Abstract
Background
Early initiation of combination antiretroviral therapy (cART) for HIV-positive pregnant women can decrease vertical transmission to less than 5%. Programmatic barriers to early cART include decentralized care, disease-stage assessment delays, and loss to follow-up.
Intervention
Our intervention had 3 components: integrated HIV and antenatal services in 1 location with 1 provider, laboratory courier to expedite CD4 counts, and community-based follow-up of women–infant pairs to improve prevention of mother-to-child transmission attendance. Preintervention HIV-positive pregnant women were referred to HIV clinics for disease-stage assessment and cART initiation for advanced disease (CD4 count <350 cells/μL or WHO stage >2).
Methods
We used a quasi-experimental design with preintervention/postintervention evaluations at 6 government antenatal clinics (ANCs) in Southern Province, Zambia. Retrospective clinical data were collected from clinic registers during a 7-month baseline period. Postintervention data were collected from all antiretroviral therapy–naive, HIV-positive pregnant women and their infants presenting to ANC from December 2011 to June 2013.
Results
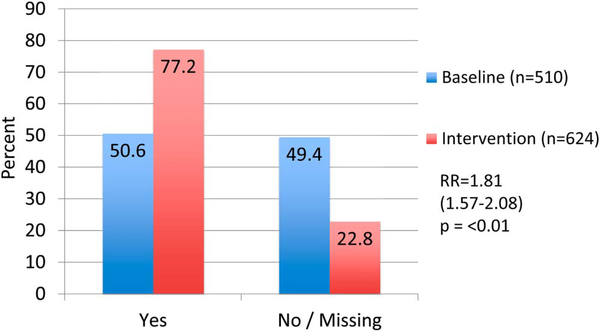
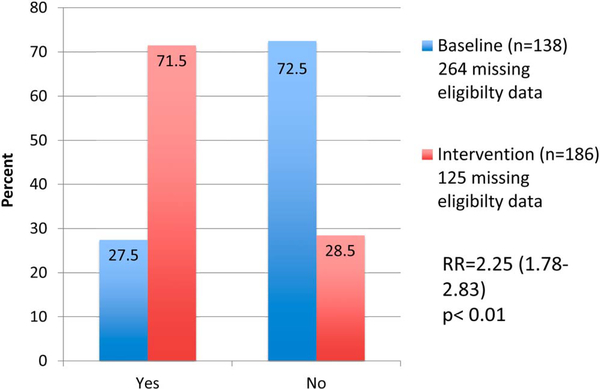
Data from 510 baseline women–infant pairs were analyzed and 624 pregnant women were enrolled during the intervention period. The proportion of HIV-positive pregnant women receiving CD4 counts increased from 50.6% to 77.2% [relative risk (RR) = 1.81; 95% confidence interval (CI): 1.57 to 2.08; P < 0.01]. The proportion of cART-eligible pregnant women initiated on cART increased from 27.5% to 71.5% (RR = 2.25; 95% CI: 1.78 to 2.83; P < 0.01). The proportion of eligible HIV-exposed infants with documented 6-week HIV PCR test increased from 41.9% to 55.8% (RR = 1.33; 95% CI: 1.18 to 1.51; P < 0.01).
Conclusions
Integration of HIV care into ANC and community-based support improved uptake of CD4 counts, proportion of cART-eligible women initiated on cART, and infants tested.
Keywords: HIV, AIDS, vertical transmission, prevention of mother-to-child transmission of HIV, integration, antenatal services, maternal and child health services, Option B+, community-based follow-up, task-shifting, attrition, loss to follow-up
INTRODUCTION
Prevention of mother-to-child transmission (PMTCT) of HIV has made significant progress in the past 2 decades, with transmission rates approaching <5% when pregnant women are initiated on combination antiretroviral therapy (cART) early in pregnancy and throughout breastfeeding.1,2 The WHO 2010 PMTCT guidelines recommended 2 options for pregnant women: Option A (cART if CD4 counts <350 cells/μL or WHO stage 3/4 and daily AZT monotherapy if CD4 >350 cells/μL plus daily nevirapine for breastfeeding infants) and Option B (cART for all HIV-positive women >14 weeks of gestation through breastfeeding plus daily nevirapine for infants through 6 weeks).3 Option A limitations include delays in obtaining initial CD4 assessment for cART initiation and frequent nevirapine redosing according to infant weight. Both options require mothers without advanced HIV disease to terminate cART at the end of breastfeeding, raising risk for antiretroviral therapy (ART) resistance, and loss to follow-up (LTFU).4,5 This concern led to “Option B+” in which lifelong cART is provided for all HIV-positive pregnant women and daily infant nevirapine for 6 weeks.6 Option B+ has been adopted by several sub-Saharan countries.7,8 Despite the intention to streamline programmatic barriers for cART initiation with Option B+, Malawi’s Option B+ program is witnessing higher rates of LTFU in the first 6 months,9 emphasizing the need for innovative delivery models that promote early cART uptake and retention.
