Abstract
Aims/hypothesis
MiR-153 is an intronic miRNA embedded in the genes that encode IA-2 (also known as PTPRN) and IA-2β (also known as PTPRN2). Islet antigen (IA)-2 and IA-2β are major autoantigens in type 1 diabetes and are important transmembrane proteins in dense core and synaptic vesicles. MiR-153 and its host genes are co-regulated in pancreas and brain. The present experiments were initiated to decipher the regulatory network between miR-153 and its host gene Ia-2β (also known as Ptprn2).
Methods
Insulin secretion was determined by ELISA. Identification of miRNA targets was assessed using luciferase assays and by quantitative real-time PCR and western blots in vitro and in vivo. Target protector was also employed to evaluate miRNA target function.
Results
Functional studies revealed that miR-153 mimic suppresses both glucose- and potassium-induced insulin secretion (GSIS and PSIS, respectively), whereas miR-153 inhibitor enhances both GSIS and PSIS. A similar effect on dopamine secretion also was observed. Using miRNA target prediction software, we found that miR-153 is predicted to target the 3′UTR region of the calcium channel gene, Cacna1c. Further studies confirmed that Cacna1c mRNA and protein are downregulated by miR-153 mimics and upregulated by miR-153 inhibitors in insulin-secreting freshly isolated mouse islets, in the insulin-secreting mouse cell line MIN6 and in the dopamine-secreting cell line PC12.
Conclusions/interpretation
miR-153 is a negative regulator of both insulin and dopamine secretion through its effect on Cacna1c expression, which suggests that IA-2β and miR-153 have opposite functional effects on the secretory pathway.
Keywords: Cav1.2, IA-2, Insulin secretion, Intronic miRNA, MicroRNAs
Introduction
Islet antigen (IA)-2 and IA-2β are major autoantigens in type 1 diabetes [1]. Previous studies have shown that they are integral transmembrane proteins of dense core vesicles (DCVs) and/or synaptic vesicles [2, 3]. IA-2 and IA-2β are widely expressed in neuroendocrine cells throughout the body (e.g. pancreatic islets, brain, adrenals and gastrointestinal cells) [2-5]. They are members of the transmembrane protein tyrosine phosphatase (PTP) family and are enzymatically inactive with standard PTP substrates owing to two critical amino acid substitutions in the PTP domain. However, recent studies suggest that IA-2β may have low phosphatidylinositol phosphatase activity [6]. IA-2 and IA-2β have been found to play important roles in the secretion of hormones and neurotransmitters such as insulin, luteinising hormone (LH) and follicular stimulating hormone (FSH), noradrenaline (norepinephrine, NE), dopamine, serotonin and renin, and reduction of these hormones results in glucose intolerance, female infertility, behavioural changes and loss of circadian rhythm [3, 7-14].
Recently, we showed that the microRNA, miR-153, is embedded in intron 19 of both IA-2 (also known as PTPRN and ICA512) and IA-2β (also known as PTPRN2) in humans and encodes two identical mature sequences (miR-153-1 and miR-153-2) [15]. In contrast, in the mouse and rat, miR-153 is found in the Ia-2β gene (also known as Ptprn2 andphogrin). The miR-153 precursor gives rise to two miRNA sequences, miR-153-3p and miR-153-5p, of which only miR-153-3p is identical to the human miR-153-2 and is highly conserved, evolutionarily, across different species [15].
It is estimated that ~37% of mammalian miRNAs are located within introns of protein coding genes [16], and co-regulation of these intragenic miRNAs and their host genes is a common biological phenomenon [15, 17]. Moreover, intronic miRNAs and their host genes, often tend to work in parallel, forming regulatory relationships between host genes and intronic miRNAs [18]. Based on the function of its host gene, Ia-2β, miR-153 is potentially linked to the secretion of hormones and neurotransmitters. Moreover, in a recent study we showed that in Ia-2β knockout mice, miR-153 is at least partly co-regulated with its host gene, Ia-2β [15]. However, the exact relationship between IA-2β, miR-153, and its target genes remains unclear. The present study was initiated to assess the functional effect of miR-153 on hormone and neurotransmitter secretion and to further our understanding of the relationship between the host gene Ia-2β, its intronic miR-153 and the gene targets of miR-153.
Methods
Mice
Targeted disruption of the individual Ia-2 and Ia-2β genes (Ia-2 KO and Ia-2β KO) and generation of Ia-2−/−/Ia-2β−/− double knockout (DKO) mice have been described previously [7, 15]. Animals used in this study were from C57/BL6 background and produced in our institute animal core facility (National Institute of Dental and Craniofacial Research [NIDCR], Bethesda, MD, USA). Lights were on from 6:00 AM to 6:00 PM and food and water were available ad libitum. All tissue samples harvested for this study were from age and sex-matched mice that were randomly selected from their home cage. Animal studies were conducted under protocols approved by the Institutional Animal Care and Use Committees of the National Institutes of Health (NIH), USA.
Cell lines
The MIN6 cell line, 293T cell line and PC12 cells culture methods are described in detail in the Electronic Supplementary Material (ESM) Methods..
Mouse islets
Islets from 3-4 month old sex-matched mice were isolated with slight modifications of the Collagenase P (Roche, Indianapolis, IN, USA) manufacturer’s protocol. See ESM Methods for further details.
Bioinformatics analysis of potential miR-153 targets
Identification of putative target genes of miR-153 was performed by two bioinformatics software programs, Targetscan 6.2 (www.targetscan.org/vert_61/, release 6.2, accessed 28 February 2013) and Pictar (http://pictar.mdc-berlin.de/, accessed 28 February 2013). We searched for miR-153 targets using the term ‘calcium’. Overlap of miR-153 putative target genes by these two software programs were selected for further analysis.
