Abstract
The 8th edition of the American Joint Committee on Cancer (AJCC) staging guidelines combine traditional TNM system with biomarkers to reflect our current understanding of tumor biology and targeted therapy. In this study, we investigated the impact of the TNM+Biomarkers staging system and the additive value of Oncotype Dx™ genomic profile recurrence score (RS) (TNM+Biomarkers+RS<11) for the staging of breast cancer (BC) using data from two tertiary referral cancer centers. Compared to TNM alone, the TNM+Biomarkers system changed the stage group in 32.7% of BCs (27% downstage, 5.7% upstage). Most (98.3%) of the downstaged BCs were estrogen receptor (ER)+/progesterone receptor (PR)+, whereas 78% of the upstaged BCs were ER-/PR-/human epidermal growth factor receptor 2 (HER2)-. Compared to TNM+Biomarkers staging, the addition of genetic profile data (TNM+Biomarker+RS<11) downstaged only <1% BCs. Our analysis suggests that for T1-T2N0 ER+/HER2- BCs Oncotype Dx™ RS <11 provides added value as a staging parameter only in a very small group of cases compared to TNM+Biomarkers alone.
Keywords: Breast cancer, Staging, Prognosis, Biomarkers, American Joint Committee on Cancer (AJCC), OncotypeDx™, Estrogen Receptor, Progesterone Receptor, Human Epidermal growth factor Receptor 2, 21-gene recurrence score assay
Introduction
Staging of breast cancer (BC) provides information useful for individual patient prognosis and for large scale analysis. The staging system is codified based on guidelines by international organizations, including the American Joint Committee on Cancer (AJCC). In the past editions of the AJCC staging guidelines, tumor size (T), lymph node status (N), and presence of distant metastasis (M) - TNM stage system – were the only parameters evaluated, providing the so called Anatomic Stage (Gabriel N. Hortobagyi, 2017). In recent years, however, the TNM system alone has become inadequate to capture the intrinsic differences in the various subtypes of BC, in particular with regard to the intrinsic differences in tumor biology and response to targeted treatment, and does not reflect the overall improved survival outcomes of patients with BC. At present, the information on estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) status of each BC is routinely used to determine the best treatment regimen. The histologic grade of BC has also been shown to significantly correlate with treatment response and patient survival (Rakha, Reis-Filho, Baehner, et al., 2010). Hence, and for the first time, the 8th edition of the AJCC BC staging guidelines integrate TNM data with the BC Biomarkers status (TNM+Biomarkers), namely histologic grade, ER, PR and HER2 status, to provide a Prognostic Stage (PS). Furthermore, the Recurrence Score (RS) generated by analysis with the 21-gene assay OncotypeDx™, the only genomic profile assay with level I evidence at the time of drafting of the AJCC 8th edition, is also included in the PS system, and any T1–2, N0, and ER+/HER- BC with RS<11 is now classified as stage group IA, independent of size (Giuliano, Edge, & Hortobagyi, 2018). In recent retrospective studies (Abdel-Rahman, 2018; Ding et al., 2017; Hu, Wei, Yi, Xin, & Liu, 2017; Lee et al., 2018; Mittendorf et al., 2017; Weiss et al., 2018; Ye et al., 2017; Zhou et al., 2017), staging BC using the TNM+Biomarkers (PS) system showed more specific correlation with clinical follow-up than staging using the TNM stage system. Most of the published studies evaluated BC patients from Asia (Hu et al., 2017; Lee et al., 2018; Wong, Wong, Lim, Lian, & Yap, 2018; Ye et al., 2017) or from the Southwest region of the USA (Weiss et al., 2018). None of the studies published so far, however, has assessed the added value of genomic profile results (RS) to staging by TNM+Biomarkers staging (PS) system. We sought to assess the impact of the 8th AJCC TNM+Biomarkers staging (PS) system in the classification of primary BCs diagnosed at two tertiary referral cancer centers in New York City, USA. Furthermore, we evaluated the contribution of genomic profile data (RS) for staging of T1–2, N0, and ER+/HER2- BCs (TNM+Biomarkers+RS<11).
Material and methods
Patient population
New York University Langone Medical Center (NYULMC) Cohort
We identified consecutive patients with diagnosis of invasive BC treated at NYULMC from January 1st, 2016 to December 31st, 2017. Information on patient age, gender, and the type of surgery was extracted from the medical records. Information regarding the characteristics of the BC (tumor size measured from excision specimen, nodal status, distant metastases, histologic subtype, Nottingham combined histologic grade (Elston & Ellis, 2002), ER, PR (Hammond et al., 2010), HER2 status (Wolff et al., 2018) and RS) was retrieved from the pathology reports.
Patients with ductal carcinoma in situ, neoadjuvant treatment and documented evidence of distant metastases were excluded from the study.
Memorial Sloan Kettering Cancer Center (MSKCC) Cohort
De-identified data from all consecutive invasive BCs treated at MSKCC between January 1st, 2011 and December 31st, 2013, with available RS was obtained. As previously described (Wen et al., 2017), all BCs in this cohort were T1-T3 (≥ 0.5 cm), N0, ER+ and HER2-. The pathology information available for the study included tumor size (measured from excision specimen), nodal status, distant metastases, histologic subtype, Nottingham combined histologic grade (Elston & Ellis, 2002), ER, PR (Hammond et al., 2010), HER2 status (Wolff et al., 2018) and RS.
Comparison of Staging using TNM versus TNM+Biomarkers versus TNM+Biomarkers+RS<11
Each BC was assigned a TNM stage and a TNM+Biomarkers stage according to the 8th edition of the AJCC guidelines (Gabriel N. Hortobagyi, 2017). Staging of multifocal BC was assigned based on the characteristics of the largest ipsilateral tumor, in accordance with the AJCC 8th ed. staging guidelines (Gabriel N. Hortobagyi, 2017). In patients with bilateral BCs, the tumors in each breast were staged separately. We used the unselected NYULMC cohort to compare staging by TNM alone versus staging by TNM+Biomarkers.