Since 2004, Boston University PMTCT Integration Project (BUPIP) has provided technical assistance to the Zambian Ministry of Health’s (MOH) PMTCT programming for over 200 clinics in Southern Province. Given the program challenges of Option A and the witnessed LTFU in Option B + programs, we developed a pilot program that addresses both concerns. To improve PMTCT ART uptake and streamline cART initiation, we integrated antenatal and HIV care at 6 government antenatal clinics (ANCs) in Southern Province, Zambia under Option A guidelines. To address adherence and retention, each mother–infant pair was linked with a community-based lay counselor to promote adherence to PMTCT components [medication and revisit adherence, early infant diagnostic testing (EID)]. Zambia adopted Option B+ as policy in January 2013, although Option B+ was not operationalized until August 2014.8 Although this pilot was implemented under Option A, the approach discussed here provides a possible prototype for Option B+ service delivery.
METHODS
Study Site and Standard of Care
From December 1, 2011, to November 30, 2013, we implemented our intervention at 6 ANCs in Southern Province, Zambia. Pilot ANCs were located in Mazabuka District (n = 3) and Livingstone District (n = 3). At the time of pilot initiation (2011), Mazabuka’s ANC HIV prevalence was 11.2% (program data, 2011) with 3002 expected live births and therefore, 336 HIV-exposed deliveries per year.10–12 Livingstone District had a 20.8% HIV ANC prevalence (program data, 2011) with 4324 expected live births and an estimated 899 HIV-exposed deliveries per year.12 Six pilot clinics were selected by convenience sample in partnership with MOH; both districts had the 2 highest HIV prevalences in Southern Province, and these 6 clinics had the highest ANC attendance within those districts.
PMTCT services before and during the intervention are described in Table S1A (see Supplemental Digital Content, http://links.lww.com/QAI/A716). Referral ART clinics were located at the same physical address for 3 of the pilot ANCs and at a separate physical addresses for the remaining 3 (range, 1–7 km); 1 facility had a CD4 FACSCount (BD Biosciences, San Jose, CA). All clinics provide routine ANC services per MOH guidelines, including folate tablets, iron tablets, tetanus toxoid injection, and sulfadoxine/pyrimethamine for intrapartum malaria treatment.13
Intervention
Our intervention had 3 components: (1) training of 132 ANC providers, (2) establishment of laboratory courier system to expedite CD4 results, and (3) follow-up of mother–infant pairs by 82 community-based lay counselors.
Training
ANC nurses and midwives (n = 132) were trained in accordance with 2010 Zambian National PMTCT/EID and Advanced ART Management Training Packages, including adult and pediatric ART initiation and management, and opportunistic infection diagnosis and management.13 Lay counselors (n = 82) were trained in National PMTCT guidelines, adherence counseling, and EID testing including dried blood spot (DBS) collection techniques. Refresher trainings for ANC providers and lay counselors occurred biannually.
cART Assessment, Initiation, and Maintenance
Pregnant women presenting to ANC were tested for HIV; if positive, blood samples were collected and couriered to the closest hub laboratory for CD4 analysis by FACS-Count. All women were provided 5 days of AZT monotherapy and instructed to return to review CD4 results and cART eligibility. If found to have CD4 <350 cells per microliter and/or stage 3/4, cART would be initiated per Option A policy; otherwise she would continue on AZT monotherapy until labor during which single-dose nevirapine followed by 7 days of lamivudine–azidothymidine was prescribed. All women were instructed to return for monthly ANC visits for ongoing HIV and antenatal care, including assessment for ARV toxicity and adjustments to ART in accordance with national practice guidelines.