MicroRNA mimic and inhibitor transfection
MIN6 cells, mouse islets and PC12 cells were transfected with a miR-153 mimic or inhibitor (Qiagen, Germantown, MD, USA) or scramble control (AllStars Negative Control siRNA, Qiagen), using HiPerFect Transfection Reagent (Qiagen) according to the manufacturer’s instructions. After 72 h, the transfected cells were further processed for glucose- or potassium-stimulated insulin secretion (GSIS or PSIS, respectively), gene expression analyses or western blotting. For further details, see ESM Methods.
GSIS or PSIS
Insulin secretion in MIN6 cells and mouse islets was measured after the cells were transfected as described above. Fold change of insulin secretion was calculated by comparing insulin levels before and after stimulation. For further details, see ESM Methods.
Dopamine secretion test
The dopamine secretion test in PC12 cells was conducted at 72 h after transfection with miR-153 mimic, miR-153 inhibitor or control. Fold change of dopamine secretion was calculated by comparing dopamine levels before and after stimulation with high K+ solution with or without phorbol-12-myristate-13-acetate (PMA). For further details, see ESM Methods.
Total RNA, miRNA and quantitative PCR
Total RNA and miRNA were extracted using a miRNeasy Mini kit (Qiagen) following manufacturer’s protocols. cDNA synthesis for both mRNA and miRNA was performed using a miScript II RT kit (Qiagen). Quantitative real-time PCR was performed using a miScript SYBR Green PCR kit (Qiagen) for miR-153 or an SYBR Green PCR Master Mix (Life Technologies, Frederick, MD, USA) for Ia-2β, Cacna1c, Cacnb4, Camk2g, Cask, Cib2 and Gapdh (primer sequences are available in ESM Table 1), and analysed on a 7500 Real-Time PCR system (Life Technologies).
Generation of Cacna1c 3′UTR reporter construct
The method for constructing the wild-type (WT) and mutant luciferase reporter plasmids for miRNA target validation was adapted from a previous report [19]. After sequencing validation, plasmid DNA was prepared using a Plasmid Miniprep kit (Qiagen). For further details, see ESM Methods.
Transfection and luciferase reporter assays
293T cells were co-transfected with luciferase reporter plasmids (either a WT or mutant plasmid) and miR-153 mimic (Qiagen) or the scramble control (Qiagen), and the Renilla luciferase control vector (Promega, Madison, WI, USA), using Lipofecatamine 2000 (Life Technologies) according to manufacturers’ instructions. The luciferase activity assay was performed 24 h after transfection, using the Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase activity was normalised to Renilla luciferase activity.
Target protector functional analysis
MiR-153 miScript target protector (miR-153-Cacna1c) was designed to be complementary to the predicted miR-153 binding site in the Cacna1c 3′UTR (Qiagen). Transfection was performed according to the manufacturer’s protocol. GSIS was performed 72 h later, together with quantitative real-time PCR for Cacna1c mRNA to assess the protective effect of the target protector. For further details, see ESM Methods.
Calcium microfluorimetry
[Ca2+]i was measured using the ratiometric dye fura-2/AM (a high affinity, intracellular calcium indicator), with procedures modified from a previous report [20]. For further details, see ESM Methods.
Protein extraction and western blot analysis
Proteins from cells or mouse tissues were isolated and then analysed by western blotting according to standard procedures. See ESM Methods for further details. Rabbit-anti-calcium channel, voltage-dependent, L type, α1C subunit (CACNA1C) antibody (1:500 dilution, Santa Cruz Biotech, Dallas, TX, USA) and mouse-anti-α-tubulin antibody (1:5000 dilution, Abcam, Cambridge, MA, USA) were employed as primary antibodies. Blots were quantified using NIH Image J Software.
Statistical analysis
Unless stated otherwise, each experiment was performed three times (n=3) and assayed in triplicate. Data are expressed as the mean ± SEM of the three experiments. The Student’s t test for two groups or ANOVA for multiple groups were used to determine statistical significance. In all cases, p<0.05 was considered significant.
Results
The effect of miR-153 on insulin and dopamine secretion
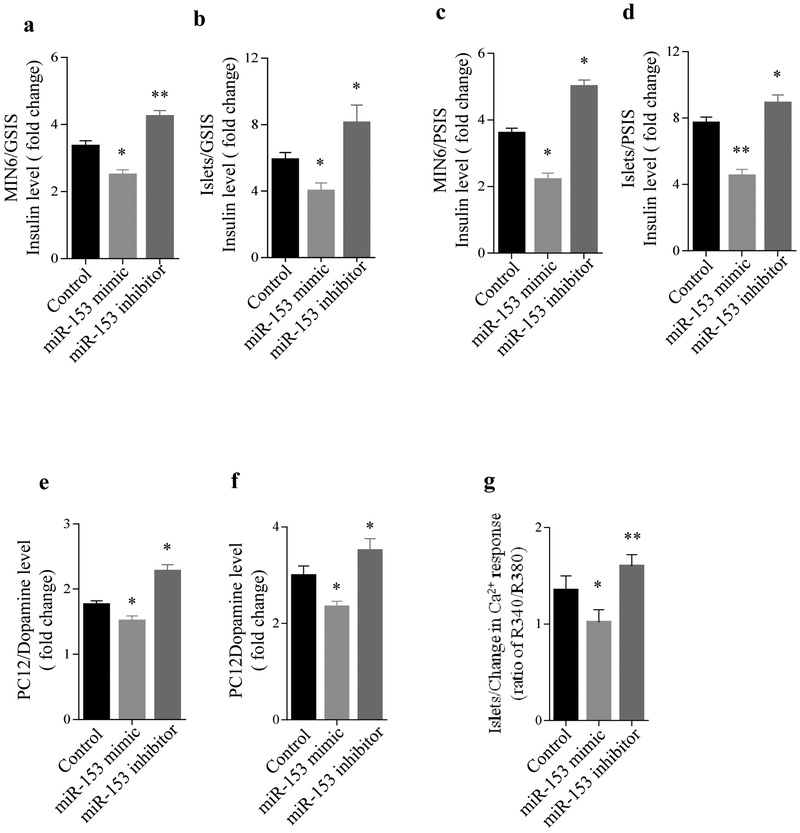
To evaluate the effect of miR-153 on insulin secretion, we used the mouse insulin-secreting cell line MIN6 and freshly isolated pancreatic islets from mice. Cells were transfected with a miR-153 mimic or inhibitor (ESM Fig. 1a,b) and analysed for GSIS and PSIS. Transfection of MIN6 cells with a miR-153 mimic, followed by glucose stimulation, led to a 25% reduction in insulin secretion. In contrast, miR-153 inhibitor led to a 30% increase in insulin secretion following glucose stimulation (Fig. 1a). Similar results were obtained using freshly isolated mouse islets (Fig. 1b). Stimulation with high potassium led to a 40-50% reduction in insulin secretion in the miR-153 mimic group and a 20-30% increase in insulin secretion in the miR-153 inhibitor group (Fig. 1c,d, respectively). These results clearly show that overexpression of miR-153 suppressed both GSIS and PSIS, whereas inhibition of endogenous miR-153 function enhanced insulin secretion.
