Abstract
Aim:
To assess trends in cytomegalovirus (CMV) infection reported among infants in California neonatal intensive care units (NICUs) during 2005–2016.
Methods:
The California Perinatal Quality Care Collaborative collects data on all very low birth weight (VLBW, birth weight ≤1500g) and acutely ill infants >1500g, representing 92% of NICUs in California. We compared clinical characteristics and length of hospital stay among infants with and without reported CMV infection (CMV-positive viral culture or polymerase chain reaction).
Results:
During 2005–2016, CMV infection was reported in 174 VLBW infants and 145 infants >1500g, or 2.7 (range: 1.5–4.7) and 1.2 (range: 0.8–1.7) per 1,000 infants, respectively (no significant annual trend). Among infants >1500g, 12 (8%) vs. 4,928 (4%) of those reported with vs. without CMV infection died (P<0.05). The median hospital stay was significantly longer among infants reported with vs. without CMV infection for both VLBW infants (98 vs. 46 days) and infants >1500g (61 vs. 14 days) (P<0.001).
Conclusions:
Reports of CMV infection remained stable over a 12-year period. Although we were not able to assess whether infection was congenital or postnatal, CMV infection among infants >1500g was associated with increased mortality.
Keywords: cytomegalovirus, congenital infection, very low birth weight infant, postnatal infection
INTRODUCTION
In the United States, congenital cytomegalovirus (CMV) infection occurs in an estimated 5 per 1,000 newborns, of whom 90% are asymptomatic and 10% present a spectrum of mild to life-threatening disease at birth, with a high risk of neurologic impairment and sensorineural hearing loss.1 Diagnosing congenital CMV infection requires testing of urine, saliva, or blood collected within 2–3 weeks of birth.2 Postnatal CMV infection, although usually asymptomatic, can cause severe disease (e.g., sepsis-like syndrome) in very low birth weight (VLBW, ≤1500g) and premature (<32 weeks) infants during the neonatal period, but has not been definitively associated with neurologic impairment or sensorineural hearing loss.3, 4 In this study, we assessed reporting rates and demographic and clinical characteristics of CMV infection among infants admitted to neonatal intensive care units (NICUs) in California during 2005–2016.
METHODS
The California Perinatal Quality Care Collaborative (CPQCC) collects data for infants admitted to a member NICU at birth or within 28 days of life. CPQCC includes 137 member hospitals, representing 92% of NICUs in California. All infants with birth weight between 401–1,500g or gestational age between 22 weeks and 31 weeks 6 days at birth (henceforth referred to as “VLBW infants”) are eligible for data collection. For infants with birth weight >1500g, CPQCC eligibility criteria include death, severe hyperbilirubinemia, early bacterial sepsis (positive blood or cerebrospinal fluid culture obtained on or before day 3 of life), and suspected encephalopathy, among other selected conditions. Physicians, nurses, and other trained personnel conduct data abstraction using a standardized questionnaire, which is electronically submitted. Standard definitions for the database were developed by the Vermont Oxford Network.5
We defined a case of CMV infection as a NICU infant with documented positive viral culture or polymerase chain reaction (PCR) assay for CMV at any time since birth. CMV laboratory testing was performed during diagnostic workup under the discretion of providers. Since not all clinical signs associated with congenital CMV disease were systematically collected, we classified infants with positive laboratory tests as having reported CMV infection. The number of infants tested for CMV was not recorded, nor were the specimen types, or specimen collection date, precluding categorization of CMV cases as congenital or postnatal.
We calculated CMV reporting rates using the number of live births meeting CPQCC eligibility criteria as a denominator. We performed univariate analyses to compare characteristics of infants with and without reported CMV infection, among VLBW infants and infants >1500g separately. We excluded infants with other reported congenital or perinatal infections, such as herpes simplex virus and HIV, from the analysis. We used the chi-square or Fisher exact test for comparison of categorical variables, the Cochran-Armitage test for trend, and the Wilcoxon test for comparison of continuous variables. We performed all analyses using SAS version 9.4 (SAS, Cary, NC). The Institutional Review Board at Stanford University approved the study.