All HIV-exposed infants were prescribed daily nevirapine for 6 weeks, and infants whose mothers were non–cART-eligible continued daily nevirapine until cessation of breastfeeding with weight-adjusted dosing occurring at 6 weeks and 6, 9, 12, and 18 months.13 HIV-exposed infants were scheduled for HIV DNA PCR at 6 weeks of age, with follow-up DNA testing of infants testing negative at 6 months, and HIV antibody testing at 12 and 18 months.
Community Follow-up
Women–infant pairs were assigned a lay counselor, a trained community member who voluntarily served to provide additional patient support and assist with navigating the health care system. Lay counselors made regular home visits throughout pregnancy and the breastfeeding period to ensure proactive timely PMTCT follow-up, refills, and adherence to infant testing schedule. Missed appointments were traced by home visit.
Study Design
We conducted a quasi-experimental nonrandomized preintervention–postintervention comparison. Preintervention (baseline) data were collected by retrospective data abstraction of clinic registers for 8 months preceding the intervention (April 2011 to November 2011). Data were collected from all HIV-positive pregnant women not on ART at the time of first ANC during dates specified. Postintervention data were collected on all ART-naive, HIV-positive women and their infants presenting to the pilot clinics during the enrollment period (December 2011 to December 2012), and then followed for the following 11 months to capture postnatal data (January 2013 to November 2013). Data were collected by retrospective abstraction quarterly for 24 months from the following registers: antenatal, PMTCT, labor and delivery, and EID log books. These times periods were chosen, as PMTCT operational policy was consistent during this time making outcomes comparable.
Data Analysis
Categorical outcomes for baseline and postintervention cohorts were compared using the χ2 tests. Risk ratios and 95% confidence intervals were also used where appropriate. Continuous outcomes were analyzed for difference in mean values using a t test. A linear regression model was used to test for trends in gestational age over time.
In addition to analysis of raw data, we performed a sensitivity analysis to account for missing data for cART-eligibility status and treatment initiation, given the differential amount of missing data between baseline and postintervention groups. To consider a worst-case scenario, we set all missing cART-eligibility values to “eligible for cART” and presumed that they were not initiated on treatment. We then calculated risk ratios and used a χ2 test to determine significance.
All data analyses were conducted using SAS 9.3.
The protocol for this study was approved by the Boston University Institutional Review Board (FWA 00008404), the ERES Converge Zambian Institutional Review Board (FWA 00011697), and Centers for Disease Control CGH ADS.
RESULTS
Of the 643 HIV-positive women presenting to ANC in the baseline cohort, 510 medical records were included in the analysis; 133 records were excluded as women were on cART treatment already. Postintervention, 872 HIV-positive women presented to ANC; 624 medical records included in the analysis; 97 women were excluded because of living outside the catchment area for the intervention, and 151 women were excluded for already being on cART (see Figure S1A, Supplemental Digital Content, http://links.lww.com/QAI/A716). Mean maternal age was known for 1065 (93.9%) women at first ANC presentation and was similar between groups (26.2 years of baseline, 26.5 years of intervention, P < 0.33). Gestational age at the first ANC presentation was known for 1098 (96.8%) women and was statistically different between groups but not of clinical relevance [baseline mean 23.4 weeks (median 23 weeks); intervention mean 22.3 weeks (median 22 weeks), P < 0.01]. Trend analysis looking for earlier gestational age at the first ANC presentation did not demonstrate a trend over time. Table 1 summarizes characteristics of the 2 groups.
TABLE 1.
Demographics of Participants (n = 1134)
| Baseline, n = 510 | Postintervention, n = 624 | Total Records With Available Data | P* | |
|---|---|---|---|---|
| Mean maternal age (SD), yrs | 26.2 (5.6) | 26.5 (5.6) | 1062 | 0.33 |
| Mean GA (SD), wk | 23.4 (6.6) | 22.3 (6.3) | 1098 | <0.01 |
| Mean CD4 count (SD), cells/μL | 347.7 (185.3) | 391.5 (201.5) | 631 | <0.01 |
| HAART eligibility, n (%) | 631 | <0.01 | ||
| CD4 cells/ μL ≥350 | 100 (47.2) | 238 (56.8) | ||
| CD4 cells/μL <350 | 112 (52.8) | 181 (43.2) | ||
| Missing cART-eligibility status, n (%) | 264 (51.8) | 134 (21.5) | 1134 | <0.01 |
Continuous outcomes analyzed for difference in mean values using t test; categorical outcomes were compared using the χ2 test.