For the analysis of possible downstaging due to genomic profile result (RS <11), we identified all T1-T2, N0, ER+/HER2- BCs with available RS in the NYULMC and MSKCC cohorts. We then assessed the tumor stage using TNM alone, TNM+Biomarkers and TNM+Biomarkers+RS<11. All changes in the aforementioned stage groups in the NYULMC and MSKCC cohorts were compared.
Ethics approval was obtained from the Institutional Review Boards (IRBs) at the two Institutions prior to study commencement, with waiver of informed consent for this retrospective analysis.
Results
NYULMC cohort analysis
We identified 1,305 consecutive primary invasive BCs from 1271 patients diagnosed at NYULMC in the study period (2016–2017). Thirty-four (2.7%) patients had synchronous bilateral BCs, and 179 (14.1%) had multifocal BCs. All patients were females, with average age of 60 years (range, 22–93). Most BCs underwent breast-conserving surgical excision (793/1305; 60.8%), and the remaining were treated by mastectomy (512/1305; 39.2%). Invasive ductal carcinoma, not otherwise specified (IDC NOS) was the most common BC subtype (1072/1305; 82.1%), followed by invasive lobular carcinoma (ILC) (170/1305; 13.0%). Most BCs were T1, N0, histologic grade 2, ER+/PR+, and HER2-. The characteristics of the NYULMC BC cohort are summarized in Table 1.
Table 1.
Clinicopathologic characteristics of Breast Carcinomas in NYULMC cohort
| Total Breast Cancers | Total n=1305 (%) |
|---|---|
| Type of surgery | |
| BCS | 793 (60.8) |
| Mastectomy | 512 (39.2) |
| Histologic Type | |
| IDC, NOS | 1072 (82.2) |
| ILC | 170 (13.0) |
| Mucinous | 22 (1.7) |
| Mixed | 21 (1.6) |
| Others | 20 (1.5) |
| Tumor size (mm) | |
| T1 (≤ 20) | 984 (75.4) |
| T2 (> 20 but ≤ 50) | 276 (21.1) |
| T3 (> 50) | 40 (3.1) |
| T4 (Any size with direct extension to the chest wall and/or to skin (ulceration or macroscopic nodules)) | 5 (0.4) |
| Node status | |
| N0 | 1047 (80.3) |
| N1 | 206 (15.8) |
| N2 | 41 (3.1) |
| N3 | 11 (0.8) |
| Tumor Grade | |
| G1 | 172 (13.2) |
| G2 | 731 (56.0) |
| G3 | 385 (29.5) |
| N/A (pT1mi) | 17 (1.3) |
| Estrogen Receptor | |
| Positive | 1152 (88.3) |
| Negative | 153 (11.7) |
| Progesterone Receptor | |
| Positive | 1019 (78.1) |
| Negative | 286 (21.9) |
| Human epidermal growth factor receptor 2 | |
| Positive | 183 (14.0) |
| Negative | 1114 (85.4) |
| Equivocal | 8 (0.6) |
| Subtypes | |
| ER+/HER2- | 1026 (78.6) |
| HER2 Positive | 183 (14.0) |
| Triple Negative | 96 (7.4) |
BCS= Breast Conserving Surgery, IDC, NOS = Invasive ductal carcinoma, Not Otherwise Specified; ILC = Invasive Lobular Carcinoma; Mixed = IDC and any other histologic types; Others include: Invasive papillary carcinoma (10 cases), Metaplastic carcinoma (4 cases), Tubular carcinoma (2 cases), Adenosquamous carcinoma (1 case), Invasive micropapillary carcinoma (1 case), Solid papillary carcinoma (1 case), and Tubulolobular carcinoma (1 case)
MSKCC cohort analysis
De-identified data, including the RS, of 999 primary invasive BCs treated at MSKCC between January 1st, 2011 and December 31st, 2013 were available for the study. All patients were females, with average age of 56 years (range, 22–84). One-hundred-thirty-five patients (13.5%) had multifocal BCs. The majority of the BC patients were managed with breast-conserving surgery (733/999; 73.4%) and remaining BCs were managed with mastectomy (266/999; 26.6%). The most common BC subtype was IDC, NOS (931/999; 93.4%), followed by ILC (32/999; 3.2%). The characteristics of the MSKCC BC cohort are detailed in Supplementary data 1.
Staging by TNM versus TNM+Biomarkers: Analysis of the NYULMC cohort
Most BCs in the NYULMC cohort were stage IA based on TNM staging alone (864/1305; 66.2%) and TNM+Biomarkers staging (968/1305; 74.2%). The TNM+Biomarkers system downstaged 352 BCs (27.0%). The most common downstage was from TNM stage group IIA to TNM+Biomarkers stage group IA. The vast majority (346/352; 98.3%) of all downstaged BCs were ER+/PR+. The TNM+Biomarkers system upstaged 75/1305 BCs (5.7%), all of which were ER-/PR-/HER2- BCs. The most common upstage was from TNM stage group IA to TNM+Biomarkers stage group IB. The complete breakdown of the staging subgroups according to TNM alone and TNM+Biomarkers in the NYULMC cohort is detailed in tables 2A and 2B.
Table 2A.
NYCLMC Cohort: Breast Cancer stage group distribution by TNM alone and TNM+Biomarkers
| Staging System |
TNM n (%) |
TNM+biomarkers n (%) |
|---|---|---|
| IA | 864 (66.2) | 969 (74.3) |
| IB | 24 (1.8) | 189 (14.5) |
| IIA | 252 (19.3) | 93 (7.1) |
| IIB | 96 (7.4) | 26 (2.0) |
| IIIA | 53 (4.1) | 14 (1.1) |
| IIIB | 5 (0.4) | 9 (0.7) |
| IIIC | 11 (0.8) | 5 (0.4) |
| Total | 1305 (100) | |
Table 2B.
