Google Scholar]
- 14. Watanabe J, Charles‐Schoeman C, Miao Y, Elashoff D, Lee YY, Katselis G, et al. Proteomic profiling following immunoaffinity capture of high‐density lipoprotein: association of acute‐phase proteins and complement factors with proinflammatory high‐density lipoprotein in rheumatoid arthritis. Arthritis Rheum 2012;64:1828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Charles‐Schoeman C, Watanabe J, Lee YY, Furst DE, Amjadi S, Elashoff D, et al. Abnormal function of high‐density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum 2009;60:2870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charles‐Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis 2012;71:1157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maradit‐Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population‐based study. Arthritis Rheum 2005;52:722–32. [DOI] [PubMed] [Google Scholar]
- 18. Maradit‐Kremers H, Nicola PJ, Crowson CS, Ballman KV, Jacobsen SJ, Roger VL, et al. Raised erythrocyte sedimentation rate signals heart failure in patients with rheumatoid arthritis. Ann Rheum Dis 2007;66:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary‐artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum 2005;52:3045–53. [DOI] [PubMed] [Google Scholar]
- 20. Del Rincón I, Williams K, Stern MP, Freeman GL, O'Leary DH, Escalante A. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum 2003;48:1833–40. [DOI] [PubMed] [Google Scholar]
- 21. Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty‐eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 22. Rao VU, Pavlov A, Klearman M, Musselman D, Giles JT, Bathon JM, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol 2015;67:372–80. [DOI] [PubMed] [Google Scholar]
- 23. Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2010;49:295–307. [DOI] [PubMed] [Google Scholar]
- 24. Westlake SL, Colebatch AN, Baird J, Curzen N, Kiely P, Quinn M, et al. Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2011;50:518–31. [DOI] [PubMed] [Google Scholar]
- 25. Giles JT, Sattar N, Gabriel SE, Ridker PM, Gay S, Warne C, et al. Comparative cardiovascular safety of tocilizumab vs etanercept in rheumatoid arthritis: results of a randomized, parallel‐group, multicenter, noninferiority, phase 4 clinical trial [abstract]. Arthritis Rheumatol 2016;68 Suppl 10 URL: http://acrabstracts.org/abstract/comparative-cardiovascular-safety-of-tocilizumab-vs-etanercept-in-rheumatoid-arthritis-results-of-a-randomized-parallel-group-multicenter-noninferiority-phase-4-clinical-trial/;. [Google Scholar]
- 26. ClinicalTrials.gov . Safety study of tofacitinib versus tumor necrosis factor (TNF) inhibitor in subjects with rheumatoid arthritis. 2014. URL: https://clinicaltrials.gov/ct2/show/NCT02092467.
- 27. Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double‐blind, placebo‐controlled phase IIa trial of three dosage levels of CP‐690,550 versus placebo. Arthritis Rheum 2009;60:1895–905. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, and the Tofacitinib Study Investigators . Phase II study of tofacitinib (CP‐690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken) 2011;63:1150–8. [DOI] [PubMed] [Google Scholar]
- 29. Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, et al. Phase IIb dose‐ranging study of the oral JAK inhibitor tofacitinib (CP‐690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease‐modifying antirheumatic drugs. Arthritis Rheum 2012;64:617–29. [DOI] [PubMed] [Google Scholar]
- 30. Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez‐Reino J, et al. A phase IIb dose‐ranging study of the oral JAK inhibitor tofacitinib (CP‐690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 2012;64:970–81. [DOI] [PubMed] [Google Scholar]
- 31. Tanaka Y, Takeuchi T, Yamanaka H, Nakamura H, Toyoizumi S, Zwillich S. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12‐week, randomized, phase 2 study. Mod Rheumatol 2015;25:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Charles‐Schoeman C, Wicker P, Gonzalez‐Gay MA, Boy M, Zuckerman A, Soma K, et al. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum 2016;46:261–71. [DOI] [PubMed] [Google Scholar]
