Abstract
Introduction
Sickle cell disease (SCD) is among the most common inherited hematologic diseases in sub-Saharan Africa (SSA). Historically, hydroxyurea administration in SSA has been restricted due to limited region-specific evidence for safety and efficacy.
Methods
We conducted a prospective observational cohort study of pediatric SCD patients in Malawi. From January 2015 to November 2017, hydroxyurea at doses of 10–20 mg/kg/day was administered to children with clinically severe disease (targeted use policy). From December 2017 to July 2018, hydroxyurea was prescribed to all patients (universal use policy).
Results
Of 187 patients with SCD, 7 (3.7%) died and 23 (12.3%) were lost-to-follow-up. The majority (135, 72.2%) were prescribed hydroxyurea; 59 (43.7%) under the targeted use policy and 76 (56.3%) under the universal use policy. There were no documented severe toxicities. Under the targeted use policy, children with SCD demonstrated absolute decreases in the rates of hospitalizations (−4.1 per 1,000 person-days; −7.2, −1.0; p = 0.004), fevers (−4.2 per 1,000 person-days; −7.2, −1.1; p = 0.002), transfusions (−2.3 per 1,000 person-days; 95% CI: −4.9, 0.3; p = 0.06), and annual school absenteeism (−51.2 per person-year; −60.1, −42.3; p < 0.0001) within 6 months of hydroxyurea commencement.
Conclusion
We successfully implemented universal administration of hydroxyurea to children with SCD at a tertiary hospital in Malawi. Similar to recently reported trials, hydroxyurea was safe and effective during routine programmatic experience, with clinical benefits particularly among high-risk children. This highlights the importance of continued widespread scale-up of hydroxyurea within SCD programs across SSA.
Keywords: Sickle cell disease, Treatment, Hydroxyurea, Pediatric, Sub-Saharan Africa, Malawi
Introduction
Sickle cell disease (SCD), one of the most common inherited hematologic diseases in the world,1,2 clinically manifests as pain, end-organ injury, and life threatening clinical events.3,4 SCD complications and mortality are high, and 50–90% of children with SCD in sub-Saharan Africa (SSA) may die before 5-years of age, often without an established diagnosis.5 The efficacy and safety of hydroxyurea to prevent complications of SCD has been demonstrated primarily in high-income countries,3,6,7,8,9,10,11 and there is limited real-world data on the use of hydroxyurea in SSA where 75% of patients with SCD worldwide are born.5,10,12,13,14
In SSA, hydroxyurea administration to patients with SCD has been historically limited due to drug access, drug familiarity, and safety concerns. In 2017, the NOHARM trial in Uganda demonstrated that hydroxyurea at a fixed dose of 20mg/kg/day did not increase malaria risk among children with SCD.10 More recently, the phase 1/2 REACH trial in four SSA countries found that hydroxyurea at 15–20 mg/kg/day followed by dose escalation was feasible, safe, and effective, resulting in decreased incidence of vaso-occlusive events, infections, malaria, transfusions, and death.12 However, these important clinical trial results have yet to be confirmed in routine clinical practice outside the context of a controlled clinical trial.10,12
In January 2015, hemoglobin electrophoresis and a dedicated SCD clinic were initiated at Kamuzu Central Hospital (KCH), through a collaboration between the Malawi Ministry of Health and University of North Carolina (UNC) Project-Malawi. The clinic aimed to establish a prospective longitudinal SCD pediatric cohort receiving standardized care, and to determine clinical characteristics and outcomes.15 Since the clinic opened, hydroxyurea use has rapidly expanded in response to published results from recent regional studies.10,12 Due to the paucity of data describing hydroxyurea use in children with SCD in SSA from routine care, non-clinical trial settings, we therefore sought to describe the safety and effectiveness of hydroxyurea use in Malawian children with SCD under real-world programmatic conditions.
Methods
Setting
KCH is a public national teaching hospital in the Malawian capital, Lilongwe. It is the only public tertiary hospital in central Malawi offering pediatric specialty care, serving an estimated population of 7.5 million, of which 3.8 million are younger than 18 years.16
Study population
From January 2015 through July 2018, patients <21 years of age with presumed SCD were screened with hemoglobin electrophoresis as previously described.15,17 Those with confirmed SCD were enrolled in an observational prospective cohort study after guardian informed consent and pediatric assent for data collection and follow up. Patients in this clinic routinely receive folic acid, sulfadoxine pyrimethamine for malaria prophylaxis, and penicillin or azithromycin as prophylaxis for bacterial infections. Infants receive pneumococcal conjugate vaccine in the first year of life per national expanded program on immunization. Booster doses of pneumococcal conjugate vaccine are prescribed every five years.