Fig. 1.
Impact of miR-153 on insulin and dopamine secretion. After stimulation with 25 mmol/l glucose (a, b) or 50 mmol/l KCl (c, d) in MIN6 cells, or primary mouse islets transfected with scrambled control, miR-153 mimic or miR-153 inhibitor for 72 h, insulin secretion was measured. Data are presented as fold change compared with the level before stimulation. Experiments were performed six times (n=6) in triplicate. (e, f) Dopamine secretion (fold change) in PC12 cells stimulated with 25 mmol/l KCl with (e) or without (f) PMA compared with basal levels, 72 h after transfection with miR-153 mimic or miR-153 inhibitor (n=3). (g) Change in mouse islet [Ca2+] responses after islet transfection with scrambled controls, miR-153 mimic or miR-153 inhibitor for 72 h, followed by stimulation with 30 mmol/l KCl. *p<0.05 and **p<0.01 vs controls
Based on the high endogenous expression of miR-153 in brain and the role of its host genes in neurotransmitter secretion, we hypothesised that miR-153 would also play a role in the secretion of neurotransmitters. To test this hypothesis, we overexpressed or inhibited miR-153 in PC12 cells (ESM Fig. 1c), a dopamine-secreting cell line, and measured dopamine secretion. PC12 cells transfected with miR-153 mimic, followed by potassium stimulation, led to a 15% reduction in dopamine secretion, whereas PC12 cells transfected with miR-153 inhibitor, followed by potassium stimulation, led to a 20% increase in dopamine secretion (Fig. 1e). Stimulation of PC12 cells with both potassium and the protein kinase C (PKC) stimulator PMA [21], led to a 20% decrease in dopamine secretion in the miR-153 mimic group and ~25% increase in the miR-153 inhibitor group (Fig. 1f). Taken together, these studies show that miR-153 is a negative regulator of both insulin and dopamine secretion.
GSIS occurs through the closure of ATP-sensitive K channels in the beta cell plasma membrane, resulting in cell depolarisation, activation of voltage-dependent calcium channels (VDCC) and a rise in islet [Ca2+]i. In order to ascertain whether miR-153 regulates the activity of VDCCs, we used FURA2/AM to measure KCl-induced changes in [Ca2+]i in control islets, or islets treated with either the miR-153 mimic or inhibitor. In the current study, we observed that 30 mmol/l KCl elicited a robust increase in [Ca2+]i in control islets, and to a larger extent in islets treated with the miR-153 inhibitor (Fig. 1g). Interestingly, the [Ca2+]i response of islets treated with the miR-153 mimic were reduced compared with control islets or to the inhibitor group (Fig. 1g), indicating that miR-153 acts on secretion at least in part by modulating VDCC activity.
Effect of miR-153 on calcium related target genes
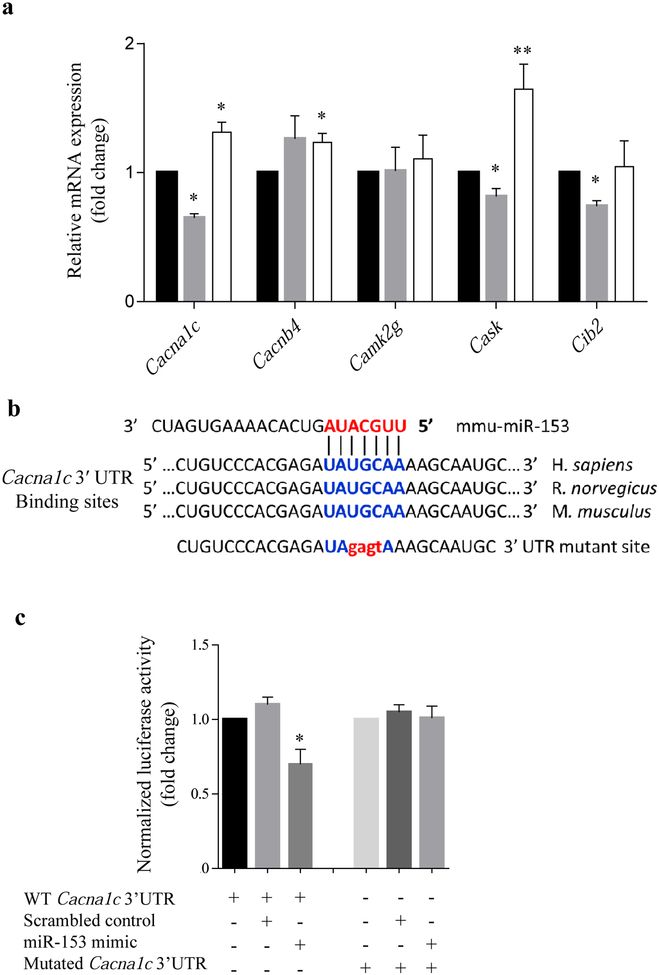
In order to identify potential targets of miR-153 that could affect insulin and dopamine secretion, the bioinformatics software Targetscan 6.2 and Pictar were employed. Within the list of miR-153 predicted targets, we found 11 calcium related targets. Five targets were identified by both software programs: Cacna1c, Cacnb4, Camk2g, Cask and Cib2. To experimentally validate the prediction, MIN6 cells were transfected with a miR-153 mimic or inhibitor. As seen in Fig. 2a, of the five targets identified, only Cacna1c and Cask had expression levels that were significantly reduced or increased after transfection with miR-153 mimic or miR-153 inhibitor. Given that the Cacna1c gene has been implicated in altering insulin secretion as well as neurotransmitters in vitro and in vivo [22-25], we chose to focus on the Cacna1c gene for further investigation.
