Abstract
Introduction
The aim of this study was to detect circulating tumor cells (CTCs) in patients with advanced renal cell carcinoma (RCC) using a novel CTC detection platform. Furthermore, we evaluated the clinical outcomes associated with a CTC-positive status.
Methods
A total of 34 patients with advanced RCC (stage III or IV) were prospectively enrolled, and 104 peripheral blood samples were analyzed for the presence of CTCs at various time points. CTCs were isolated using a tapered-slit filter, which captures CTCs based on size and deformability. The presence of CTCs was confirmed using both staining and morphological criteria. CTC status was then correlated with clinical characteristics and survival outcomes.
Results
CTCs were detected in 62% of patients during the pretreatment period, and the median CTC count was 2 (interquartile range 1–3). During the followup period, CTCs were detected in 56% (18/32), 65% (20/31), and 41% (7/17) of patients at one week, one month, and three months after treatment, respectively. Overall, CTCs were found in 57.9% (66/114) of blood samples in the range of 1–7 cells. Although no statistical significance was found, CTC detection in patients with stage IV disease was more common than in patients with stage III disease (68.4% vs. 53.3%). Two-year progression-free survival and cancer-specific survival tended to be lower in CTC-positive patients compared with CTC-negative patients.
Conclusions
The tapered-slit filter is an efficient technique to detect CTCs in advanced RCC.
Introduction
Worldwide, approximately 350 000 cases of renal cell carcinoma (RCC) are diagnosed annually, and more than 140 000 deaths are attributed to the disease.1,2 Although many RCCs are now detected earlier in the course of disease, about 30% of patients present with locally advanced or metastatic disease, and the five-year relative survival rates for locally advanced RCC and metastatic RCC are 72.4% and 13.9%, respectively.3 For patients with localized RCC, complete resection of the primary tumor is the only curative treatment. In contrast, nephrectomy is often a palliative treatment for patients with advanced RCC. An integrated management approach with surgery and systemic therapies is the standard strategy to enhance cancer control for both locally advanced RCC and metastatic RCC.
To date, treatment decisions regarding RCC have depended solely on clinical criteria. A better understanding of the molecular biology of disseminated tumor cells might lead to the identification of biomarkers that more accurately determine diagnosis and prognosis, as well as aiding in selecting patients likely to benefit from the chosen therapy. Circulating tumor cells (CTCs) are malignant cells in the peripheral blood originating from the primary tumor site that are responsible for metastatic sites. Detection of CTCs using a minimally invasive liquid biopsy may obviate the need for invasive biopsies of metastatic sites and can be conveniently used as a clinical monitoring tool for cancer prognosis. Several clinical studies have shown that detection of CTCs has a close relationship to survival and metastatic potential in solid tumors.4–6 Despite considerable effort toward using CTCs as biomarkers for RCC, the clinical relevance of CTC detection in advanced RCC patients is still controversial, and the prognostic impact of their detection has not yet been determined.7,8
Various techniques are available to detect CTCs from peripheral blood using their unique properties, including tissue-specific nucleic sequences, cell-surface markers, and physical properties.9 However, each technique has specific merits and limitations. Recently, new methods to isolate CTCs have been developed using a photosensitive polymer-based microfilter.10 This uniquely designed membrane filter, termed a tapered-slit filter (TSF), simply connects to commercially available syringes to capture the CTCs from unprocessed and label-free blood samples. However, no studies have investigated the efficacy of this technique in RCC patients. Thus, the aim of the present study was to evaluate the detection of CTCs in patients with advanced RCC using a novel TSF-based detection process. We also investigated the clinical characteristics and prognostic significance of the presence of CTCs.