RESULTS
From 2005 to 2010, reporting rates of CMV infection among infants admitted to California NICUs increased from 1.5 to 4.6 per 1,000 VLBW infants.6 However, the increasing trend was no longer significant when data from 2011–2016 were included in the analysis. CMV infection was reported in 2.7 per 1,000 VLBW infants and 1.2 per 1,000 eligible infants >1500g admitted to California NICUs during 2005–2016. Figure 1 shows 3-year moving average of reporting rates of CMV infection for both groups.
Figure 1.
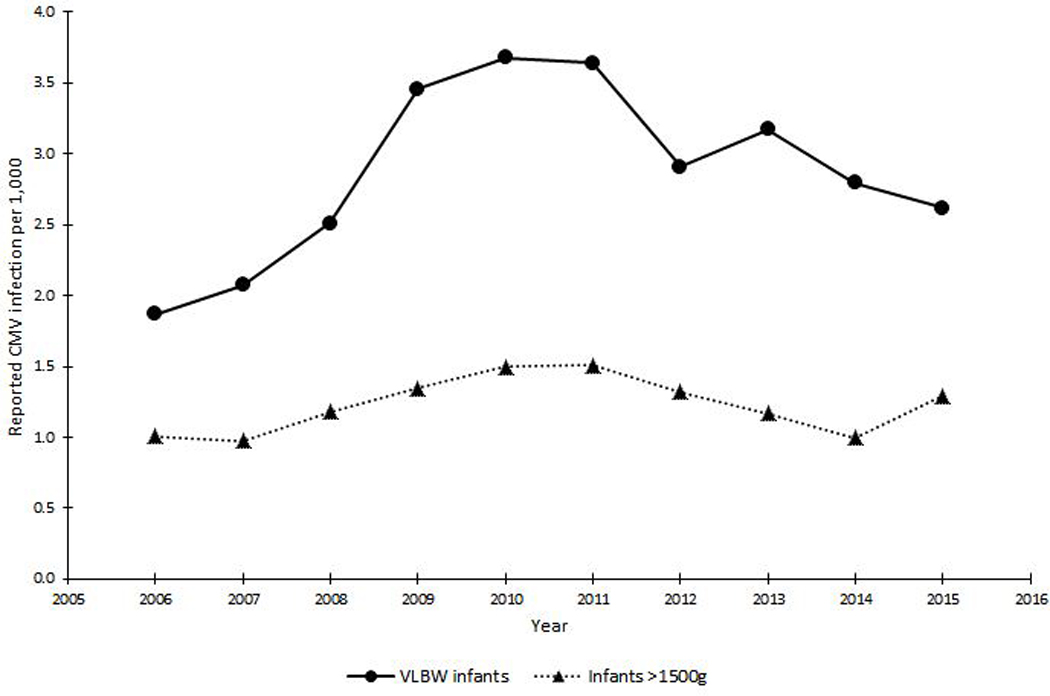
Three-year moving average of reporting rates of CMV infection among VLBW infants and infants >1500g in neonatal intensive care units, California, 2006–2015
During 2005–2016, the majority of infants reported with CMV infection (61% of VLBW infants and 52% of infants >1500g) were born at hospitals with community NICUs. As defined by the California Children’s Services Program, community NICUs have the capability of providing a full range of neonatal care services (intensive, intermediate, and continuing care) for severely ill neonates and infants, which corresponds to level III of neonatal care by the American Academy of Pediatrics.7, 8 Reported CMV infection was significantly more likely among VLBW infants born to Asian/Pacific Islander mothers than White mothers (odds ratio [OR] = 2.1; 95% confidence interval [CI] = 1.5–3.2) (P<0.05). Reported CMV infection was more likely among infants >1500g born to mothers aged <20 years (OR = 3.6; 95% CI = 2.2–5.8) and ≥40 years (OR = 1.8; 95% CI = 1.1–3.1) compared to those born to mothers aged 30–39 years (P<0.05). We observed no significant differences by maternal race among infants >1500g, by maternal age among VLBW infants, or by maternal Hispanic origin in either infant groups.
A minority of infants reported with CMV infection, 9 (5%) VLBW infants and 44 (30%) infants >1500g, were discharged or died within 21 days of birth. Most VLBW infants and about half of infants <1500g were delivered by C-section (Table 1). About half of the infants reported with CMV infection were ever fed breastmilk during NICU stay; this proportion was significantly different only between infants >1500g reported with and without CMV infection (53% vs. 71%) (Table 1).
Table 1.