NYCLMC Cohort: Shifts in Breast Cancer stage groups observed using TNM+Biomarkers over TNM alone
| Staging by TNM Cases (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage Group |
IA | IB | IIA | IIB | IIIA | IIIB | IIIC | ||
| Staging by TNM + Biomarkers cases (%) |
IA | 796 (61.0)= | 23 (1.8)▼ | 147 (11.3)▼ | 3 (0.2) ▼ | Total Downstaged 352 (27.0) |
|||
| IB | 68 (5.3)▲ | 1 (0.1)= | 49 (3.8)▼ | 47 (3.6)▼ | 24 (1.8) ▼ | ||||
| IIA | 55 (4.2)= | 29 (2.2)▼ | 9 (0.7) ▼ | ||||||
| IIB | 1 (0.1)▲ | 14 (1.1)= | 11 (0.8)▼ | ||||||
| IIIA | 3 (0.2)▲ | 6 (0.5)= | 1 (0.1)▼ | 4 (0.3)=▼ | |||||
| IIIB | 4 (0.3)= | 5 (0.4)▼ | |||||||
| IIIC | 3 (0.2)▲ | 2 (0.2)= | |||||||
| Total Upstaged 75 (5.7) |
Total Unchanged 878 (67.3) |
||||||||
Values in orange shaded boxes indicate cases (n(%)▲) that are upstaged; green shaded boxes indicate cases (n(%)▼) that are downstaged.
Impact of Recurrence Score <11 on staging of T1-T2, N0, ER+/HER2- Breast Carcinomas: Analysis of the NYULMC cohort and MSKCC cohort
In the NYULMC cohort, there were 342/1305 (26.2%) T1-T2, N0, ER+/HER2- BCs with available RS. Only 53/342 BCs (15.5%) had RS<11. All 999 BCs in the MSKCC cohort had available RS. A total of 993 BCs (99.4%) were T1-T2, N0, ER+/HER2- and 232/993 (23.4%) had RS<11.
The two groups combined yield a total of 285 T1-T2, N0, ER+/HER2- BCs with RS<11 (53 from NYULMC and 232 BCs from MSKCC), suitable for TNM+Biomarkers+RS<11 staging. The mean patient age was 60.4 years old (range, 36–84). The majority of BC patients were managed with breast-conserving surgery (197/285; 69.1%) and the remaining patients were managed with mastectomy (88/285; 30.9%). The most common BC subtype was IDC, NOS (237/285; 83.2%). The majority of the BCs (254/285; 89.1%) were T1 (≤ 20 mm) and approximately half of the BCs were histologic grade 2 (154/285; 54.0%). Table 3 summarizes the characteristics of NYULMC and MSKCC BCs with T1-T2, N0, ER+/HER2- and RS<11.
Table 3.
Clinicopathologic characteristics of T1-T2N0, ER-positive, HER2-negative, RS<11 BCs
| NYULMC (2016–2017) (n=53) |
MSKCC (2011–2013) (n=232) |
Total BCs (n=282) |
|
|---|---|---|---|
| Breast Cancer | n(%) | n (%) | n (%) |
| Mean age (in years) [range] | 61.5 [36–84] | 58.6 [41–84] | 60.4 [36–84] |
| Primary surgery | |||
| BCS | 36 (67.9) | 161 (69.4) | 197 (69.1) |
| Mastectomy | 17 (32.1) | 71 (30.6) | 88 (30.9) |
| Histologic Type | |||
| IDC | 41 (77.4) | 196 (84.5) | 237 (83.2) |
| ILC | 6 (11.3) | 7 (3.0) | 13 (4.5) |
| Others | 6 (11.3) | 29 (12.5) | 35 (12.3) |
| Tumor size (mm) | |||
| T1 (≤ 20) | 41 (77.4) | 214 (92.2) | 255 (89.5) |
| T2 (> 20 but ≤ 50) | 12 (22.6) | 18 (7.8) | 30 (10.5) |
| Histologic Grade | |||
| G1 | 14 (26.4) | 106 (45.7) | 120 (42.1) |
| G2 | 34 (64.2) | 120 (51.7) | 154 (54.0) |
| G3 | 5 (9.4) | 6 (2.6) | 11 (3.9) |
| Progesterone Receptor | |||
| Positive | 53 (100) | 223 (96.1) | 276 (96.8) |
| Negative | 0 (0) | 9 (3.9) | 9 (3.2) |
BCS= Breast Conserving Surgery, IDC, NOS = Invasive ductal carcinoma, Not Otherwise Specified; ILC = Invasive Lobular Carcinoma; Mixed = IDC and any other histologic types;
In combined two cohorts, the most common TNM stage group was IA (255/285; 89.5%), followed by stage IIA (30/285; 10.5%). When the TNM+Biomarkers system was applied 28 of the 30 BCs (93.3%) were downstaged from stage IIA to IA and 2 BCs (6.7%) were downstaged to group IB. A total of 283/285 (99.3%) BCs were stage group IA by TNM+Biomarkers system. When OncotypeDx™ score was incorporated, the remaining two BCs (2/285; 0.7%) were further downstaged from IB to IA based on TNM+Biomarkers+RS<11 (Figures 1 and 2 illustrate the two cases). In brief, case 1 is a grade 3 invasive ductal carcinoma with focal (<50%) micropapillary and mucinous features and case 2 is a grade 3 invasive ductal carcinoma, not otherwise specified, with a predominant solid architecture. Detailed staging analysis is summarized in Table 4.
Figure 1a. Case 1.