Methods
Study population
This study was approved by the Institutional Review Board of our institution. A total of 34 patients with locally advanced or metastatic RCC (stage III or IV) were enrolled in this study after obtaining informed consent. All patients underwent surgery or initiation of targeted therapy between August 2015 and May 2016. Patients who had a bilateral tumor and/or synchronous malignancies were excluded from this study. Demographic and clinical data were assessed, including medical history, physical examination, radiographic findings, and pathological reports. For the pre-treatment radiological evaluation, all patients underwent bone scintigraphy scans and computed tomography scans of the abdomen, pelvis, and chest. Other radiographic imaging studies, including positron emission tomography and brain magnetic resonance imaging, were performed when clinically indicated. Distal metastases were verified using appropriate imaging studies and pathological results from biopsies. Tumor staging was assessed according to the current 8th tumor-node-metastasis staging classification from the American Joint Committee on Cancer.11
CTC collection
Peripheral blood samples from each patient were collected before the initiation of treatment and during the followup period. Pre-treatment samples were collected one day prior to the initiation of treatment. During the followup period, blood samples were collected at one week, one month, and three months after surgery or initiation of targeted therapy. A total of 5 mL of peripheral blood was collected in a BD Vacutainer® tube for each sample. All blood samples were transferred to the Korea Advanced Institute of Science and Technology (KAIST) for identification and counting of CTCs. To avoid cell lysis and destruction during delivery, the collection tubes were packed with ice packs in a foam cooler and delivered within six hours of collection.
Identification and counting of CTCs
CTC isolation and counting were performed using a TSF device that had been optimized for this use as previously reported.10,12 The TSF isolates CTCs based on their physical properties, such as size and deformability, regardless of their surface protein expression. In addition, its unique design, with a wide cell entrance and a gradually narrowing slit exit, increases the sample flow rate with minimal cell stress, thus rapidly isolating viable, heterogeneous CTCs from clinical samples. Five milliliters of whole blood from the patient were diluted in 10 ml of phosphate-buffered saline solution without any pretreatment (e.g., cell fixation, erythrocyte lysis, or Ficoll® separation) and directly processed through the TSF device by withdrawing the syringe plunger. After sample processing, the cells captured by the TSF were gently released by applying a reverse flow of phosphate-buffered saline solution, and the released cells were cytospun on a glass slide using a cytocentrifuge (Shandon Cytospin III, Thermo Scientific, Wilmington, DE, U.S.).
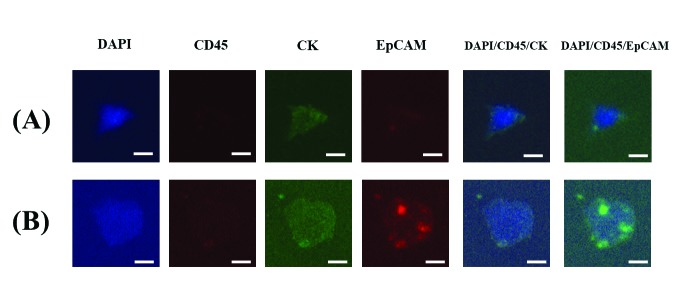
The cells captured by the TSF were analyzed using immunofluorescence staining. The immunostaining protocol was optimized for this use as described previously.12 Briefly, the slide glass with mounted cells was fixed, permeabilized, blocked, and immunofluorescently stained. Fluorescent images were then acquired and examined using MetaMorph® software (Molecular Devices, Sunnyvale, CA, U.S.). The immunofluorescent cells were classified as CTCs from RCC if they met both the staining criteria (4′,6-diamidino-2-phenylindole [DAPI]+, cluster of differentiation 45 [CD45]−, cytokeratin [CK]+, and epithelial cell adhesion molecules [EpCAM]±) and the morphological criteria (bigger size, higher nucleus-to-cytoplasm ratio, and higher degree of irregularity than background blood cells) (Fig. 1). The CTCs were identified and counted by two independent researchers who were blinded to the detailed clinical information.
Fig. 1.
The representative images of the circulating tumour cells captured by microfilter and stained by immunofluorescent staining. The bar represents 10 μm. (A) EpCAM negative circulating tumor cells, DAPI+/CD45−/CK+/EpCAM-; (B) EpCAM positive circulating tumor cells, DAPI+/CD45−/CK+/EpCAM+. CD45: cluster of differentiation 45; CK: cytokeratin; DAPI: 4′,6-diamidino-2-phenylindole; EpCAM: epithelial cell adhesion molecules.
