SUMMARY
microRNAs (miRNAs) broadly regulate gene expression through association with Argonaute (Ago), which also protects miRNAs from degradation. However, miRNA stability is known to vary and is regulated by poorly understood mechanisms. A major emerging process, termed target-directed miRNA degradation (TDMD), employs specialized target RNAs to selectively bind to miRNAs and induce their decay. Here, we report structures of human Ago2 (hAgo2) bound to miRNAs and TDMD-inducing targets. miRNA and target form a bipartite duplex with an unpaired flexible linker. hAgo2 cannot physically accommodate the RNA, causing the duplex to bend at the linker and display the miRNA 3′-end for enzymatic attack. Altering 3′-end display by changing linker flexibility, 3′-end complementarity, or mutationally inducing 3′-end release impacts TDMD efficiency leading to production of distinct 3′-miRNA isoforms in cells. Our results uncover the mechanism driving TDMD and reveal 3′-end display as a key determinant regulating miRNA activity via 3′-remodeling and/or degradation.
eTOC Blurb
microRNAs (miRNAs) shape post-transcriptional gene expression by repressing messenger RNAs . Conversely, certain target RNAs induce miRNA decay through a process called target-directed miRNA degradation (TDMD). Sheu-Gruttadauria and Pawlica, et al., show how these targets expose the miRNA 3′end for enzymatic attack, enabling 3′-end remodeling and degradation.
INTRODUCTION
miRNAs are small, single-stranded RNAs that shape the expression of most messenger RNAs (mRNAs) and are critical for physiological homeostasis and development in metazoans (Bartel, 2018). miRNAs function through a class of proteins called Ago, of which there are four in humans (hAgo1-hAgo4). Ago uses the miRNA as a guide to locate complementary sequences in RNA targets. These targets are then silenced through either the inherent endonucleolytic cleavage activity of hAgo2 or, more typically, through inducing translational suppression and/or mRNA degradation.
Despite the pervasive biological role of miRNAs and detailed understanding of their biogenesis, mechanisms driving miRNA turnover remain largely enigmatic (Bartel, 2018). However, it is known that miRNA stability can vary dramatically between specific miRNAs and cellular contexts and therefore appears to be regulated (Bartel, 2018; Duffy et al., 2015; Marzi et al., 2016; Ruegger and Grosshans, 2012). Recently, it has been demonstrated that Tudor-SN cleaves and downregulates certain miRNAs (Elbarbary et al., 2017). An additional mechanism for turnover is driven by highly complementary target RNAs that, upon binding to their cognate miRNA, result in miRNA decay instead of target repression through a process known as target-directed miRNA degradation (TDMD) (Ameres et al., 2010; Ameres et al., 2011; Baccarini et al., 2011; Cazalla et al., 2010; Lee et al., 2013; Libri et al., 2012; Marcinowski et al., 2012; Xie et al., 2012). Initial insights into TDMD arose from artificial targets (Ameres et al., 2010) and studies of HSUR1, a viral non-coding RNA that binds host miR-27 and induces its decay (Cazalla et al., 2010). The discovery of additional viral and cellular transcripts that also provoke miRNA degradation has revealed that TDMD is a widespread mechanism for regulating miRNA abundance (Bitetti et al., 2018; Ghini et al., 2018; Kleaveland et al., 2018; Lee et al., 2013; Libri et al., 2012; Marcinowski et al., 2012).
Unlike ordinary miRNA targets that pair predominantly with the miRNA seed region (nts 2-8 from the 5′ end), which is sometimes accompanied by supplementary interactions (with nts 12-17), TDMD targets exhibit central mismatches and extensive complementarity to the miRNA 3′ end (Table S1). TDMD is often accompanied by miRNA 3′-terminal tailing and trimming (here referred to as remodeling) (Ameres et al., 2010; Ameres et al., 2011; de la Mata et al., 2015; Kleaveland et al., 2018; Marcinowski et al., 2012). Ago typically sequesters its guide RNA within a central RNA binding cleft and shields both miRNA termini, suggesting that extensive pairing with TDMD targets must expose the miRNA 3′ end to terminal nucleotidyl transferases and to 3′-to-5′ exonucleases.
Current structural insights into Ago-mediated miRNA targeting in animals indicate that Ago creates distinct chambers within its central cleft that facilitate binding with certain regions of its guide RNA, effectively dividing the miRNA into functional domains. Ago first creates a “seed chamber” for efficient and stable association with seed-paired target RNAs (Schirle et al., 2014). hAgo2 also creates a “supplementary chamber,” which houses the supplementary nucleotides and creates an energetically favorable site for initial interactions with the guide 3′ half (Sheu-Gruttadauria et al., 2019). These two chambers are separated by a “central gate,” which occludes the central region of the miRNA guide and narrows the central nucleic acid binding cleft. Beyond the supplementary chamber, which can only accommodate a duplex ≤5 base pairs, the miRNA tail threads through a narrow N-PAZ channel with the 3′ end securely bound within the PAZ domain. Therefore, how hAgo2 accommodates extensive 3′ miRNA base-pairing beyond the supplementary region and how the miRNA 3′ end might become exposed during TDMD remains unknown.
Here, we report crystal structures of hAgo2 bound simultaneously to miRNAs and TDMD targets. The complex adopts a previously unseen conformation in which the central gate of hAgo2 is open, miRNA and target are wrapped around each other, and the miRNA 3′ end is released from its binding pocket. The shape of the Ago2 central cleft and distortions in the miRNA-target duplex, mediated by the presence of central mismatches, allow display of the miRNA 3′ end for enzymatic attack. Target mutagenesis demonstrates that for efficient TDMD, a miRNA and a target must form a flexible bipartite duplex. Importantly, the structure of the duplex, including 3′-pairing features and flexibility between paired regions, substantially influences the 3′-remodeling outcome, suggesting that these features determine accessibility to cellular enzymes. Finally, through Ago mutagenesis we show that once miRNA 3′-end binding is compromised, the miRNA 3′ end is available for tailing and trimming by yet unknown cellular enzymes. The combined results reveal how human Ago engages targets with extended complementarity, how TDMD targets enable miRNA decay, and structural features that direct miRNA 3′-remodeling. These insights may inform both TDMD target identification and determination of the enzymes responsible for decay.
RESULTS
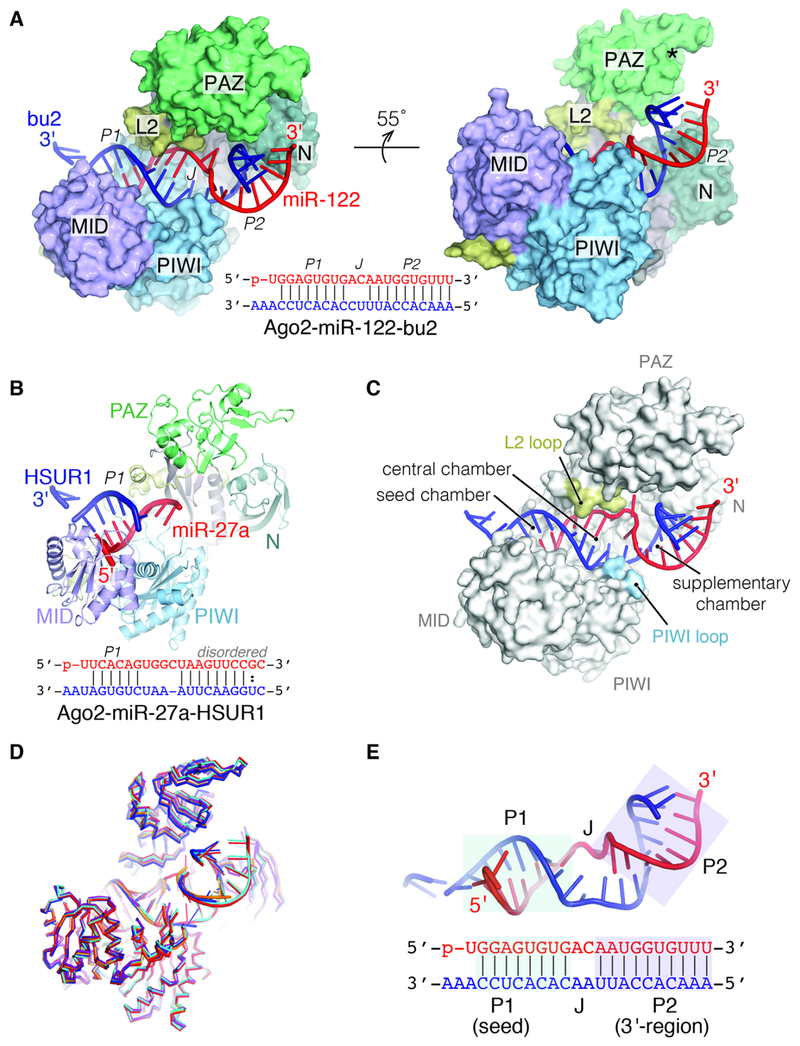
Structure of Human Ago2–miRNA–TDMD target complex
To explore how human Ago accommodates extended 3′ miRNA base-pairing beyond the supplementary region and how the miRNA 3′ end might become exposed during TDMD, we determined crystal structures of hAgo2 bound to miRNAs and TDMD targets (Table 1). We examined HSUR1 in complex with miR-27a, as well as several artificial targets with extensive complementarity to miR-122 (bu2, bu3, and bu4, reflecting the number of central mismatches). Notably, bu4 has been previously shown to drive TDMD of miR-122 when overexpressed in hepatocytes (Denzler et al., 2016). In all determined structures, hAgo2 adopts an extended conformation that exhibits a wider central cleft than ever observed in a eukaryotic Argonaute (Figs. 1 and S1). The central gate, composed of loops PIWI (P601–G607) and L2 (G349–T357), is open, which allows the miRNA-target duplex to extend into a now accessible “central chamber” (Fig. 1C). All structures show unambiguous electron density for an RNA duplex (called P1) formed by the miRNA seed-pairing to the target (Fig. 1D). Electron density for a second RNA duplex (P2), composed of the target and miRNA 3′ end, was also observed in most structures (Fig. 1D). P1 and P2 are connected by an unpaired joining region (J), which renders the miRNA-target duplex bent and partially under-wound at its center (Fig. 1E). Importantly, all structures showed unambiguous release of the miRNA 3′ end from the PAZ binding pocket, which instead is occupied by the 3′ terminus of the target RNA from a neighboring molecule in the crystal lattice.
Table 1. Crystallographic data and refinement statistics.
Numbers in parentheses represent statistics in highest resolution shell. Rmerge = (ΣhΣi|Ih − Ihi| / ΣhΣiIhi) × 100, where Ih is the mean of Ihi observations of reflection h. R-factor and Rfree = Σ||Fo| − |Fc|| / Σ|Fo| × 100 for 95% of recorded data (R-factor) or 5% of data (Rfree).
