Abstract
Down syndrome (trisomy 21) is characterized by genome-wide imbalances that result in a range of phenotypic manifestations. Altered expression of DYRK1A in the trisomic context has been linked to some Down syndrome phenotypes. DYRK1A regulates the splicing of cardiac troponin (TNNT2) through a pathway mediated by the master splicing factor SRSF6. Here, we documented the expression of the DYRK1A-SRSF6-TNNT2 pathway in a collection of myocardial samples from persons with and without Down syndrome. Results suggest that “gene dosage effect” may drive the expression of DYRK1A mRNA but has no effect on DYRK1A protein levels in trisomic myocardium. The levels of phosphorylated DYRK1A-Tyr321 tended to be higher (~35%) in myocardial samples from donors with Down syndrome. The levels of phosphorylated SRSF6 were 2.6-fold higher in trisomic myocardium. In line, the expression of fetal TNNT2 variants was higher in myocardial tissue with trisomy 21. These data provide a representative picture on the extent of inter-individual variation in myocardial DYRK1A-SRSF6-TNNT2 expression in the context of Down syndrome.
Keywords: Dual Specificity Tyrosine Phosphorylation Regulated Kinase 1A (DYRK1A), Down syndrome, cardiac troponin, alternative splicing
1. Introduction
Down syndrome is caused by the presence of an extra total or partial copy of chromosome 21 (trisomy 21) (Patterson, 2009). Down syndrome is characterized by genome-wide imbalances that result in a range of phenotypic manifestations (Ait Yahya-Graison et al., 2007). Individuals with Down syndrome exhibit various degrees of neurological, skeletal, immunological, and cardiovascular abnormalities (Freeman et al., 1998; Roper and Reeves, 2006). The altered expression of certain chromosome 21 genes may be responsible for a subset of Down syndrome traits that includes cardiovascular phenotypes (Barlow et al., 2001; Shapiro, 1999). For example, DYRK1A (dual specificity tyrosine phosphorylation regulated kinase 1A) participates in the control of cardiomyocyte proliferation and differentiation during fetal and early neonatal development (Hille et al., 2016). DYRK1A also participates in suppression of myocardial hypertrophy through a mechanism involving the calcineurin/nuclear factor of activated T cells (NFAT) signaling pathway (Arron et al., 2006; da Costa Martins et al., 2010; Grebe et al., 2011). A recent study showed that DYRK1A impacts the splicing of cardiac troponin transcript variants (TNNT2, alternative name: cTnT) in human cell lines and hearts from Ts65Dn mice - a murine model of Down syndrome - (Lu and Yin, 2016). Increased expression of DYRK1A promotes the inclusion of exon five into the TNNT2 transcript through phosphorylation of the splicing factor SRSF6 (alternative name: SRp55). This is relevant because cardiac myofibrils containing protein troponin isoforms with exon 5 insertion - i.e. the fetal isoforms cTnT1 and cTnT2 - are more sensitive to Ca2+ (Briggs et al., 1994; Gomes et al., 2002; Gomes et al., 2004). The coexistence of fetal cTnT1 and adult cTnT isoforms (e.g., cTnT3) in ventricular muscle with dilated cardiomyopathy has been linked to decreases in myocardial contractility and pumping efficiency (Anderson et al., 1995; Biesiadecki et al., 2002).
There is a paucity of studies documenting the expression of DYRK1A and its prominent targets SRSF6 and TNNT2 in trisomic myocardium. Thus, the goal of this study was to document the expression of the DYRK1A-SRSF6-TNNT2 pathway in a collection of myocardial tissue samples from subjects with and without Down syndrome.