Clinical data collection
Patients were followed with routine clinic visits every 3 months and more frequently as clinically indicated. During clinic visits, all patients underwent an assessment by a healthcare provider, including a thorough history and physical exam. Routinely collected data included the occurrence of SCD related acute clinical crises, blood transfusions, hospitalizations, malaria infection, fever, and absenteeism from school due to SCD-related illness. Patient information was recorded on paper case report forms and stored in individual patient files. Key clinical variables were abstracted from paper forms at the time of analysis and linked with an electronic database of laboratory results.
Laboratory assays
Every 6 months, routine laboratory testing was performed including full blood count, serum chemistries including renal function and liver function, and urinalysis. Reticulocyte counts and HbF levels were not measured. Point of care tests were available in clinic for malaria rapid diagnostic tests (SD Bioline, Alere) and hemoglobin (Hb301 Hemacue analyzer), if malaria or clinically significant anemia were suspected. Malaria microscopy blood slides were also performed on suspected malaria cases. All laboratory tests were performed at the UNC Project-Malawi laboratory, located on the KCH campus.
Hydroxyurea use
From January 2015 until November 2017, hydroxyurea at 10–20 mg/kg/day was prescribed to children with severe or recurrent sickle-related clinical complications such as painful vaso-occlusive crises, blood transfusions, hospitalization for sepsis or malaria, as well as high-risk designation based on prior cerebrovascular accident, acute chest syndrome, or other life-threatening event. This period of hydroxyurea application for clinically higher-risk patients only will subsequently be referred to as the targeted use policy.
Starting in December 2017, after publication of the NOHARM study results10 and increased availability of hydroxyurea at KCH, we prescribed hydroxyurea to all patients in this cohort with the explicit goal of achieving universal coverage for all children with SCD. Patients initiating hydroxyurea were recommended to have a full blood count after 4–6 weeks of commencing hydroxyurea to monitor for myelosuppression whenever possible. The more recent period of hydroxyurea application will subsequently be referred to as the universal use policy.
During the study period hydroxyurea was only available in the form of 500 mg capsules. The number of capsules administered per week depended on the weight of the patient. The total dose in milligrams per week was calculated per patient and divided by 500 to determine the number of capsules administered per week for each patient with split dosing days.
Hydroxyurea was not initiated until at least 6 months of age. Hydroxyurea was administered to young children by opening the capsule and dissolving the contents in liquids or foods. Patients were started on a dose of 10 mg/kg/day. The dose was subsequently escalated to 15 mg/kg/day if there were ongoing sickle cell crises. If patients experienced events at a dose of 15 mg/kg/day, the dose was escalated to 20 mg/kg/day. Hydroxyurea and other medicines were provided to patients from the KCH pharmacy free of charge, and care provided free of charge as per hospital policy. Intermittent stock out of medications were supported with supply from UNC-Project Malawi.
Statistical methods
Laboratory results for full blood counts, renal and liver function tests were aggregated and stratified by number of months pre- and post-hydroxyurea initiation. Differences in means were tested with two-sample t-tests. Count data on hospitalizations, blood transfusions, and school absences were summed over eligible person-time to estimate rates during the time period 6 months before and 6 months after the hydroxyurea start date.
The total person-time contributed by each participant was the summation of the number of days between each clinic visit. Participants who died or who were lost to follow-up contributed person-time and events to the analysis up until their last recorded study visit. If participants began taking hydroxyurea on a date between two clinic visits, the resulting person-time from that period and corresponding adverse events were excluded from analysis. If any of the adverse outcomes were not recorded, we assumed that there had been none since the previous visit. Fever and pain crises were assessed as binary variables. To derive a crude rate estimate, we assumed that any reported fever or pain crisis corresponded to one event during that time period. Rates of school absences were restricted to school aged participants between the ages of 6 and 18, and person-time occurring during school vacation (August-September) was excluded. Primary and secondary education in Malawi are free of cost, so the majority of children go to school. Five school aged participants who reported that school attendance was “not applicable” at any point during follow-up were excluded from calculation of school-absence rates, as it was assumed to be a clerical error. To account for bias caused by outliers reporting high rates of school absences, we repeated our analyses, excluding the patient with the highest rate of absences reported under each policy. An alpha of 0.05 was used as the cutoff for statistical significance. All analyses were conducted in SAS 9.4 (SAS Institute Inc., Cary, NC).