| hAgo2 crystal | miR-27a-HSUR1 | miR-122–bu2 | miR-122–bu3 | miR-122–bu4 |
|---|---|---|---|---|
| PDB ID | 6MFN | 6MDZ | 6MFR | 6NIT |
| Beamline | APS 23IDD | SSRL 12-2 | SSRL 12-2 | SSRL 12-2 |
| Space Group | P22121 | P212121 | P212121 | P212121 |
| Unit Cell Dimensions | ||||
| a (Å) | 66.95 | 107.68 | 109.79 | 107.43 |
| b (Å) | 104.21 | 138.47 | 138.43 | 138.04 |
| c (Å) | 152.86 | 152.76 | 153.81 | 154.86 |
| α (°) | 90.00 | 90.00 | 90.00 | 90.00 |
| β (°) | 90.00 | 90.00 | 90.00 | 90.00 |
| γ (°) | 90.00 | 90.00 | 90.00 | 90.00 |
| hAgo2 Molecules per ASU | 1 | 2 | 2 | 2 |
| Data Collection | ||||
| Wavelength (Å) | 1.033230 | 0.979500 | 0.979500 | 0.979500 |
| Resolution (Å) | 28.59-2.5 | 39.50-3.40 | 39.57-3.59 | 39.56-3.79 |
| (2.60-2.50) | (3.58-3.40) | (3.81-3.59) | (4.10-3.79) | |
| No Reflections | ||||
| Total | 125207 | 167924 | 204429 | 167916 |
| Unique | 37628 | 31940 | 27731 | 23149 |
| Completeness (%) | 99.3 (98.5) | 99.5 (99.0) | 98.7 (92.6) | 98.9 (97.0) |
| Redundancy | 3.3 (3.4) | 5.3 (5.4) | 7.4 (7.1) | 7.3 (6.9) |
| I/σI | 13.9 (2.4) | 8.4 (3.0) | 8.5 (2.9) | 5.8 (1.9) |
| Rmerge | 6.1 (51.5) | 16.6 (56.4) | 19.3 (78.0) | 28.3 (100.68) |
| Rpim | 3.9 (32.3) | 8.0 (26.8) | 7.6 (31.1) | 11.2 (43.0) |
| Refinement | ||||
| Resolution (Å) | 28.59-2.50 | 38.29-3.40 | 39.42-3.60 | 39.30-3.80 |
| R–free/R–factor | 24.68/20.90 | 26.43/21.97 | 28.27/23.92 | 29.41/24.27 |
| R.M.S. Deviation | ||||
| Bond Distances (Å) | 0.003 | 0.002 | 0.003 | 0.003 |
| Bond Angles (°) | 0.71 | 0.57 | 0.85 | 0.82 |
| Number of Atoms | ||||
| Non-hydrogen, protein | 6459 | 12810 | 12913 | 12798 |
| Non-hydrogen, RNA | 379 | 1498 | 1297 | 1297 |
| Phenol | 0 | 35 | 0 | 0 |
| Phosphate | 0 | 15 | 0 | 0 |
| Mg | 1 | 3 | 0 | 0 |
| Water | 31 | 0 | 0 | 0 |
| Ramachandran Plot | ||||
| Most Favored Regions | 95.6% | 94.7% | 94.3% | 95.1% |
| Additionally Allowed | 4.3% | 5.2% | 5.6% | 4.8% |
| Generously Allowed | 0.1% | 0.1% | 0.1% | 0.1% |
Figure 1. The TDMD conformation of hAgo2.
(A) 3.4 Å structure of hAgo2 (surface representation) bound to miRNA-122 (red) and a target RNA with extensive complementarity (bu2, blue). hAgo2 domains are labeled. Asterisk indicates location of the 3′ binding pocket in the PAZ domain. Below is miR-122–bu2 duplex schematic. (B) 2.5 Å structure of hAgo2 (cartoon representation) bound to miR-27a (red) and the HSUR1 target region (blue). miR-27a-HSUR1 duplex schematic is shown below, with disordered nucleotides indicated. (C) Surface representation of TDMD conformation highlighting open central cleft. Seed, central, and supplementary chambers are labeled. The open central gate formed by L2 and PIWI loops is shown. (D) Superposition of all hAgo2–miRNA–TDMD target structures (7 structures, including all unique copies in the asymmetric units of the 4 obtained crystal forms) demonstrating a common overall conformation. (E) The miR-122–bu2 duplex (schematic shown below with miRNA sequence in red, and TDMD target sequence in blue) composed of P1 and P2 connected through kinked region J.
A structural model for extended miRNA-target pairing and 3′-end release
Initial miRNA–target interactions generally occur through the seed region and therefore, P1 is likely established first during TDMD target recognition. However, a key feature in TDMD-inducing targets are mismatches within the central region (Table S1). Therefore, it is unlikely that formation of P2 proceeds through the central nucleotides, as is the case for Thermus thermophilus Ago (Wang et al., 2008) (see Fig. S1), but is instead established first within the supplementary region (Sheu-Gruttadauria et al., 2019).
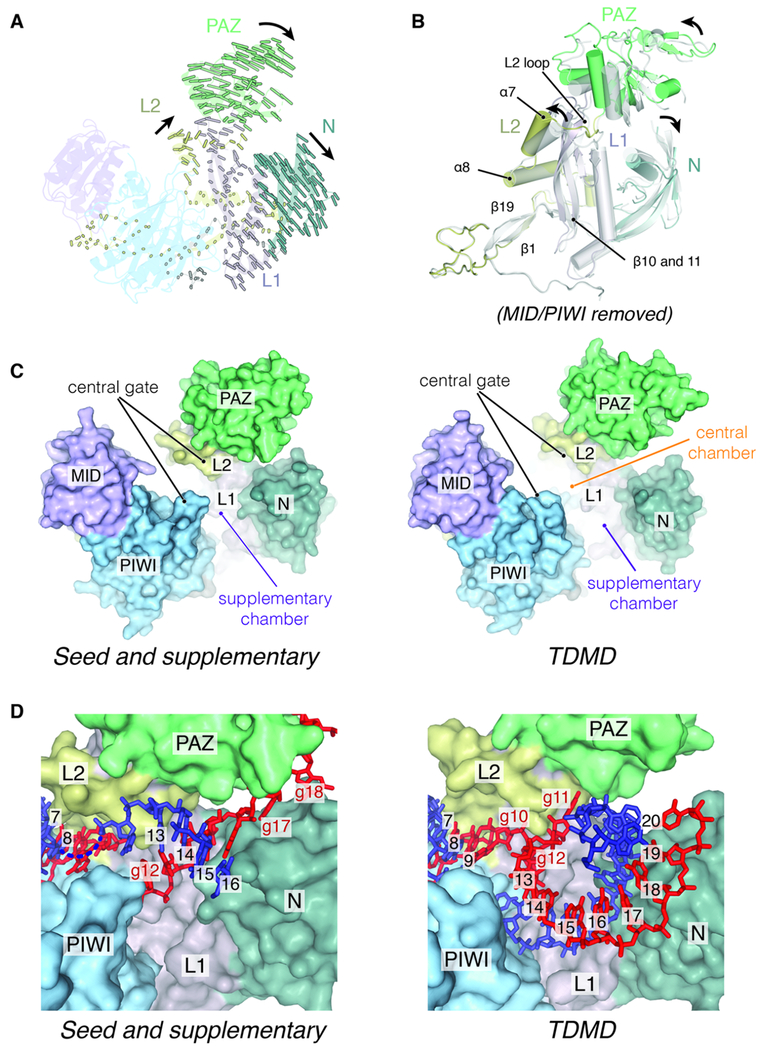
Comparison of the TDMD structures with the seed plus supplementary-paired complex reveals conformational changes in Ago2 protein associated with pairing beyond the supplementary region into the miRNA 3′ tail. The central cleft opens through a complex hinge where the N and PAZ domains shift away from the MID/PIWI lobe and away from each other (Figs. 2A and S2A) along a pivot point formed by the sole β-sheet in the L1 domain. This hinge resides at the interface of the MID/PIWI and N/PAZ lobes, along α-helix-8, β-strand-1 (in the N-terminus) and β-strand-19 (in L2) (Fig. 2B). Notably, a phosphorylation site (S387), which is proposed to stimulate miRNA-based silencing and repress endonucleolytic activity, maps to this hinge region (Horman et al., 2013; Zeng et al., 2008). Target cleavage by hAgo2 requires extensive pairing, especially within the central and supplementary regions (Elbashir et al., 2001). Therefore, this hinge may be essential for regulating hAgo2’s ability to open its central cleft, achieve extended pairing, and access a catalytically-competent state.
Figure 2. A structural model for extended miRNA-target pairing.
(A) Inter-Cα distances between L1 (gray-purple), L2 (mustard), PAZ (green), and N (teal) domains in seed plus supplementary and TDMD conformations, aligned to the MID/PIWI lobe, shown as colored lines. Cartoons of the seed and supplementary conformations are shown for reference. Directions of movements are indicated with arrows. (B) Seed plus supplementary (gray) and TDMD (domain colored) conformations aligned to the MID/PIWI lobe, with the MID/PIWI lobe removed for clarity. Structural elements involved in the hinges are labeled. Movements of the PAZ and N domains are indicated with arrows. (C) Surface representation of the seed and supplementary (left) and TDMD (right) conformations, with RNA removed for clarity to show shape of the central cleft. The central gate is highlighted to show opening and creation of the central chamber. The widened supplementary chamber is indicated. (D) Supplementary chambers of the seed and supplementary (left) and TDMD (right) conformations. RNA base-pairs are labeled and non-paired miRNA nucleotides indicated.
The hAgo2 hinge lies at the base of α-helix-7, which senses pairing in the seed region (Klum et al., 2018; Schirle et al., 2014) and feeds into the L2 loop portion of the central gate. Movements of the PAZ domain cause α-helix-7 to shift away from the seed region and the central gate to open (Fig S2B), creating a central chamber through which the miRNA–target duplex is wound (Figs. 1 and 2C). Based on these observations, we suggest that extension of pairing beyond the supplementary region into the 3′ tail may induce release of the 3′ end, which breaks the tether between the N/PAZ and MID/PIWI lobes formed by the miRNA, allowing the PAZ domain to shift ~8 Å and open the central gate (Fig. 2A, C and Movie S1). The MID/PIWI domain is largely the same between both conformations except for the PIWI loop in the central gate, which is disordered in the TDMD conformation (Fig. S2C). These conformational changes reveal how pairing status of the guide 3′ end can be coupled to opening of the central cleft, even in the absence of complementarity to the central region.
The hAgo2 N domain also shifts away from both the PAZ domain and MID/PIWI lobe (Figs. 2A, B and S2A), further widening the central cleft to expand the supplementary chamber (Fig. 2D). Movements between the PAZ and N domains occur through an additional hinge that resides in L1, across α-helix-3 and the β-sheet (Fig. S2D). Superimposing the seed plus supplementary conformation of hAgo2 and the TDMD RNA duplex reveals that P2 would clash with loops in the N domain formed by residues D95-N99 and E64-R69 (here called N loop 1 and loop 2) (Fig. S2E). These loops shift ~ 4 Å to accommodate the P2 duplex, which may propagate to the positional movements of the N domain. N loop 1 positions itself close to the minor groove of the P2 duplex and both loops contain several positively charged residues that may contribute to the position of the RNA (Fig. S2F).
TDMD target features facilitate stable miRNA 3′-end display
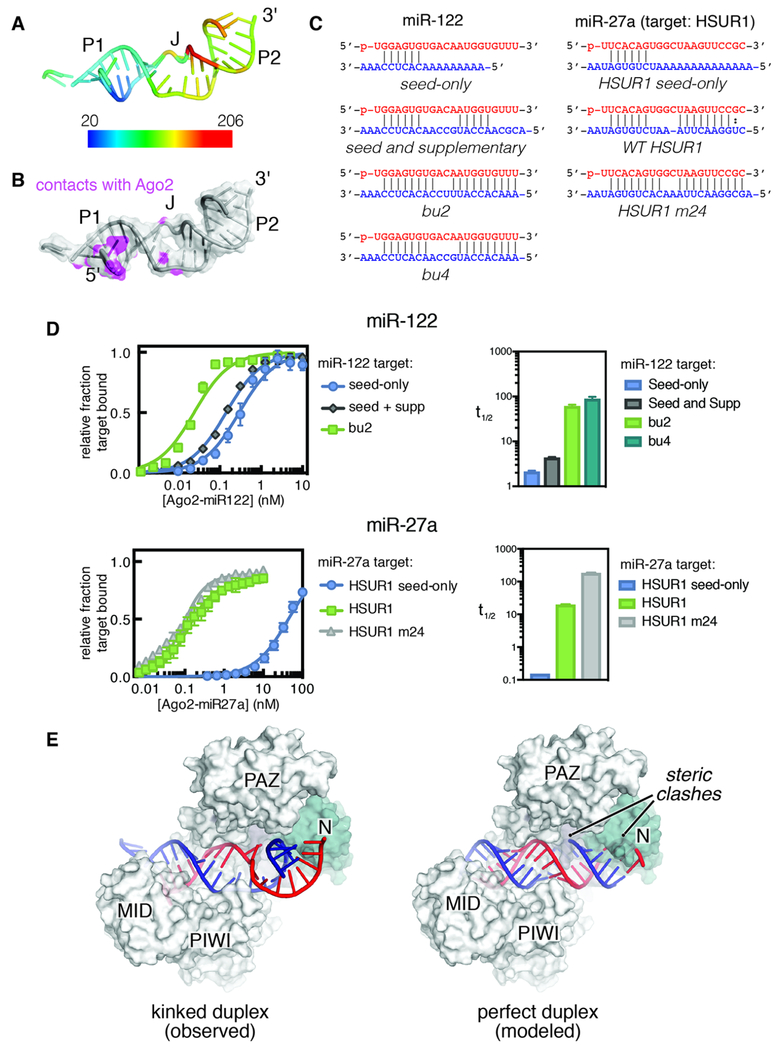
Known TDMD targets (Table S1) harbor common features that likely stabilize the TDMD conformation and promote 3′-end display. First, pairing to the miRNA seed region forms P1, which makes the most contacts with hAgo2 and exhibits the lowest crystallographic B-factors in the RNA (Fig. 3A, B). Thus, P1 likely anchors the miRNA-target duplex on hAgo2. Second, TDMD targets contain extensive complementarity to the miRNA 3′ tail region, forming P2. In contrast to P1, electron density for P2 was relatively weak, indicating mobility within the complex (Figs. 3A and S3A). Despite this mobility, binding measurements reveal that target RNAs capable of forming an extended P2 associate with hAgo2–miRNA complexes >40 times more stably than length-matched RNAs with complementarity restricted to the seed region (Fig. 3C, D). Thus, P2 formation produces an extraordinarily long-lived hAgo2–miRNA–target ternary complex. Extending our analysis to let-7a, the best biochemically characterized miRNA (Salomon et al., 2015; Wee et al., 2012), indicates that increased affinity associated with P2 formation is generally applicable to all miRNA sequences, and results in ternary complexes with stabilities exceeding those of any miRNA–target interaction reported previously (Tables S2 and S3). The remarkable stability of the ternary complex may derive from wrapping of the target around the miRNA within the central cleft (Fig. 1A), as well as the repositioning of α-helix-7 (Fig. S2C), which functions in target release by breaking seed-pairing (Klum et al., 2017) and is held away from the seed in the TDMD conformation. In addition, a stable P2 likely prevents re-binding of the miRNA by the PAZ domain, allowing long-lived exposure of the miRNA 3′ end.