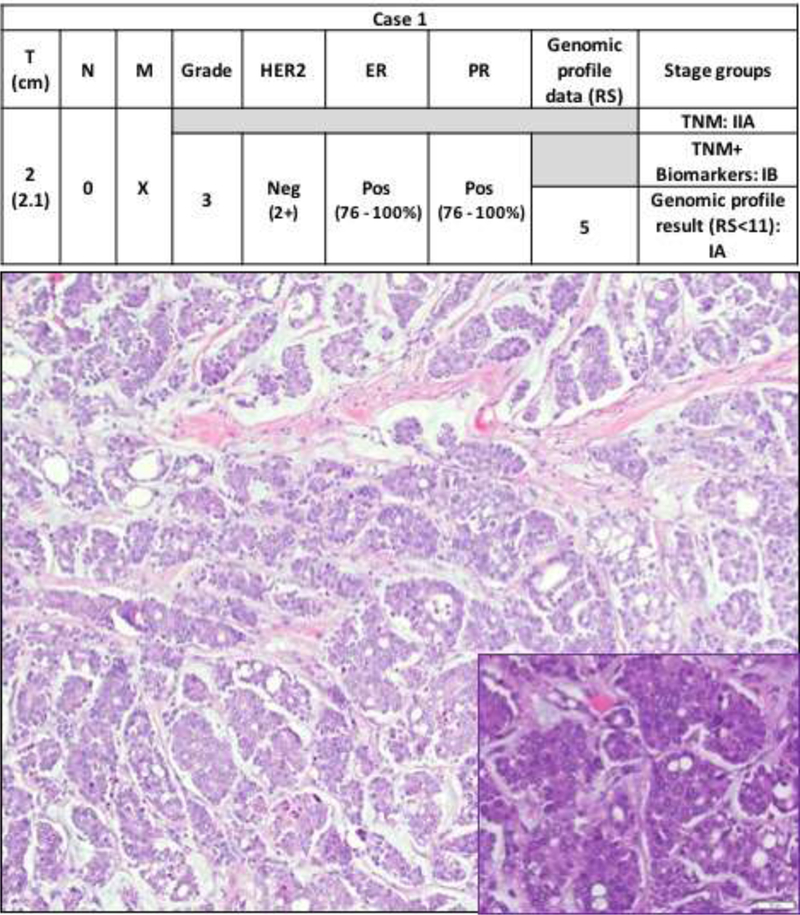
Step-wise downstaging of BC by different staging systems: TNM, TNM+biomarkers and finally TNM+biomarkers+genomic profile result (RS<11). Tumor size (T), Node status (N), Distant metastasis (M), human epidermal growth receptor2 (HER2), estrogen receptor (ER), progesterone receptor (PR), Neg (negative), Pos (Positive). The photomicrograph shows an invasive ductal carcinoma with micropapillary architecture in a mucinous background (hematoxylin and eosin, 10X). The inset shows the micropapillary clusters with high nuclear grade (hematoxylin and eosin, 40X).
Figure 2. Case 2.
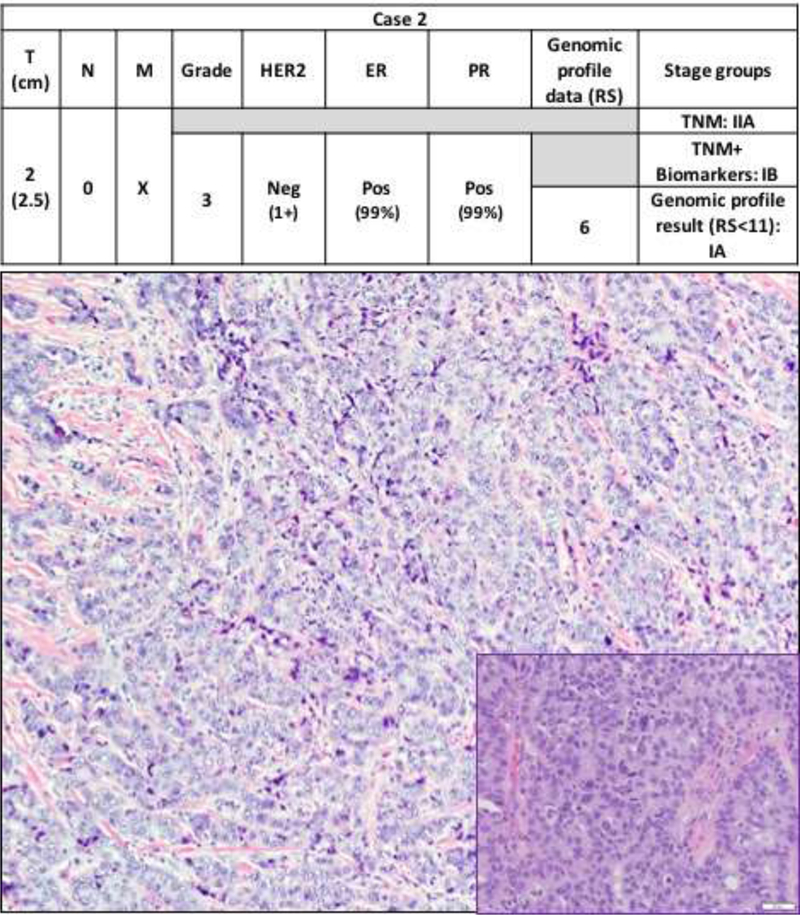
Step-wise downstaging of BC by different staging systems: TNM, TNM+biomarkers and finally TNM+biomarkers+genomic profile result (RS<11). Tumor size (T), Node status (N), Distant metastasis (M), human epidermal growth receptor2 (HER2), estrogen receptor (ER), progesterone receptor (PR), Neg (negative), Pos (Positive). The photomicrograph shows an invasive ductal carcinoma with solid growth pattern (hematoxylin and eosin, 10X). The inset shows sheets of invasive ductal carcinoma cells with high nuclear grade with occasional mitotic figures (hematoxylin and eosin, 40X).
Table 4.