Characteristics of very low birth weight infants and infants >1500g reported with and without CMV infection in neonatal intensive care units, California, 2005–2016
| Characteristics | Very low birth weight infants (401g–1500g) | Infants >1500g | ||||
|---|---|---|---|---|---|---|
| Reported with CMV infection (N = 174) |
Reported without CMV infection a (N = 64,101) |
OR (95% CI) | Reported with CMV infection (N = 145) |
Reported without CMV infection a (N = 116,416) |
OR (95% CI) | |
| n (%) | n (%) | n (%) | n (%) | |||
| IUGR | 50 (29) | 10,020 (16) | 2.1 (1.5–3.0)* | 25 (17) | 4,908 (4) | 4.8 (3.1–7.3)* |
| Anomaly diagnosed prior to birth | 6 (3) | 2,091 (3) | 1.0 (0.5–2.4) | 37 (26) | 15,771 (14) | 2.2 (1.5–3.2)* |
| Birth at <32 weeks gestational age | 148 (85) | 55,795 (87) | 0.8 (0.6–1.3) | 12 (8) | 11,317 (10) | 0.8 (0.4–1.5) |
| Delivered by C-section | 136 (78) | 45,284 (71) | 1.5 ( 1.0–2.2) | 69 (48) | 64,111 (55) | 0.7 ( 0.6–1.0) |
| Breastmilk intake b | 92 (53) | 35,240 (59) | 0.8 (0.6–1.1) | 77 (53) | 81,994 (71) | 0.5 (0.3–0.6)* |
| Head circumference ≤10th percentile | 41 (24) | 7,001 (11) | 2.1 (1.5–3.0)* | 36 (25) | 7,270 (6) | 4.5 (3.0–6.7)* |
| Seizure | 6 (3) | 2,040 (3) | 1.0 (0.4–2.3) | 10 (7) | 6,268 (5) | 1.3 (0.7–2.5) |
| Neuroimaging evaluation | 172 (99) | 56,214 (88) | 5.9 (1.5–23.9)* | 132 (91) | 51,445 (44) | 12.6 (7.1–22.3)* |
| Cystic periventricular leukomalacia | 5 (3) | 1,446 (2) | 1.1 (0.5–2.8) | 11 (8) | 715 (1) | 6.5 (3.5–12.0)* |
| Periventricular-intraventricular hemorrhage, Grades 1–4 | 52 (30) | 13,817 (22) | 1.3 (1.0–1.8) | 26 (18) | 4,362 (4) | 2.7 (1.8–4.2)* |
| Death c | 15 (9) | 6,171 (10) | 0.9 (0.5–1.5) | 12 (8) | 4,929 (4) | 2.0 (1.1–3.7)* |
Abbreviations: OR = odds ratio; CI = confidence interval; IUGR = intrauterine growth restriction.
Note: Periventricular-intraventricular hemorrhage graded as follows: 0 for no subependymal or intraventricular hemorrhage, which constituted the reference group; 1 for subependymal germinal matrix hemorrhage only; 2 for intraventricular blood, without ventricular dilation; 3 for intraventricular blood with ventricular dilation; and 4 for intraparenchymal hemorrhage.
Excludes infants with other congenital or perinatal infections.
Infant ever noted to have been fed breastmilk during NICU hospital course.
Neonatal death between age 1–28 days in CPQCC NICUs; deaths in the delivery room or within 12 hours of admission were excluded from the denominator and numerator.
Significant difference at the P<0.05 level using the Chi-square test.
Among infants reported with CMV infection, the median birth weight was 845g (interquartile range [IQR]: 690–1120g) for VLBW infants and 2429g (IQR: 1990–3002g) for infants >1500 g, which was significantly lower than for those without CMV infection (1070g [IQR: 780–1310g], and 2820g [IQR: 2160–3390g], respectively) (P<0.001). Among both VLBW infants and infants >1500g, those reported with vs. without CMV infection were more likely to have had intrauterine growth restriction and head circumference <10th percentile (P<0.05) (Table 1). Among infants >1500g, CMV infection was also associated with an anomaly detected prior to birth, and neuroimaging abnormalities (cystic periventricular leukomalacia and periventricular-intraventricular hemorrhage grades 1–3) (P<0.05) (Table 1).