Fig. 2.
Effect of miR-153 mimic or inhibitor on calcium genes: putative miR-153 target site in the Cacna1c 3′-UTR. (a) Expression analysis of miR-153 predicted targets by quantitative real-time PCR 72 h after transfection with scrambled control (black bars), miR-153 mimic (grey bars) or miR-153 inhibitor (white bars). All data were normalised to Gapdh (n=3). (b) MiR-153 predicted target site in mouse Cacna1c 3′-UTR and mutated 3′-UTR sequence with four nucleotide replacements (lower case letters). There is strong sequence conservation in mice, rats and humans. (c) Luciferase reporter assay demonstrating functional activity of miR-153 mimic on WT Cacna1c 3′-UTR in 293T cells, but not on mutated Cacna1c 3′-UTR. Normalised to Renilla luciferase activity (n=3). *p<0.05 and **p<0.01 vs controls
Validation of a miR-153 target site in the Cacna1c 3′UTR
To confirm that Cacna1c can be directly regulated by miR-153, a portion of the mouse Cacna1c gene 3′-UTR was cloned into a luciferase reporter vector. A mutated construct was also generated, in which the putative miR-153 binding site UAUGCAA was mutated into UAgagtA and then cloned into a luciferase reporter vector (Fig. 2b). When compared with the reporter vector alone, luciferase activity was significantly reduced following transient co-transfection of miR-153 mimic with luciferase expression plasmid in 293T cells (Fig. 2c). In contrast, transfection of 293Tcells with scrambled control did not have any effect on the luciferase activity. However, mutations within the seed sequence binding site of Cacna1c abrogated the effect of miR-153 mimic (Fig. 2c), thereby confirming that Cacna1c is a direct target of miR-153.
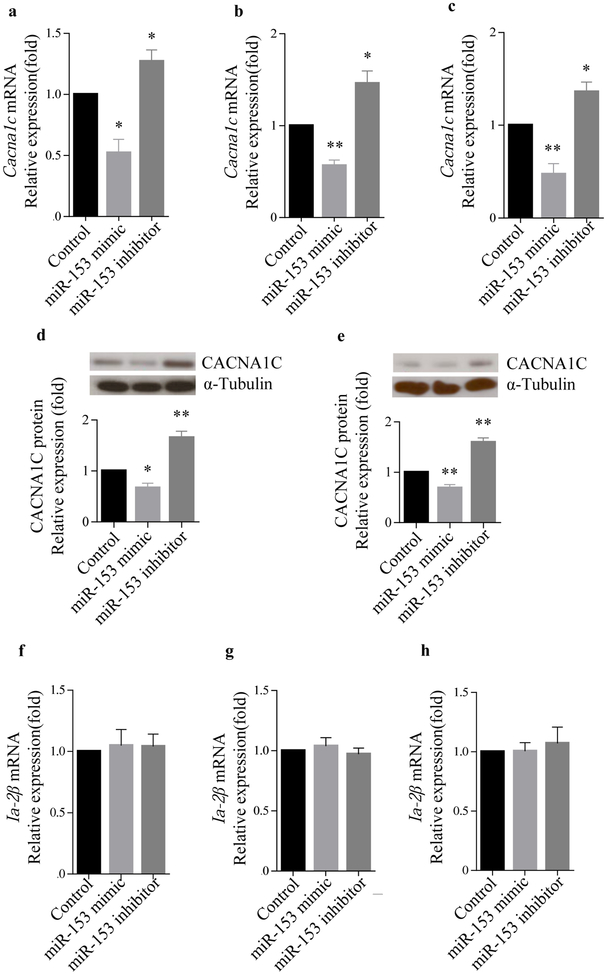
Regulation of endogenous Cacna1c expression by miR-153 in different cell lines
Transfection of cell lines with a miR-153 mimic significantly reduced Cacna1c mRNA expression by nearly 50% as compared with the control in MIN6 cells, normal mouse islets and PC12 cells. In contrast, a significant increase in Cacna1c mRNA expression was observed in all three of these cells following transfection with the miR-153 inhibitor (Fig. 3a–c). Western blot analysis confirmed the effects of miR-153 on the CACNA1C protein level in MIN6 cells and PC12 cells (Fig. 3d,e). To rule out the possibility that miR-153 was exerting its effect through changes in the expression of its host gene, Ia-2β, we determined Ia-2β mRNA levels following transfection of the different cell lines with either miR-153 mimic or miR-153 inhibitor (Fig. 3f,g). Our results showed that miR-153 has no effect on Ia-2β mRNA levels.
Fig. 3.