Comparison of stage groups in T1-T2, N0, ER-positive, HER2-negative tumors by TNM alone, TNM+Biomarkers, and TNM+Biomarkers+RS<11
| NYULMC 2016–2017 (n=53) N (%) |
MSKCC 2011–2013 (n=232) N (%) |
|||||
|---|---|---|---|---|---|---|
| Stage Group |
TNM | TNM+Biomarkers | TNM+Biomarker+RS<11 | TNM | TNM+Biomarkers | TNM+Biomarker+RS<11 |
| IA | 41 (77.4) | 51 (96.2) | 53 (100) | 214 (92.2) | 232 (100) | 232 (100) |
| IB | 0 (0) | 2 (3.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IIA | 12 (22.6) | 0 (0) | 0 (0) | 18 (7.8) | 0 (0) | 0 (0) |
Discussion
The staging guidelines for BC introduced in the AJCC 8th ed. manual incorporate anatomic parameters (TNM status) as well as biologic parameters (tumor histologic grade, ER, PR, HER2 status) with the intent to provide a more accurate staging classification that takes into account tumor biology and the advantages of targeted therapy. The 8th ed. AJCC staging guidelines also include a special provision for staging T1–2, N0, ER+/HER2- BCs with RS<11 as stage group IA, independent of tumor size and grade. After the release of the AJCC 8th ed staging system, few groups validated its biologic and prognostic significance by restaging BCs in series with long-term follow-up data. In particular, Weiss et al. (Weiss et al., 2018) examined 3,327 BC patients with TNM stage groups I through IIIC treated at the MD Anderson Cancer Center between 2007 and 2013, and 54,727 BC patients with TNM stage groups I to IV reported to the California Cancer Registry between 2005 and 2009. Using the TNM+Biomarkers system, 807 patients (28.1%) in the MD Anderson Cancer Center cohort were downstaged, and 849 patients (29.5%) upstaged (Weiss et al., 2018). In the California Cancer Registry cohort, 10,488 patients (20.6%) were downstaged, and 15,794 patients (31.0%) upstaged (Weiss et al., 2018). In our study, the TNM+Biomarkers system changed the stage of 427 (32.7%) BCs in the NYULMC cohort: 352 (27.0%) BCs were downstaged, and 75 (5.7%) BCs were upstaged. Some variation in the downstaging and upstaging rates, in part, is attributable to differences in ethnicity and social status of the patient populations in different geographic areas. Overall, the analysis documents a substantial impact in BC staging using the TNM+Biomarkers over TNM alone. Compared to Weiss’s study (Weiss et al., 2018), the different rates of upstage and downstage in our cohort may be due to differences in tumor size, grade, and biomarker status, with the NYULMC BCs having overall more favorable characteristics compared to those analyzed in Weiss’s study (Weiss et al., 2018). For example, 60.5% and 63.3% of the two groups of BCs analyzed in Weiss’s study were ≤20 mm (Weiss et al., 2018), whereas 75.4% of BCs in the NYULMC cohort were ≤20 mm. The proportions of HER2+ BCs also differ: in the MD Anderson cohort, 9.2% of BCs were HER2+, in contrast to 14.0% BCs in NYULMC cohort. Even though HER2+ BCs are a biologically aggressive subgroup, and carry a poor prognosis (Burstein, 2005), HER2-targeted therapy has dramatically improved the outcome of HER2+ BCs (Cserni, Chmielik, Cserni, & Tot, 2018; Mittendorf et al., 2017; Nitta et al., 2016; Ross et al., 2009). Currently, HER2+ BCs are regarded as a “favorable” subgroup, provided that the patients receive appropriate anti HER2-targeted treatment. Therefore, the higher proportion of HER2+ BCs in the NYULMC cohort (14%) also contributed to the overall higher percentage of BCs that were downstaged in our study compared to Weiss’s study (9%).
A study by Mittendorf et al. which outlines the bioscores, also demonstrated that HER2+ BCs have added benefit compared to HER2- BCs in overall improved disease-specific survival because of anti-HER2-therapy (Mittendorf et al., 2017). In accordance with Mittendorf’s study, using the TNM+Biomarkers system, HER2+ BCs are generally assigned into lower stage groups compared to various TNM stage groups. Therefore, the efficacy of anti-HER2-treatment seems to offset the intrinsically unfavorable outcome in patients with HER2+ BCs across most TNM stage groups and improve their prognosis. Furthermore, ER-/PR-/HER2+ BCs of various sizes and histologic grades are assigned in a lower stage groups compared to ER-/PR-/HER2- (triple negative) BCs of same size and histologic grades. In our experience, when considering ER-/PR-/HER2+ BCs, 1/45 (2.2%) ER-/PR-/HER2+ BCs was downstaged from TNM stage group IIIC to TNM+Biomarkers stage group IIIB and the remaining 44/45 (97.8%) ER-/PR-/HER2+ BCs did not change their stage groups. However, 75/96 (78%) ER-/PR-HER2- BCs were upstaged using TNM+Biomarkers system.
Triple negative (TN) BCs constitute 10–17% of BCs and, in general, carry a poor prognosis (Badve et al., 2011; Fallahpour, Navaneelan, De, & Borgo, 2017; Rakha & Ellis, 2009; Rigon et al., 2015). Because of their intrinsic aggressive behavior, and the lack of targeted therapy, TN status has a global upstaging effect in the TNM+Biomarkers system across all stage groups as seen in our study and several other published studies (Cserni et al., 2018; Gabriel N. Hortobagyi, 2017; Hu et al., 2017; Lee et al., 2018; Weiss et al., 2018). The TNM+Biomarkers system considers all TNBCs as one homogenous group of BCs with equally poor prognosis (Gabriel N. Hortobagyi, 2017). However, TNBCs comprise a heterogeneous group with varied genetic, histologic and clinical features, ranging from low grade, locally aggressive neoplasm with low if any metastatic potential, to high grade BCs developing visceral metastasis within 2–3 years from diagnosis and having a highly aggressive behavior (Geyer et al., 2017; Pareja et al., 2016; Schmadeka, Harmon, & Singh, 2014). Despite the histopathologic and clinical heterogeneity in TNBCs, the TNM+Biomarkers system does not account for the biologic diversity and less aggressive behavior of some special types of TNBCs, such as adenoid cystic carcinoma. All that said, our patient cohort did not include significant number of special histologic subtypes of TNBCs. More studies are needed to refine the TNM+Biomarkers (PS) system for a more accurate prognostic stratification of this heterogeneous group of tumors.