2. Material and Methods
2.1. Human heart samples
The Institutional Review Board of the State University of New York at Buffalo approved this research. Heart samples from donors with (n = 14) and without Down syndrome (n = 16) were procured from The National Disease Research Interchange (NDRI, funded by the National Center for Research Resources), The Cooperative Human Tissue Network (CHTN, funded by the National Cancer Institute), and the National Institute of Child Health and Human Development (NICHD) Brain and Tissue Bank. The postmortem to tissue recovery interval was ≤10 h. Samples (2 – 20 g, myocardium, left ventricle only) were frozen immediately after recovery and stored in liquid nitrogen until further processing. Down syndrome status was confirmed by array comparative genomic hybridization (aCGH) as described (Quinones-Lombrana et al., 2014). Heart samples were processed following standardized procedures to isolate DNA and RNA as described (Quinones-Lombrana et al., 2014). The main demographics from donors with and without DS are summarized in Table S1.
2.2. Quantification of myocardial mRNA expression
The expression of DYRK1A mRNA and TNNT2 transcript variants was analyzed by quantitative real time polymerase chain reaction with specific primers (Table 1) following the MIQE guidelines (Bustin et al., 2009). The stability of four candidate reference genes: 18s rRNA, ACTB, B2M, and SNORD47 was evaluated in a subset of myocardial samples using GeNorm V3. According to GeNorm, the most stably expressed genes were ACTB and 18s rRNA. However, variability analysis showed unacceptable Cq value variability for 18s rRNA (22.02 ± 0.96), B2M (22.10 ± 1.34), and SNORD47 (24.89 ± 1.26) when compared to the Cq variability of ACTB (23.59 ± 0.51. Figure 1). Thus, ACTB was considered a suitable normalizer based on comparatively low Cq variability in myocardial tissue. Total RNA (50 ng) was reverse transcribed and amplified with the iTaq Universal SYBR Green One-Step Kit (Bio-Rad, Hercules, CA). TNNT2 transcript variants, DYRK1A, and the reference gene ACTB were amplified in parallel in a CFX96 Touch Real-Time PCR Detection System (Bio-Rad) with the following cycling parameters: 50°C for 10 min (reverse transcription), 95°C for 1 min, followed by 40 cycles of 95°C for 10 s, 58°C for 30 s and 72°C for 30 s. The expression of DYRK1A mRNA in individual myocardial samples was expressed relative to the averaged expression of DYRK1A mRNA in the group of myocardial samples from donors without Down syndrome (n = 16), which was assigned an arbitrary value of 1.00.
Table 1.
List of primers for real time quantitative PCR
| Linearity | Efficiency | |||
|---|---|---|---|---|
| cTnT1/cTnT2 | Forward | 5’-GGAGGACTGGAGAGAGGAC-3’ | 0.96 | 96% |
| Reverse | 5’-CACCAAGTTGGGCATGAACG-3’ | |||
| cTnT3 | Forward | 5’-CTGTTGAAGAGCAGGAGGAG-3’ | 0.98 | 110% |
| Reverse | 5’-CCGACGTCTCTCGATCCTG-3’ | |||
| cTnT4 | Forward | 5’-AGCAGGAAGAGCAGGAGGAG-3’ | 0.99 | 96% |
| Reverse | 5’-CCGACGTCTCTCGATCCTG-3’ | |||
| DYRK1A | Forward | 5’-GGACAGGTTGTAAAGGCATATG-3’ | 0.99 | 104% |
| Reverse | 5’-GCGTTTCAAATGCACTATGTAG-3’ | |||
| ACTB | Forward | 5’-GGACTTCGAGCAAGAGATGG-3’ | 0.96 | 103% |
| Reverse | 5’-AGCACTGTGTTGGCGTACAG-3’ |
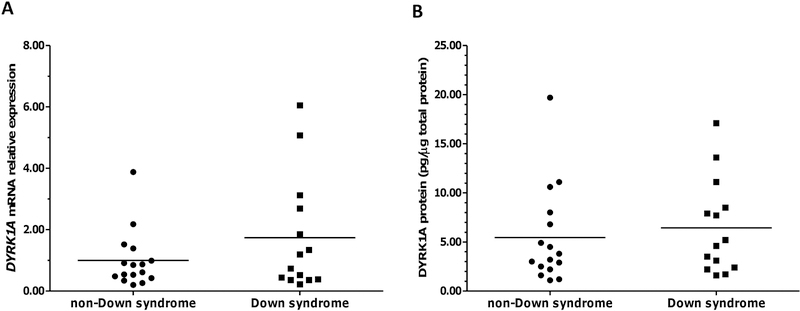
Fig. 1. DYRK1A expression in myocardium from donors with and without Down syndrome.