A total of 740 participants across both groups obtained CD4 counts. There was a statistically significant difference in CD4 uptake between baseline women (50.6%, n = 510) versus postintervention (77.2%, n = 624); relative risk (RR) of 1.81 [95% confidence interval (CI): 1.57 to 2.08; P < 0.01] (Fig. 1). CD4 count values were available for 631 women total (85.2%) and mean CD4 counts were significantly lower in the baseline (347.7 cells/μL) compared with postintervention (mean 391.5 cells/μL), P < 0.01. When applying a cART initiation threshold of less than 350 cells per microliter, 52.8% baseline women with CD4 value available (n = 112) were cART eligible and 43.2% postintervention women with CD4 value available (n = 181) met cART-eligibility criteria (RR = 0.82; 95% CI: 0.69 to 0.97; P = 0.02). WHO staging was rarely reported in the medical record (0 baseline, 321/624 postintervention). Fifteen of 321 medical records with WHO staging recorded were cART eligibile on the basis of WHO stage alone (stage 3 or 4).
FIGURE 1.
Proportion of HIV+ pregnant women receiving a CD4 count at baseline compared with postintervention.
Of the known cART-eligible women, 27.5% of baseline women compared with 71.5% of postintervention women were initiated on cART (RR = 2.25; 95% CI: 1.78 to 2.83; P < 0.01) (Fig. 2). There was differential missing data between baseline and postintervention, with 51.8% of baseline women missing cART-eligibility data (eg, CD4 count value or WHO staging), compared with 21.5% of intervention women (P < 0.01) (Table 1). Given the disproportionate amount of missing data in the 2 groups, a sensitivity analysis was performed in which we assumed the worst-case scenario in that all women from both baseline and postintervention with missing eligibility criteria (CD4 and/or WHO staging data) were cART eligible and not initiated on cART. Under these worst-case scenario assumptions, the uptake of maternal cART for all cART-eligible women would have been 13.7% at baseline (n = 402) compared with 54.6% postintervention (n = 311) for an RR of 2.62 (95% CI: 2.23 to 3.06; P < 0.01). Alternatively, assuming a “best-case” scenario in which all women with missing eligibility data followed a similar distribution of CD4 counts that we observed in those for whom we had data, the uptake of maternal cART would have been 19% at baseline compared with 72% at postintervention, RR of 3.73 (95% CI: 2.90 to 4.79; P < 0.01).
FIGURE 2.
Proportion of HIV+ cART-eligible women who initiated cART at baseline compared with postintervention.
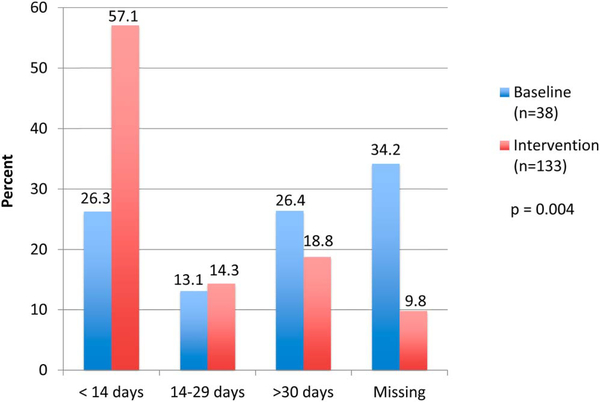
The time from HIV diagnosis to cART initiation was known for 144 of 170 patients initiated on cART (25/38 baseline, 119/132 postintervention). The mean time measured in days from HIV diagnosis to cART initiation was 31.8 days at baseline (range, 21–142 days; SD, 38.3) and 22.2 days of postintervention (range, 5–245; SD, 39.0) P < 0.26. When we categorize the length of time from HIV diagnosis to cART initiation into less than 14 days, less than 30 days, or greater than 30 days, a greater proportion of postintervention women initiated on cART in less than 14 days (57.1%) compared with baseline (26.3%, P = 0.004). These narrow time intervals depicted in Figure 3 were chosen as cutoffs to categorize what proportion of women were initiating cART before their next scheduled monthly antenatal/HIV appointment (Fig. 3).
FIGURE 3.
Time from HIV diagnosis to HAART initiation.
