Summary and Perspectives
The twin goals of determining how neural secretion is unique and how proteins in the presynaptic terminal can be targeted in the treatment of neurological disorders are both met in the study of SV2. Unlike the machinery of vesicle fusion, SV2 is unique to regulated secretion and has no functional homolog in the vesicles that mediate membrane trafficking at other cellular locations.
SV2 is, to date, the only synaptic vesicle protein that is a viable therapeutic target in the treatment of nervous system diseases. This may be linked to the fact that its function(s) are limited to quiescent synapses. By playing an essential role in regulating initial release probability, SV2 action has special relevance to neural network synchrony. Given the role of aberrant excitability in numerous neurological disorders, the relevance of SV2 to drug development will likely only grow.
It’s been 30 years since neuroscientists began identifying the protein components of synaptic vesicles. The goal of determining the molecular events that produce and regulate neurotransmitter release promised greater understanding of normal brain function as well as new therapeutic targets to treat nervous system dysfunction.
The unique size and morphology of the vesicles observed in electron micrographs of synapses suggested that neurotransmitter secretion was mediated by a unique organelle. The abundance of these vesicles inspired the development of purification strategies that provided the material for generating antibodies that, in turn, were used to initiate the molecular characterization of neurotransmitter release. This work revealed that synaptic vesicles share features common to all types of membrane trafficking, and thus are not the singular organelle first imagined.
Yet transmitter-secreting vesicles do contain unique components that provide tight control of their action and potential targets for new therapies to treat disorders of the nervous system. Synaptic Vesicle Protein 2 (SV2) is perhaps the best example of this class of protein. It is specific to vesicles that undergo calcium-regulated secretion and is the receptor for a class of anti-epileptic drugs that shows promise in the treatment of a range of nervous system disorders. In this review we consider what we’ve learned about the function of SV2 since it was first reported in 1988 [1], how what we’ve learned contributes to our understanding of the evolution of synaptic transmission, and how targeting SV2 action can be leveraged in the development of new therapies.
SV2 is small family of membrane glycoproteins with the features of solute transporters and additional unique domains
SV2 was identified with a monoclonal antibody generated against synaptic vesicles purified from the electric organ of the electric ray Discopyge ommata [1]. The antibody specifically labeled small clear vesicles of the size observed in electron micrographs of synapses, and the protein containing the epitope was named Synaptic Vesicle Protein 2. Biochemical studies revealed SV2 to be a large glycoprotein. Interestingly, the SV2 antibody also labeled all neural and endocrine vesicles surveyed, suggesting it performs a function specific to calcium-regulated secretion.
Isolation and sequencing of cDNAs encoding SV2 revealed it to be a 73 KDa protein with the signature motifs of a family of structurally and phylogenetically-related proteins termed Major Facilitator Superfamily (MFS) transporters [2–5]. These motifs include 12 predicted TM domains that appear to have arisen from the duplication of a single six TM domain protein [3, 6], a basic RXGRR motif between TM2 and TM3, conserved glycine residues in TM4 and TM5, and a sequence of prolines and charged residues following TM domains 6 and 12 [7]. Of the MFS proteins, SV2 is most similar to the SLC22 subfamily of organic ion transporters [8]. The SLC22 transporters share with SV2 a glycine rich domain in the 4th TM segment and functionally important residues corresponding to Y328 and W320 of PiPT, a SLC22-related phosphate transporter for which structural data has been obtained [9]. Although the structure of SV2 has yet to be solved at high resolution, structural models have been generated based on its predicted similarity to the solved structures of other members of the MFS family [10]. Low resolution tomography studies indicate that, like MFS transporters [11], SV2 exists in two major structural conformations: one presenting a pore-like opening towards the cytoplasm, and another with a cleft-like opening facing the intravesicular space [12].