(A) DYRK1A mRNA expression. (B) DYRK1A protein expression. Each symbol depicts the average of individual samples. Horizontal lines indicate group means. Samples were analyzed in triplicates.
For the absolute quantification of TNNT2 transcript variants, cloned PCR products were serially diluted to generate standards for calibration curves. In all cases, the regression coefficients were r2 ≥ 0.96. The amplification efficiencies for TNNT2 transcript variants and ACTB were comparable and ranged between 96 and 110%. Experimental samples and standards for calibration curves were analyzed in triplicate. For each myocardial sample, the copy number of each TNNT2 transcript variant and ACTB were calculated using the average of Cq values and direct extrapolation from calibration curves. Specificity was evaluated by electrophoresis analysis (2% agarose gels stained with SYBR safe, Thermo Fisher Scientific, Waltham, MA), melting curve analysis, and DNA sequencing of TNNT2 amplicons. In all cases, myocardial mRNA levels were expressed as copy number ratios using the following expression:
2.3. Quantification of DYRK1A protein expression
Total DYRK1A protein content in homogenates of myocardium was measured with the human DYRK1A ELISA kit (Lifespan Biosciences, Seattle, WA) as per the manufacturer’s instructions.
2.4. Immunoblotting
For the analysis of TNNT2, SRSF6, and DYRK1A phosphorylation status, myocardial tissue was homogenized as described (Quinones-Lombrana et al., 2017). Myocardium lysates (20 μg) were denatured with NuPAGE LDS sample buffer (Thermo Fisher Scientific) containing NuPAGE sample reducing agent (Thermo Fisher Scientific), protease inhibitor cocktail (Thermo Fisher Scientific), and Halt phosphatase inhibitor (Thermo Fisher Scientific), and boiled at 70°C for 10 min prior to use. Proteins were separated by gel electrophoresis using NuPAGE Novex 4 – 12% Bis-Tris precast gels (Thermo Fisher Scientific) and transferred onto PVDF membranes using the iBlot Gel Transfer Device (Thermo Fisher Scientific). Membranes were blocked with 5% non-fat milk in 0.2% Tween 20-PBS for 1 h at room temperature and then probed with specific antihuman antibodies for SRSF6 (Santa Cruz Biotechnology, Dallas, TX), TNNT2 (Santa Cruz Biotechnology) or Tyr321 phosphorylated DYRK1A (Thermo Fisher Scientific) overnight at 4°C. Next, membranes were incubated with a secondary mouse or rabbit anti-IgG antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology) for 1.5 h at room temperature. To normalize for protein loading, membranes were stripped with Restore Western Blot Stripping Buffer (Thermo Fisher Scientific) and re-probed with anti-ACTB antibody (Santa Cruz Biotechnology). Immunoreactive bands were visualized with the ECL Plus Western blotting substrate (GEHealthcare, Chicago, IL) in a ChemiDoc MP gel imaging system (Bio-Rad). Densitometric analysis was performed using Image Lab software (Bio-Rad).
2.5. Phosphatase treatment
SRSF6 phosphorylation status was assessed by incubating myocardial lysates (15 μg) with 25 units of calf intestine alkaline phosphatase (Promega, Madison, WI) in a 20 μl reaction volume containing 50 mM Tris/HCl (pH 9.3) for 1 h at 37°C. Changes in banding patterns due to SRSF6 de-phosphorylation were detected by immunoblotting as described.
2.6. Data analysis
Statistics were computed with Excel 2013 (Microsoft Office; Microsoft, Redmond, WA) and GraphPad Prism version 4.03 (GraphPad Software Inc., La Jolla, CA). The D’Agostino-Pearson test was used to analyze the normality of datasets. The Mann-Whitney U test or Student’s t-test were used to compare group means. Cohen’s d test was used to calculate the effect size. Spearman’s coefficient of correlation (rs) was used to analyze data sets with non-normal distributions. Data are expressed as the mean ± standard deviation (SD). Differences between means were considered significant at p<0.05.