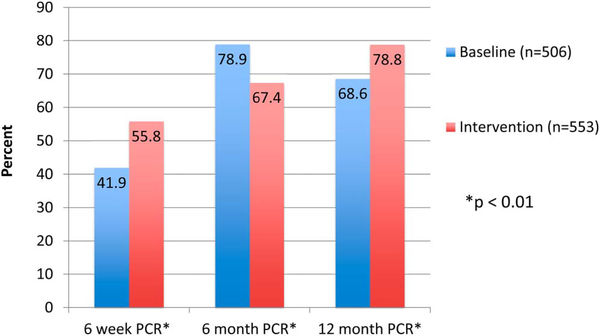
Six-week DBS DNA-PCR results were reported on 41.9% of baseline (n = 506) compared with 55.8% (n = 553) of postintervention infants (P < 0.01), RR = 1.33 (95% CI: 1.18 to 1.5). Median age of infants at the time of DBS collection was 6.9 weeks (baseline) versus 6.7 weeks (postintervention). Six-month DNA-PCR results were reported on 78.9% of baseline (n = 506) compared with 67.8% (n = 553) of postintervention infants (P < 0.01), RR = 0.85 (95% CI: 0.79 to 0.92). Finally, for infants who initially tested negative but were still breastfeeding at 12 months, rapid HIV antibody testing was performed on 68.6% of baseline infants (n = 506) compared with 78.8% (n = 553) of postintervention infants (P < 0.01), RR = 1.15 (95% CI: 1.06 to 1.24) (Fig. 4). Seroprevalence of those with reported results at any age was 5.1% at baseline versus 4.8% postintervention (P = 0.87).
FIGURE 4.
Proportion of HIV-exposed infants with early infant testing reported.
Although there are variable definitions of LFTU, pilot clinicians documented patients as LTFU using provider-specific LTFU definitions (2–6 months of missing appointments); 24.7% of the baseline cohort were labeled as LTFU compared with 14.7% in the postintervention cohort for an RR of 1.58 (95% CI: 1.23 to 2.03; P < 0.01).
Delivery of each of the antenatal care services recommended by MOH guidelines was measured at baseline and postintervention. Results were stable to slightly improved in the postintervention cohort as reported in Table 2.
TABLE 2.
Antenatal Service Delivery Indicators at Baseline Compared With Postintervention
| Baseline | Postintervention | Total, N | P for Difference in Mean Values | |
|---|---|---|---|---|
| Maternal ANC visits | ||||
| N | 499 | 585 | 1084 | |
| Mean (SD) | 2.03 (1.1) | 2.28 (1.1) | 2.16 (1.1) | <0.0001 |
| Median (IQR) | 2 (2) | 2(2) | 2 (2) | |
| No. ANC visits in which mothers received folate supplementation | ||||
| N | 499 | 585 | 1084 | |
| Mean (SD) | 1.52 (1.1) | 1.84 (1.2) | 1.69 (1.1) | <0.0001 |
| Median (IQR) | 1(1) | 2 (2) | 1 (2) | |
| No. ANC visits in which mothers received iron supplementation | ||||
| N | 499 | 584 | 1083 | |
| Mean (SD) | 1.47 (1.1) | 1.83 (1.2) | 1.66 (1.2) | <0.0001 |
| Median (IQR) | 1(1) | 2 (2) | 1 (2) | |
| No. ANC visits in which mothers received intrapartum malaria treatment | ||||
| N | 498 | 545 | 1043 | |
| Mean (SD) | 1.02 (1.0) | 1.86 (1.8) | 1.46 (1.5) | <0.0001 |
| Median (IQR) | 1 (2) | 1 (4) | 1 (3) | |
| No. TT doses administered | ||||
| N | 498 | 580 | 1078 | |
| Mean (SD) | 2.91 (1.8) | 2.99 (1.7) | 2.95 (1.7) | 0.4832 |
| Median (IQR) | 3 (4) | 3 (2) | 3(3) |
DISCUSSION
Options A, B, and B+ for PMTCT services all introduce the provision of cART within PMTCT services, requiring new models of care that increase efficiency of cART initiation and reduce attrition. The success of the integrated ANC/ART approach we present here has a number of advantages. First, for countries using Option A policy, a “one stop shop” theoretically can streamline cART initiation by consolidating service provision along a continuum of care and potentially decreasing time gaps observed in referral models. Second, regardless of policy option, an integrated model with 1 location and 1 provider may reduce stigma or fear of public disclosure as the clinic offers maternal/child health services in addition to HIV care; this is important to consider for family-centric HIV care models. Third, the addition of a community-based lay counselor who follows a mother–infant pair through pregnancy, delivery, and breastfeeding has the potential to boost maternal and infant ART adherence, reduce attrition, and provide much needed linkage to chronic HIV care—addressing a reported need in Option B+ models. This model was able to increase cART uptake for cART-eligible women to nearly 72%, which represents roughly 186 HIV-exposed infants with reduced MTCT risk.