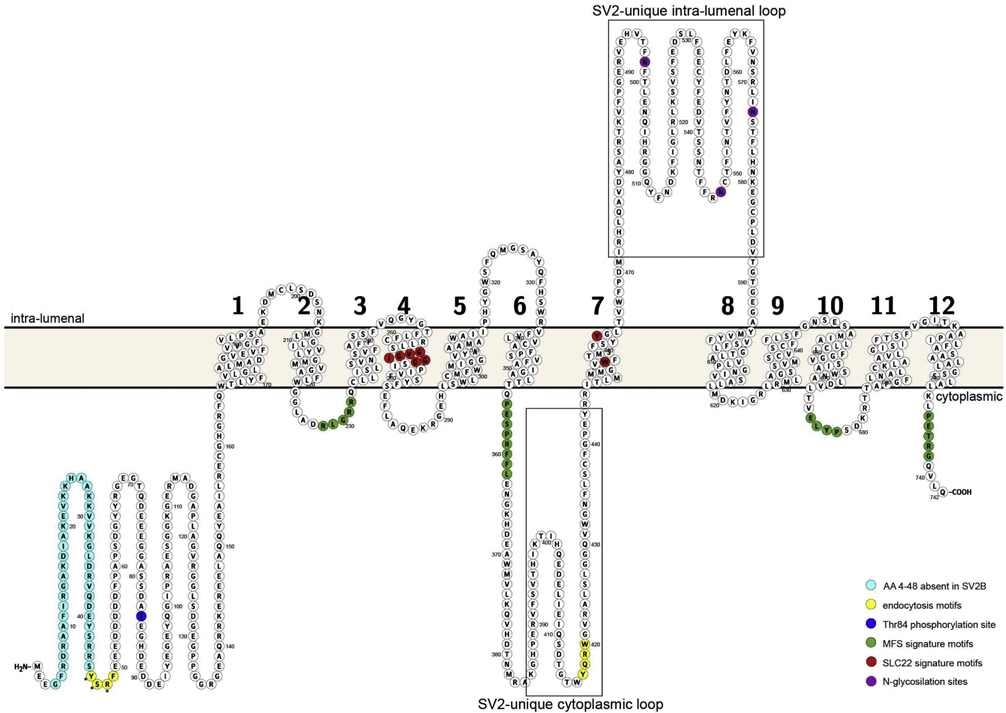
SV2 differs from other MFS proteins by having a long, cytoplasmic amino terminus and a large, lumenal loop between TM domains 7 and 8 that contain three N-linked glycosylation sites. Analysis of SV2 glycosylation revealed long keratan sulfate additions [13], suggesting that SV2 contributes significant mass to the vesicle lumen.
Two other SV2 genes were subsequently identified along with a related protein termed SVOP [14–16]. The three SV2 paralogs are denoted SV2A, SV2B, and SV2C. They share about 60% sequence similarity and are all recognized by the monoclonal antibody that originally defined SV2 (Figure 1). All three SV2s have an extended amino terminus and a large domain between TM domains 7 and 8 that contain the three N-linked glycosylation sites. Mutations of these N-linked glycosylation sites affected SV2A expression levels and trafficking to synapses suggesting that glycosylation is important for ER quality control of SV2 folding [17, 18]. Interestingly, the amino acid sequences of SV2s differ most in these two regions that distinguish them from other MFS proteins. Notably, SV2B has a shorter N-terminus than SV2A and SV2C, lacking the portion corresponding to amino acids 4–48 of mammalian SV2A. This region in SV2A contains an accessory binding domain for the synaptic vesicle protein synaptotagmin [19] (discussed below), and a tyrosine-based endocytosis motif [20].
Figure 1. The SV2 proteins.
Shown is a schematic drawing of human SV2A (NCBI accession: NP_001315603) depicting the position of transmembrane domains and domains of interest. Each circle denotes an amino acid, and the orange bar the vesicle membrane. Amino terminal residues unique to SV2A and SV2C are colored green. The sequences conserved in Major Facilitator Transporters are colored magenta. Residues conserved in SV2A and SV2C (and which differ in SV2B) are colored red. Residues specific to cytoplasmic domains of SV2s are colored cyan. Tyrosine based endocytosis motifs are colored yellow. Phosphorylation and glycosylation sites are represented as dark squares. The illustration was generated using Protter tool [135]
One or more SV2 paralog is present in all synapses, consistent with SV2 performing a function required by all types of neurotransmission. While SV2A is expressed in nearly all neurons, SV2B is principally expressed in glutamatergic neurons, making SV2A the only paralog expressed in GABAergic neurons. SV2C expression is largely limited to striatum, midbrain and hindbrain neurons and is the primary SV2 paralog in dopaminergic neurons [16, 21–25] [(2004) Allen Institute for Brain Science. Allen Mouse Brain Atlas. Available from: mouse.brain-map.org]. The functional significance of three, very similar but non-identical SV2 genes is still not fully understood. All three can rescue normal neurotransmission when expressed in neurons lacking SV2 demonstrating functional redundancy [18]. The utility of having three SV2 genes could rest in differential regulation of expression, or post transcriptional/translational regulation across different types of neurons. Indeed, developmental regulation of SV2 expression [26] differs between paralogs [21].