Estrogen and progesterone- positive BCs tend to have lower histologic grade than ER-/PR- BCs and respond well to hormonal therapy (Bae et al., 2015; Chen, Linden, Anderson, & Li, 2014; Rakha, Reis-Filho, & Ellis, 2010). In general, the TNM+Biomarkers system downstages N0–1M0, ER+/PR+ BCs (Cserni et al., 2018; Hu et al., 2017; Lee et al., 2018; Weiss et al., 2018; Ye et al., 2017). For example, T2N1M0/T3N0M0 BCs fall in TNM stage group IIB, but using the TNM+Biomarkers system, the ER+/PR+ status shifts the tumors to either stage IA or IIA, depending on the histologic grade (Gabriel N. Hortobagyi, 2017; Giuliano et al., 2018; Weiss et al., 2018). In our analysis, ER+/PR+ BCs contributed to 98.3% of all downstaging BCs. In contrast, ER+/PR- or ER-/PR+ BCs were infrequently downstaged (3 ER+/PR- and 2 ER-/PR+). Single hormone positive BCs have less favorable prognosis compared to ER+/PR+ BCs (Bae et al., 2015; Chen et al., 2014; Dunnwald, Rossing, & Li, 2007; Fallahpour et al., 2017). Thus, the TNM+Biomarkers system downstages ER+/PR+ BCs and conveys the survival advantage associated with ER+/PR+ status versus positivity for only one of the two hormone receptors (Cserni et al., 2018).
For the first time, BC genomic profile is integrated into the 8th AJCC staging system. The Breast Cancer Guideline Committee on the National Comprehensive Cancer Network and American Society of Clinical Oncology state genomic profile results provide additional prognostic and predictive information (Harris et al., 2007; Leung et al., 2016). At this time, OncotypeDx™ (Genomic Health Inc., Redwood City, CA) is the only multigene assay with level I evidence to support its use in addition to the TNM-Biomarkers system (Enewold, Geiger, Zujewski, & Harlan, 2015; Leung et al., 2016). The Trial Assigning Individualized Option for Treatment (TAILORx) documented very low rates of recurrence at 5 years (<1% for distant recurrence, and <2% for any recurrence) for BCs with favorable gene-expression profile (defined as RS < 11) treated with endocrine therapy alone (Sparano et al., 2015). Consequently, the 8th ed AJCC staging guidelines assign T1-T2N0, ER-positive, HER2-negative BCs with RS<11 to stage group IA, independent of tumor grade and PR status. In our analysis of the 285 T1-T2 N0 ER+/HER2- BCs with RS<11, only 2 BCs (< 1%) were further downstaged from TNM+Biomarkers stage group IB to final stage group IA based on RS<11. Although the data are limited, and more information is needed, our analysis suggests that, despite its clinical validity as a predictive tool, OncotypeDx™ RS <11 has only limited added value as a prognostic marker in T1-T2N0 ER+/HER2- BCs. Several studies have shown that histologic grade, ER/PR/HER2 status and Ki-67 proliferation index correlate well with RS (Allison, Kandalaft, Sitlani, Dintzis, & Gown, 2012; Auerbach, Kim, & Fineberg, 2010; Bomeisl, Thompson, Harris, & Gilmore, 2015; Cuzick et al., 2011; Flanagan, Dabbs, Brufsky, Beriwal, & Bhargava, 2008; Geradts, Bean, Bentley, & Barry, 2010; Hou, Tozbikian, Zynger, & Li, 2017; Klein et al., 2013). Our data also show that the combination of TNM and biomarker status provides prognostic information comparable to the RS in over 99% of T1–2N0, ER+/HER2- BCs. Of note, two invasive lobular BCs in the NYULMC cohort were T3N0, histologic grade 2, ER+/HER2- BCs and both had low RS (10 and 5, respectively). Despite having low RS, these BCs could not be downstaged based on genomic profile due to their large size (T3).
We acknowledge that our study has limitations. Our analysis focused on patients treated at two cancer centers in a geographic area where most of the population has good access to health care and breast cancer screening. Our data is current and provides information that is applicable to contemporary BCs, however no follow-up information is available. In particular, correlation of the staging data with follow-up information in the MSKCC BC cohort could not be performed, due to the favorable biology of the tumors and lack of any event (data not shown). As we reported previously, the rate of distant metastasis for BCs with RS<10 is very low (1 of 510 patients; 0.2%) (Sparano et al., 2015; Wen et al., 2017). The 8th edition AJCC staging guidelines do not separate different subtypes of BC and therefore we did not perform separate analysis to see if there are any differences in impact between histologic subtypes. Nevertheless, it would be interesting to investigate if histologic subtypes impact the TNM+Biomarkers system.
In conclusion, our study demonstrated that the TNM+Biomarkers system changes tumor stage groups in approximately one third of all BCs. In our cohort, the TNM+Biomarkers system resulted in more downstaging than upstaging. Most of the downstaging was from TNM stage group IIA to TNM+Biomarkers stage group IA. Most triple negative BCs will upstage with few TNBCs staying in the same stage groups reflecting the limited treatment options. Lastly, TNM+Biomarkers system accurately classifies over 99% of T1-T2N0, ER+, HER2- BCs. The genomic profile results (RS<11) seem to provide little additional value for staging T1-T2N0, ER+, HER2- BCs. To our knowledge, this is the first and only study that evaluates the impact of genomic profile data in TNM+Biomarkers system.
Supplementary Material
Footnotes
Disclosure/conflict
The authors declare no conflict of interest.