Regulation of Cacna1c expression by miR-153. Effects of miR-153 mimic and miR-153 inhibitor on Cacna1c expression by quantitative real-time PCR: (a) MIN6 cells; (b) mouse islets; (c) PC12 cells. (n=3). Western blots for CACNA1C and α-tubulin protein levels in MIN6 cells (d) and PC12 cells (e). The relative expression values were determined by Image J (n=3). Ia-2β mRNA levels were unchanged in MIN6 cells (f), mouse islets (g) and PC12 cells (h) following transfection with miR-153 mimic or inhibitor. (n=3), *p<0.05 and **p<0.01 vs controls
Effect of miR-153-Cacna1c target protector
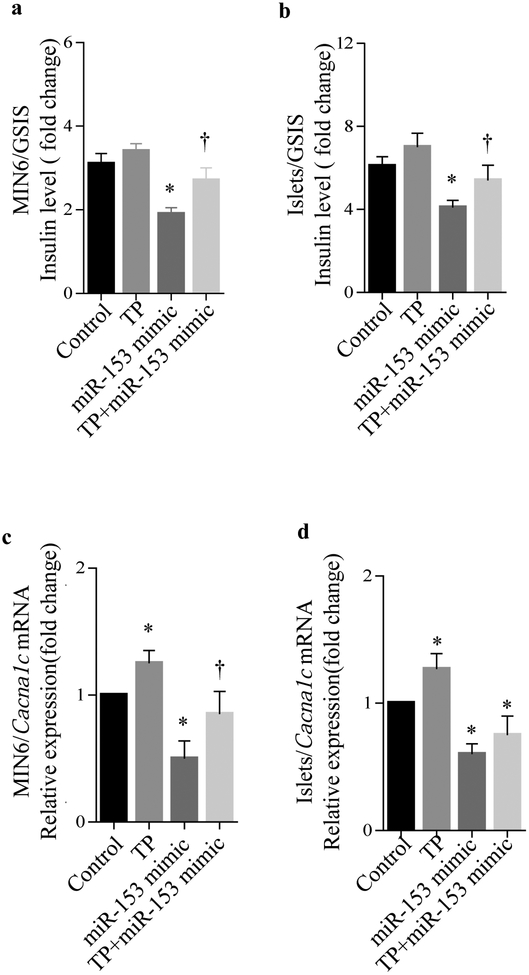
To confirm that the reduction of GSIS was due to miR-153 acting through its effect on the Cacna1c gene, a miR-153-Cacna1c target protector was employed. The target protector is a single-stranded modified RNA that is complementary to the miR-153 binding site on the Cacna1c 3′UTR and specifically disrupts the interaction between miR-153 and Cacna1c 3′UTR. As seen in MIN6 cells (Fig. 4a) and freshly isolated mouse islets (Fig. 4b), transfection with the miR-153-Cacna1c target protector abrogated, at least in part, the suppressive effect of exogenous miR-153 mimic on GSIS. This provides further evidence that the effect of miR-153 mimic on insulin secretion is mediated, at least in part, through Cacna1c gene regulation.
Fig. 4.
Effect of miR-153-Cacna1c target protector. Glucose stimulates insulin secretion (fold change) in MIN6 cells (a) and mouse islets (b), after transfection with miR-153 mimic with and without miR-153-Cacna1c target protector (TP), showing that the target protector partly abrogated the inhibitory effect of miR-153 on GSIS (n=3). Cacna1c expression analysis by quantitative real-time PCR in MIN6 cells (c) and mouse islets (d) after transfection with miR-153 mimic with and without its target protector. All data were normalised to Gapdh. n=3, *p<0.05 vs controls, †p<0.05, miR-153 mimic vs TP+miR-153 mimic
To determine the effect of miR-153-Cacna1c target protector on Cacna1c mRNA expression, quantitative real-time PCR was employed. In MIN6 cells (Fig. 4c) and freshly isolated mouse islets (Fig. 4d), co-transfection of cells with miR-153 mimic and miR-153-Cacna1c target protector also abrogated, at least in part, the suppressive effect of exogenous miR-153 mimic on Cacna1c mRNA levels. Although in freshly isolated mouse islets, co-transfection of miR-153 mimic with miR-153-Cacna1c target protector did not fully restore Cacna1c mRNA expression to normal levels, a positive trend was observed (Fig. 4d). The most likely explanation for the failure of the protector to fully restore GSIS is that it is specific to Cacna1c and therefore could not protect the degradation of other potential miR-153 targets involved in secretion.