Ethical approval and consent
The study was approved by the UNC-Chapel Hill institutional review board and the Malawi National Health Science Research Committee. Written informed consent was obtained from parents of all enrolled children. Children aged 7–17 years also provided informed assent.
Results
Baseline characteristics
From January 2015 to July 2018 our sickle cell clinic enrolled 187 pediatric patients with SCD (HbSS: 177, HbFS: 4, HbSC: 1, no confirmed SCD hemoglobin electrophoresis result: 5). The ages at diagnosis of the patients with the HbFS results were 5 months, 2 years, 11 years, and 14 years. Ninety-eight (52%) participants were male and 86 were female (46%). The mean age at enrollment was 7.3 years (standard deviation (SD): 4.8). Mean baseline laboratory values were: hemoglobin 7.6 g/dL (SD: 1.4), lactate dehydrogenase 642.1 U/L (SD: 217.1), mean corpuscular volume 87.4 fL (SD: 9.2), neutrophils 5.2 × 103 /µL (1.9), platelets 467.6 × 103 /µL (SD: 181.4), and white blood count 15.5 × 103 /µL (SD: 5.8). Seven (3.7%) patients died and 23 (12.3%) were lost-to-follow-up: 10 relocated to other clinics, two were alive at the end of the study period but had defaulted on treatment, and 11 could not be reached by phone to assess vital status.
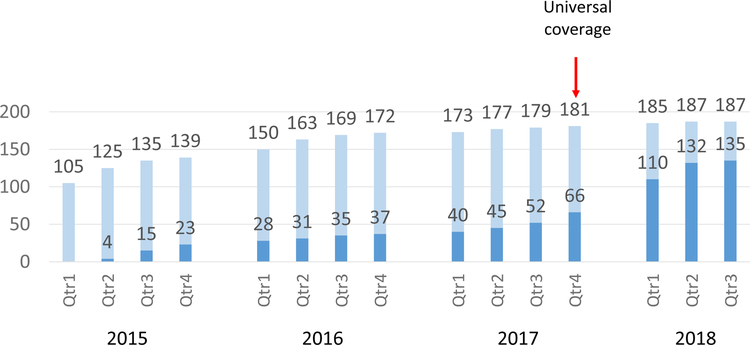
The majority (135, 72.2%) were prescribed hydroxyurea; 59 (43.7%) began taking it under the targeted use policy prior to December 2017 and 76 (56.3%) under the universal use policy (Figure 1). Although we did not have recorded dosage information at every clinic visit, the mean dosage prescribed during the 6 months following commencement of hydroxyurea was 9.98 (SD: 3.09, N: 37) mg/kg/day under the targeted use policy and 9.23 (SD: 2.31, N: 22) mg/kg/day under the universal use policy. The mean age at hydroxyurea start was 8.4 years (SD: 5.1) under the targeted use policy, and 9.5 years (SD: 4.7) under the universal use policy, with a similar gender distribution and family history of SCD between both groups (Table 1). Reflecting their clinical severity, children initiating hydroxyurea under the targeted use policy had a lower mean hemoglobin in the 6 months prior to treatment as compared to children under the universal use policy (7.2 g/dL, SD: 1.1; vs. 7.9 g/dL, SD: 1.3; p=0.01), and a higher mean lactate dehydrogenase (721.8 U/L, SD: 241.5; vs. 626.0 U/L, SD: 229.4; p=0.02).
Figure 1.
Cumulative count of actively followed children with sickle cell disease at Kamuzu Central Hospital in Malawi from 2015 to 2018.
Lighters bars represent all children (N=187), darker bars represent children on hydroxyurea (N = 135).
Table 1.
Characteristics of children with sickle cell disease taking hydroxyurea at Kamuzu Central Hospital in Malawi (N = 135).