References
- Abdel-Rahman O (2018). Validation of the 8th AJCC prognostic staging system for breast cancer in a population-based setting. Breast Cancer Res Treat, 168(1), 269–275. doi: 10.1007/s10549-017-4577-x [DOI] [PubMed] [Google Scholar]
- Allison KH, Kandalaft PL, Sitlani CM, Dintzis SM, & Gown AM (2012). Routine pathologic parameters can predict Oncotype DX recurrence scores in subsets of ER positive patients: who does not always need testing? Breast Cancer Res Treat, 131(2), 413–424. doi: 10.1007/s10549-011-1416-3 [DOI] [PubMed] [Google Scholar]
- Auerbach J, Kim M, & Fineberg S (2010). Can features evaluated in the routine pathologic assessment of lymph node-negative estrogen receptor-positive stage I or II invasive breast cancer be used to predict the Oncotype DX recurrence score? Arch Pathol Lab Med, 134(11), 1697–1701. doi: 10.1043/2009-0439-OAR.1 [DOI] [PubMed] [Google Scholar]
- Badve S, Dabbs DJ, Schnitt SJ, Baehner FL, Decker T, Eusebi V, … Reis-Filho JS (2011). Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol, 24(2), 157–167. doi: 10.1038/modpathol.2010.200 [DOI] [PubMed] [Google Scholar]
- Bae SY, Kim S, Lee JH, Lee HC, Lee SK, Kil WH, … Nam SJ (2015). Poor prognosis of single hormone receptor- positive breast cancer: similar outcome as triple-negative breast cancer. BMC Cancer, 15, 138. doi: 10.1186/s12885-015-1121-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomeisl PE, Thompson CL, Harris LN, & Gilmore HL (2015). Comparison of Oncotype DX Recurrence Score by Histologic Types of Breast Carcinoma. Arch Pathol Lab Med, 139(12), 1546–1549. doi: 10.5858/arpa.2014-0557-OA [DOI] [PubMed] [Google Scholar]
- Burstein HJ (2005). The distinctive nature of HER2-positive breast cancers. N Engl J Med, 353(16), 1652–1654. doi: 10.1056/NEJMp058197 [DOI] [PubMed] [Google Scholar]
- Chen L, Linden HM, Anderson BO, & Li CI (2014). Trends in 5-year survival rates among breast cancer patients by hormone receptor status and stage. Breast Cancer Res Treat, 147(3), 609–616. doi: 10.1007/s10549-014-3112-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserni G, Chmielik E, Cserni B, & Tot T (2018). The new TNM-based staging of breast cancer. Virchows Arch. doi: 10.1007/s00428-018-2301-9 [DOI] [PubMed] [Google Scholar]
- Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, … Forbes, J. F. (2011). Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol, 29(32), 4273–4278. doi: 10.1200/JCO.2010.31.2835 [DOI] [PubMed] [Google Scholar]
- Ding J, Wu W, Fang J, Chu Y, Zheng S, & Jiang L (2017). Changes of breast cancer staging when AJCC prognostic staging manual is used: a retrospective analysis of a Chinese cohort. Int J Biol Markers, 0. doi: 10.5301/ijbm.5000302 [DOI] [PubMed] [Google Scholar]
- Dunnwald LK, Rossing MA, & Li CI (2007). Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res, 9(1), R6. doi: 10.1186/bcr1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston CW, & Ellis IO (2002). Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Elston CW & Ellis. IO Histopathology 1991; 19; 403–410. Histopathology, 41(3A), 151–152, discussion 152–153. [DOI] [PubMed] [Google Scholar]
- Enewold L, Geiger AM, Zujewski J, & Harlan LC (2015). Oncotype Dx assay and breast cancer in the United States: usage and concordance with chemotherapy. Breast Cancer Res Treat, 151(1), 149–156. doi: 10.1007/s10549-015-3366-7 [DOI] [PubMed] [Google Scholar]
- Fallahpour S, Navaneelan T, De P, & Borgo A (2017). Breast cancer survival by molecular subtype: a population-based analysis of cancer registry data. CMAJ Open, 5(3), E734–E739. doi: 10.9778/cmajo.20170030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan MB, Dabbs DJ, Brufsky AM, Beriwal S, & Bhargava R (2008). Histopathologic variables predict Oncotype DX recurrence score. Mod Pathol, 21(10), 1255–1261. doi: 10.1038/modpathol.2008.54 [DOI] [PubMed] [Google Scholar]
- Hortobagyi Gabriel N., C. JL, D’Orsi Carl J., Edge Stephen B., Mittendorf Elizabeth A., Rugo Hope S., Solin Lawrence J., Weaver Donald L., Winchester David J., Giuliano Armando. (2017). Updated AJCC 8th Ed Breast Cancer Staging System AJCC Cancer Staging Manual, Eighth Edition: The American College of Surgeons (ACS). [Google Scholar]
- Geradts J, Bean SM, Bentley RC, & Barry WT (2010). The oncotype DX recurrence score is correlated with a composite index including routinely reported pathobiologic features. Cancer Invest, 28(9), 969–977. doi: 10.3109/07357907.2010.512600 [DOI] [PubMed] [Google Scholar]
- Geyer FC, Pareja F, Weigelt B, Rakha E, Ellis IO, Schnitt SJ, & Reis-Filho JS (2017). The Spectrum of Triple-Negative Breast Disease: High- and Low-Grade Lesions. Am J Pathol, 187(10), 2139–2151. doi: 10.1016/j.ajpath.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AE, Edge SB, & Hortobagyi GN (2018). Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann Surg Oncol. doi: 10.1245/s10434-018-6486-6 [DOI] [PubMed] [Google Scholar]
- Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, … College of American, P. (2010). American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med, 134(7), e48–72. doi: 10.1043/1543-2165-134.7.e48 [DOI] [PubMed] [Google Scholar]
- Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, … American Society of Clinical, O. (2007). American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol, 25(33), 5287–5312. doi: 10.1200/JCO.2007.14.2364 [DOI] [PubMed] [Google Scholar]