Integration of PMTCT services into routine antenatal care has previously been evaluated to measure its impact on HIV testing uptake and ART initiation.14–17 A 2008 retrospective cohort study in South Africa compared 3 different models of HIV/antenatal care for 14,617 pregnant HIV-positive women: (1) integrated antenatal and HIV care; (2) referral to onsite ART clinic (proximal); and (3) referral to an off-site ART clinic (distal).18 They found significant differences in the proportion of treatment eligible-women initiating cART between the integrated model (55%) and the distal referral model (45%), but there was still a 45% of cART-eligible women not initiated on optimal treatment.18 In 2009, an integrated ART in ANC model was piloted in Lusaka, Zambia, and similarly provided pregnant mothers a single provider for all antenatal and ART care. While this demonstrated improved uptake of cART for cART-eligible pregnant women from 14.4% to 32.9%, more than 67% of cART-eligible women remained on short course ART or no therapy at all. Time to cART initiation did not differ between groups.19
Our integrated model was tested under Option A policy, which required a CD4 count or WHO clinical staging to determine cART eligibility.21 Our model differs from previous Option A studies in that we instituted a laboratory courier system to obtain CD4 counts with a goal turnaround of 5 days to enable more rapid cART assessment and initiation. In our model, the proportion of women initiated on cART in <14 days increased from 26.3% to 57.1% (P = 0.0004), with 71.4% of women were initiated on cART in <30 days. In contrast, the integrated model in the South African study had less than 10% initiated on cART by 20 days, and the study by Killam et al showed a mean time of 5 weeks from the first ANC until cART initiation with no difference between study arms.18,19 The need for expedited CD4 counts was of particular importance under Option A. Although a CD4 count is not required for cART under Option B+, a similar courier laboratory system could be applied to improve turnaround time for DBS testing or for CD4 counts for HIV-positive family members.
In August 2014, Zambia began to operationalize Option B+ guidelines and initiating every HIV-positive pregnant woman on cART regardless of CD4/disease stage. Though Option B+ simplifies the programmatic barriers of cART initiation, the challenge of addressing attrition and linking mother–baby pairs to chronic HIV care becomes critical. The reality of these challenges has been witnessed in the Malawi national program, which experienced higher LTFU rates among Option B+ patients. Women who started cART in pregnancy were 5 times more likely to not return to clinic after initiating cART as compared with pregnant women starting cART for advanced HIV disease (odds ratio = 5.0; 95% CI: 4.2 to 6.1).9 The lay counselor component of our intervention with active follow-up of mother–infant dyads from time of maternal HIV diagnosis through linkage to chronic services may offer a promising strategy to address LTFU. We demonstrated a decrease in ANC LTFU rates from 24.7% to 14.7% (RR = 1.58; 95% CI: 1.23 to 2.03), although the lay counselor model needs to be tested at scale and evaluated for economic feasibility.
In our study, roughly 55.8%, 67.4%, and 78.8% of HIV-exposed infants had a reported DBS result at 6 weeks, 6 months, and 12 months, respectively, which was an improvement at the 6-week and 12-month mark but a decrease at the 6-month time mark. There were notable national stockouts of the DBS cards for several months during the postintervention period, which may explain this decrease. None of the aforementioned studies evaluating the integrated ART/ANC model followed mother–infant pairs beyond delivery; therefore, we are unable to compare our retention to theirs at 6 months. Two cluster-randomized control trials that use an integrated HIV/ANC model and a community cadre are underway in Kenya and Nigeria. These studies will follow mother–infant pairs through 6 and 3 months, respectively, and offer evidence as to whether this approach impacts LFTU rates.22,23 In addition, retention data for mothers who “graduate from PMTCT” to chronic ART care are needed.