Statistical analyses
The median and interquartile range (IQR) were used to describe the quantitative variables, and frequency and percentage were used for the qualitative variables. The Shapiro-Wilk normality test was used to investigate the normal distribution of the continuous variables. The demographic and clinical data were compared according to CTC positivity. The continuous variables were compared using the Mann-Whitney U-test, and the categorical variables were compared using either the Pearson’s Chi-square test or linear-by-linear association. To assess the clinical characteristics associated with the detection of CTCs, the correlations between CTC positivity and the clinical characteristics of the patients were analyzed. Followup outcomes, including progression-free survival (PFS) and cancer-specific survival (CSS), were evaluated to better understand the relationships between CTC status and clinical outcomes. Kaplan-Meier curves were constructed to illustrate PFS and CSS rates according to pre-treatment CTC positivity, and differences were assessed using the log-rank test. A p-value <0.05 was taken to indicate statistical significance. Statistical analyses were performed using SPSS® for Windows, version 21.0. (IBM Corporation, NY, U.S.).
Results
Patient characteristics
The characteristics of the enrolled patients are summarized in Table 1. Each patient’s age, gender, clinical stage, treatment modality, pathological T stage, and duration of followup were documented. The median age of the study cohort was 61 years (IQR 54–68) and 19 (55.9%) were male. The study included 15 (44.1%) patients with stage III RCC and 19 (55.9%) patients with stage IV RCC, with 30 (88.2%) patients receiving surgery, such as nephrectomy or metastasectomy. The median followup duration was 19.5 months (IQR 16.8–23.3). Histology results were available for all patients, with conventional clear-cell RCC seen in 31 (91.2%) patients, chromophobe RCC in one (2.9%) patient, clear-cells and papillary RCC in one (2.9%) patient, and clear-cell RCC with a sarcomatoid component in one (2.9%) patient.
Table 1.
Characteristics of the study cohort who having the advanced renal cell carcinoma (n=34)
| Characteristic | Value |
|---|---|
| Age, years | 61.0 (54.0–68.0) |
| Gender, n (%) | |
| Male | 19 (55.9) |
| Female | 15 (44.1) |
| Clinical TNM grouping, n (%) | |
| III | 15 (44.1) |
| IV | 19 (55.9) |
| Prior nephrectomy performed, n (%) | 4 (11.8) |
| Treatment, n (%) | |
| Nephrectomy and/or metastasectomy | 19 (55.9) |
| Targeted therapy after renal biopsy | 4 (11.8) |
| Nephrectomy and adjuvant treatment | 11 (32.4) |
| *Pathological T stage, n (%) | |
| pT1 | 4 (14.8) |
| pT2 | 4 (14.8) |
| pT3a | 16 (59.3) |
| pT3b | 2 (7.4) |
| pT3c | 1 (3.7) |
| pT4 | 0 (0) |
| Length of followup, months | 19.5 (16.8–23.3) |
Data are presented as median (interquartile range [IQR]) or n (%).
Patients who underwent nephrectomy (n=27).
TNM grouping III = T3 N0 M0 or T1~3 N1 M0; TNM grouping IV = T4 any N M0 or any T N2 M0 or any T any N M1.
CTC detection and count
CTCs were detected in 61.8% (21/34) of patients during the pre-treatment period, with a median CTC count of 2 (range 1–6; IQR 1–3). When patients were grouped according to clinical stage, the percentage of patients with ≥1 CTC was higher in stage IV (68.4%, 13/19) patients than in stage III (53.3%, 8/15) patients (Table 2). Of the 19 patients with metastases to reginal lymph nodes or a distant site, 14 (73.7%) had detectable CTCs and four (35.7%) had ≥3 CTCs. CTCs were detected in 56.3% (18/32), 64.5% (20/31), and 41.2% (7/17) of patients at one week, one month, and three months after treatment, respectively. Overall, CTCs were found in 66 of 114 blood samples (57.9%) in the range of 1–7 cells (median 1). During the followup period, a decrease in CTC count was seen in 40.6% (13/32), 35.5% (11/31), and 47.1% (8/17) of patients at one week, one month, and three months, respectively. The detailed clinicopathologic characteristics of the 21 patients who were positive for CTCs are summarized in Table 3. Of these 21 patients, 14 (66.7%) had metastases to reginal lymph nodes or a distant site. Although no significant associations were found, male gender and stage IV disease were more common in those with detectable CTCs (66.7% and 61.9%, respectively) compared with those without detectable CTCs (38.5% and 46.2%, respectively).