| Targeted use policy (N = 59) |
Universal use policy (N = 76) |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | Standard Deviation | N | Mean | Standard Deviation | p-value* | ||
| Age at enrollment (years) | 59 | 7.4 | 5.1 | 76 | 7.1 | 4.7 | 0.7 | |
| Age at Hydroxyurea start (years) | 59 | 8.4 | 5.1 | 76 | 9.5 | 4.7 | 0.2 | |
| Sex | ||||||||
| Female | 27 (46%) | - | - | 42 (55%) | - | - | 0.3 | |
| Male | 32 (54%) | - | - | 34 (45%) | - | - | ||
| Length of follow-up time at KCH (months) | 59 | 16.4 | 10.9 | 76 | 32.2 | 10.3 | <0.0001 | |
| District | ||||||||
| Lilongwe | 48 (81%) | - | - | 52 (68%) | - | - | 0.2 | |
| Outside Lilongwe | 4 (7%) | - | - | 9 (12%) | - | - | ||
| Unknown | 7 (12%) | - | - | 15 (20%) | - | - | - | |
| Family history of SCD | ||||||||
| Yes | 17 (29%) | - | - | 21 (28%) | - | - | 0.9 | |
| No | 40 (68%) | - | - | 51 (67%) | - | - | ||
| Unknown | 2 (3%) | - | - | 4 (5%) | - | - | - | |
| Diagnosed with SCD prior to enrollment | ||||||||
| Yes | 46 (78%) | - | - | 58 (76%) | - | - | 0.9 | |
| No | 11 (19%) | - | - | 14 (18%) | - | - | ||
| Unknown | 2 (3%) | - | - | 4 (5%) | - | - | - | |
| Treated for SCD prior to enrollment | ||||||||
| Yes | 46 (78%) | - | - | 62 (82%) | - | - | 0.6 | |
| No | 11 (19%) | - | - | 10 (13%) | - | - | ||
| Unknown | 2 (3%) | - | - | 4 (5%) | - | - | - | |
| Average hemoglobin in the 6 months before starting hydroxyurea (g/dL) | 40 | 7.2 | 1.1 | 42 | 7.9 | 1.3 | 0.01 | |
| Average lactate dehydrogenase in the 6 months before starting hydroxyurea (U/L) | 36 | 721.8 | 241.5 | 39 | 626.0 | 229.4 | 0.2 | |
Two-sample population proportions z-score or t-test comparing group means
Abbreviations: KCH, Kamuzu Central Hospital: SCD, sickle cell disease
Of the 47 (28%) participants with confirmed SCD and who were not prescribed hydroxyurea, 5 died and 16 were lost to follow-up before the universal policy began, 11 had not yet had another clinic visit after the universal policy began, and 15 had a clinic visit after the policy changed but were not prescribed hydroxyurea. Of the 23 participants who were lost to follow-up, six (26%) were prescribed hydroxyurea, and of the seven participants who died, two (29%) were prescribed hydroxyurea.
Laboratory monitoring
Table 2 shows a comparison of mean laboratory values before and after starting hydroxyurea. Under the targeted use policy, the mean ANC decreased from 6.2 × 103 /µL (SD: 1.7) to 4.8 × 103 /µL (SD: 2.0) (p = 0.02) between pre-treatment and 4–6 months post-treatment, while there was a non-significant increase in ANC under the universal policy. There were no significant changes in mean hemoglobin after starting hydroxyurea, but a decreasing trend in lactate dehydrogenase was observed with both policies. In the universal use policy group, there was an increase in mean MCV from 91.7 fL (SD: 9.0) to 97.5 fL (SD: 8.1) (p = 0.04), with a non-significant increase in the targeted group.
Table 2.
Mean lab results for children with sickle cell disease before and after being prescribed hydroxyurea at Kamuzu Central Hospital in Malawi (N = 135).
| Targeted Policy | ||||
|---|---|---|---|---|
| Pre-hydroxyurea (any time in the 6 months before treatment) |
Post-hydroxyurea (4–6 months after treatment) |
|||
| Laboratory Result | N | Mean (SD) | N | Mean (SD) |
| Neutrophil count (103/µL) | 40 | 6.2 (1.9) | 22 | 4.8 (2.0) |
| White blood cells (103/µL) | 40 | 16.7 (5.2) | 22 | 14.0 (5.8) |
| Platelets (103/µL) | 40 | 477.3 (164.8) | 22 | 459.2 (159.2) |
| Hemoglobin (g/dL) | 40 | 7.2 (1.1) | 22 | 7.1 (1.4) |
| Lactate Dehydrogenase (U/L) | 36 | 721.8 (241.5) | 18 | 649.2 (265.6) |
| Mean corpuscular volume (fL) | 40 | 90.6 (8.2) | 22 | 95.6 (12.8) |
| Universal Policy | ||||
|
Pre-hydroxyurea (any time in the 6 months before treatment) |
Post-hydroxyurea (4–6 months after treatment) |
|||
| Laboratory Result | N | Mean (SD) | N | Mean (SD) |
| Neutrophil count (103/µL) | 42 | 5.9 (2.9) | 13 | 7.2 (3.2) |
| White blood cells (103/µL) | 42 | 14.8 (5.3) | 13 | 18.3 (7.1) |
| Platelets (103/µL) | 42 | 375.1 (120.2) | 13 | 438.2 (135.8) |
| Hemoglobin (g/dL) | 42 | 7.9 (1.3) | 13 | 8.2 (1.2) |
| Lactate Dehydrogenase (U/L) | 39 | 651.4 (229.4) | 11 | 594.1 (144.4) |
| Mean corpuscular volume (fL) | 42 | 91.7 (9.0) | 13 | 97.5 (8.1) |
If an individual had more than one lab result per time period, these results were averaged prior to finding the overall population mean so that each child only contributed at most one value per time period.