Figure 3. TDMD targets facilitate stable display of the miRNA 3′ end.
(A) Temperature factors (rainbow colors) indicate that P1 is the most stable region of the RNA duplex. (B) The miR-122–bu2 duplex, with atoms that directly contact hAgo2 (purple). (C) Schematics of miRNA–target sequences used in binding and dissociation assays. (D) TDMD targets bind hAgo2 with significantly higher affinity and longer half-lives than seed-only or seed plus supplementary target RNAs. Shown for miR-122 (top) and miR-27a (bottom). Equilibrium binding data (left) and half-lives of ternary complex (right) are shown. Averages with standard errors are shown, n=3. Equilibrium binding data were normalized to Bmax for each curve for clarity. (E) Comparison of the observed miRNA–TDMD target duplex (left) and an ideal RNA duplex (right) shows how kinked region J prevents steric clashes with Ago2 in the TDMD conformation.
The J region that connects P1 and P2 is also a recurrent feature of miRNA–TDMD target interactions. Structurally, J facilitates RNA duplex bending, which appears to be an essential aspect of the TDMD conformation. Modeling of an undistorted miRNA–target duplex onto the TDMD structure reveals steric clashes with the PIWI, L1, and N domains of hAgo2 that would block miRNA-target pairing beyond miRNA nucleotide 11 (Figs. 3E and S3B). Thus, for siRNA-mediated target cleavage, which requires central and 3′-pairing (Elbashir et al., 2001), hAgo2 likely employs a distinct “slicing” conformation that is further opened in the central and supplementary chambers. This conformation may be relatively unstable, as suggested by previous observations demonstrating that highly-complementary targets, especially those with strong central and 3′-pairing, lead to destabilization of the ternary complex and release (unloading) of the miRNA–target duplex from Ago (De et al., 2013; Jo et al., 2015; Park et al., 2017). Indeed, central mismatches identical to those of crystallized constructs substantially impede unloading even in the presence of strong 3′-complementarity (Fig. S3C, D). These central mismatches also inhibit slicing (Fig. S3E), further indicating that slicing proceeds through a conformation distinct from TDMD. Together, these findings suggest that the unpaired J region and deformation near the center of the miRNA–target duplex is essential for accessing the TDMD conformation and stable formation of the P2 duplex.
Finally, the position of the N domain and the shallow supplementary chamber in the TDMD conformation prevent hAgo2 from enveloping the entire miRNA–TDMD target duplex (Figs. 1A and 2D). The bend at the J region therefore allows P2 to extend out of the central cleft with the miRNA 3′-end solvent exposed and displayed, which likely facilitates attack by terminal transferases and exonucleases.
HSUR1 mutagenesis corroborates the TDMD structural model
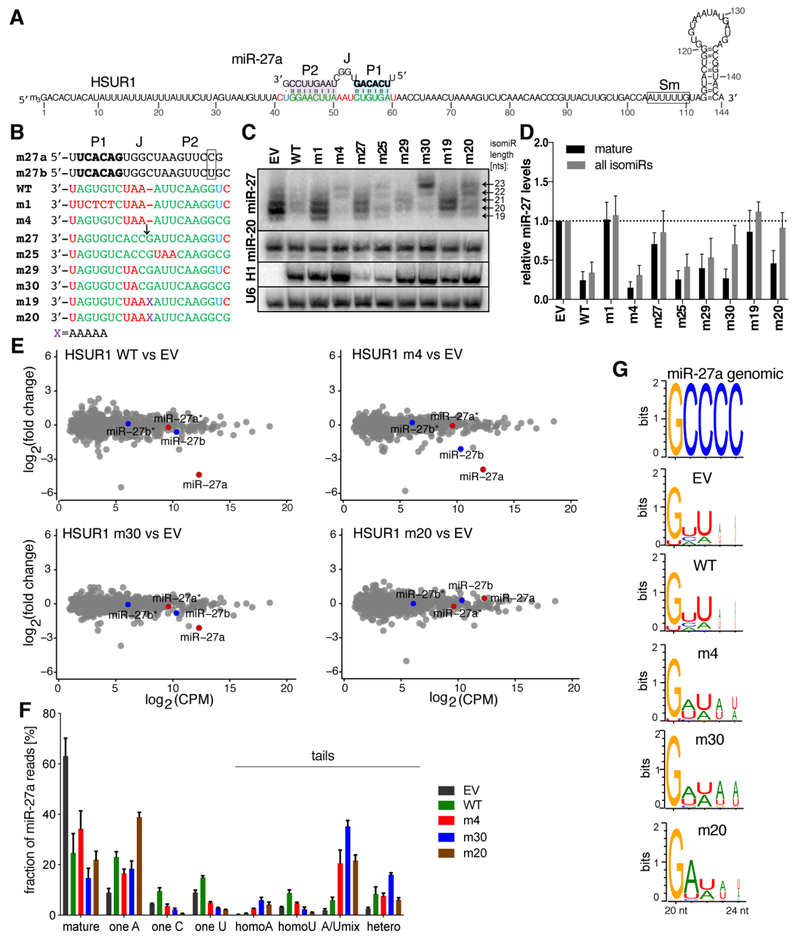
To assess our structural model for TDMD, we determined how mutations in the P1, P2, and J forming regions of HSUR1 affect miR-27 levels in BJAB cells by Northern blot (Figs. 4A–D and S4). Notably, Northern blotting detects both members of miR-27 family (miR-27a and miR-27b). Loss of pairing to the seed region inhibited TDMD, even in the presence of strong complementarity to the 3′ region, indicating that P1 is necessary for establishing the TDMD conformation (mutant m1, Fig. 4C, D). This observation agrees with previous reports that show seed-pairing to be required for TDMD (Ameres et al., 2010; Cazalla et al., 2010; de la Mata et al., 2015; Ghini et al., 2018; Kleaveland et al., 2018; Lee et al., 2013; Libri et al., 2012; Marcinowski et al., 2012).
Figure 4. Functional validation of the TDMD structural model.
(A) An open HSUR1 structure showing the predicted miR-27a interactions. Sm – the Sm protein binding site, bolded nts – miRNA seed sequence, green nts – HSUR1 nts complementary to miRNA, red – mismatched nts, blue – non-canonical base-pairs. (B) Sequences of WT HSUR1 and its mutants; nts 41 to 60-66 are depicted 3′-5′ to show complementarity to miR-27a; EV – empty vector, purple X = AAAAA, ↓ - predicted Ago2-cleavage site. Boxed is miRNA position 19, which differs between miR-27a and miR-27b. Colors as in (A). (C) Northern blot showing the impact of HSUR1 mutations on endogenous miR-27 (probe detects both miR-27a and miR-27b) and miR-20 levels in BJAB cells stably expressing the indicated mutants. The sizes of miR-27 isomiRs are indicated by arrows. H1 – HSUR1. (D) Quantification of (C). The levels of mature miR-27 (the 20-nt long band) and its isomiRs (bands between 18 and 24 nts) were normalized to the geometric mean of miR-20 and miR-16, and are reported relative to the empty vector control (error bars, s.d.; n=6). (E) The impact of HSUR1 construct expression on total miRNA levels in BJAB cells analyzed by high-throughput sequencing. Shown are log2 fold changes in miRNA means as a function of expression (n=3). miR-27a and miR-27a* are indicated in red, while miR-27b and miR-27b*, in blue. CPM – counts per million. (F) Frequency of various nt additions to miR-27a in the presence of indicated HSUR1 mutants (n=3). (G) Identity and frequency of nt additions to miR-27a HSUR1 mutants.
In addition to P1, TDMD requires formation of a sufficiently strong P2 (mutant m2, Fig. S4). Analysis of HSUR1 mutants indicates that P2 must be at least 6 base pairs in length and have hybridization energy < −11 kcal/mol to induce robust decay (Fig. S4, Table S4). Notably, the supplementary chamber of hAgo2 cannot contain a duplex longer than 5 base pairs (Sheu-Gruttadauria et al., 2019), suggesting that exceeding the 3′-pairing capacity of the supplementary chamber may be connected to establishing the TDMD conformation.
The J region is also functionally important for TDMD. Moderate perturbations (i.e. m29) or positional shifts (i.e. m25) in this region were well-tolerated, demonstrating flexibility in the structural requirements in this region (Fig. 4C, D). However, lengthening J substantially impeded miRNA decay (m19). miRNA remodeling in this mutant was partially restored by compensatory mutations extending the length of P2 (m20), revealing an interplay between the architecture of J and formation of P2 (Table S4). A perfectly complementary target (m27), lacking a J region, was notably less efficient in inducing TDMD. The levels of the perfectly complementary target were also lower, likely due to miR-27-guided slicing by hAgo2 (Figs. 4C and S4D). This result contrasts some previous reports demonstrating that perfectly complementary targets, when overexpressed, can induce robust miRNA decay (Ameres et al., 2010; Baccarini et al., 2011). We suggest that hAgo2 samples both slicing-competent and TDMD conformations in the presence of fully-complementary targets. Therefore, the ubiquitous presence of a J region in natural TDMD targets (Fig. S4) may ensure that the TDMD conformation is favored in addition to inhibiting slicing of the target.
Certain TDMD targets induce generation of miRNA isomiRs
Surprisingly, HSUR1 mutants bearing complementarity to the very 3′ end of miR-27 (i.e. m4, m20, and m30; Figs. 4 and S4) induced accumulation of elongated miR-27 isoforms, or isomiRs (Desvignes et al., 2015; McCall et al., 2017; Neilsen et al., 2012). This effect appears to be directly connected to the 3′-pairing status as elongated isomiRs were not induced by related HSUR1 mutants bearing mismatches to the miR-27 3′ end (i.e. WT vs. m4, m19 vs. m20, and m29 vs. m30). The levels to which elongated isomiRs accumulated varied with the size and position of the J region. For m30, which has a small, 2 base-pair mismatch opposite positions 8–9 of miR-27, the level of mature miR-27 was reduced in favor of pervasive remodeling (Fig. 4C), indicating that although the miR-27 3′ end was exposed, the efficiency of degradation was impaired. Moving the J region further into the central chamber of hAgo2 by either shifting the mismatches to position 10–11 of miR-27 (m24), increasing the number of central mismatches (m4 and m9), or creating a bulge in the central region of miR-27 (m16) reestablished efficient miRNA decay. These results demonstrate that miRNA 3′-pairing status, in conjunction with the structure of J, can strongly impact the outcome of engaging the HSUR1 TDMD target.