Attention to maintaining and improving routine service delivery while integrating new service delivery is imperative.24 Our model did not demonstrate any decrease in standard ANC service provision, and there was a slight measurable improvement in provision of folate, iron, and intrapartum malaria treatment. Further research is needed to investigate the burden of work placed on ANC staff and quality indicators for HIV care delivered in this task-shifting model. Task shifting for HIV care has been demonstrated to maintain quality in several contexts.25–27
Limitations of this evaluation include (1) lack of a comparison arm, as this evaluation is a predesign/postdesign; (2) retrospective data abstraction from service delivery registers which contributes to the differential amount of missing data in the preintervention versus postintervention arms; and (3) 3 interventions (training of midwives in HIV care, laboratoy courier system for CD4 counts, and community lay counselors) implemented simultaneously making interpretation of results difficult to attribute to 1 intervention. The predesign/postdesign was chosen because of funding limitations. To address the differential amounts of missing data in the preintervention and postintervention comparison groups, we performed a sensitivity analysis on primary outcomes, which assumed both a best- and worst-case scenario for the missing data. The differential missingness of the data is likely due to health system improvements due to our training and intervention, which could be considered a process outcome itself. Analyses from either scenario still show a statistically significant increase of cART initiation with our intervention, suggesting that our pilot does demonstrate improvement in services delivered and not just improvement in clinical documentation. We recognize that it is impossible to attribute the increase in proportion of women with CD4 counts obtained or proportion of cART-eligible women initiated on cART to any 1 variable. Therefore, these 3 interventions must be considered as a package of interventions to obtain the effect demonstrated here. That said, if a program is challenged with poor or delayed cART uptake, consider investing in the integration of HIV and ANC services as it streamlines this process. If programs have relatively good cART uptake but poor adherence and high LTFU, the active tracing and adherence counseling provided by community lay counselors may be a higher priority investment.
Programmatic challenges included (1) intermittent national-level stockouts of critical commodities (ie, DBS cards, HIV rapid tests), (2) lack of incentive structure for lay counselors resulting in frequent turnover, and (3) shifts of trained personnel in ANCs. The programmatic realities of high turnover and staffing shifts are challenges that all largescale programs face as recently reported in Kenya.22 Our training model of refresher onsite mentorship attempts to alleviate gaps in staff training. The Government of Zambia is considering professionalization of the lay counselor cadre, which may decrease attrition.
As low- and middle-income countries chose between Option A and B+, health service models that provide decentralized, streamlined, family-centric care, and strong community-level support to reduce attrition throughout the PMTCT cascade are needed. The next step in implementation is to evaluate different strategies for HIV care retention, including support from Community Health Workers, role of SMS reminders, incentives, family-centric HIV care, and exploring motivations for seeking care in a pregnant population. Our model of integrated HIV/antenatal care, coupled with community lay counselors, demonstrates measurable improvement in PMTCT process outcomes while maintaining antenatal care service quality.
Supplementary Material
Acknowledgments
Supported by Cooperative Agreement Number 5U2GPS001418 from the Centers for Disease Control and Prevention, with support from the Coordinating Office of Global Health.
Footnotes
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362: 2282–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–180. Available at: http://www.sciencedirect.com/science/article/pii/S1473309910702887. Accessed October 2, 2013. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization; Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants: Recommendations for a Public Health Approach. Geneva, Switzerland, WHO Press; 2010. [PubMed] [Google Scholar]
- 4.Danel C, Moh R, Minga A, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in West Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367: 1981–1989. [DOI] [PubMed] [Google Scholar]
- 5.Danel C, Moh R, Chaix ML, et al. Two-months-off, four-months-on antiretroviral regimen increases the risk of resistance, compared with continuous therapy: a randomized trial involving West African adults. J Infect Dis. 2009;199:66–76. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Programmatic Update: Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants: Recommendations for a Public Health Approach. 2012. Available at: www.who.int/hiv/pub/mtct/programmatic_update2012/en/. Accessed September 1, 2015. [PubMed]