Overall, there were no cases of thrombocytopenia (platelet counts < 80 × 103/μL), moderate neutropenia (ANC 0.5–1.5 × 103/μL), or severe neutropenia (ANC < 0.5 × 103/μL) over the study period. Four individuals had mild neutropenia (ANC 1.5–2.0 × 103/μL), two under the targeted use policy, and two under the universal use policy. Nineteen malaria tests were performed on children presenting with fever, one was positive, obtained from a child taking hydroxyurea under the universal use policy.
Clinical events
Under the targeted use policy, children with SCD demonstrated a decrease in the rate of SCD-related complications (Table 3), with a pre-hydroxyurea versus post-hydroxyurea rate difference of −4.1 (95% CI: −7.2, −1.0; p = 0.004) per 1,000 person-days for hospitalizations, −4.2 (95% CI: −7.2, −1.1; p = 0.002) per 1,000 person-days for fever, and −2.3 (95% confidence interval [CI]): −4.9, 0.3; p = 0.06) per 1,000 person-days for transfusions when comparing the 6 months pre-treatment to 6 months post-treatment. Rates of pain crises also decreased, with a rate difference of −2.8 (95% CI: −6.5, 0.9; p-value: 0.1) per 1,000 person-days, although this was not statistically significant.
Table 3.
Rate of disease-related complications among children with sickle cell disease at Kamuzu Central Hospital in Malawi stratified by hydroxyurea administration policy (N = 135).
| Targeted use policy | Universal use policy | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Hydroxyurea | Post-Hydroxyurea | Effect estimate | Pre-Hydroxyurea | Post-Hydroxyurea | Effect estimate | ||||||||||
| N | Rate per 1,000 PD | N | Rate per 1,000 PD | Rate difference | 95% CI | P-value | N | Rate per 1,000 PD | N | Rate per 1,000 PD | Rate difference | 95% CI | P-value | ||
| Person days (PD) | 3,186 | - | 5,206 | - | - | - | - | 8,167 | - | 4,670 | - | - | - | - | |
| Transfusions | 14 | 4.4 | 11 | 2.1 | −2.3 | (−4.9, 0.3) | 0.06 | 4 | 0.5 | 2 | 0.4 | −0.1 | (−0.8, 0.7) | 0.9 | |
| Hospitalizations | 21 | 6.6 | 13 | 2.5 | −4.1 | (−7.2, −1.0) | 0.004 | 10 | 1.2 | 5 | 1.1 | −0.2 | (−1.4, 1.1) | 0.8 | |
| Pain crises* | 26 | 8.2 | 28 | 5.4 | −2.8 | (−6.5, 0.9) | 0.1 | 35 | 4.3 | 22 | 4.7 | 0.4 | (−2.0, 2.9) | 0.7 | |
| Fever* | 20 | 6.3 | 11 | 2.1 | −4.2 | (−7.2, −1.1) | 0.002 | 23 | 2.8 | 13 | 2.8 | 0.0 | (−1.9, 1.9) | 1.0 | |
| N | Rate per 365.25 PD | N | Rate per 365.25 PD | Rate difference | P-value | N | Rate per 365.25 PD | N | Rate per 365.25 PD | Rate difference | P-value | ||||
| Person days (PD) | 1,127 | - | 2,064 | - | - | - | - | 4,194 | - | 2,992 | - | - | - | - | |
| School absences† | 182 | 59.0 | 44 | 7.8 | −51.2 | (−60.1, −42.3) | <0.001 | 104 | 9.1 | 104 | 12.7 | 3.6 | (−0.6, 6.6) | 0.01 | |
Fever and pain crises were evaluated as a yes/no binary outcome at each visit. We assumed that each reported “yes” corresponded to one fever or pain crisis.
Excludes children that reported any school “Not Applicable” who were not attending school at any point during the study period, and any person time that may have overlapped with school break during August and September of each year.