Table 2.
Number of pre-treatment CTCs per 5 ml peripheral blood sample according to clinical TNM grouping
| Number of CTCs in TSF | Stage III (n=15) | Stage IV (n=19) |
|---|---|---|
| 0 | 7 (46.7) | 6 (31.6) |
| 1 | 5 (33.3) | 5 (26.3) |
| 2 | 0 (0) | 3 (15.8) |
| 3 | 1 (6.7) | 3 (15.8) |
| 4 | 1 (6.7) | 0 (0) |
| 5 | 1 (6.7) | 1 (5.3) |
| >5 | 0 (0) | 1 (5.3) |
Data are presented as n (%), except where otherwise stated. CTCs: circulating tumor cells; IQR: interquartile range; TSF: tapered-slit filter.
Table 3.
Characterization of patients positive for CTCs in pre-treatment (n=21)
| Patient number | Gender | Age | TNM grouping | Histologic diagnosis | Metastasis sites | Treatment | Number of CTCs in TSF |
|---|---|---|---|---|---|---|---|
| 1 | Male | 74 | III | Clear-cell | — | Nephrectomy | 4 |
| 2 | Male | 32 | IV | Clear-cell | Lung, adrenal gland | Metastasectomy, targeted therapy | 1 |
| 3 | Male | 67 | III | Clear-cell | — | Nephrectomy | 1 |
| 4 | Female | 70 | IV | Chromophobe | Lymph nodes | Nephrectomy | 3 |
| 5 | Male | 61 | IV | Clear-cell | Lung | Nephrectomy, targeted therapy | 2 |
| 6 | Female | 70 | IV | Clear-cell | Lymph node, lung, pelvic mass | Nephrectomy, targeted therapy | 1 |
| 7 | Male | 61 | III | Clear-cell | Lymph node | Nephrectomy | 1 |
| 8 | Male | 66 | III | Clear-cell | — | Nephrectomy | 1 |
| 9 | Male | 53 | IV | Clear-cell | Lung, psoas muscle | Nephrectomy, targeted therapy | 3 |
| 10 | Female | 62 | III | Clear-cell | — | Nephrectomy | 1 |
| 11 | Male | 58 | III | Clear-cell | — | Nephrectomy | 3 |
| 12 | Male | 67 | IV | Clear-cell, papillary | Lymph node, bone, liver | Nephrectomy, targeted therapy | 1 |
| 13 | Male | 47 | IV | Clear-cell | Adrenal gland | Metastasectomy | 2 |
| 14 | Male | 70 | IV | Clear-cell with sarcomatoid component | Lymph nodes, lung | Nephrectomy, targeted therapy | 6 |
| 15 | Male | 67 | III | Clear-cell | — | Nephrectomy, targeted therapy | 5 |
| 16 | Female | 77 | IV | Clear-cell | Lymph nodes, lung | Targeted therapy | 3 |
| 17 | Female | 67 | IV | Clear-cell | Lung | Nephrectomy | 5 |
| 18 | Male | 58 | IV | Clear-cell | Lung, adrenal gland | Metastasectomy, targeted therapy | 2 |
| 19 | Male | 40 | IV | Clear-cell | Lung | Nephrectomy, targeted therapy | 1 |
| 20 | Female | 53 | IV | Clear-cell | Lung, liver | Nephrectomy, targeted therapy | 1 |
| 21 | Female | 51 | III | Clear-cell | — | Nephrectomy | 1 |
CTCs: circulating tumor cells; TNM: tumor-node-metastasis; TSF: tapered-slit filter.