3. Results and Discussion
The myocardial expression of DYRK1A mRNA and DYRK1A protein was examined in samples from donors with and without Down syndrome. Myocardial DYRK1A mRNA expression displayed considerable interindividual variability (DSDYRK1AmRNA range: 0.22 – 6.05 relative fold; non-DSDYRK1AmRNA range: 0.20 – 3.88 relative fold). On average, the expression of DYRK1A mRNA was 74% higher in samples from donors with Down syndrome (DSDYRK1AmRNA: 1.74 ± 1.86 relative fold vs. non-DSDYRK1AmRNA: 1.00 ± 0.93; Mann-Whitney test p = 0.547; Cohen’s d = 0.502. Fig. 1A). Although the difference between means did not reach statistical significance at p < 0.05 due to interindividual variability, the effect size is consistent with tissular “gene dosage effect” due to trisomy 21 (Ait Yahya-Graison et al., 2007; Arron et al., 2006; Duchon and Herault, 2016; Guimera et al., 1999). The expression of DYRK1A protein was similar in myocardial tissue from donors with and without Down syndrome (DSDYRK1Aprotein: 6.44 ± 4.80 pg/μg total protein vs. non-DSDYRK1Aprotein: 5.44 ± 4.92 pg/μg total protein; Mann-Whitney test p = 0.454; Cohen’s d = 0.206. Fig. 1B). There were no significant correlations between DYRK1A mRNA and DYRK1A protein levels in myocardial samples from subjects with and without DS (Table 2). The expression of DYRK1A protein in trisomic myocardium is variable and does not exhibit a trend consistent with gene dosage effect. Previous reports based on the analysis of transcriptomes and proteomes from aneuploid cells showed that while global transcriptional levels in trisomic and tetrasomic cells tended to reflect copy number changes (i.e., “gene dosage effect”), the expression of some proteins was reduced towards diploid levels by compensatory mechanisms such as autophagy activation (Aivazidis et al., 2017; Spellman et al., 2013; Stingele et al., 2012).
Table 2.
Linear regression analysis of DYRK1A-SRSF6-TNNT2 expression in myocardium from subjects with and without DS
| non Down syndrome | Down syndrome | |||
|---|---|---|---|---|
| Spearman r | p value | Spearman r | p value | |
| DYRK1A mRNA vs DYRK1A protein | 0.326 | 0.222 | 0.119 | 0.686 |
| DYRK1A protein vs Tyr321-phosphorylated DYRK1A | −0.495 | 0.072 | −0.163* | 0.577 |
| Phosphorylated SRSF6 vs cTnT1,2 copy number ratio | 0.118 | 0.664 | −0.323 | 0.256 |
coefficient Pearson’s
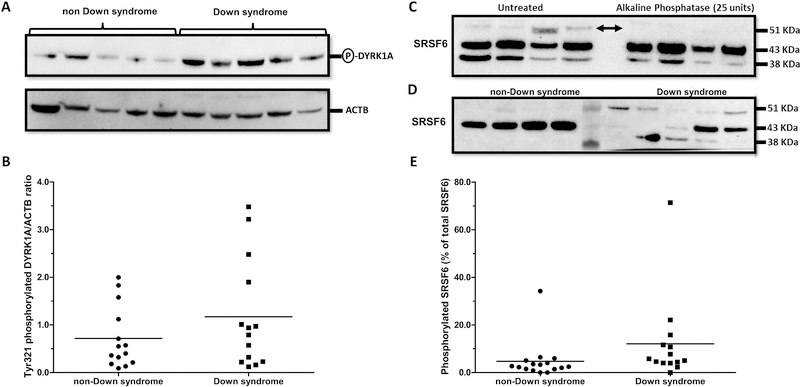