Children beginning hydroxyurea under the universal use policy showed minimal changes in rates pre- and post-treatment with a rate difference of −0.1 (95% CI: −0.8, 0.7; p = 0.9) per 1,000 person-days for transfusions, −0.2 (95% CI: −1.4, 1.1; p = 0.8) per 1,000 person-days for hospitalizations, 0.4 (95% CI: −2.0, 2.9; p = 0.7) per 1,000 person-days for pain crises, and 0.0 (95% CI: −1.9, 1.9; p = 1.0) per 1,000 person-days for fever. However, participants under the universal policy reported a lower rate of SCD-related events pre-treatment than the targeted use policy cohort.
Participants under the targeted use policy also showed a dramatically lower rate difference of −51.2 (95% CI: −60.1, −42.3; p < 0.0001) school absences per person-year when taking hydroxyurea as compared to pre-treatment. Children under the universal use policy demonstrated a significant increase in school absences, with a rate difference of 3.6 (95% CI: 0.6, 6.6; p-value: 0.01) absences per person-year, though they began with a much lower baseline rate of school absences, as compared to the targeted use policy group (9.1 vs. 59.0 absences per person-year). To assess the influence of outliers, we performed a sensitivity analysis, excluding the one patient from each policy who had the highest rate of school absences. The significant decrease in the targeted use policy remained, −49.4 (95% CI: −58.2, −40.5; p-value: <0.0001), and the increase in school absences with the universal use policy was no longer significant 2.4 (95% CI: −0.5, 5.3; p-value: 0.10).
Discussion
Replicating results from recently conducted trials in SSA, our study demonstrates that use of hydroxyurea in children with SCD in Malawi is safe and effective, particularly among high-risk children with clinical indications to initiate hydroxyurea. Importantly, our study was conducted in a real-world, programmatic, non-clinical trial setting, lending support to the generalizability of recent findings from clinical trials evaluating hydroxyurea in SSA.
No remarkable laboratory toxicities were noted in children on hydroxyurea. Four children had mild neutropenia and there were no cases of moderate or severe neutropenia. The occurrence of neutropenia was uncommon in our study similar to the NOHARM study, although a slightly higher rate was observed in the REACH study. This difference could be due to the hydroxyurea dose escalation beyond the levels used in our study and longer duration of follow up in the REACH study. Children under the targeted use policy had lower ANC at 4–6 months after commencing hydroxyurea in contrast to patients in the universal use policy with higher ANC. This may be attributed to higher doses of hydroxyurea used in the targeted policy due to dose escalation following recurrent illnesses unlike in the universal policy group where patients were more likely to be clinically well, resulting in under-dosing or possibly decreased adherence. Of the 19 malaria blood microscopy tests performed, only one was positive. With such a small number of patients tested by microscopy, we are unable to confidently analyze the risk of malaria with hydroxyurea use. However, the NOHARM and REACH studies reported no increased risk of malaria with hydroxyurea use.10,12
Hydroxyurea use for children with SCD in Malawi was effective and resulted in improved clinical benefits, especially among high-risk patients who had reductions in non-malarial febrile illness, hospitalization, and blood transfusions. Similar trends of reduced adverse event rates under hydroxyurea have been reported in multiple studies.3,10,12,18,19 Lower-risk children under the universal use policy had minimal changes in blood transfusions and hospitalization. A study in Brazil also demonstrated greater benefit of hydroxyurea use in patients with a severe SCD phenotype.18 Furthermore, significantly reduced school absenteeism was noted amongst patients in the targeted use policy, possibly reflecting broader quality of life benefits beyond clinical and laboratory improvements, as demonstrated elsewhere.20
Based on these findings, and not withstanding more clearly observed benefits among higher-risk children in our cohort, we advocate for a hydroxyurea universal use approach for all patients in resource-limited settings similar to Malawi. SCD is a chronic condition with ongoing multiorgan pathology even when asymptomatic, and efforts should be made to treat SCD patients early before developing multi-organ damage.21 Hence the use of hydroxyurea with the universal use approach is recommended to prevent development of poor health. Evidence-based guidelines from the National Heart Lung and Blood Institute similarly recommend offering hydroxyurea treatment to all patients with SCD starting at 9 months of age,22 and the World Health Organization lists hydroxyurea on its essential medicines list.23 Therefore, continued regional advocacy will remain critical for all patients with SCD across SSA to access hydroxyurea to prevent morbidity and mortality, particularly in light of our programmatic data and data from recently reported clinical trials.