Among VLBW infants, 15 (8%) vs. 6,171 (10%) of those reported with vs. without CMV infection died (P>0.05) (Table 1). Among infants >1500g, 12 (8%) vs. 4,928 (4%) of those reported with vs. without CMV infection died (P<0.05). Among surviving infants reported with CMV infection, the median length of hospital stay was 98 days (interquartile range: 70–123) for VLBW infants and 46 days (IQR: 18–76) for infants >1500 g, which was significantly longer than for those without CMV infection (61 days [IQR: 42–87], and 14 days [IQR: 8–28], respectively) (P<0.001).
DISCUSSION
Our study includes >180,000 infants admitted to California NICUs from 2005 to 2016; CMV infection was reported in 2.7 per 1,000 VLBW infants and 1.2 per 1,000 infants >1500g. As expected, CMV reporting rates were lower than the prevalence observed in studies that screened all infants for CMV infection. CMV screening among NICU infants may identify additional infants with congenital CMV disease that could benefit from antiviral therapy, regular monitoring, and early intervention. VLBW infants with congenital CMV infection have an increased risk of neurologic impairment and sensorineural hearing loss compared with VLBW infants without congenital CMV infection.9 Data on safety and efficacy of antiviral treatment for VLBW with CMV infection are lacking and additional studies may be warranted given the high morbidity in this population.10
CPQCC collects data on all VLBW infants, among whom 2.7 per 1,000 were reported with CMV infection during 2005–2016. In comparison, a study in Alabama during 1993–2008 found a rate of 7.4 per 1,000 (based on adding together reported rates of 3.9 per 1,000 VLBW infants with congenital CMV infection based on universal screening, and 3.5 per 1,000 VLBW infants with postnatal CMV infection based on clinical indication for additional testing).9 Cases of CMV infection in VLBW infants may have been missed because laboratory testing was likely conducted based on clinical suspicion. Population risk factors for congenital and postnatal CMV infection may vary among states.
Assessing whether reported CMV infection was congenital or postnatal in our cohort, particularly among VLBW infants, is nearly impossible because the date of specimen collection was not recorded. Infants who died or were discharged after 21 days of birth could have had peri- or postnatal CMV infection. Perinatal CMV infection may occur in up to 50% of infants exposed to CMV in cervicovaginal secretions.11 However, the proportion of CMV-seropositive women shedding virus in cervicovaginal secretions is relatively low (3% to 13%) in the third trimester of pregnancy.11, 12 In our study, 78% of VLBW infants and 48% of infants >1500g with reported CMV infection were delivered by C-section. Postnatal CMV infection acquired via breastfeeding can cause disease in VLBW and premature (<32 weeks) infants because their immune system is relatively immature and most placental transfer of maternal antibodies occurs during the third trimester.13 Most of our VLBW infants but only 8% of the infants >1500g reported with CMV infection were born at <32 weeks gestational age. Although half of the infants with reported CMV infection were fed breastmilk during NICU stay, data collected did not distinguish between mother’s own milk or donor milk. The proportion who ever received breastmilk increased from 52% to 64% among VLBW infants, and from 63% to 76% among infants >1500g, during 2005–2016 (data not shown). However, maternal CMV serostatus was unknown. The quality of information could be improved by collecting additional data to allow characterization of congenital and postnatal CMV infection in the NICU population.
Reported CMV infection was associated with significantly longer hospital stays, and among infants >1500g, with a 2-fold mortality compared to infants without reported CMV infection. CMV testing was performed at the discretion of the clinicians providing care and may have varied by NICU. Additionally, not all infants >1500g admitted to the NICU are eligible for CPQCC data collection and many infants with symptomatic congenital CMV disease may not have severe enough manifestations to require NICU hospitalization. Thus, infants with more severe CMV disease were likely identified. Among infants >1500g, an anomaly detected prior to birth, head circumference <10th percentile, and abnormal neuroimaging findings were more likely among those reported with CMV infection than without. The likelihood of cystic periventricular leukomalacia, which is a strong predictor of neurological impairment in preterm infants and has also been associated with cerebral palsy,14, 15 was 6.5-fold higher among infants >1500g reported with CMV infection than without. Linkage of CPQCC data to the California Children’s Service High-Risk Infant Follow-up Program databases may enable future assessment of long-term neurodevelopmental outcomes and health care resources utilized by eligible infants.16
CPQCC data have been useful to monitor trends in CMV infection among NICU infants in California and identify demographic groups at higher risk of disease, such as VLBW infants born to Asian/Pacific islanders and infants >1500g born to mothers <20 years or >40 years of age. However, data from California may not be generalizable to other U.S. states. Perinatal Quality Care Collaborative Initiatives in other U.S. states and NICUs around the world participating in the Vermont Oxford Network could provide useful information on CMV trends and higher risk groups, if the data are collected. Increasing awareness of CMV disease and its impact on public health is important to move forward with the development of CMV vaccines and successful implementation of a vaccination program in the future.