Survival outcomes
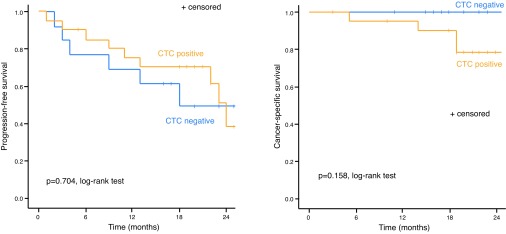
The median followup duration was 20.0 (IQR 18.5–23.5) and 18.0 (IQR 15.5–24.0) months for CTC-positive and -negative patients, respectively (p=0.649). During the followup period, disease progression occurred in 44.1% (15/34) of patients, with rates of 53.8% (6/13) in CTC-negative patients and 42.9% (9/21) in CTC-positive patients. Overall, four patients died during the study, all of whom were CTC-positive. Although there were no significant differences in PFS and CSS according to CTC positivity, both the two-year PFS and CSS of CTC-negative patients were higher compared with those of CTC-positive patients (49.2% and 100% vs. 38.5% and 81.0%, respectively) (Fig. 2).
Fig. 2.
Cumulative survival of the study cohort by pre-treatment CTC status: (A) progression-free survival; (B) cancer-specific survival. CTC: circulating tumor cell
Discussion
In this study, we investigated the clinical efficacy of the TSF technique for CTC detection in advanced RCC. The results of our study demonstrated that CTCs are frequently detected in patients with advanced RCC. Of the 34 patients, 21 (62%) had one or more CTCs detected in the pre-treatment period. CTCs were isolated from 74% (14/19) of patients with metastases to reginal lymph nodes or a distant site. The CTC detection rate in patients with stage IV disease tended to be higher than in patients with stage III disease. To identify changes in the CTC level in individual patients after initiation of treatment, 104 peripheral blood samples from 34 advanced RCC patients were drawn at various time points. CTCs were detected in 56%, 65%, and 41% of patients at one week, one month, and three months after treatment, respectively.
Tumor biomarkers can indicate important clinical parameters, such as disease occurrence, recurrence, progression, and survival, although biomarkers obtained from tumor tissues have several drawbacks. One of the main disadvantages is that they may not accurately reflect genetic heterogeneity, as they are only a small part of the tumor. In contrast, blood-based platforms could provide a more comprehensive view of tumors. Several circulating cell types have been studied as biomarkers related to RCC. Circulating endothelial cells and circulating endothelial progenitors, which are related to tumor vascularization, were found more frequently in patients with RCC than in healthy control subjects.13,14 Several studies have demonstrated that peripheral blood from patients with advanced RCC contains relatively high numbers of myeloid-derived suppressor cells, which may contribute to tumor progression by facilitating angiogenesis, immunosuppression, and metastasis.15,16 However, these markers are limited in their use as a tool in routine clinical applications because of the difficulty in reliably quantifying them, and their exact role as prognostic biomarkers remains to be determined.
CTCs are tumor cells in the blood that are disseminated from the site of a primary or metastatic tumor. The detection and characterization of CTCs as a complementary biomarker can be helpful for diagnosis, risk assessment, prediction of benefits from a specific treatment, and evaluation of recurrence or progression of cancer.4–6 Although studies of CTCs in RCC patients are limited, CTCs are commonly present in very low numbers in the blood of patients with RCC, even those with metastases. Likewise, the role of CTCs as a pre-treatment marker has been assessed in a limited manner in RCC. Efforts to reliably detect RCC cells in blood have been limited by the absence of biomarkers that are widely and specifically expressed in RCC cells relative to background blood cells. Epithelial markers, such as EpCAM and CK, are useful for differentiating most CTCs in patients with other solid tumors, but RCC cells often lack epithelial differentiation.17 Thus, technical challenges in the detection and characterization of CTCs have hindered the widespread integration of CTC-based techniques in standard clinical practice. Although a considerable number of methods have been developed to detect and analyze CTCs from peripheral blood, most of them are not yet standardized, and there remains some controversy regarding the usefulness of each method. In previous studies, CTCs were detected in 16–53% of RCC patients, and the detection rate can vary widely based on methodology used to capture CTCs (Table 4).7,18–23
Table 4.