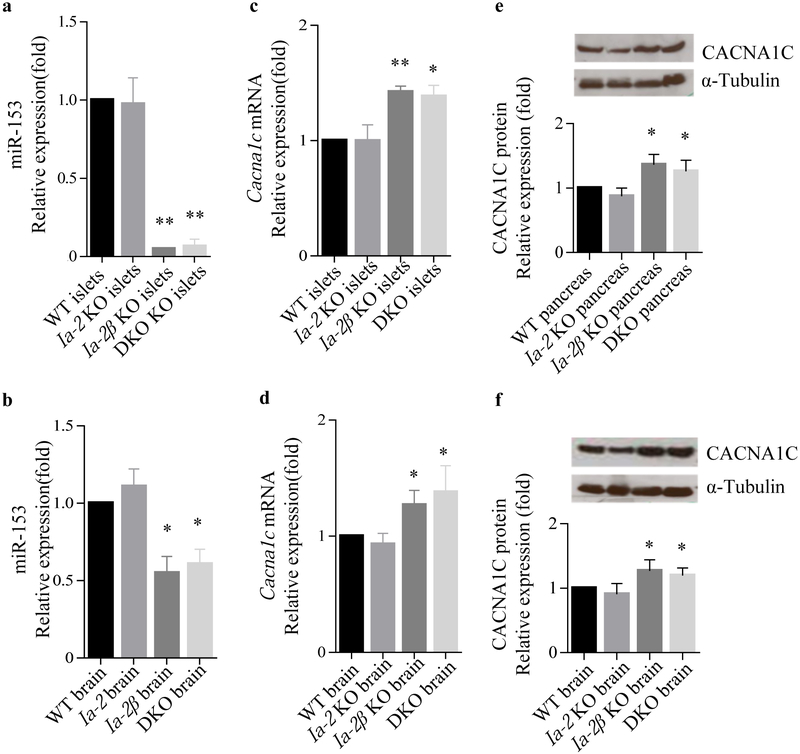
MiR-153 and Cacna1c expression in Ia-2β KO mice
To determine whether the expression of Cacna1c is controlled by miR-153 in vivo, Ia-2β KO and DKO mice, in which miR-153 levels are dramatically reduced [15], were used for expression correlation analysis. Our results showed that miR-153 levels are dramatically reduced in pancreatic islets from Ia-2β KO and DKO mice (Fig. 5a) and also—although to a lesser extent—in the brain from these mice (Fig. 5b). These observations are consistent with previous studies [15]. In contrast, miR-153 levels remain unchanged in pancreatic islets and brain from Ia-2 KO mice, thus supporting the idea that in mice miR-153 is only expressed from Ia-2β. Based on this information, we speculated that Cacna1c mRNA would be elevated in islets and brains from Ia-2β KO and DKO mice due to low expression of miR-153, but would not be elevated in Ia-2 KO mice. As anticipated, we found a significant increase in Cacna1c mRNA (Fig. 5c,d) and protein (Fig. 5e,f) in both the islets and brain of Ia-2β KO and DKO mice, whereas no obvious change was observed in Ia-2 KO mice (Fig. 5c–f). These findings support the contention that the expression of Cacna1c in vivo is regulated, at least in part, by miR-153.
Fig. 5.
miR-153 and Cacna1c expression in Ia-2β KO mice. Quantitative real-time PCR analysis of miR-153 levels (a, b) and Cacna1c mRNA (c, d) in brain and islets from WT, Ia-2 KO, Ia-2β KO and DKO mice, normalised to Gapdh. (e, f) CACNA1C expression was determined by western blot in pancreas and brain from WT, Ia-2 KO, Ia-2β KO and DKO mice. The relative expression values were determined by Image J. n=5, *p<0.05 and **p<0.01 vs controls
Discussion
Previously we showed that the knockout of the DCV transmembrane genes, Ia-2 and Ia-2β in mice, led to a decrease in the number of DCVs and resulted in a decrease in the secretion of hormones and neurotransmitters [3, 7, 10-12]. This in turn results in a variety of pathophysiological changes including glucose intolerance, female infertility, learning and behavioural disorders and dysregulation of circadian rhythm [3, 7, 9-11, 14]. The present experiments add support to previous studies that showed that the knockout of Ia-2β, but not Ia-2, results in a decrease in miR-153, which is embedded in intron 19 of the Ia-2β gene [15]. The current experiments also show that miR-153 mimics depress hormone and neurotransmitter (e.g. insulin and dopamine) secretion, whereas miR-153 inhibitors enhance hormone and neuroendocrine secretion.
Since miRNA function is mediated through its effect on a specific set of target genes, we undertook a bioinformatics search to identify miR-153 predicted target genes that might play a role in secretion. Our search identified 11 calcium channel related genes. Five of these gene targets were common in the two different prediction software algorithms that we used. Validation experiments in MIN6 cells confirmed the effect of miR-153 on the endogenous levels of two of these target genes, Cacna1c and Cask.
We focused on Cacna1c because of its well-known involvement in secretion and found that miR-153 mimic downregulated the expression of Cacna1c mRNA and protein, whereas miR-153 inhibitor upregulated the expression of Cacna1c mRNA and protein in MIN6 cells, normal mouse islets and dopamine-secreting PC12 cells. Thus, there is a complex relationship between IA-2β and miR-153.
Hormone exocytosis and neurotransmitter release are fundamental cell biology processes and their regulation is essential for maintaining normal islet and brain function. The release of insulin and dopamine is tightly regulated and dependent on calcium entering cells following cell membrane depolarisation, with subsequent activation of voltage-gated calcium channels [24-28]. Cacna1c is a well-studied gene. The global knockout of Cacna1c in mice results in death at birth [29], whereas beta cell-specific Cacna1c knockout does not result in death, but does result in strong inhibition of GSIS [23]. Other studies have shown that CACNA1C is physically coupled to a number of vesicle-release machinery proteins including the Rab3-interacting molecules (RIMs), Snap-25 and Syntaxin-1[30]. Interestingly, Snap25 and Syntaxin-1 also are predicted targets of miR-153 [15, 31]. To determine the in vivo importance of this microRNA on the secretion of insulin and dopamine, specific knockout or overexpression of miR-153 in mice is required. The development of these knockout and transgenic mice is underway in our laboratory, but the already existing Ia-2β knockout mice may provide some clues as to what to expect. The Ia-2β, but not the Ia-2, knockout mice, showed a significant decrease of miR-153 and a significant increase of both Cacna1c mRNA and protein in the islets and brain. Thus, it is not unreasonable to expect an increase of CACNA1C in vivo following the specific knockout of miR-153. However, this prediction must be viewed with caution since miR-153 targets a number of genes unrelated to calcium channel genes which could directly or indirectly affect secretion.
Although in the current study we focused on the role of miR-153 on insulin and dopamine secretion, miR-153 has been shown to both suppress and enhance tumour growth and play a role in cell proliferation, migration and invasion [32-37]. In addition, miR-153 is dysregulated in some cases of Parkinson’s disease and Alzheimer’s disease where it affects the expression of several disease-related targets such as α-synuclein, amyloid precursor protein and amyloid precursor-like protein 2 [38-41].