Phosphorylation of DYRK1A in Tyr321 impacts protein kinase activity (Becker and Sippl, 2011; Duchon and Herault, 2016; Walte et al., 2013). The expression of Tyr321-phosphorylated DYRK1A was variable (DSDYRK1A range: 0.12 – 3.48 relative fold; non-DSDYRK1A range: 0.09 – 2.00 relative fold) and tended to be higher (~35%) in myocardial samples from donors with Down syndrome (DS: 1.17 ± 1.15 vs. non-DS: 0.72 ± 0.65; Mann-Whitney test p = 0.208; Cohen’s d = 0.488. Fig. 2A and 2B). There were no significant correlations between DYRK1A and Tyr321-phosphorylated DYRK1A in samples from subjects with and without DS (Table 2). In the cell nucleus, DYRK1A modifies the splicing pattern of target genes through selective phosphorylation of members of the SR family of splicing factors (Alvarez et al., 2003; Naro and Sette, 2013). For example, phosphorylation of SRSF6 by DYRK1A suppresses the inclusion of exon 10 in transcripts encoding the neuronal microtubule-associated protein Tau, and the resulting imbalance of Tau isoforms has been linked to the development of tauopathies (Shi et al., 2008; Yin et al., 2012). Interestingly, Lu et al. showed that phosphorylation of SRSF6 by DYRK1A promotes the inclusion of exon 5 into TNNT2 transcripts (i.e., increased expression of fetal TNNT2 transcripts) (Lu and Yin, 2016). Thus, we investigated the expression of myocardial SRSF6 by immunoblotting with an anti-SRSF6 antibody and alkaline phosphatase treatment (Fig. 2C). The levels of phosphorylated SRSF6 relative to total SRSF6 in myocardium from subjects with Down syndrome were higher in comparison to the levels of phosphorylated SRSF6 in samples from donors without Down syndrome (DS%phosphoSRSF6: 12.09 ± 18.03 vs. non-DS%phosphoSRSF6: 4.73 ± 8.10; Mann-Whitney test p = 0.016; Cohen’s d = 0.527. Fig. 2D and 2E).
Fig. 2. Analysis of DYRK1A and SRSF6 phosphorylation status in myocardium from donors with and without Down syndrome.
(A) Representative immunoblots for Tyr321-phosphorylated DYRK1A and ACTB. (B) Tyr321-phosphorylated DYRK1A expression ratios in myocardial samples. (C) Detection of phosphorylated SRSF6 by alkaline phosphatase treatment and immunoblotting. Arrows indicate phosphorylated SRSF6 (D) Analysis of myocardial SRSF6 expression by immunoblotting. (E) Expression of phosphorylated SRSF6 in myocardial samples. Each symbol depicts the average of individual samples. Horizontal lines indicate group means. Samples were analyzed in triplicates.
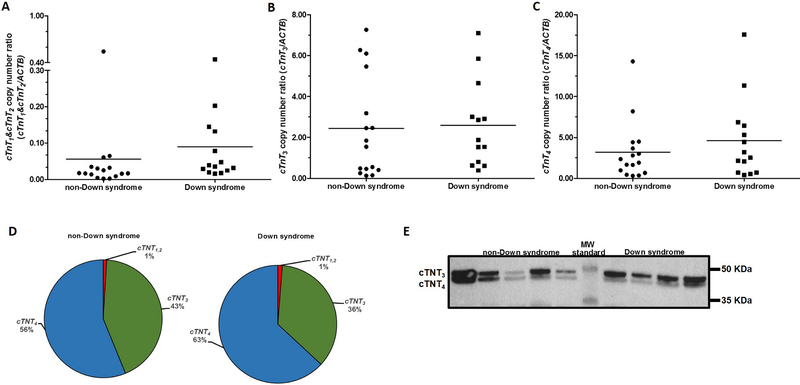