Detection rates of circulating tumour cells in renal cell carcinoma with various techniques
| Method | Blood sample volume per patient | Detection rate (%) | CTC count per blood sample | Stage | References | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Sample | Patients | ||||||
| PCR based approach | RT-PCR | — | — | 48.6% (18/37) | — | Any stage | 18 |
| RT-PCR | — | — | 52.9 % (46/87) | — | Any stage | 19 | |
| RT-PCR | 8 ml | — | 45.7% (21/46) | — | Any stage | 20 | |
| Immunocytochemistry-based approach | MACS technique | 8 ml | 27.9% (29/104) | 32.2% (19/59) | Median 8 (range 1–38) | Any stage | 21 |
| MACS technique | — | 28.9% (105/363) | 37.4% (80/214) | Median 5 (range 1–51) | Any stage | 22 | |
| MACS technique | 16 ml | 41.2% (96/233) | 52.6% (81/154) | Mean 6 (range 1–51) | Any stage | 7 | |
| CellSearch | 7.5 ml | — | 16.0% (4/25) | Mean 1 (range 1–4) | Stage IV | 23 | |
| Morphological based approach | TSF technique | 5 ml | 57.9% (66/114) | 61.8% (21/34) | Median 1 (range 1–7) | Stage III or IV | Present study |
CTC: circulating tumour cell; MACS: magnetic cell sorting; PCR: polymerase chain reaction; RT-PCR: reverse transcription-polymerase chain reaction; TSF: tapered-slit filter.
Our study reports a relatively high detection rate (62%) using the TSF platform to analyze CTCs compared with previous studies. Notably, CTCs were found in only 16% of patients with metastatic RCC in a study of 25 patients using the CellSearch® platform (Janssen Diagnostics, LLC, Raritan, NJ, U.S.).23 The CellSearch® platform has only been approved by the U.S. Food and Drug Administration for prognostic use in patients with metastatic breast, colorectal, and prostate cancer. This platform and most subsequent techniques use magnetic beads to selectively bind to antibodies for EpCAM on CTCs. However, these platforms are limited in that they cannot detect EpCAM-weak or -negative CTCs in epithelial-mesenchymal transition or non-epithelial tumor types, and a recent study reported that a significant portion of CTCs are EpCAM-negative.24 Due to irreversible antibody interactions and suppression of cell proliferation, magnetic bead-based methods have difficulty in capturing viable cells.25 Moreover, the requirement for additional chemical treatment and a controlled experiment setup for downstream analysis make it difficult to capture and examine CTCs in conditions with limited resources, which can be a barrier for its application in routine clinical practice.26
Alternatively, new technologies using a physical property-based system or microfilters have been developed to overcome the disadvantages of prior platforms and have shown comparable results with the CellSearch® platform.27–29 Among those platforms, Kang et al10 introduced the microfilter (TSF), with a wide cell entrance and gradually narrowing exit to both reduce stress on captured cells and to specifically capture CTCs by taking advantage of both their size and deformability. This simple, rapid, label-free device is ideal for further investigating the functional and molecular properties of CTCs.10,12 In addition, a CTC detection method using a combination of the TSF platform and surface-marker expression with confirmatory morphologic criteria could be useful for rapid cancer diagnosis and prognosis assessment because it enriches CTCs from patients’ blood samples in a label-free and simple manner that is independent of surface-marker expression, such as EpCAM. Reliable performance regarding CTC detection using a TSF platform has been reported for nine different types of cancer cells, including RCC, which showed a CTC detection rate of 78%.12,30 Those studies were extended to verify the device’s potential for clinical use with blood samples from 18 cancer patients with four different types of cancer cells, including two RCC patients. Briefly, of the 18 cancer patients, 11 (61.1%) showed at least one CTC using immunofluorescence staining. The average number of CTCs was 2.5 and ranged from 1–8 in all types of cancers. On the other hand, no CTCs were detected from the two healthy control patients. Furthermore, even in an extremely low cell concentration (5 cells/ml), the device successfully captured over 79% of the spiked cancer cells for each of the four different cancer types, verifying the potential for CTC detection at extremely rare cell concentrations.12 In a recently published study on the differential diagnosis of adnexal masses using the TSF platform, 77.4% (24/31) of patients were positive for CTCs, with a 100% (10/10) detection rate in early-stage patients and a 66.7% (14/21) detection rate in advanced-stage patients.30
To better understand the relationships between CTCs and clinical outcomes, we analyzed the clinical characteristics and survival outcomes of patients with respect to CTC status. Unfortunately, there were no statistically significant differences in clinical characteristics, PFS, or CSS between patients who were CTC-positive or CTC-negative. However, these results should be interpreted with caution because they might be affected by the small size of the study cohort and the short followup duration. To investigate whether the detection of CTCs in patients with advanced RCC is associated with an increased risk of progression or cancer-specific death, future long-term followup evaluations are warranted.