Abbreviations:
- CMV
cytomegalovirus
- CPQCC
California Perinatal Quality Care Collaborative
- IUGR
intrauterine growth restriction
- NICU
neonatal intensive care unit
- VLBW
very low birth weight
Footnotes
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of Interest: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Fowler KB, McCollister FP, Sabo DL, et al. A Targeted Approach for Congenital Cytomegalovirus Screening Within Newborn Hearing Screening. Pediatrics. 2017;139(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazzarotto T, Guerra B, Lanari M, Gabrielli L, Landini MP. New advances in the diagnosis of congenital cytomegalovirus infection. J Clin Virol. 2008;41(3):192–7. [DOI] [PubMed] [Google Scholar]
- 3.Gunkel J, Wolfs TF, de Vries LS, Nijman J. Predictors of severity for postnatal cytomegalovirus infection in preterm infants and implications for treatment. Expert Rev Anti Infect Ther. 2014;12(11):1345–55. [DOI] [PubMed] [Google Scholar]
- 4.Gunkel J, de Vries LS, Jongmans M, et al. Outcome of Preterm Infants With Postnatal Cytomegalovirus Infection. Pediatrics. 2018;141(2). [DOI] [PubMed] [Google Scholar]
- 5.Vermont Oxford Network. Available at: http://www.vtoxford.org/about/about.aspx Accessed September 16, 2018.
- 6.Lanzieri TM, Bialek SR, Bennett MV, Gould JB. Cytomegalovirus infection among infants in California neonatal intensive care units, 2005–2010. J Perinat Med. 2014;42(3):393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.California Children’s Services Manual of Procedures. Chapter 3.25.2 - Standards for neonatal intensive care units (NICUs). Community NICU. Available at: https://www.dhcs.ca.gov/services/ccs/Documents/CommunityNICU.pdf Accessed February 4, 2019.
- 8.Stark AR, American Academy of Pediatrics Committee on F, Newborn. Levels of neonatal care. Pediatrics. 2004;114(5):1341–7. [DOI] [PubMed] [Google Scholar]
- 9.Turner KM, Lee HC, Boppana SB, Carlo WA, Randolph DA. Incidence and impact of CMV infection in very low birth weight infants. Pediatrics. 2014;133(3):e609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimberlin DW, Lin CY, Sanchez PJ, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143(1):16–25. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds DW, Stagno S, Hosty TS, Tiller M, Alford CA, Jr. Maternal cytomegalovirus excretion and perinatal infection. N Engl J Med. 1973;289(1):1–5. [DOI] [PubMed] [Google Scholar]
- 12.Barbosa NG, Yamamoto AY, Duarte G, et al. Cytomegalovirus Shedding in Seropositive Pregnant Women From a High-Seroprevalence Population: The Brazilian Cytomegalovirus Hearing and Maternal Secondary Infection Study. Clin Infect Dis. 2018;67(5):743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mussi-Pinhata MM, Pinto PC, Yamamoto AY, et al. Placental transfer of naturally acquired, maternal cytomegalovirus antibodies in term and preterm neonates. J Med Virol. 2003;69(2):232–9. [DOI] [PubMed] [Google Scholar]
- 14.van Wezel-Meijler G, Steggerda SJ, Leijser LM. Cranial ultrasonography in neonates: role and limitations. Semin Perinatol. 2010;34(1):28–38. [DOI] [PubMed] [Google Scholar]
- 15.De Vries LS, Van Haastert IL, Rademaker KJ, Koopman C, Groenendaal F. Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr. 2004;144(6):815–20. [DOI] [PubMed] [Google Scholar]
- 16.California Department of Health Care Services. High Risk Infant Follow Up. Available at: http://www.dhcs.ca.gov/services/ccs/Pages/HRIF.aspx#overview Accessed September 16, 2018.