Adenylates and uridylates predominate among residues added to 3′ ends of miR-27
To determine the identity of the isomiRs generated in the presence of HSUR1 mutants, we performed high-throughput RNA sequencing of small RNAs (sRNA-seq) from BJAB cells transduced with one of the following vectors: EV, HSUR1 WT, m4, m30, or m20. In the presence of WT HSUR1, the levels of total miR-27a, but not miR-27b, decreased significantly (Fig. 4E). Interestingly, initially HSUR1 was reported to induce decay of both miR-27a and miR-27b (Cazalla et al., 2010); however, this was based on RT-qPCR, which is less sensitive than sRNA-seq. Mutant m4 was also able to decrease the levels of miR-27b, but to a lesser extent than of miR-27a. Similar to the results in Fig. 4C, D, mutants m30 and m20 did not greatly affect the total levels of miR-27, but affected their length distribution (Fig. S5C). The presence of the mutants altered the length distribution of miR-27 isomiRs in the sRNA-seq data, although not perfectly mirroring species detected by Northern blotting (Fig. S5A). It is possible that the 3′ end of some isomiRs is modified or assumes a secondary structure that inhibits ligation and/or reverse transcription during sRNA-seq library preparation (Hafner et al., 2011; Zhuang et al., 2012). In all obtained reads miR-27a was almost exclusively modified at the 3′ end (<0.2% of editing was observed at the 5′ ends). Interestingly, in the presence of HSUR1 WT, the longer miR-27a reads – similarly to the EV control – are enriched in sequences with U tailing, while in the presence of HSUR1 mutants, tails are most often composed of mixed A and U residues (Fig. 4F, G). In addition, in the presence of HSUR1 mutants, miR-27b tails are comprised mostly of A residues and to a lesser extent of U residues (Fig. S5C). These A-additions do not reflect the upstream sequence of HSUR1 and thus are non-templated (Fig. S5D). Notably, the presence of mixed A/U tails on miRNAs during TDMD has been reported by others (de la Mata et al., 2015; Ghini et al., 2018; Marcinowski et al., 2012). These findings, combined with the observed accumulation of extended miR-27 isomiRs, suggest that during TDMD, miRNA uridylation triggers degradation (as in the case of HSUR1 WT), while mixed A/U tails do not.
Extended isomiRs are sequestered in stable complexes with Ago and target RNAs and associate with all four human Agos
During TDMD, miR-27 can be found in association with Ago and HSUR1 (in the α-Sm IP) (Cazalla et al., 2010) (Fig. 5A). Similarly, miR-27 isomiRs induced by mutants m30 and m20 also co-immunoprecipitate both with Ago proteins and with their targets. These observations suggest that long-lived ternary complexes composed of Ago, miRNA, and TDMD target might exist in cells, as observed in vitro (Figs. 2D and S2). If true, we would expect that the mutant HSUR1s effectively function as miR-27 sponges and thereby render extended isomiRs incompetent for silencing. To investigate this possibility, we used luciferase reporters bearing either perfectly complementary sites to miR-27a or sites cloned from a miR-27a natural target, SEMA7A (Guo et al., 2014), to test siRNA- and miRNA-mediated silencing, respectively. The longer isomiRs generated in the presence of mutant m30 were incompetent for siRNA- and miRNA-induced silencing (Fig. 5B), corroborating the notion that these isomiRs are sequestered in ternary complexes with Ago protein and m30 RNA. Alternatively, it is possible that this decrease in miR-27 activity stems from slightly reduced miRNA levels (Fig. 4). Interestingly, miR-27 isomiRs generated in the presence of mutant m20 are competent for siRNA-induced silencing, but only partially for miRNA-induced silencing (Fig. 5B). The competence in siRNA-induced silencing may be the result of the shorter miR-27 isomiRs, which are enriched in the α-Ago IP, but not the α-Sm IP (Fig. 5A). Importantly, the longer miR-27 isomiRs associate with all four human Agos (Fig. 5C), indicating that all these Ago proteins can adopt a common TDMD conformation.
Figure 5. miR-27a isomiRs are associated with all four Ago proteins and with HSUR1 mutants.
(A) Northern blot showing anti-pan Ago or anti-Sm (recognizes the HSUR1 RNP) immunoprecipitates from extracts of BJAB cells stably expressing HSUR1 mutants (see Fig.4B). I – input, 2%; S – supernatant, 2%; P – pellet (50% for Ago and 100% for Sm), H1 – HSUR1, n=3. (B) miR-27 activity in BJAB cells stably expressing HSUR1 mutants was measured using a Renilla luciferase reporter with either four perfectly complementary miR-27a binding sites (left) or four miR-27a binding sites cloned from the SEMA7A gene (right) in its 3′ UTR. The levels of Renilla luminescence are reported relative to the empty vector (EV) control (error bars, s.d.; n≥3). (C) Northern blot showing anti-FLAG co-immunoprecipitation of RNAs with FLAG-HA-tagged Ago variants (1 to 4) stably expressed in BJAB cells that were concomitantly transduced with either EV or HSUR1 (WT or m30). I – input, 10%; P – pellet, 100%, n=3.
Disruption of 3′-end miRNA binding by Ago results in tailing and trimming of the miRNA
To test the hypothesis that the exposure of the miRNA 3′ end drives its degradation, we examined miRNAs bound to hAgo2 containing a PAZ domain mutation that disrupts normal interactions with the miRNA 3′ end (Ma et al., 2004). Strikingly, in the absence of HSUR1, all investigated miRNAs bound by the PAZ mutant exhibited a substantial increase in tailed and trimmed species (Fig. 6B, C). Trimmed miRNA species were more abundant than tailed species, and all isomiRs were more prevalent than during TDMD driven by HSUR1. Indeed, expression of HSUR1 WT led to the specific loss of modified miR-27 species (Fig. 6B), indicating that pairing to the TDMD target enables decay of the released 3′ end. Transfected 5′ 32P-labeled miR-27a duplexes exhibited a similar pattern of predominantly trimmed isomiRs bound to the hAgo2 PAZ mutant (Fig. 6D; also true for the analogous hAgo1 PAZ mutant – Fig. S5E), confirming that these modifications occur on the 3′ end of mature miRNAs. The results indicate that releasing the miRNA 3′ end from the PAZ domain is sufficient to render it susceptible to enzymatic attack. This notion is in agreement with a previous study suggesting that the PAZ domain plays a role in protecting the miRNA 3′ end (Hur et al., 2013). Taken together with the finding that changes in base-pairing features in the J region and P2 end can lead to the formation of isomiRs, these results suggest that the TDMD target serves two functions: first, to induce stable removal of the miRNA 3′ end from the PAZ domain, which renders it susceptible to enzymatic attack and destruction, and second, to present a substrate that is amenable to specific enzymes that mediate efficient decay.
Figure 6. The Ago2 F294A PAZ domain mutant mimics TDMD-induced mature miRNA tailing and trimming.
(A) Structure of the hAgo2-miRNA complex (PDB ID: 4w5n) showing how F294 interacts with the miRNA 3′ end. (B) Northern blot showing anti-FLAG immunoprecipitates of FLAG-HA-tagged Ago2 variants (WT or F294A) stably expressed in BJAB cells concomitantly transduced with empty vector (EV) or WT HSUR1. I – input, 10%; S – supernatant, 10%; P – pellet, 100%, H1 – HSUR1. (C) Quantification of (B) in cells transduced with EV. Levels of mature, tailed, and trimmed miRNAs are presented as fraction of total miRNA levels in the pellets (error bars, s.d.; n=3). (D) miRNA modifications occur on mature miRNAs. 32P-radiolabeled miR-27a duplexes were transfected into BJAB cells expressing either empty vector (EV_2) or Ago2 variants (WT or F294A); 24 h later, anti-FLAG immunoprecipitation was performed, n=3.
DISCUSSION
Although first identified almost a decade ago, the mechanism underlying TDMD has remained largely uncharacterized. Efforts have been hindered by a lack of information on how mammalian Argonautes accommodate extended miRNA pairing, which is a hallmark of TDMD target RNAs. Our structural insights explain why TDMD requires tandem segments of miRNA–target complementarity separated by an unpaired region and identify features most important for decay.
Our structures also reveal how the shape of the central RNA binding cleft of hAgo2 governs miRNA–target association. Previous work showed that guide–target duplex propagation in prokaryotic Ago occurs linearly from seed to 3′ end (Wang et al., 2009). This simple mechanism is enabled by the structure of TtAgo, which has a deep, but short central cleft that appears to open readily upon seed-pairing and likely evolved to encapsulate a fully-paired, 16-nt long ideal duplex (Fig. S1). In comparison, hAgo2 has a shallower central cleft that is divided into the seed and supplementary chambers by the central gate. The physical connection between the gate and the PAZ domain allows opening of the central cleft to be coupled to 3′ release. Therefore, pairing likely proceeds in a discontinuous, step-wise manner with several checkpoints that must be surpassed before achieving a slicing-competent state. These checkpoints may manifest as low-energy conformations with pairing to the seed, supplementary, and 3′-end regions of the guide RNA. We suggest that TDMD targets leverage one of these conformations to allow prolonged release and display of the 3′ end in the absence of central pairing. The multi-step opening mechanism used by hAgo2 may also explain the evolution of diverse target site types that can be recognized by animal miRNAs (Bartel, 2018).
This work also provides structural insight into other hAgo2 mechanisms, including target slicing and duplex loading. Analysis of the TDMD structure indicates that an additional conformational barrier must be overcome before achieving a slicing-competent state, which likely has widened central and supplementary chambers that can accommodate a fully-paired duplex (Fig. 3). Reaching this state almost certainly requires further movement of the N domain, which may explain previous domain-swapping results demonstrating that regions responsible for governing N domain movements, including the N-terminal and L1 hinge, are essential for restoring enzymatic activity in the catalytically inactive hAgos 1, 3, and 4 (Faehnle et al., 2013; Hauptmann et al., 2013; Nakanishi et al., 2013). The N domain also serves as a wedge to enable unwinding of guide–passenger duplexes during loading of small RNAs into hAgo2 (Kwak and Tomari, 2012). Mutations associated with unwinding defects map to the hinge region that mediates movements between the PAZ and N domains as well as the N loops that move to accommodate P2 (Kwak and Tomari, 2012). Moreover, miRNA duplexes with central mismatches, like those creating the J region in our TDMD structures, are preferentially loaded into hAgo2 (Kawamata et al., 2009). We therefore suggest that a TDMD-like conformation may also be used during the loading of small RNA duplexes into hAgo2.
Our mutational analysis of HSUR1 underscores the necessity of P1, a sufficiently strong P2, and the presence of a correctly placed and flexible J to achieve the TDMD conformation (Figs. 4 and S4). Within these criteria, sequence complementarity requirements for TDMD are somewhat flexible, which agrees with reports of known TDMD targets whose base-pairing features vary (Fuchs Wightman et al., 2018) (Table S1). It is possible that additional unidentified RNA motifs beyond the miRNA binding site are necessary for TDMD (Kleaveland et al., 2018; Lee et al., 2013). In addition, a lack of secondary structure within TDMD targets is essential for miRNA decay (Pawlica et al., 2016).
Our mutational analysis of HSUR1 also demonstrates that the structure of J, in combination with 3′-pairing status, can substantially impact the outcome of engaging the TDMD target (Figs. 4 and S4). Based on the structure of the complex, we suggest that the position and flexibility of J determine the angle at which P2 extends from the hAgo2 central cleft, as well as how the helical turn of the miRNA–target duplex is disrupted between P1 and P2. Combined with P2 length, these features determine the position of the exposed 3′ end within the complex (Fig. 1). Docking structures of cellular enzymes implicated in miRNA 3′-end remodeling onto the hAgo2–miR-122–bu2 structure indicates that the position of the exposed 3′ end within the complex likely influences which enzyme can most readily attack (Fig. S6). HSUR1 mutagenesis also suggests that different enzymes, or enzymatic activities, are recruited to the miRNA 3′ end depending on its base-pairing status. Interestingly, at least one human terminal transferase, TUT7, has different activity depending on the 3′-pairing status of its substrate (Kim et al., 2015). We therefore suggest that, a combination of 3′-pairing status, P2 length, and structure of J determine which enzymes will engage the released miRNA 3′ end. This model may explain the previous findings showing TDMD of miR-7 by the non-coding RNA Cyrano (Kleaveland et al., 2018), and TDMD of let-7e by miRNA ‘tough decoys’ was inhibited by mutations predicted to shift J away from the central chamber (Xie et al., 2012). Based on this model, we further suggest that a TDMD-like mechanism, in which recruitment of remodeling enzymes is favored over degradation enzymes, may be used during generation of post-Dicer-processing 3′ isomiRs, which are widespread and developmentally controlled in animals (Gebert and MacRae, 2018).