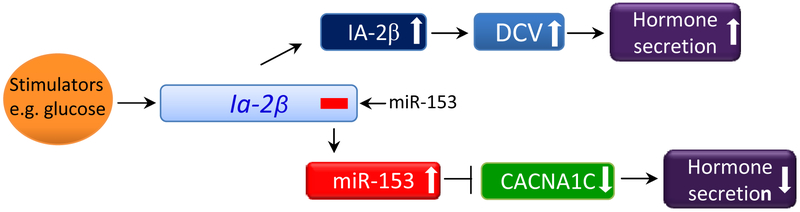
In conclusion, stimulation of the Ia-2β gene increases both IA-2β protein and miR-153 expression (Fig. 6). The increase in IA-2β protein increases the number of DCV and in turn neuroendocrine secretion [3, 7, 10, 42]. In contrast, the increase in miR-153, which is a negative regulator, decreases Cacna1c expression and in turn suppresses neuroendocrine secretion. Thus, IA-2β and miR-153 have opposite functional effects on the secretory pathway.
Fig. 6.
Model illustrating the dual, but opposite, effects on secretion resulting from the stimulation of both the Ia-2β gene and the expression of its intronic microRNA, miR-153. The increase in IA-2β protein facilitates secretion by increasing the number of DCV, whereas an increase in the expression of miR-153, a negative regulator, decreases secretion by inhibiting the expression of calcium channel gene, Cacna1c
Supplementary Material
Acknowledgements
The authors thank T. Cai (NIDCR, NIH, Bethesda, MD, USA) for his advice and meaningful discussions.
Funding
This work was supported by the Intramural Research Program of the NIDCR, NIH, Bethesda, MD, USA. Research in LSS’s lab is funded by RO1DK46409.
Abbreviations
- CACNA1C
Calcium channel, voltage-dependent, L type, α1C subunit
- DCV
Dense core vesicle
- DKO
Ia-2/Ia-2β double knockout
- GSIS
Glucose-stimulated insulin secretion
- IA
Islet antigen
- Ia-2 KO
Ia-2 knockout mice
- Ia-2β KO
IA-2β knockout mice
- miRNA
MicroRNA
- NIDCR
National Institute of Dental and Craniofacial Research
- PMA
Phorbol-12-myristate-13-acetate
- PSIS
Potassium-stimulated insulin secretion
- PTP
Protein tyrosine phosphatase
- TP
Target protector
- VDCC
Voltage-dependent calcium channels
- WT
Wild-type
Footnotes
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Notkins AL, Lernmark A (2001) Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest 108: 1247–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solimena M, Dirkx R, Hermel JM, et al. (1996) ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J 15: 2102–2114 [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura T, Kubosaki A, Ito Y, Notkins AL (2009) Disturbances in the secretion of neurotransmitters in IA-2/IA-2beta null mice: changes in behavior, learning and lifespan. Neuroscience 159: 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomi H, Kubota-Murata C, Yasui T, Tsukise A, Torii S (2013) Immunohistochemical analysis of IA-2 family of protein tyrosine phosphatases in rat gastrointestinal endocrine cells. J Histochem Cytochem 61: 156–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeyama N, Ano Y, Wu G, et al. (2009) Localization of insulinoma associated protein 2, IA-2 in mouse neuroendocrine tissues using two novel monoclonal antibodies. Life Sci 84: 678–687 [DOI] [PubMed] [Google Scholar]
- 6.Caromile LA, Oganesian A, Coats SA, Seifert RA, Bowen-Pope DF (2010) The neurosecretory vesicle protein phogrin functions as a phosphatidylinositol phosphatase to regulate insulin secretion. J Biol Chem 285: 10487–10496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai T, Hirai H, Zhang G, et al. (2011) Deletion of Ia-2 and/or Ia-2beta in mice decreases insulin secretion by reducing the number of dense core vesicles. Diabetologia 54: 2347–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SM, Theilig F, Qin Y, et al. (2009) Dense-core vesicle proteins IA-2 and IA-2β affect renin synthesis and secretion through the β-adrenergic pathway. Am J Physiol Renal Physiol 296: F382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubosaki A, Nakamura S, Notkins AL (2005) Dense core vesicle proteins IA-2 and IA-2beta: metabolic alterations in double knockout mice. Diabetes 54(Suppl 2): S46–S51 [DOI] [PubMed] [Google Scholar]
- 10.Kubosaki A, Gross S, Miura J, et al. (2004) Targeted disruption of the IA-2beta gene causes glucose intolerance and impairs insulin secretion but does not prevent the development of diabetes in NOD mice. Diabetes 53: 1684–1691 [DOI] [PubMed] [Google Scholar]
- 11.Saeki K, Zhu M, Kubosaki A, Xie J, Lan MS, Notkins AL (2002) Targeted disruption of the protein tyrosine phosphatase-like molecule IA-2 results in alterations in glucose tolerance tests and insulin secretion. Diabetes 51: 1842–1850 [DOI] [PubMed] [Google Scholar]
- 12.Kubosaki A, Nakamura S, Clark A, Morris JF, Notkins AL (2006) Disruption of the transmembrane dense core vesicle proteins IA-2 and IA-2beta causes female infertility. Endocrinology 147: 811–815 [DOI] [PubMed] [Google Scholar]
- 13.Carmona GN, Nishimura T, Schindler CW, Panlilio LV, Notkins AL (2014) The dense core vesicle protein IA-2, but not IA-2beta, is required for active avoidance learning. Neuroscience 269: 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SM, Power A, Brown TM, et al. (2009) Deletion of the secretory vesicle proteins IA-2 and IA-2beta disrupts circadian rhythms of cardiovascular and physical activity. FASEB J 23: 3226–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandemakers W, Abuhatzira L, Xu H, et al. (2013) Co-regulation of intragenic microRNA miR-153 and its host gene Ia-2 beta: identification of miR-153 target genes with functions related to IA-2beta in pancreas and brain. Diabetologia 56: 1547–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10: 126–139 [DOI] [PubMed] [Google Scholar]