- 7.Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378:282–284. [DOI] [PubMed] [Google Scholar]
- 8.Republic of Zambia Ministry of Health. Lifelong Antiretroviral Drugs (ARVs) for All HIV Positive Pregnant Women in Zambia Policy Guidelines for Health Facilities in Zambia. Lusaka, Zambia, Republic of Zambia Ministry of Health; 2013. [Google Scholar]
- 9.Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (“Option B+”) in Malawi. AIDS. 2014;28:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Republic of Zambia, National AIDS Council of Zambia. Zambia Country Report: Monitoring the Declaration of Commitment on HIV and AIDS and the Universal Access Lusaka, Zambia, Republic of Zambia Ministry of Health: 2012. [Google Scholar]
- 11.Central Statistical Office (CSO), Ministry of Health (MOH), Tropical Diseases Research Unit (TDRC) MII. Demographic and Health Survey Zambia 2007. Calverton, MD: CSO and Macro International Inc; 2009. [Google Scholar]
- 12.Central Statistical Office. National Census: Southern Province Fact Sheet 2010. Lusaka, Zambia: 2010. Available at: http://www.zamstats.gov.zm/report/Census/2010/2010SummaryCensusWallChart-SouthernProvince.pdf. Accessed October 7, 2013. [Google Scholar]
- 13.Republic of Zambia Ministry of Health. 2010 National Protocol Guidelines: Integrated Prevention of Mother to Child Transmission. Lusaka, Zambia, Republic of Zambia Ministry of Health; 2010. [Google Scholar]
- 14.Tudor Car L, Van Velthoven MHMMT, Brusamento S, et al. Integrating prevention of mother-to-child HIV transmission programs to improve uptake: a systematic review. PLoS One. 2012;7:e35268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vo BN, Cohen CR, Smith RM, et al. Patient satisfaction with integrated HIV and antenatal care services in rural Kenya. AIDS Care. 2012;37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tudor Car L, van-Velthoven MHMMT, Brusamento S, Elmoniry H,Car J, Majeed A, Atun R Integrating prevention of mother-to-child HIV transmission (PMTCT) programmes with other health services for preventing HIV infection and improve-ing HIV outcomes in developing countries. Cochrane Database of Systematic Reviews 2011, Issue 6 Art. No.: CD008741. DOI: 10.1002/14651858.CD008741.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ong’ech JO, Hoffman HJ, Kose J, et al. Provision of services and care for HIV-exposed infants: a comparison of maternal and child health (MCH) clinic and HIV comprehensive care clinic (CCC) models. J Acquir Immune Defic Syndr. 2012;61:83–89. [DOI] [PubMed] [Google Scholar]
- 18.Stinson K, Jennings K, Myer L. Integration of antiretroviral therapy services into antenatal care increases treatment initiation during pregnancy: a cohort study. PLoS One. 2013;8:e63328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killam WP, Tambatamba BC, Chintu N, et al. Antiretroviral therapy in antenatal care to increase treatment initiation in HIV-infected pregnant women: a stepped-wedge evaluation. AIDS. 2010;24:85–91. [DOI] [PubMed] [Google Scholar]
- 20.Kalembo FW, Zgambo M. Loss to follow-up: a major challenge to successful implementation of prevention of mother-to-child transmission of HIV-1 programs in sub-Saharan Africa. ISRN AIDS. 2012;2012:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Republic of Zambia Ministry of Health. Republic of Zambia Ministry of Health Guidelines for Antiretroviral Therapy for HIV in Infants and Children in Zambia. 2010.
- 22.Turan JM, Steinfeld RL, Onono M, et al. The study of HIV and antenatal care integration in pregnancy in Kenya: design, methods, and baseline results of a cluster-randomized controlled trial. PLoS One. 2012;7: e44181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aliyu MH, Blevins M, Audet C, et al. Optimizing PMTCT service delivery in rural North-Central Nigeria: protocol and design for a cluster randomized study. Contemp Clin Trials. 2013;36:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi BH, Adler MR, Bolu O, et al. Progress, challenges, and new opportunities for the prevention of mother-to-child transmission of HIV under the US President’s Emergency Plan for AIDS Relief. J Acquir Immune Defic Syndr. 2012;60:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fairall L, Bachmann MO, Lombard C, et al. Articles task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet. 2012;380:889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callaghan M, Ford N, Schneider H. Review a systematic review of task-shifting for HIV treatment and care in Africa. Hum Resour Health. 2010; 8 Available at: http://www.biomedcentral.com/content/pdf/1478-4491-8-8.pdf. Accessed October 7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long L, Brennan A, Fox MP, et al. Treatment outcomes and cost-effectiveness of shifting management of stable ART patients to nurses in South Africa: an observational cohort. Plos Med. 2011;8:e1001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.