TDMD is often accompanied by tailing, which has been hypothesized to be a prerequisite for degradation (Ameres et al., 2010; Ameres et al., 2011; de la Mata et al., 2015; Ghini et al., 2018; Marcinowski et al., 2012). Indeed, cellular RNAs are often ‘tagged’ for degradation by terminal nucleotidyl transferases before being degraded by 3′–to–5′ exonucleases (Song et al., 2015). However, tailing during TDMD is not always observed (Bitetti et al., 2018), and one recent report demonstrated that PAPD4 (also known as TUT2, TENT2)-mediated adenylation is not required for Cyrano-induced miR-7 decay (Kleaveland et al., 2018). Our observations of extended isomiRs in the presence of either HSUR1 mutants or an Ago PAZ domain mutation (Figs 4, 6, S4, and S5) suggest that during TDMD the miRNA 3′-end is subjected to tailing, but do not demonstrate that this step is required for miRNA decay. We suggest that the isomiRs observed in the presence of HSUR1 mutants may represent off-pathway stabilized populations. Indeed, our sRNA-seq results indicate that in the presence of HSUR1 WT miR-27a reads are enriched in U-tailing, while in the presence of the HSUR1 mutants A/U mixed tails appear. This may point to a TDMD mechanism in which the miRNA is uridylated before decay, but if A residues are incorporated the miRNA is stabilized. Interestingly, monoadenylation can stabilize miRNAs (D’Ambrogio et al., 2012; Katoh et al., 2009). However, judging from steady-state miRNA levels alone we cannot distinguish which isomiRs are intermediates versus failed products of TDMD.
In summary, we have discovered a target-driven conformation for human hAgo2 that enables exposure of the miRNA 3′ end, rendering it susceptible to enzymatic attack. Although the enzymes responsible for catalyzing TDMD remain to be identified, and it has been suggested that multiple pathways may exist (Kleaveland et al., 2018), the finding that all known TDMD targets are predicted to form P1, P2, and J structures (Table S1), as we observe with HSUR1, indicates that the TDMD conformation of Argonaute is a core feature in all target-driven miRNA decay and 3′-remodeling processes.
STAR Methods
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Bacterial strains and plasmids
Bacteria used for cloning were chemically competent E. coli either OminMAX™ (Thermo Fisher) or Stbl2™(Thermo Fisher). Bacteria used for production of bacmid were DH10Bac™ chemically competent E. coli (Thermo Fisher). Plasmids are listed in Key Resources table.
KEY RESOURCES TABLE
| REAGENT OR RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-pan Ago | Sigma Millipore | Cat# MABE56 |
| anti-Sm | Lerner et al., 1981 | N/A |
| anti-HA.11 | BioLegend | Cat# 16B12 |
| anti-GAPDH | Cell Signaling | Cat# 2118 |
| Chemicals and Reagents | ||
| TransIT-293T | Mirus Bio | Cat# MIR 2704 |
| Western Lightning Plus-ECL | Perkin Elmer | Cat# NEL103E001EA |
| cOmplete™ Mini EDTA-free Protease Inhibitor tablets | Roche | Cat# 4693159001 |
| Murine RNase Inhibitor | New England Biolabs | Cat# M0314S |
| Ribonucleic acid, transfer from baker’s yeast | Sigma | Cat# 9014-25-9 |
| Ni-NTA Agarose | Qiagen | Cat# 30210 |
| StrepTactin Sepharose HP | GE Healthcare Life Sciences | Cat# 28935599 |
| High Capacity NeutrAvidin™ Agarose | Pierce | Cat# 29202 |
| Q-Sepharose Fast Flow | GE Healthcare Life Sciences | Cat# 17051001 |
| TRIzol™ Reagent | Sigma | Cat# 15596026 |
| [γ-32P]ATP | PerkinElmer | Cat# NEG035C |
| Amersham Hybond-NX membrane | GE Healthcare | Cat# 95038-412 |
| 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) | Sigma | Cat# E6383 |
| Phusion® High-Fidelity DNA Polymerase | NEB | Cat# M0530 |
| NotI, and | NEB | Cat# R3189 |
| XhoI | NEB | Cat# R0146 |
| EcoRI | NEB | Cat# R3101 |
| XbaI | NEB | Cat# R0145 |
| Protein A-Sepharose® CL-4B | GE Healthcare | Cat# GE17-0780-01 |
| ANTI-FLAG® M2 Affinity Gel | Sigma | Cat# A2220 |
| NEBNext® Small RNA Library Prep Set for Illumina | NEB | Cat # E7300 |
| SF Cell Line 4D-NucleofectorTM X Kit L | Lonza | Cat# V4XC-2012 |
| PVDF membrane | BioRad | Cat# 88518 |
| OminMAX™ | Thermo Fisher | Cat# C854003 |
| Stbl2™ | Thermo Fisher | Cat# 10268019 |
| DH10Bac™ | Thermo Fisher | Cat# 10361012 |
| Critical Commercial Kits | ||
| Dual-Luciferase Reporter Assay System | Promega | Cat# E1910 |
| Cell Lines | ||
| HEK293T (human, female) | Steitz lab | N/A |
| BJAB (human, female) | Steitz lab | N/A |
| Sf9 (insect) | Expression Systems | 94-001S |
| Oligonucleotides | ||
| See Table S5 for oligonucleotide sequences used in this study | This study | |
| Recombinant DNA | ||
| pFastBac HT A | Thermo Fisher | Cat# 10584027 |
| pFastBac His-Flag-TEV hAgo2 3xD;2xA WT | (Schirle and MacRae, 2012) | N/A |
| pFastBac His-Flag-TEV hAgo2 3xD;2xA D669A | This study | N/A |
| pAGM-HSUR1 wild-type and its mutants | Guo et al., 2014, Pawlica et al., 2016 and this paper | N/A |
| psPAX2 | a gift from Didier Trono | Addgene Cat#12260 |
| pMD2.G | a gift from Didier Trono | Addgene Cat#12259 |
| pLVX-Puro | Clontech | Cat# 632164 |
| pIRESneo-FLAG/HA Ago1 | (Meister et al., 2004) | Addgene Cat# 10820 |
| pIRESneo-FLAG/HA Ago2 | (Meister et al., 2004) | Addgene Cat# 10822 |
| pIRESneo-FLAG/HA Ago3 | (Meister et al., 2004) | Addgene Cat# 10823 |
| pIRESneo-FLAG/HA Ago4 | (Meister et al., 2004) | Addgene Cat# 10824 |
| pLVX-Ago1, pLVX-Ago2, pLVX-Ago3, pLVX-Ago4, pLVX-F294A Ago2, pLVX-F292A Ago1 | This paper | N/A |
| psiCHECK-2 | Promega | Cat# C8011 |
| psiCHECK-2-miR-27a | This paper | N/A |
| psiCHECK-2-SEMA7A | This paper | N/A |
| Software | ||
| Prism 6 and 7 | Graphpad | https://www.graphpad.com/scientific-software/prism/ |
| Coot | (Emsley et al., 2010) | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| Phenix | (Adams et al., 2010) | http://phenix-online.org |
| CCP4 | (Winn et al., 2011) | http://www.ccp4.ac.uk |
| PyMOL | Schrödinger, LLC | https://pymol.org/2/ |
| ImageQuant TL | GE Healthcare Life Sciences | Cat# 290006 05 |
| Imagej | https://imagej.net | |
| RNAstructure, version 5.8.1 | Reuter and Mathews, 2010 | https://rna.urmc.rochester.edu/RNAstructure.html |
| Atomic coordinates | ||
| hAgo2–miR-27a–HSUR1 | This study | PDB ID 6MFN |
| hAgo2–miR-122–bu2 | This study | PDB ID 6MDZ |
| hAgo2–miR-122–bu3 | This study | PDB ID 6MFR |
| hAgo2–miR-122–bu4 | This study | PDB ID 6NIT |
| sRNA-seq data | ||
| miRNA-seq from BJAB cells transduced with either empty vector control or HSUR1 constructs | This study | NCBI Gene Expression Omnibus (GEO) accession number GSE130632 |
| Data images | ||
| Unprocessed and uncompressed data images | This study | http://dx.doi.org/10.17632/tcx8spf6c6.1 |
Bacterial media and growth conditions
All bacterial cultures were grown in Luria-Bertani (LB) medium at 37°C. When applicable, media was supplemented with one or more of the following at the indicated concentrations: ampicillin (100 μg/mL), kanamycin (40 μg/mL), tetracycline (5 μg/mL), gentamycin (7 μg/mL), 5-Bromo-4-Chloro-3-Indolyl β-D-Galactopyranoside (X-gal, 20 μg/mL in dimethylformamide), and/or IPTG (1 mM).
Insect cell media and growth conditions
Sf9 cells were grown in Lonza Insect XPRESS™ medium supplemented with penicillin (100 units/mL), streptomycin (100 μg/mL), L-glutamine (2.92 mg/mL) at 27°C in suspension.
Human cell culture growth conditions
HEK293T cells and BJAB cells were grown at 37°C wit h 5% CO2 in DMEM and RPMI medium, respectively, containing 10% fetal bovine serum, 2 mM L-glutamine, 100 I.U./mL penicillin, and 100 μg/mL streptomycin. BJAB cells were maintained at densities between 0.5 and 1.8 x 106 cells/ml. The cell lines exhibit expected morphology and are mycoplasma-negative.
METHOD DETAILS
Purification of hAgo2 bound to miR-122 and miR-27a for crystallization and binding assays
Human Ago2 proteins loaded with miR-122 or miR-27a were purified as described previously (Schirle et al., 2014). Briefly, His-tagged hAgo2 was purified from Sf9 cells using a baculovirus system (Invitrogen). Cells were lysed in Lysis Buffer (300mM NaCl, 0.5 mM TCEP, 50 mM Tris, pH 8) using a single pass through a M-110P lab homogenizer (Microfluidics). Lysate was cleared and the soluble fraction was applied to Ni-NTA resin (Qiagen) and incubated for 1.5 h. The resin was washed with Nickel Wash Buffer (300 mM NaCl, 300 mM imidazole, 0.5 mM TCEP, 50 mM Tris, pH 8). Co-purifying cellular RNAs were degraded with micrococcal nuclease (Clontech) on-resin in Nickel Wash Buffer supplemented with 5mM CaCl2. The resin was washed again with Nickel Wash Buffer and then eluted in four column volumes of Nickel Elution Buffer (300 mM NaCl, 300 mM imidazole, 0.5 mM TCEP, 50 mM Tris, pH 8). Eluted hAgo2 was incubated with synthetic miRNA concurrent with overnight cleavage of the N-terminal His6 tags using TEV protease and dialysis against Hi-Trap Dialysis Buffer (300 mM NaCl, 15 mM imidazole, 0.5 mM TCEP, 50 mM Tris, pH 8) at 4°C. The dialyzed protein was then passed through a Hi-Trap Chelating column (GE Healthcare) and the unbound material was collected. The hAgo2 molecules loaded with the desired miRNA were purified using a modified Arpon method (Flores-Jasso et al., 2013). Loaded molecules were further purified by size exclusion using a Superdex 200 Increase 10/300 column (GE Healthcare) in High Salt SEC Buffer (1M NaCl, 0.5mM TCEP, 50mM Tris pH 8). The final protein was dialized into low salt for crystallography, concentrated, aliquoted, flash frozen and stored at −80°C. Samples were thawed slowly on ice for all experiments. Concentration was determined by absorbance at λ=280 nm.
Crystallization and data collection
Ternary hAgo2–miRNA–target complexes were formed by adding 1.2 molar equivalents of synthetic target RNA to purified hAgo2 and incubation on ice for 5 min. Protein was then diluted to 1.5 mg/mL for crystallization screens. Crystals were grown using hanging drop vapor diffusion at 20°C. Drops contained a 1:1 ratio of protein to reservoir solution. Crystals of the Ago2–miR-122–bu2 were grown in the following reservoir solution: 16% PEG3350, 50mM Tris pH8, 10mM MgCl2, 75mM Phenol. These crystals were used iteratively as seeds to grow larger crystals in drops that contained a 1:1:0.2 ratio of protein to reservoir (10% PEG3350, 50mM Tris pH8, 20mM MgCl2, 75mM Phenol) to seeds. Ago2–miR-122–bu2 crystals were also used as seeds to grow all other complexes in the same reservoir solution. Data were collected under cryogenic conditions remotely at beam lines 12-2 at the Stanford Synchrotron Radiation Lightsource (SSRL) and 23IDD at the Argonne National Laboratory Advanced Photon Source. Data were processed using XDS and Scala (Kabsch, 2010; Winn et al., 2011).