- Hou Y, Tozbikian G, Zynger DL, & Li Z (2017). Using the Modified Magee Equation to Identify Patients Unlikely to Benefit From the 21-Gene Recurrence Score Assay (Oncotype DX Assay). Am J Clin Pathol, 147(6), 541–548. doi: 10.1093/ajcp/aqx008 [DOI] [PubMed] [Google Scholar]
- Hu H, Wei W, Yi X, Xin L, & Liu Y (2017). A Retrospective Analysis of Clinical Utility of AJCC 8th Edition Cancer Staging System for Breast Cancer. World J Oncol, 8(3), 71–75. doi: 10.14740/wjon1039e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein ME, Dabbs DJ, Shuai Y, Brufsky AM, Jankowitz R, Puhalla SL, & Bhargava R (2013). Prediction of the Oncotype DX recurrence score: use of pathology-generated equations derived by linear regression analysis. Mod Pathol, 26(5), 658–664. doi: 10.1038/modpathol.2013.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Sohn G, Kim J, Chung IY, Lee JW, Kim HJ, … Ahn SH (2018). A retrospective prognostic evaluation analysis using the 8th edition of the American Joint Committee on Cancer staging system for breast cancer Breast Cancer Res Treat. doi: 10.1007/s10549-018-4682-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung RC, Yau TC, Chan MC, Chan SW, Chan TW, Tsang YY, … Cheung PS (2016). The Impact of the Oncotype DX Breast Cancer Assay on Treatment Decisions for Women With Estrogen Receptor-Positive, Node-Negative Breast Carcinoma in Hong Kong. Clin Breast Cancer, 16(5), 372–378. doi: 10.1016/j.clbc.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Mittendorf EA, Chavez-MacGregor M, Vila J, Yi M, Lichtensztajn DY, Clarke CA, … Hunt KK (2017). Bioscore: A Staging System for Breast Cancer Patients that Reflects the Prognostic Significance of Underlying Tumor Biology. Ann Surg Oncol, 24(12), 3502–3509. doi: 10.1245/s10434-017-6009-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta H, Kelly BD, Allred C, Jewell S, Banks P, Dennis E, & Grogan TM (2016). The assessment of HER2 status in breast cancer: the past, the present, and the future. Pathol Int, 66(6), 313–324. doi: 10.1111/pin.12407 [DOI] [PubMed] [Google Scholar]
- Pareja F, Geyer FC, Marchio C, Burke KA, Weigelt B, & Reis-Filho JS (2016). Triple-negative breast cancer: the importance of molecular and histologic subtyping, and recognition of low-grade variants. NPJ Breast Cancer, 2, 16036. doi: 10.1038/npjbcancer.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakha EA, & Ellis IO (2009). Triple-negative/basal-like breast cancer: review. Pathology, 41(1), 40–47. doi: 10.1080/00313020802563510 [DOI] [PubMed] [Google Scholar]
- Rakha EA, Reis-Filho JS, Baehner F, Dabbs DJ, Decker T, Eusebi V, … Ellis IO (2010). Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res, 12(4), 207. doi: 10.1186/bcr2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakha EA, Reis-Filho JS, & Ellis IO (2010). Combinatorial biomarker expression in breast cancer. Breast Cancer Res Treat, 120(2), 293–308. doi: 10.1007/s10549-010-0746-x [DOI] [PubMed] [Google Scholar]
- Rigon E, Saggia C, Rossi V, Genestroni S, Gaudino E, Campisi P, … Alabiso O (2015). FISH in triple-negative breast cancer: a possible strategy for the future? Future Oncol, 11(7), 1023–1026. doi: 10.2217/fon.15.25 [DOI] [PubMed] [Google Scholar]
- Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, & Hortobagyi GN (2009). The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist, 14(4), 320–368. doi: 10.1634/theoncologist.2008-0230 [DOI] [PubMed] [Google Scholar]
- Schmadeka R, Harmon BE, & Singh M (2014). Triple-negative breast carcinoma: current and emerging concepts. Am J Clin Pathol, 141(4), 462–477. doi: 10.1309/AJCPQN8GZ8SILKGN [DOI] [PubMed] [Google Scholar]
- Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, … Sledge GW (2015). Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med, 373(21), 2005–2014. doi: 10.1056/NEJMoa1510764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Chavez-MacGregor M, Lichtensztajn DY, Yi M, Tadros A, Hortobagyi GN, … Mittendorf EA,. (2018). Validation Study of the American Joint Committee on Cancer Eighth Edition Prognostic Stage Compared With the Anatomic Stage in Breast Cancer. JAMA Oncol, 4(2), 203–209. doi: 10.1001/jamaoncol.2017.4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen HY, Krystel-Whittemore M, Patil S, Pareja F, Bowser ZL, Dickler MN, … Brogi E (2017). Breast carcinoma with an Oncotype Dx recurrence score <18: Rate of distant metastases in a large series with clinical follow-up. Cancer, 123(1), 131–137. doi: 10.1002/cncr.30271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, … Dowsett M (2018). Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. doi: 10.5858/arpa.2018-0902-SA [DOI] [PubMed] [Google Scholar]
- Wong RX, Wong FY, Lim J, Lian WX, & Yap YS (2018). Validation of the AJCC 8th prognostic system for breast cancer in an Asian healthcare setting. Breast, 40, 38–44. doi: 10.1016/j.breast.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Ye J, Wang W, Xu L, Duan X, Cheng Y, Xin L, … Liu Y (2017). A retrospective prognostic evaluation analysis using the 8th edition of American Joint Committee on Cancer (AJCC) cancer staging system for luminal A breast cancer. Chin J Cancer Res, 29(4), 351–360. doi: 10.21147/j.issn.1000-9604.2017.04.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Xu L, Ye J, Xin L, Duan X, & Liu Y (2017). The Prognostic Value of the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging System in HER2-Enriched Subtype Breast Cancer, a Retrospective Analysis. Anticancer Res, 37(8), 4615–4621. doi: 10.21873/anticanres.11862 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