As noted above, this study has several limitations that must be acknowledged. For one, the study was prospective, but it included a relatively small number (n=34) of patients at a single institution, which likely limited the statistical power. Future work will focus on expanding the cohort and optimizing CTC detection. Second, the median followup time (19.5 months) was too short for an in-depth analysis of the survival outcomes. Overall, the relationship between CTC status and prognosis in advanced RCC requires further study. Despite these limitations, our study results show the reliability and potential benefits of detecting CTCs in advanced RCC using a novel TSF-based platform.
Conclusions
Our study demonstrates that CTCs are frequently detected in patients with advanced RCC using a novel TSF platform. Our findings suggest that this method may be useful for diagnosis and determining a prognosis for RCC. Additional progress is needed to more accurately estimate prognosis, and larger studies of TSF platforms are required to further define the clinical significance of CTCs captured in advanced RCC.
Acknowledgements/funding
This research was supported by the Converging Research Center Program funded by the Ministry of Science, Information and Communications Technology (ICT) and Future Planning of Korea (Project No.2015054201).
Footnotes
Competing interests: The authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–21. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods, and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Jung KW, Won YJ, Oh CM, et al. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017;49:292–305. doi: 10.4143/crt.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–21. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 7.Bluemke K, Bilkenroth U, Meye A, et al. Detection of circulating tumor cells in peripheral blood of patients with renal cell carcinoma correlates with prognosis. Cancer Epidemiol Biomarkers Prev. 2009;18:2190–4. doi: 10.1158/1055-9965.EPI-08-1178. [DOI] [PubMed] [Google Scholar]
- 8.Tan KV, Namdarian B, Costello AJ, et al. Potential use of circulating endothelial cells as a biomarker of renal cell carcinoma. Urol Oncol. 2011;29:237–43. doi: 10.1016/j.urolonc.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Small AC, Gong Y, Oh WK, et al. The emerging role of circulating tumor cell detection in genitourinary cancer. J Urol. 2012;188:21–6. doi: 10.1016/j.juro.2012.02.2558. [DOI] [PubMed] [Google Scholar]
- 10.Kang YT, Doh I, Cho YH. Tapered-slit membrane filters for high-throughput viable circulating tumor cell isolation. Biomed Microdevices. 2015;17:45. doi: 10.1007/s10544-015-9949-6. [DOI] [PubMed] [Google Scholar]
- 11.Paner GP, Stadler WM, Hansel DE, et al. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur Urol. 2018;73:560–9. doi: 10.1016/j.eururo.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Kang YT, Doh I, Byun J, et al. Label-free rapid viable enrichment of circulating tumor cell by photosensitive polymer-based microfilter device. Theranostics. 2017;7:3179–91. doi: 10.7150/thno.19686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatt RS, Zurita AJ, O’Neill A, et al. Increased mobilization of circulating endothelial progenitors in von Hippel-Lindau disease and renal cell carcinoma. Br J Cancer. 2011;105:112–7. doi: 10.1038/bjc.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruenwald V, Beutel G, Schuch-Jantsch S, et al. Circulating endothelial cells are an early predictor in renal cell carcinoma for tumor response to sunitinib. BMC Cancer. 