The major strength of our study is that it provides additional real-world evidence from a programmatic setting for the safety and efficacy of hydroxyurea in children with SCD in SSA to supplement the results of the NOHARM and REACH studies. Notably, despite the absence of pediatric formulations of hydroxyurea in our setting, we nevertheless successfully administered capsules to a routine care pediatric population by adjusting the number of days per week for administration in relation to patient weight. However, there are also some limitations to our study. First, data analysis was limited to a period of 6 months prior to and 6 months after starting hydroxyurea. The benefits of hydroxyurea have been previously demonstrated even at 6 months,10,12,19 although maximal effects have been reported to occur between 12 and 18 months on treatment.6 Second, our study used a low-to-medium dose of hydroxyurea without dose escalation to high doses, as this was a new drug being upscaled in Malawi and studies had shown that this dose was safe and effective.1 The NOHARM and REACH studies have shown higher doses to also be safe and effective in malaria-endemic regions of SSA.10,12 A third limitation was our inability to robustly analyze malaria occurrence due to low number of malaria cases, perhaps partially due to high levels of malaria prophylaxis in our population, although other recent studies have demonstrated similar or lower malaria incidence for those on hydroxyurea versus placebo.10,12 A fourth limitation was that we did not quantify HbF levels after commencement of hydroxyurea as a biomarker of adherence and efficacy. Reflecting the programmatic nature of the study, we assessed hydroxyurea adherence primarily by guardian self-report, although observed laboratory trends demonstrating increased MCV and decreased lactate dehydrogenase post-treatment are all consistent with reasonable levels of cohort-level hydroxyurea adherence.4,10,12,18,21,20
To conclude, this study demonstrates low-to-medium dose hydroxyurea use is safe and effective in reducing the occurrence of clinical adverse events in children with SCD in Malawi. Our clinic has upscaled hydroxyurea use to near universal coverage at a national teaching hospital, highlighting the importance of building capacity to improve access to hydroxyurea for children with SCD across SSA, where the majority of patients with SCD live. Although greater impact was demonstrated in the targeted use policy group of higher-risk patients, we advocate hydroxyurea initiation at a medium dose of 20mg/kg/day, with possible dose escalation depending on the clinical setting, for all children with SCD to prevent SCD-related adverse events and improve survival. Future regional priorities should include continued evaluation of hydroxyurea dose escalation in routine care settings with longer term follow-up to provide real-world evidence of generalizability of recent clinical trial results.10,12 Additionally, widely available and effective treatment in the form of hydroxyurea further highlights the need for Malawi and other SSA countries to adopt newborn screening for SCD, so effective treatments can be initiated before potentially irreversible complications arise.
Acknowledgments
This study was funded by NIH Grant UO1HL11765 (NK, KIA). KDW, JBH and GE received support from NIH Research Training Grant # D43TW009340 funded by the NIH Fogarty International Center and the Fulbright US Student Program, NINDS, NIMH and NHBLI. KDW is also supported by the National Institute of General Medical Sciences of the NIH under Award # T32GM086330. JSC received support from NIH/NIAID (T32 AI070114). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to acknowledge Russell Ware for his critical review of the draft manuscript.
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Abbreviations:
- ANC
Absolute Neutrophil Count
- CI
Confidence Interval
- HBF
Fetal Hemoglobin
- KCH
Kamuzu Central Hospital
- MCV
Mean Corpuscular Volume
- SCD
Sickle Cell Disease
- SD
Standard Deviation
- SSA
Sub-Saharan Africa
- UNC
University of North Carolina
Footnotes
Conflict of Interest Statement
None of the authors have any have conflicts of interest to disclose.