Next, we examined the myocardial expression of adult TNNT2 splicing variants (i.e., cTnT3 and cTnT4) and the fetal variants cTnT1 and cTnT2 (Mesnard-Rouiller et al., 1997). A forward PCR primer targeting exon 5 was used to analyze the expression of cTnT1 and cTnT2 (Fig. 3). The expression ratios of the fetal cTnT1 and cTnT2 variants was 50% higher in myocardial tissue with trisomy 21 (DScTnT1,2mRNA: 0.09 ± 0.12 vs. non-DScTnT1,2mRNA: 0.06 ± 0.13; Mann-Whitney test p = 0.026; Cohen’s d = 0.278 Fig. 4A). The expression of cTnT3 was similar in samples from donors with and without Down syndrome (DScTnT3mRNA: 2.59 ± 2.12 vs. non-DScTnT3mRNA: 2.44 ± 2.49; Mann-Whitney test p = 0.497; Cohen’s d = 0.067 Fig. 4B). The expression of cTnT4 was 44% higher in myocardial samples from donors with Down syndrome (DScTNT4Mrna: 4.58 ± 4.86 vs. non-DScTNT4mRNA: 3.19 ± 3.60; Mann-Whitney test p = 0.350; Cohen’s d = 0.389 Fig. 4C), but the difference between means did not reach statistical significance at p<0.05. The most abundant TNNT2 transcript variant in myocardial tissue from subjects with and without Down syndrome was cTnT4. cTnT4 represented ≈60% of the total TNNT2 transcript variants while both fetal variants represented ≈1% of the total variants (Fig. 4D). There were no significant correlations between myocardial levels of phosphorylated SRSF6 and the expression of fetal TNNT2 variants in samples from subjects with and without DS (Table 2). In terms of protein expression, the adult isoform cTnT3 showed the highest average expression in myocardial tissue from both groups, representing ≈60% of the total TNNT2 protein. The expression levels of cTnT3 and cTnT4 isoforms did not differ between myocardial samples from donors with and without Down syndrome. Expression of the fetal cTnT1 and cTnT2 protein isoforms of TNNT2 was not detectable in myocardium from subjects with and without Down syndrome (Fig. 4E). This result is in line with previous studies reporting low to undetectable levels of fetal TNNT2 protein isoforms by immunoblotting in adult myocardium (Anderson et al., 1995; Anderson et al., 1991; Nassar et al., 2005).
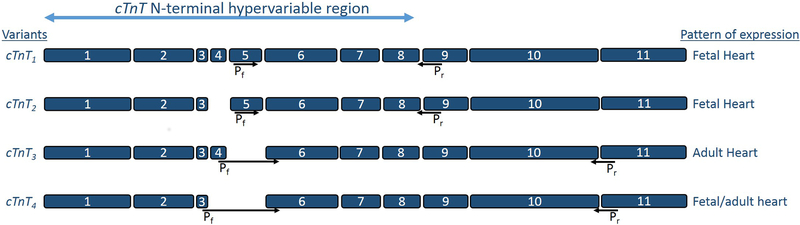
Fig. 3. Schematic representation of TNNT2 transcript variants (cTnT1, cTnT2, cTnT3, and cTnT4) expressed in human myocardium.
Arrows indicate the locations of specific forward and reverse PCR primers.
Fig. 4. Expression of TNNT2 splicing variants in myocardium from donors with and without Down syndrome.
(A) Expression of the fetal TNNT2 transcript variants cTnT1 and cTnT2. (B) Expression of the cTnT3 variant. (C) Expression of the cTnT4 variant. Each symbol depicts the average of individual samples. Horizontal lines indicate group means. Samples were analyzed in triplicates. (D) Relative abundance of TNNT2 transcript variants in myocardial tissue (E) Analysis of TNNT2 protein expression by immunoblotting.