2010;10:695. doi: 10.1186/1471-2407-10-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 16.Kusmartsev S, Su Z, Heiser A, et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14:8270–8. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Yanez M, Heymach JV, Zurita AJ. Circulating biomarkers in advanced renal cell carcinoma: Clinical applications. Curr Oncol Rep. 2012;14:221–9. doi: 10.1007/s11912-012-0231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKiernan JM, Buttyan R, Bander NH, et al. The detection of renal carcinoma cells in the peripheral blood with an enhanced reverse transcriptase-polymerase chain reaction assay for MN/CA9. Cancer. 1999;86:492–7. doi: 10.1002/(SICI)1097-0142(19990801)86:3<492::AID-CNCR18>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 19.Shimazui T, Yoshikawa K, Uemura H, et al. Detection of cadherin-6 mRNA by nested RT-PCR as a potential marker for circulating cancer cells in renal cell carcinoma. Int J Oncol. 2003;23:1049–54. doi: 10.3892/ijo.23.4.1049. [DOI] [PubMed] [Google Scholar]
- 20.Li G, Passebosc-Faure K, Gentil-Perret A, et al. Cadherin-6 gene expression in conventional renal cell carcinoma: A useful marker to detect circulating tumor cells. Anticancer Res. 2005;25:377–81. [PubMed] [Google Scholar]
- 21.Bilkenroth U, Taubert H, Riemann D, et al. Detection and enrichment of disseminated renal carcinoma cells from peripheral blood by immunomagnetic cell separation. Int J Cancer. 2001;92:577–82. doi: 10.1002/ijc.1217. [DOI] [PubMed] [Google Scholar]
- 22.Blumke K, Bilkenroth U, Schmidt U, et al. Detection of circulating tumor cells from renal carcinoma patients: Experiences of a two-center study. Oncol Rep. 2005;14:895–9. doi: 10.3892/or.14.4.895. [DOI] [PubMed] [Google Scholar]
- 23.Gradilone A, Iacovelli R, Cortesi E, et al. Circulating tumor cells and «suspicious objects» evaluated through CellSearch(R) in metastatic renal cell carcinoma. Anticancer Res. 2011;31:4219–21. [PubMed] [Google Scholar]
- 24.Giordano A, Gao H, Anfossi S, et al. Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol Cancer Ther. 2012;11:2526–34. doi: 10.1158/1535-7163.MCT-12-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiwari A, Punshon G, Kidane A, et al. Magnetic beads (Dynabead) toxicity to endothelial cells at high bead concentration: implication for tissue engineering of vascular prosthesis. Cell Biol Toxicol. 2003;19:265–72. doi: 10.1023/B:CBTO.0000004929.37511.ed. [DOI] [PubMed] [Google Scholar]
- 26.Shen Q, Xu L, Zhao L, et al. Specific capture and release of circulating tumor cells using aptamer-modified nanosubstrates. Adv Mater. 2013;25:2368–73. doi: 10.1002/adma.201300082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang T, Jia CP, Jun Y, et al. Highly sensitive enumeration of circulating tumor cells in lung cancer patients using a size-based filtration microfluidic chip. Biosens Bioelectron. 2014;51:213–8. doi: 10.1016/j.bios.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 28.Farace F, Massard C, Vimond N, et al. A direct comparison of CellSearch and ISET for circulating tumor-cell detection in patients with metastatic carcinomas. Br J Cancer. 2011;105:847–53. doi: 10.1038/bjc.2011.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams DL, Stefansson S, Haudenschild C, et al. Cytometric characterization of circulating tumor cells captured by microfiltration and their correlation to the CellSearch((R)) CTC test. Cytometry A. 2015;87:137–44. doi: 10.1002/cyto.a.22613. [DOI] [PubMed] [Google Scholar]
- 30.Suh DH, Kim M, Choi JY, et al. Circulating tumor cells in the differential diagnosis of adnexal masses. Oncotarget. 2017;8:77195–206. doi: 10.18632/oncotarget.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]