- 33. Burmester GR, Blanco R, Charles‐Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP‐690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 34. Fleischmann R, Kremer J, Cush J, Schulze‐Koops H, Connell CA, Bradley JD, et al. Placebo‐controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 35. Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin‐Mola E, et al. Tofacitinib in combination with nonbiologic disease‐modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 36. Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 37. Van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP‐690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve‐month data from a twenty‐four–month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 38. Van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 39. Wollenhaupt J, Lee EB, Curtis JR, Silverfield J, Terry K, Soma K, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open‐label, long‐term extension study. Arthritis Res Ther 2019;21:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wollenhaupt J, Silverfield J, Lee EB, Curtis JR, Wood SP, Soma K, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open‐label, long term extension studies. J Rheumatol 2014;41:837–52. [DOI] [PubMed] [Google Scholar]
- 41. Yamanaka H, Tanaka Y, Takeuchi T, Sugiyama N, Yuasa H, Toyoizumi S, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open‐label, long‐term extension study. Arthritis Res Ther 2016;18:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 43. Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, et al. Long‐term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liao KP, Liu J, Lu B, Solomon DH, Kim SC. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non–rheumatoid arthritis patients. Arthritis Rheumatol 2015;67:2004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28. [DOI] [PubMed] [Google Scholar]
- 46. Navarro‐Millán I, Yang S, DuVall SL, Chen L, Baddley J, Cannon GW, et al. Association of hyperlipidaemia, inflammation and serological status and coronary heart disease among patients with rheumatoid arthritis: data from the National Veterans Health Administration. Ann Rheum Dis 2016;75:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Andersson C, Lyass A, Vasan RS, Massaro JM, D'Agostino RB Sr, Robins SJ. Long‐term risk of cardiovascular events across a spectrum of adverse major plasma lipid combinations in the Framingham Heart Study. Am Heart J 2014;168:878–83. [DOI] [PubMed] [Google Scholar]
- 48. Kosmas CE, DeJesus E, Rosario D, Vittorio TJ. CETP inhibition: past failures and future hopes. Clin Med Insights Cardiol 2016;10:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gebhard C, Rhainds D, Tardif JC. HDL and cardiovascular risk: is cholesterol in particle subclasses relevant? [editorial]. Eur Heart J 2015;36:10–2. [DOI] [PubMed] [Google Scholar]
- 50. Charles‐Schoeman C, Gonzalez‐Gay MA, Kaplan I, Boy M, Geier J, Luo Z, et al. Effects of tofacitinib and other DMARDs on lipid profiles in rheumatoid arthritis: implications for the rheumatologist. Semin Arthritis Rheum 2016;46:71–80. [DOI] [PubMed] [Google Scholar]
- 51. McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD, et al. Effect of interleukin‐6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo‐controlled study. Ann Rheum Dis 2015;74:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Raterman HG, Levels H, Voskuyl AE, Lems WF, Dijkmans BA, Nurmohamed MT. HDL protein composition alters from proatherogenic into less atherogenic and proinflammatory in rheumatoid arthritis patients responding to rituximab. Ann Rheum Dis 2013;72:560–5. [DOI] [PubMed] [Google Scholar]
- 53. Charles‐Schoeman C, Fleischmann R, Davignon J, Schwartz H, Turner SM, Beysen C, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol 2015;67:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin‐6 and its signalling in atherosclerosis. Thromb Haemost 2009;102:215–22. [DOI] [PubMed] [Google Scholar]
- 55. Hicks KA, Hung HM, Mahaffey KW, Mehran R, Nissen SE, Stockbridge NL, et al, on behalf of the Standardized Data Collection for Cardiovascular Trials Initiative . Standardized definitions for cardiovascular and stroke endpoint events in clinical trials. 2014. URL: https://www.cdisc.org/system/files/all/standard/Draft%20Definitions%20for%20CDISC%20August%2020%2C%202014.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