4. Conclusions
Research on fundamental issues concerning the pathobiology of DS continues to be hampered by the scarcity of good quality tissue samples from donors with DS. Our observations are limited by the relatively small number of myocardial tissue samples and the extent of interindividual variability. Sample size limitations also precluded evaluating the impact of age and/or gender on myocardial mRNA and protein expression. This set of samples is representative of the current life span for persons with Down syndrome (Table S1), and the data may reflect the range of variation in myocardial DYRK1A- SRSF6-TNNT2 expression in the context of Down syndrome (Head et al., 2016). Our findings suggest that relatively subtle increases in the levels of phosphorylated DYRK1A and phosphorylated SRSF6 may impact the splicing pattern of TNNT2 in trisomic myocardium. In this regard, a recent analysis of the role of SRSF6 in pancreatic β-cells showed that SRSF6 is involved in the splicing of over 4,000 genes (Juan-Mateu et al., 2018). Thus, it will be of interest to examine whether altered SRSF6 expression in trisomic myocardium results in alternative splicing alterations in gene networks associated to cardiac structure and function.
Supplementary Material
Highlights.
SRSF6 phosphorylation levels were higher in trisomic myocardium.
Expression of fetal TNNT2 splicing variants was higher in trisomic myocardium.
Subtle increases in DYRK1A and SRSF6 may impact TNNT2 splicing.
Acknowledgments
Funding
This study was supported by the National Institute of General Medical Sciences (award GM073646).
Abbreviations
- aCGH
array comparative genomic hybridization
- ACTB
actin B
- cTnT
cardiac muscle troponin T
- DS
Down syndrome
- DYRK1A
dual specificity tyrosine phosphorylation regulated kinase 1A
- ELISA
enzyme linked immunosorbent assay
- NFAT
nuclear factor of activated T cells
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PVDF
polyvinylidene difluoride
- SRSF6
serine and arginine rich splicing factor 6
- TNNT2
troponin T2 cardiac type
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ait Yahya-Graison E, et al. , 2007. Classification of human chromosome 21 gene-expression variations in Down syndrome: impact on disease phenotypes. Am J Hum Genet. 81, 475–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aivazidis S, et al. , 2017. The burden of trisomy 21 disrupts the proteostasis network in Down syndrome. Plos One. 12, e0176307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez M, et al. , 2003. DYRK1A accumulates in splicing speckles through a novel targeting signal and induces speckle disassembly. J Cell Sci. 116, 3099–107. [DOI] [PubMed] [Google Scholar]
- Anderson PA, et al. , 1995. Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circ Res. 76, 681–6. [DOI] [PubMed] [Google Scholar]
- Anderson PA, et al. , 1991. Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ Res. 69, 1226–33. [DOI] [PubMed] [Google Scholar]
- Arron JR, et al. , 2006. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 441, 595–600. [DOI] [PubMed] [Google Scholar]
- Barlow GM, et al. , 2001. Down syndrome congenital heart disease: a narrowed region and a candidate gene. Genet Med. 3, 91–101. [DOI] [PubMed] [Google Scholar]
- Becker W, Sippl W, 2011. Activation, regulation, and inhibition of DYRK1A. Febs Journal. 278, 246–256. [DOI] [PubMed] [Google Scholar]
- Biesiadecki BJ, et al. , 2002. Cardiac troponin T variants produced by aberrant splicing of multiple exons in animals with high instances of dilated cardiomyopathy. J Biol Chem. 277, 50275–85. [DOI] [PubMed] [Google Scholar]
- Briggs MM, et al. , 1994. Identification of a fetal exon in the human fast troponin T gene. FEBS Lett. 350, 37–40. [DOI] [PubMed] [Google Scholar]
- Bustin SA, et al. , 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 55, 611–22. [DOI] [PubMed] [Google Scholar]
- da Costa Martins PA, et al. , 2010. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol. 12, 1220–7. [DOI] [PubMed] [Google Scholar]
- Duchon A, Herault Y, 2016. DYRK1A, a Dosage-Sensitive gene involved in neurodevelopmental disorders, is a target for drug development in Down syndrome. Front Behav Neurosci. 10, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SB, et al. , 1998. Population-based study of congenital heart defects in Down syndrome. Am J Med Genet. 80, 213–7. [PubMed] [Google Scholar]