Model determination and refinement
hAgo2–miRNA–target ternary complex structures were solved by molecular replacement using the MID-PIWI lobe and the PAZ and N domains of the seed-paired target structure (4W5O) as sequential search models with Phaser-MR in the PHENIX graphical interface (McCoy et al., 2007). Models were built using Coot and submitted to XYZ coordinate, TLS, and B-factor refinement using PHENIX (Adams et al., 2010). Model building and refinement continued iteratively until all interpretable electron density was modeled. Refinement of the miR-122 structures used a common Rfree data set. All structure figures were generated using PyMOL (Schrödinger, LLC) or Chimera (UCSF).
Equilibrium binding assays
Equilibrium dissociation constants were determined as described previously (Schirle et al., 2014). Briefly, miRNA-loaded Ago2 samples were incubated with 32P 5′-radiolabeled target RNA in binding reaction buffer (30 mM tris pH 8.0, 100 mM potassium acetate, 2 mM magnesium acetate, 0.5 mM TCEP, 0.005% (v/v) NP-40, 0.01 mg/mL baker’s yeast tRNA), in a reaction volume of 100 μL at room temperature.
Ago2 and radiolabeled target concentrations, as well as equilibration times, depended on the target RNA. For HSUR1 ΔP2, miR-27a-loaded Ago2 ranged from 0-100 nM; for all others, loaded Ago2 ranged from 0-10 nM. miR-122-loaded Ago2 was incubated with 0.1 nM radiolabeled miR-122 targets for 45 min; miR-27a-loaded Ago2 was incubated with 0.05 nM radiolabeled HSUR1 targets for 60 min.
Using a dot-blot apparatus (GE Healthcare), protein-RNA complexes were captured on Protran nitrocellulose membrane (0.45 μm pore size, Whatman, GE Healthcare Life Sciences) and unbound RNA on Hybond Nylon membrane (Amersham, GE Healthcare). Samples were applied with vacuum and then washed using 100 μL of ice-cold wash buffer (30 mM Tris pH 8.0, 0.1 M potassium acetate, 2 mM magnesium acetate, 0.5 mM TCEP). Membranes were air-dried and signals visualized by phosphorimaging.
Target dissociation assays
Target dissociation rates were determined as previously reported (Klum et al., 2018). Briefly, miRNA-loaded Ago2 samples were incubated with 0.1 nM 32P 5′-radiolabeled target RNA in binding reaction buffer (30 mM tris pH 8.0, 100 mM potassium acetate, 2 mM magnesium acetate, 0.5 mM TCEP, 0.005% (v/v) NP-40, 0.01 mg/mL baker’s yeast tRNA) in a reaction master mix with a volume of 100 μL per time point at room temperature.
Ago2 concentrations, as well as equilibration times, depended on the target RNA. For HSUR1 ΔP2, miR-27a-loaded Ago2 was 20 nM; for all others, Ago2 was 1 nM. miR-122-loaded Ago2 was incubated for 45 min; miR-27a-loaded Ago2 was incubated for 60 min.
After equilibration, a “zero” time point 100 μL aliquot was applied to a dot-blot apparatus under vacuum, then chased with 100 μL of ice-cold wash buffer. After addition of excess unlabeled target RNA to a final concentration of 100 nM, 100 μL aliquots taken at time points were applied to a dot-blot apparatus under vacuum, then chased with 100 μL of ice-cold wash buffer as before. All miR-122-loaded time points were 1-320 min, miR-27a-loaded HSUR1 ΔP2 time points were 0.25-20 min, and miR-27a-loaded HSUR1 and HSUR1 19.2 time points were 5-200 min. Membranes were air-dried and signals visualized by phosphorimaging. Quantification was performed using ImageQuant.
Preparation of 32P-labeled miR-122–Ago2 complexes for unloading assays
Loaded Ago2 was purified similarly to a protocol described previously above although typically on a smaller scale (using ~500x106 Sf9 cells infected with His6-FLAG-TEV-Ago2 baculovirus as starting material). Briefly, the cell pellet was lysed and the clarified lysate was purified by Ni-NTA resin (Qiagen). After micrococcal nuclease treatment, the Ni-NTA resin was washed again and the protein was eluted from the resin by imidazole addition. Ethylene glycol tetraacetic acid (EGTA), pH 8.0 was added to the eluate to a final concentration of 5 mM to chelate any remaining calcium ions. 32P-radiolabeled miRNA was then added to the nickel-purified Ago2 and the resulting Ago2–miR-122 mixture was dialyzed overnight at 4°C into wash buffer without imidazole (50 mM tris, pH 8.0, 300 mM NaCl, 0.5 mM TCEP).
Ago2 molecules correctly loaded with 32P-labeled miR-122 were then isolated from those bound to co-purifying cellular small RNAs using the immobilized capture oligo, as described above. Excess biotinylated competitor DNA was removed by incubation with High Capacity NeutrAvidin agarose resin (Thermo Fisher). To remove any remaining contaminating nucleic acid, 32P-miR122-loaded Ago2 was then diluted to reach a final salt concentration of 150 mM by adding Q resin equilibration buffer (to a final concentration of 20 mM tris, pH 8.0, 0.01% CHAPS, 0.5 mM TCEP), and then applied to Q Sepharose® Fast Flow resin (GE Healthcare Life Sciences), 50 μL packed per 15 nmol competitor DNA. The resin was washed with 5 column volumes of Q resin wash buffer (20 mM tris, pH 8.0, 250 mM NaCl, 0.5 mM TCEP), and the Ago2-containing flow-through and wash were combined in a separate tube.
For use in subsequent unloading assays, the purified Ago2–miRNA complexes were bound to ANTI-FLAG® M2 affinity agarose resin (Sigma-Aldrich®), 150 μL packed per pellet, by rocking for 1.5-2 h at 4°C. The resin was then wash ed with 30 column volumes FLAG wash buffer (20 mM tris, pH 8.0, 150 mM NaCl, 5 U/mL murine RNase inhibitor, 0.5 mM TCEP), 30 column volumes high-salt FLAG wash buffer (20 mM tris, pH 8.0, 1 M NaCl, 5 U/mL murine RNase inhibitor, 0.5 mM TCEP), and 30 column volumes FLAG wash buffer.
Ago2–miR-122 complexes were quantified by removing two samples, each 1-5% of the washed ANTI-FLAG resin, and running them alongside bovine serum albumin standards on an SDS-PAGE gel. Bands were quantified using ImageJ.
miRNA unloading assays
Prior to the unloading assays, wild-type Ago2 loaded with 32P 5′-end labeled miRNA RNA and purified as described above was bound to α-FLAG resin. Subsequent to washing with buffer (20 mM tris pH 8.0, 150 mM NaCI, 0.5 mM TCEP, 1 U/mL murine RNase inhibitor), the total resin volume was adjusted to a concentration of 0.69 pmol Ago2–miR-122 per μL resin.
Each unloading assay consisted of 5 μL Ago2–miR-122 FLAG resin and 10 μL buffer pre-warmed to 37°C. Unloading was initiated by addi ng 34.5 pmol target RNA and allowed to proceed for 20 min at 37°C. After brief centrifugat ion, the supernatant solution was moved to a tube containing 100% formamide and the resin was washed and resuspended with 100% formamide, as described previously. Supernatant (unloaded) and pellet (bound) samples were then run on denaturing PAGE and 32P quantified by phosphorimaging. The fraction miR-122 released was calculated by dividing the number of counts in the unloaded sample by the total number of counts in the unloaded and bound samples.
hAgo2 slicing assay
Purified hAgo2–miR-122 complex (~20 nM, final concentration) was incubated at 37°C with complementary 5′-32P-labeled target RNAs (~100 nM, final concentrations) containing various mismatches to the miRNA-122 central region in reaction buffer composed of 30 mM Tris pH 8.0, 0.1 M potassium acetate, 2 mM magnesium acetate, 0.5 mM TCEP, 0.01 mg/mL baker’s yeast tRNA. Target cleavage was stopped at various times by mixing aliquots of each reaction with an equal volume of denaturing gel loading buffer (98% w/v formamide, 0.025% xylene cyanol, 0.025% w/v bromophenol blue, 10 mM EDTA pH 8.0) and incubating at 95°C for 3 min. Intact and cleaved target RNAs were resolved by denaturing PAGE (15%) and visualized by phosphorimaging.
Cloning and mutagenesis
pAGM-HSUR1 plasmid was previously generated (Guo et al., 2014) by amplifying the HSUR1 sequence expressed by a U1 promoter from the pUc-U1-HSUR 1 plasmid (Fan et al., 1997) and inserting it into a PacI site of the lentiviral plasmid pAGM (Pertel et al., 2011). pAGM plasmid expresses ZsGeen protein allowing fluorescence-based selection.
For HSUR1 mutagenesis, mutations were introduced into the pAGM-HSUR1 by “megaprimer” site-directed mutagenesis using Phusion polymerase (NEB). Briefly, two PCR reaction were performed: 1) using a HSUR1_F flanking primer and a mutation-containing reverse primer, and 2) using a HSUR1_R flanking primer and a mutation-containing forward primer. The two gel-isolated PCR products served as a template for a third overlap extension PCR reaction using HSUR1_F primer and HSUR1_R primer. The final product (megaprimer) was used for site-directed mutagenesis of pAGM-HSUR1. Primer sequences are available upon request.
Four human Argonaute FLAG-HA-tagged proteins were cloned from pIRESneo-FLAG/HA-Ago plasmids (Addgene) into pLVX-Puro (Clontech) using EcoRI and XbaI cloning sites. F292A Ago1, F294A Ago2 mutations were introduced using site-directed mutagenesis on the appropriate pLVX-Ago plasmids. Primer sequences are available upon request. Luciferase reporters were obtained by inserting sequences containing four miR-27a binding sites separated by a 10-nt linkers downstream of Renilla luciferase into the NotI and XhoI sites of the psiCHECK-2 vector (Promega). miR-27a binding sites were either perfectly complementary
(gcggccgcGCGGAACTTAGCCACTGTGAActaatcaagaGCGGAACTTAGCCACTGTGAAtgcgtcttaGCGGAACTTAGCCACTGTGAActaatcaagaGCGGAACTTAGCCACTGTGAActcgag) or from SEMA7A
(gcggccgcTTATAACTTAGGCTAAACTGTGAActaatcaagaTTATAACTTAGGCTAAACTGTGAAtgcgtcttaTTATAACTTAGGCTAAACTGTGAActaatcaagaTTATAACTTAGGCTAAACTGTGAActc gag).
All mutations were confirmed by Sanger sequencing (Keck, Yale).
Lentiviral transduction and stable cell line generation
To produce viral vectors, 6-well plates of sub-confluent HEK293T cells were co-transfected using TransIT-293T (Mirus Bio) with 2 μg psPAX2 (Addgene), 1 μg pMDG.2 (Addgene) and 2 μg of the appropriate either HSUR1-expressing pAGM (Guo et al., 2014; Pawlica et al., 2016) or Argonaute-expressing pLVX (this paper). The virus-containing supernatants were collected 2 days later and passed through 0.45 μm filters. For stable transduction, BJAB cells were infected at multiplicity of infection ~10 for the viruses described above, and several days later subjected to selection. For pAGM-based lentiviruses, the cells were sorted for green fluorescence using the same gates across cell lines on Bio-Rad’s S3e Cell Sorter. For pLVX-based lentiviruses, the cells were selected using 1μg/ml puromycin (Gibco). For the cells expressing both pAGM- and pLVX-based constructs, first transduction with viruses containing pLVX-based constructs was performed, followed by transduction with viruses carrying pAGM-based constructs. Stable cell lines were maintained for the maximum of four months. For HSUR1 mutants, the transduction was repeated three independent times and for Ago mutants, two independent times.