- 17.Baskerville S, Bartel DP (2005) Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA 11: 241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao X, Qiao Y, Han D, Zhang Y, Ma N (2012) Enemy or partner: relationship between intronic micrornas and their host genes. IUBMB life 64: 835–840 [DOI] [PubMed] [Google Scholar]
- 19.Nicolas FE (2011) Experimental validation of microRNA targets using a luciferase reporter system. Methods in molecular biology 732: 139–152 [DOI] [PubMed] [Google Scholar]
- 20.Nunemaker CS, Zhang M, Wasserman DH, et al. (2005) Individual mice can be distinguished by the period of their islet calcium oscillations: is there an intrinsic islet period that is imprinted in vivo? Diabetes 54: 3517–3522 [DOI] [PubMed] [Google Scholar]
- 21.Nishimura T, Harashima S, Yafang H, Notkins AL (2010) IA-2 modulates dopamine secretion in PC12 cells. Mol Cell Endocrinol 315: 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitert MD, Nagorny CL, Wendt A, Eliasson L, Mulder H (2008) CaV1.2 rather than CaV1.3 is coupled to glucose-stimulated insulin secretion in INS-1 832/13 cells. J Mol Endocrinol 41: 1–11 [DOI] [PubMed] [Google Scholar]
- 23.Schulla V, Renstrom E, Feil R, et al. (2003) Impaired insulin secretion and glucose tolerance in beta cell-selective Ca(v)1.2 Ca2+ channel null mice. EMBO J 22: 3844–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortensen OV (2013) MKP3 eliminates depolarization-dependent neurotransmitter release through downregulation of L-type calcium channel Cav1.2 expression. Cell Calcium 53: 224–230 [DOI] [PubMed] [Google Scholar]
- 25.Hofmann F, Flockerzi V, Kahl S, Wegener JW (2014) L-type CaV1.2 calcium channels: from in vitro findings to in vivo function. Physiol Rev 94: 303–326 [DOI] [PubMed] [Google Scholar]
- 26.Gaisano HY (2014) Here come the newcomer granules, better late than never. Trends Endocrinol Metab 25: 381–388 [DOI] [PubMed] [Google Scholar]
- 27.Sudhof TC (2012) Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol 4: a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rorsman P, Braun M, Zhang Q (2012) Regulation of calcium in pancreatic alpha- and beta-cells in health and disease. Cell Calcium 51: 300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seisenberger C, Specht V, Welling A, et al. (2000) Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem 275: 39193–39199 [DOI] [PubMed] [Google Scholar]
- 30.Gandini MA, Felix R (2012) Functional interactions between voltage-gated Ca(2+) channels and Rab3-interacting molecules (RIMs): new insights into stimulus-secretion coupling. Biochim Biophys Acta 1818: 551–558 [DOI] [PubMed] [Google Scholar]
- 31.Wei C, Thatcher EJ, Olena AF, et al. (2013) miR-153 regulates SNAP-25, synaptic transmission, and neuronal development. PLoS One 8: e57080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anaya-Ruiz M, Cebada J, Delgado-Lopez G, Sanchez-Vazquez ML, Perez-Santos JL (2013) miR-153 silencing induces apoptosis in the MDA-MB-231 breast cancer cell line. Asian Pac J Cancer Prev 14: 2983–2986 [PubMed] [Google Scholar]
- 33.Wu Z, He B, He J, Mao X (2013) Upregulation of miR-153 promotes cell proliferation via downregulation of the PTEN tumor suppressor gene in human prostate cancer. Prostate 73: 596–604 [DOI] [PubMed] [Google Scholar]
- 34.Xu Q, Sun Q, Zhang J, Yu J, Chen W, Zhang Z (2013) Downregulation of miR-153 contributes to epithelial-mesenchymal transition and tumor metastasis in human epithelial cancer. Carcinogenesis 34: 539–549 [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Pickard K, Jenei V, et al. (2013) miR-153 supports colorectal cancer progression via pleiotropic effects that enhance invasion and chemotherapeutic resistance. Cancer Res 73: 6435–6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan Y, Du W, Wang Y, et al. (2015) Suppression of AKT expression by miR-153 produced anti-tumor activity in lung cancer. Int J Cancer 136: 1333–1340 [DOI] [PubMed] [Google Scholar]
- 37.Shan N, Shen L, Wang J, He D, Duan C (2015) MiR-153 inhibits migration and invasion of human non-small-cell lung cancer by targeting ADAM19. Biochem Biophys Res Commun 456: 385–391 [DOI] [PubMed] [Google Scholar]
- 38.Liang C, Zhu H, Xu Y, et al. (2012) MicroRNA-153 negatively regulates the expression of amyloid precursor protein and amyloid precursor-like protein 2. Brain Res 1455: 103–113 [DOI] [PubMed] [Google Scholar]
- 39.Doxakis E (2010) Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. The Journal of biological chemistry 285: 12726–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long JM, Ray B, Lahiri DK (2012) MicroRNA-153 physiologically inhibits expression of amyloid-beta precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J Biol Chem 287: 31298–31310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HJ, Park G, Jeon BS, Park WY, Kim YE (2013) A mir-153 binding site variation in SNCA in a patient with Parkinson's disease. Mov Disord 28: 1755–1756 [DOI] [PubMed] [Google Scholar]
- 42.Harashima S, Clark A, Christie MR, Notkins AL (2005) The dense core transmembrane vesicle protein IA-2 is a regulator of vesicle number and insulin secretion. Proc Natl Acad Sci U S A 102: 8704–8709 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.