- Gomes AV, et al. , 2002. Cardiac troponin T isoforms affect the Ca2+ sensitivity and inhibition of force development. Insights into the role of troponin T isoforms in the heart. J Biol Chem. 277, 35341–9. [DOI] [PubMed] [Google Scholar]
- Gomes AV, et al. , 2004. Cardiac troponin T isoforms affect the Ca(2+) sensitivity of force development in the presence of slow skeletal troponin I: insights into the role of troponin T isoforms in the fetal heart. J Biol Chem. 279, 49579–87. [DOI] [PubMed] [Google Scholar]
- Grebe C, et al. , 2011. Enhanced expression of DYRK1A in cardiomyocytes inhibits acute NFAT activation but does not prevent hypertrophy in vivo. Cardiovascular Research. 90, 521–8. [DOI] [PubMed] [Google Scholar]
- Guimera J, et al. , 1999. Human minibrain homologue (MNBH/DYRK1): characterization, alternative splicing, differential tissue expression, and overexpression in Down syndrome. Genomics. 57, 407–18. [DOI] [PubMed] [Google Scholar]
- Head E, et al. , 2016. Aging in Down syndrome and the development of Alzheimer’s disease neuropathology. Curr Alzheimer Res. 13, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille S, et al. , 2016. Dyrk1a regulates the cardiomyocyte cell cycle via D-cyclin-dependent Rb/E2f-signalling. Cardiovascular Research. 110, 381–394. [DOI] [PubMed] [Google Scholar]
- Juan-Mateu J, et al. , 2018. SRp55 Regulates a Splicing Network that controls human pancreatic beta-cell function and survival. Diabetes. 67, 423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Yin XM, 2016. Overexpression of Dyrk1A regulates cardiac troponin T splicing in cells and mice. Biochem Biophys Res Commun. 473, 993–998. [DOI] [PubMed] [Google Scholar]
- Mesnard-Rouiller L, et al. , 1997. Troponin T mRNA and protein isoforms in the human left ventricle: pattern of expression in failing and control hearts. Journal of Molecular and Cellular Cardiology. 29, 3043–55. [DOI] [PubMed] [Google Scholar]
- Naro C, Sette C, 2013. Phosphorylation-mediated regulation of alternative splicing in cancer. Int J Cell Biol. 2013, 151839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar R, et al. , 2005. cTnT1, a cardiac troponin T isoform, decreases myofilament tension and affects the left ventricular pressure waveform. Am J Physiol Heart Circ Physiol. 288, H1147–56. [DOI] [PubMed] [Google Scholar]
- Patterson D, 2009. Molecular genetic analysis of Down syndrome. Hum Genet. 126, 195–214. [DOI] [PubMed] [Google Scholar]
- Quinones-Lombrana A, et al. , 2017. Investigation of the role of DNA methylation in the expression of ERBB2 in human myocardium. Gene. 628, 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Lombrana A, et al. , 2014. Interindividual variability in the cardiac expression of anthracycline reductases in donors with and without Down syndrome. Pharm Res. 31, 1644–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper RJ, Reeves RH, 2006. Understanding the basis for Down syndrome phenotypes. PLoS Genet. 2, e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro BL, 1999. The Down syndrome critical region. J Neural Transm Suppl. 57, 41–60. [DOI] [PubMed] [Google Scholar]
- Shi J, et al. , 2008. Increased dosage of Dyrk1A alters alternative splicing factor (ASF)-regulated alternative splicing of tau in Down syndrome. J Biol Chem. 283, 28660–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman C, et al. , 2013. Expression of trisomic proteins in Down syndrome model systems. Gene. 512, 219–25. [DOI] [PubMed] [Google Scholar]
- Stingele S, et al. , 2012. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol Syst Biol. 8, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walte A, et al. , 2013. Mechanism of dual specificity kinase activity of DYRK1A. Febs Journal. 280, 4495–511. [DOI] [PubMed] [Google Scholar]
- Yin X, et al. , 2012. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A) modulates serine/arginine-rich protein 55 (SRp55)-promoted Tau exon 10 inclusion. J Biol Chem. 287, 30497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.