Northern blot analysis
Total RNAs from BJAB cells were TRIzol (Thermo Fisher) isolated and separated by 15% 8M urea-PAGE (15 μg per lane). The RNA was electrotransferred to Hybond-NX membrane (Amersham), which was then cut at the position of Xylene Cylenol (which comigrates with ~40 bp long dsDNA); the bottom was 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) crosslinked for higher miRNA sensitivity (Pall and Hamilton, 2008), while the top was UV crosslinked. miRNAs, HSUR1, and U6 were detected by 5′ end-labeling with [γ32P]ATP (PerkinElmer) DNA probes. Radioactive signals were detected using a Storm 840 Phosphorimager (Molecular Dynamics).
Small RNA sequencing
Total RNA was separated on 15% urea-PAGE and small RNA was excised from gel between bromophenol blue and xylene cyanol. For each sample, 200 ng of gel purified small RNA was used to prepare cDNA library using NEBNext® Multiplex Small RNA Library Prep for Illumina (NEB) according to manufacturer’s guide. The cDNA libraries were on the Illumina HiSeq 2500 platform using 75 bp single-end reads. The reads were trimmed of adaptors using fastx_clipper (FASTX-Toolkit; http://hannonlab.cshl.edu/fastx_toolkit) and filtered using fastq_quality_filter (FASTX-Toolkit) with parameters -q 20 and -p 80.
To asses differential expression of miRNAs, reads were aligned to mature miRNAs sequences from miRbase_v21 using STAR (Dobin et al., 2013) with settings: -- outFilterMismatchNoverLmax 0.05 --outFilterMatchNmin 14 --outFilterScoreMinOverLread 0 -- outFilterMatchNminOverLread 0 --alignIntronMax 1. Then SAMtools (Dobin et al., 2013) were used to count aligned reads. Differential expression was determined using edgeR (Robinson et al., 2010).
To analyze miR-27a and miR-27b isomiRs, first the 19 nucleotides of miRNAs were string matched, allowing for one mismatch at positions 1 through 18 using custom perl scripts. miRNA additions and lengths were determined by custom perl scripts. To calculate fraction of reads with certain additions or of lengths, their numbers were divided by the total number of miRNA reads. The most abundant isomiR was considered mature.
For sequence logo representations, reads between 22 and 26 nt-long of miR-27a and b were aligned using mafft_v6 (Katoh et al., 2002) and sequence logos were generated using WebLogo_v3.5 (Crooks et al., 2004).
Immunoprecipitation
3 x 107 cells were washed once in PBS, resuspended in 500 μl of NET-2 buffer (50 mM Tris [pH 7.5], 150 mM NaCl, and 0.05% NP-40) and sonicated 10 x 30s using a water sonicator Bioruptor Pico (Diagenode). Lysates were pre-cleared by centrifugation at 16000g for 10 min at 4°C. For anti-Sm (Lerner et al., 1981) and anti-pan Ago (Sigma Millipore, MABE56) immunoprecipitation, lysates were incubated with nutation for 4 h at 4°C with 5 μg of antibody previously immobilized on protein A-sepharose (Amersham). For anti-FLAG immunoprecipitation, lysates were incubated with nutation for 4 h at 4°C with 20 μl of ANTI-FLAG® M2 Gel (Sigma Millipore). Samples were then washed six times with NET-2 buffer; RNA was TRIzol extracted and analyzed by Northern blot as described above.
For transfection of radiolabeled miR-27a, 2 x 107 cells were nucleofected with 10 pmol of 5′ end-labeled synthetic miR-27a annealed to a passenger strand. Nucleofection was carried out in 100 μl of SF solution using 4D-Nucleofector (Lonza). 24 h later anti-FLAG immunoprecipitation was performed as described above.
Luciferase reporter assays
3 x 107 cells were nucleofected with 20 ng psiCHECK-2 reporter and 2 μg pBlueScript II (Stratagene) in 100 μl of SF solution using 4D-Nucleofector (Lonza). 24 h post-transfection, Firefly and Renilla luciferase activities were measured by Dual-Luciferase Reporter Assay System (Promega) on a GloMax-Multi+ Microplate Multimode Reader (Promega) according to the manufacturer’s instructions.
Western blot analysis
3 x 107 of cells were lysed in 100 μl of NET-2 buffer supplemented with complete EDTA-free proteinase inhibitor cocktail tablet (Roche), sonicated as described above, and the supernatants were mixed with 35 μl 4X SDS-PAGE loading buffer. Typically, 25 μl (corresponding to ~40 μg total protein) were separated on a 10% SDS-PAGE gel, and electrotransferred to a PVDF membrane (BioRad). After blocking with 10% milk in 1× TBST (20 mM Tris [pH 7.5], 150 mM NaCl, 0.1% Tween 20), the membrane was probed with the appropriate antibodies and detected with Western Lightning Plus-ECL (PerkinElmer) using a GBox (SYNGENE). Primary antibodies used were anti-pan Ago (Sigma Millipore), anti-HA.11 (BioLegend) and anti-GAPDH (Cell Signaling).
QUANTIFICATION AND STATISTICAL ANALYSIS
Target RNA equilibrium binding experiments
Fraction target RNA bound was determined by calculating the ratio of bound to total (bound + free) target RNA for various concentrations of Ago2–miRNA complexes. RNA was quantified by phosphorimaging using ImageQuant (GE Healthcare). N=3 for each binding experiment, where N represents number of experimental replications. Dissociation constants calculated using Prism version 6.0g (GraphPad Software, Inc.), with the following formula that accounts for potential ligand depletion (Wee et al., 2012):
where F = fraction of target bound, Bmax = calculated maximum number of binding sites, [ET] = total enzyme concentration, [ST] = total target concentration, and KD = apparent equilibrium dissociation constant.
Target dissociation assays
Fraction target RNA bound was determined by calculating the ratio of bound to total (bound + free) target RNA for various concentrations of Ago2-miRNA complexes. RNA was quantified by phosphorimaging using ImageQuant (GE Healthcare Life Sciences). N=3 for each binding experiment, where N represents number of experimental replications. Dissociation rates calculated by fitting data to a one-phase exponential decay using Prism version 6.0g (GraphPad Software, Inc.).
Minimum folding energy and Z-score calculations
Structure predictions and free energy calculations were performed with RNAstructure, version 5.8.1 (Reuter and Mathews, 2010). Thermodynamic Z-scores were calculated by determining the average difference in folding free energy predicted for a native sequence versus 200 randomized “mutants” and normalizing that value by the standard deviation of the entire set.
Northern blot quantification
Densitometry was performed using ImageQuant (GE Healthcare). For mature miR-27 quantification bands corresponding to 20 nt were analyzed, and for all miR-27 isomiRs quantification bands between 18 and 24 nt were summed. Levels of miR-27 were normalized to the levels of miR-20 and miR-16 and to the empty vector control. Levels of HSUR1 were normalized to US and to HSUR1 WT.
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contacts, Joan A. Steitz (joan.steitz@yale.edu) and Ian J. MacRae (macrae@scripps.edu).
Supplementary Material
Figure S1. Related to Figure 1. Structural comparison of extended pairing in TtAgo and hAgo2.
Figure S2. Related to Figure 2. Conformational changes that open the central cleft upon extended pairing and 3′ release.
Figure S3. Related to Figure 3. TDMD targets form extraordinarily stable complexes with hAgo2–miRNA.
Figure S4. Related to Figure 4. Base-pairing patterns determine the outcome of TDMD.
Figure S5. Related to Figures 4, 5 and 6. Further analysis of 3′ end release.
Figure S6. Related to Figure 6. Access of the hAgo-bound miRNA 3′ end to enzymes implicated in miRNA 3′ modification.
Table S1. Related to Figure 1. Known miRNA-TDMD target interactions.
Table S2. Related to Figure 3. miRNA–target duplexes used in biochemical studies.
Table S3. Related to Figure 3. Biochemical data for target RNAs binding and dissociating from the hAgo2–miRNA complex.
Table S4. Related to Figure 4. Stability metrics of HSUR1 mutants.
Table S5. Related to Key Resource Table. Oligonucleotides used in this study.
Movie S1. Related to Figure 2. Conformational changes for extended miRNA-target pairing. Illustration of differences between the seed plus supplementary paired (PDB ID 6N4O) and TDMD (PDB ID 6MDZ) conformations of hAgo2. Conformational differences are depicted as a series of discrete steps for clarity. hAgo2 is displayed as a surface representation in gray. miRNA and target are displayed as ribbons colored red and blue, respectively.
Highlights.
Structural and mutational analyses reveal mechanism of target directed miRNA degradation
TDMD targets trap Ago2 in a conformation with miRNA 3′-end displayed for enzymatic attack
miRNA-TDMD target pairing features dictate miRNA 3′-end remodeling and fate
miRNA 3′-end display is a mechanism that controls miRNA stability and activity
ACKNOWLEDGMENTS
We thank the members of the MacRae and Steitz labs for advice and support. We are grateful to Stefan Niekamp for help with Pymol scripts used to visualize inter-Cα distances, W. Moss for help with Z-score calculations, and Y. E. Guo for providing the psiCHEK-miR-27a plasmid. Diffraction data were collected at beamlines 12-2 at the Stanford Synchrotron Radiation Lightsource, supported by the U.S. Department of Energy (contract DE-AC02-76SF00515) and the National Institute of General Medical Sciences (grant P41GM103393), and 23IDD at the Advanced Photon Source, supported by the U.S. Department of Energy (contract DE-AC02-06CH11357). This work was supported by National Institutes of Health grants GM115649, GM104475, and GM127090 (I.J.M.), and CA016038 (J.A.S.). J.S.-G. was a Pre-doctoral Fellow of the American Heart Association and an Abrams Charitable Trust Award recipient. P.P. was funded by the American Cancer Society (TESARO, Inc., PF-18-1124-01-RMC) and is supported by NIH fellowship K99/R00 (K99GM129412). J.A.S. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
Authors declare no competing interests.