References
- 1.Jain DL, Apte M, Colah R, et al. Efficacy of Fixed Low Dose Hydroxyurea in Indian Children with Sickle Cell Anemia: A Single Center Experience. Indian Pediatr 2013;5:929–933. doi: 10.1007/s13312-013-0264-0. [DOI] [PubMed] [Google Scholar]
- 2.Piel FB, Patil AP, Howes RE, et al. Global epidemiology of Sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet 2013;381(9861):142–151. doi: 10.1016/S0140-6736(12)61229-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: A multicentre, randomised, controlled trial (BABY HUG). Lancet 2011;377(9778):1663–1672. doi: 10.1016/S0140-6736(11)60355-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hankins JS, Ware RE, Rogers ZR, et al. Long-term hydroxyurea therapy for infants with sickle cell anemia: The HUSOFT extension study. Blood 2005;106(7):2269–2275. doi: 10.1182/blood-2004-12-4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa: A neglected cause of early childhood mortality. Am J Prev Med 2011;41(6 SUPPL.4):S398–S405. doi: 10.1016/j.amepre.2011.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinney TR, Helms RW, O’Branski EE, et al. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Pediatric Hydroxyurea Group. Blood 1999;94(5):1550–1554. doi: 10.1016/0016-7037(91)90154- [DOI] [PubMed] [Google Scholar]
- 7.Thompson BW, Miller ST, Rogers ZR, et al. The pediatric hydroxyurea phase III clinical trial (BABY HUG): Challenges of study design. Pediatr Blood Cancer 2010;54(2):250–255. doi: 10.1002/pbc.22269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware RE, Davis BR, Schultz WH, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia - TCD with Transfusions Changing to Hydroxyurea (TWiTCH): A multicentre, open-label, phase 3, non-inferiority trial. Lancet 2016;387(10019):661–670. doi: 10.1016/S0140-6736(15)01041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornburg CD, Files BA, Luo Z, et al. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood 2012;120(22):4304–4310. doi: 10.1182/blood-2012-03-419879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opoka RO, Ndugwa CM, Latham TS, et al. Novel use Of Hydroxyurea in an African Region with Malaria ( NOHARM ): a trial for children with sickle cell anemia 2018;130(24):2585–2594. doi: 10.1182/blood-2017-06-788935 [DOI] [PubMed] [Google Scholar]
- 11.Voskaridou E, Christoulas D, Bilalis A, et al. The Effect of Prolonged Administration of Hydroxyurea on Morbidity and Mortality in Adult Patients with Sickle Cell Syndromes: Results of a 17-Year, Single-Center Trial (LaSHS). Blood 2010. 115 (12): 2354–63. doi: 10.1182/blood-2009-05-221333 [DOI] [PubMed] [Google Scholar]
- 12.Tshilolo L, Tomlinson G, Williams TN, et al. Hydroxyurea for Children with Sickle Cell Anemia in Sub-Saharan Africa. N Engl J Med 2018;380 (2): 121–131. doi: 10.1056/NEJMoa1813598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGann PT, Williams TN, Olupot-Olupot P, et al. Realizing effectiveness across continents with hydroxyurea: Enrollment and baseline characteristics of the multicenter REACH study in Sub-Saharan Africa. Am J Hematol 2018;93(4):537–545. doi: 10.1002/ajh.25034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galadanci NA, Umar Abdullahi S, Vance LD, et al. Feasibility trial for primary stroke prevention in children with sickle cell anemia in Nigeria (SPIN trial). Am J Hematol 2017;92(8):780–788. doi: 10.1002/ajh.24770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heimlich JB, Chipoka G, Kamthunzi P, et al. Establishing sickle cell diagnostics and characterizing a paediatric sickle cell disease cohort in Malawi. Br J Haematol 2016;174(2):325–329. doi: 10.1111/bjh.13769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malawi National Statistics Office. 2018 Malawi Population & Housing Census; 2018. http://www.nsomalawi.mw/images/stories/data_on_line/demography/census_2018/2018%20Population%20and%20Housing%20Census%20Preliminary%20Report.pdf.
- 17.Kamthunzi P, Topazian H, Mvalo T, et al. Development of sickle cell diagnostics and a pediatric sickle cell clinic in Malawi. Blood Advances 2018; 2 (Supplement 1), 14–16. 10.1182/bloodadvances.2018gs110913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopes de Castro Lobo C, Pinto JFC, Nascimento EM, Moura PG, Cardoso GP, Hankins JS. The effect of hydroxcarbamide therapy on survival of children with sickle cell disease. Br J Haematol 2013;161(6):852–860. doi: 10.1111/bjh.12323 [DOI] [PubMed] [Google Scholar]
- 19.Silva-Pinto AC, Angulo IL, Brunetta DM, et al. Efeitos clínicos e hematológicos do tratamento com hidroxiureia em pacientes falciformes: Experiência de um centro no Brasil. Sao Paulo Med J 2013;131(4):238–243. doi: 10.1590/1516-3180.2013.1314467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nwenyi E, Leafman J, Mathieson K, et al. Differences in quality of life between pediatric sickle cell patients who used hydroxyurea and those who did not. International Journal of Health Care Quality Assurance 2014; 27(6), 468–481. 10.1108/IJHCQA-01-2013-000821. [DOI] [PubMed] [Google Scholar]
- 21.Ware RE. How I treat How I use hydroxyurea to treat young patients with sickle cell anemia. Cell 2010;115(26):5300–5311. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: Summary of the 2014 evidence-based report by expert panel members. JAMA - J Am Med Assoc 2014;312(10):1033–1048. doi: 10.1001/jama.2014.10517 [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. WHO Model List of Essential Medicines WHO Med web http//:www.who.int/medicines/publications/EML. 2017;15(March):8–10. doi: 10.1016/S1473-3099(14)70780-7 [DOI] [Google Scholar]