REFERENCES
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta crystallographica Section D, Biological crystallography 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameres SL, Horwich MD, Hung JH, Xu J, Ghildiyal M, Weng Z, and Zamore PD (2010). Target RNA-directed trimming and tailing of small silencing RNAs. Science 328, 1534–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameres SL, Hung JH, Xu J, Weng Z, and Zamore PD (2011). Target RNA-directed tailing and trimming purifies the sorting of endo-siRNAs between the two Drosophila Argonaute proteins. RNA 17, 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarini A, Chauhan H, Gardner TJ, Jayaprakash AD, Sachidanandam R, and Brown BD (2011). Kinetic analysis reveals the fate of a microRNA following target regulation in mammalian cells. Current biology : CB 21, 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2018). Metazoan MicroRNAs. Cell 173, 20–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitetti A, Mallory AC, Golini E, Carrieri C, Carreno Gutierrez H, Perlas E, Perez-Rico YA, Tocchini-Valentini GP, Enright AJ, Norton WHJ, et al. (2018). MicroRNA degradation by a conserved target RNA regulates animal behavior. Nature structural & molecular biology 25, 244–251. [DOI] [PubMed] [Google Scholar]
- Cazalla D, Yario T, and Steitz JA (2010). Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science 328, 1563–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, and Brenner SE (2004). WebLogo: a sequence logo generator. Genome research 14, 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrogio A, Gu W, Udagawa T, Mello CC, and Richter JD (2012). Specific miRNA stabilization by Gld2-catalyzed monoadenylation. Cell Rep 2, 1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mata M, Gaidatzis D, Vitanescu M, Stadler MB, Wentzel C, Scheiffele P, Filipowicz W, and Grosshans H (2015). Potent degradation of neuronal miRNAs induced by highly complementary targets. EMBO reports 16, 500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De N, Young L, Lau PW, Meisner NC, Morrissey DV, and Macrae IJ (2013). Highly Complementary Target RNAs Promote Release of Guide RNAs from Human Argonaute2. Molecular cell 50, 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzler R, McGeary SE, Title AC, Agarwal V, Bartel DP, and Stoffel M (2016). Impact of MicroRNA Levels, Target-Site Complementarity, and Cooperativity on Competing Endogenous RNA-Regulated Gene Expression. Molecular cell 64, 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvignes T, Batzel P, Berezikov E, Eilbeck K, Eppig JT, McAndrews MS, Singer A, and Postlethwait JH (2015). miRNA Nomenclature: A View Incorporating Genetic Origins, Biosynthetic Pathways, and Sequence Variants. Trends in genetics : TIG 31, 613–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy EE, Rutenberg-Schoenberg M, Stark CD, Kitchen RR, Gerstein MB, and Simon MD (2015). Tracking Distinct RNA Populations Using Efficient and Reversible Covalent Chemistry. Molecular cell 59, 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbarbary RA, Miyoshi K, Myers JR, Du P, Ashton JM, Tian B, and Maquat LE (2017). Tudor-SN-mediated endonucleolytic decay of human cell microRNAs promotes G1/S phase transition. Science 356, 859–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Martinez J, Patkaniowska A, Lendeckel W, and Tuschl T (2001). Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. The EMBO journal 20, 6877–6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, and Cowtan K (2010). Features and development of Coot. Acta crystallographica Section D, Biological crystallography 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faehnle CR, Elkayam E, Haase AD, Hannon GJ, and Joshua-Tor L (2013). The making of a slicer: activation of human Argonaute-1. Cell Rep 3, 1901–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan XC, Myer VE, and Steitz JA (1997). AU-rich elements target small nuclear RNAs as well as mRNAs for rapid degradation. Genes & development 11, 2557–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Jasso CF, Salomon WE, and Zamore PD (2013). Rapid and specific purification of Argonaute-small RNA complexes from crude cell lysates. RNA 19, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Wightman F, Giono LE, Fededa JP, and de la Mata M (2018). Target RNAs Strike Back on MicroRNAs. Frontiers in Genetics 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebert LFR, and MacRae IJ (2018). Regulation of microRNA function in animals. Nature reviews Molecular cell biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghini F, Rubolino C, Climent M, Simeone I, Marzi MJ, and Nicassio F (2018). Endogenous transcripts control miRNA levels and activity in mammalian cells by target-directed miRNA degradation. Nature communications 9, 3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YE, Riley KJ, Iwasaki A, and Steitz JA (2014). Alternative capture of noncoding RNAs or protein-coding genes by herpesviruses to alter host T cell function. Molecular cell 54, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Renwick N, Brown M, Mihailovic A, Holoch D, Lin C, Pena JT, Nusbaum JD, Morozov P, Ludwig J, et al. (2011). RNA-ligase-dependent biases in miRNA representation in deep-sequenced small RNA cDNA libraries. RNA 17, 1697–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann J, Dueck A, Harlander S, Pfaff J, Merkl R, and Meister G (2013). Turning catalytically inactive human Argonaute proteins into active slicer enzymes. Nature structural & molecular biology. [DOI] [PubMed] [Google Scholar]
- Horman SR, Janas MM, Litterst C, Wang B, Macrae IJ, Sever MJ, Morrissey DV, Graves P, Luo B, Umesalma S, et al. (2013). Akt-Mediated Phosphorylation of Argonaute 2 Downregulates Cleavage and Upregulates Translational Repression of MicroRNA Targets. Molecular cell 50, 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur JK, Zinchenko MK, Djuranovic S, and Green R (2013). Regulation of Argonaute Slicer Activity by Guide RNA 3′ End Interactions with the N-terminal Lobe. The Journal of biological chemistry 288, 7829–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo MH, Shin S, Jung SR, Kim E, Song JJ, and Hohng S (2015). Human Argonaute 2 Has Diverse Reaction Pathways on Target RNAs. Molecular cell 59, 117–124. [DOI] [PubMed] [Google Scholar]
- Kabsch W (2010). Xds. Acta crystallographica Section D, Biological crystallography 66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, and Miyata T (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30, 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh T, Sakaguchi Y, Miyauchi K, Suzuki T, Kashiwabara S, Baba T, and Suzuki T (2009). Selective stabilization of mammalian microRNAs by 3′ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes & development 23, 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata T, Seitz H, and Tomari Y (2009). Structural determinants of miRNAs for RISC loading and slicer-independent unwinding. Nature structural & molecular biology 16, 953–960. [DOI] [PubMed] [Google Scholar]
- Kim B, Ha M, Loeff L, Chang H, Simanshu DK, Li S, Fareh M, Patel DJ, Joo C, and Kim VN (2015). TUT7 controls the fate of precursor microRNAs by using three different uridylation mechanisms. The EMBO journal 34, 1801–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleaveland B, Shi CY, Stefano J, and Bartel DP (2018). A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klum SM, Chandradoss SD, Schirle NT, Joo C, and MacRae IJ (2017). Helix-7 in Argonaute2 shapes the microRNA seed region for rapid target recognition. The EMBO journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klum SM, Chandradoss SD, Schirle NT, Joo C, and MacRae IJ (2018). Helix-7 in Argonaute2 shapes the microRNA seed region for rapid target recognition. The EMBO journal 37, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak PB, and Tomari Y (2012). The N domain of Argonaute drives duplex unwinding during RISC assembly. Nature structural & molecular biology. [DOI] [PubMed] [Google Scholar]
- Lee S, Song J, Kim S, Kim J, Hong Y, Kim Y, Kim D, Baek D, and Ahn K (2013). Selective degradation of host MicroRNAs by an intergenic HCMV noncoding RNA accelerates virus production. Cell host & microbe 13, 678–690. [DOI] [PubMed] [Google Scholar]
- Lerner EA, Lerner MR, Janeway CA Jr., and Steitz JA (1981). Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proceedings of the National Academy of Sciences of the United States of America 78, 2737–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libri V, Helwak A, Miesen P, Santhakumar D, Borger JG, Kudla G, Grey F, Tollervey D, and Buck AH (2012). Murine cytomegalovirus encodes a miR-27 inhibitor disguised as a target. Proceedings of the National Academy of Sciences of the United States of America 109, 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JB, Ye K, and Patel DJ (2004). Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain. Nature 429, 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinowski L, Tanguy M, Krmpotic A, Radle B, Lisnic VJ, Tuddenham L, Chane-Woon-Ming B, Ruzsics Z, Erhard F, Benkartek C, et al. (2012). Degradation of cellular mir-27 by a novel, highly abundant viral transcript is important for efficient virus replication in vivo. PLoS pathogens 8, e1002510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi MJ, Ghini F, Cerruti B, de Pretis S, Bonetti P, Giacomelli C, Gorski MM, Kress T, Pelizzola M, Muller H, et al. (2016). Degradation dynamics of microRNAs revealed by a novel pulse-chase approach. Genome research 26, 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall MN, Kim MS, Adil M, Patil AH, Lu Y, Mitchell CJ, Leal-Rojas P, Xu J, Kumar M, Dawson VL, et al. (2017). Toward the human cellular microRNAome. Genome research 27, 1769–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, and Read RJ (2007). Phaser crystallographic software. J Appl Crystallogr 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, and Tuschl T (2004). Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Molecular cell 15, 185–197. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Ascano M, Gogakos T, Ishibe-Murakami S, Serganov AA, Briskin D, Morozov P, Tuschl T, and Patel DJ (2013). Eukaryote-specific insertion elements control human ARGONAUTE slicer activity. Cell Rep 3, 1893–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakel K, Bonneau F, Eckmann CR, and Conti E (2015). Structural basis for the activation of the C. elegans noncanonical cytoplasmic poly(A)-polymerase GLD-2 by GLD-3. Proceedings of the National Academy of Sciences of the United States of America 112, 8614–8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilsen CT, Goodall GJ, and Bracken CP (2012). IsomiRs--the overlooked repertoire in the dynamic microRNAome. Trends in genetics : TIG 28, 544–549. [DOI] [PubMed] [Google Scholar]
- Pall GS, and Hamilton AJ (2008). Improved northern blot method for enhanced detection of small RNA. Nat Protoc 3, 1077–1084. [DOI] [PubMed] [Google Scholar]
- Park JH, Shin SY, and Shin C (2017). Non-canonical targets destabilize microRNAs in human Argonautes. Nucleic acids research 45, 1569–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlica P, Moss WN, and Steitz JA (2016). Host miRNA degradation by Herpesvirus saimiri small nuclear RNA requires an unstructured interacting region. RNA 22, 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertel T, Hausmann S, Morger D, Zuger S, Guerra J, Lascano J, Reinhard C, Santoni FA, Uchil PD, Chatel L, et al. (2011). TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 472, 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajappa-Titu L, Suematsu T, Munoz-Tello P, Long M, Demir O, Cheng KJ, Stagno JR, Luecke H, Amaro RE, Aphasizheva I, et al. (2016). RNA Editing TUTase 1: structural foundation of substrate recognition, complex interactions and drug targeting. Nucleic acids research 44, 10862–10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter JS, and Mathews DH (2010). RNAstructure: software for RNA secondary structure prediction and analysis. BMC bioinformatics 11, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, and Smyth GK (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger S, and Grosshans H (2012). MicroRNA turnover: when, how, and why. Trends Biochem Sci 37, 436–446. [DOI] [PubMed] [Google Scholar]
- Salomon WE, Jolly SM, Moore MJ, Zamore PD, and Serebrov V (2015). Single-Molecule Imaging Reveals that Argonaute Reshapes the Binding Properties of Its Nucleic Acid Guides. Cell 162, 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirle NT, and MacRae IJ (2012). The crystal structure of human Argonaute2. Science 336, 1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirle NT, Sheu-Gruttadauria J, and MacRae IJ (2014). Structural basis for microRNA targeting. Science 346, 608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu-Gruttadauria J, Xiao Y, Gebert LF, and MacRae IJ (2019). Beyond the seed: structural basis for supplementary microRNA targeting by human Argonaute2. The EMBO journal, e101153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Song J, Mo B, and Chen X (2015). Uridylation and adenylation of RNAs. Sci China Life Sci 58, 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Juranek S, Li H, Sheng G, Tuschl T, and Patel DJ (2008). Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature 456, 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Juranek S, Li H, Sheng G, Wardle GS, Tuschl T, and Patel DJ (2009). Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature 461, 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee LM, Flores-Jasso CF, Salomon WE, and Zamore PD (2012). Argonaute divides its RNA guide into domains with distinct functions and RNA-binding properties. Cell 151, 1055–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, et al. (2011). Overview of the CCP4 suite and current developments. Acta crystallographica Section D, Biological crystallography 67, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Reuter M, Lilie H, Liu Y, Wahle E, and Song H (2005). Structural insight into poly(A) binding and catalytic mechanism of human PARN. The EMBO journal 24, 4082–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Ameres SL, Friedline R, Hung JH, Zhang Y, Xie Q, Zhong L, Su Q, He R, Li M, et al. (2012). Long-term, efficient inhibition of microRNA function in mice using rAAV vectors. Nature methods 9, 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Sankala H, Zhang X, and Graves PR (2008). Phosphorylation of Argonaute 2 at serine-387 facilitates its localization to processing bodies. The Biochemical journal 413, 429–436. [DOI] [PubMed] [Google Scholar]
- Zhuang F, Fuchs RT, Sun Z, Zheng Y, and Robb GB (2012). Structural bias in T4 RNA ligase-mediated 3′-adapter ligation. Nucleic Acids Res 40, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Related to Figure 1. Structural comparison of extended pairing in TtAgo and hAgo2.
Figure S2. Related to Figure 2. Conformational changes that open the central cleft upon extended pairing and 3′ release.
Figure S3. Related to Figure 3. TDMD targets form extraordinarily stable complexes with hAgo2–miRNA.
Figure S4. Related to Figure 4. Base-pairing patterns determine the outcome of TDMD.
Figure S5. Related to Figures 4, 5 and 6. Further analysis of 3′ end release.
Figure S6. Related to Figure 6. Access of the hAgo-bound miRNA 3′ end to enzymes implicated in miRNA 3′ modification.
Table S1. Related to Figure 1. Known miRNA-TDMD target interactions.
Table S2. Related to Figure 3. miRNA–target duplexes used in biochemical studies.
Table S3. Related to Figure 3. Biochemical data for target RNAs binding and dissociating from the hAgo2–miRNA complex.
Table S4. Related to Figure 4. Stability metrics of HSUR1 mutants.
Table S5. Related to Key Resource Table. Oligonucleotides used in this study.
Movie S1. Related to Figure 2. Conformational changes for extended miRNA-target pairing. Illustration of differences between the seed plus supplementary paired (PDB ID 6N4O) and TDMD (PDB ID 6MDZ) conformations of hAgo2. Conformational differences are depicted as a series of discrete steps for clarity. hAgo2 is displayed as a surface representation in gray. miRNA and target are displayed as ribbons colored red and blue, respectively.