An especially interesting aspect of SV2 biology is the apparent precision with which it is trafficked to synaptic vesicles. Of seven proteins quantified using single vesicle analysis, SV2 was the most uniformly dispersed, with over 95% of all vesicles having five copies of SV2 [27]. Such tight regulation of protein sorting suggests that SV2 expression levels have the potential to influence synaptic function. This conjecture is supported by two observations; 1) overexpression of SV2A produces synaptic deficits [28], and 2) SV2A expression is a major target in microRNA regulation of homeostatic synaptic plasticity [29].
Bona fide SV2 emerges in vertebrates
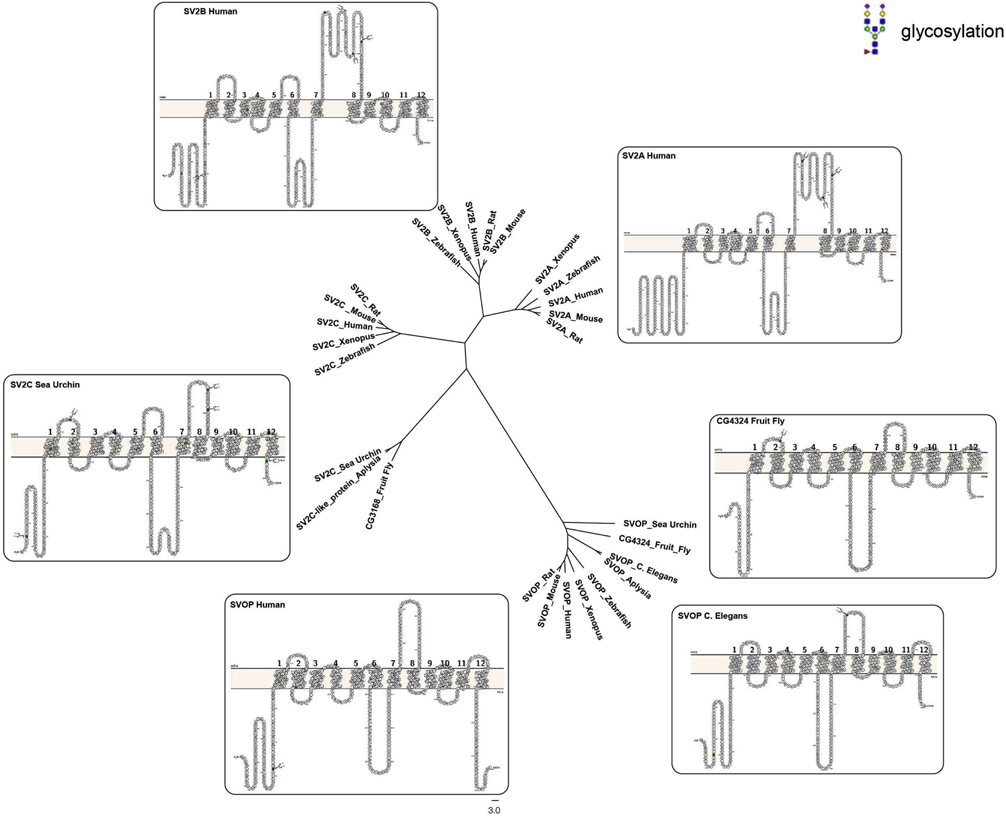
Transporter-like proteins with some SV2-defining motifs can be found in single cell flagellates (e.g. Salpingoceca Rosetta PTSG_08752 (NCBI accession: XP_004990136)) and invertebrate animals (e.g. Drosophila SV2C-related protein CG14691). While these may be functional precursors of SV2, they lack the glycosylated, long intra-lumenal loop characteristic of vertebrate SV2s (Figure 2), a domain that appears crucial to SV2 trafficking to secretory vesicles. Concordantly, SV2-like proteins with glycosylation sites in the intralumenal loop appear well after the emergence of calcium-regulated secretion suggesting glycosylation serves a regulatory role. Putative SV2 precursors also lack the extended N-terminus characteristic of SV2s. As described later in this review, this domain contains motifs that contribute to SV2’s role in trafficking other synaptic vesicle proteins. These additions to the MFS scaffold raise the possibility that this ancient class of proteins acquired new functions or even evolved into a functionally distinct class of proteins. Supporting this conjecture is the fact that the only known function of the transporter-like protein unc93b is trafficking Toll-like receptors to endosomes [30, 31]. To date, there is no functional information on the putative SV2 homologs in invertebrates, and thus bona fide SV2 is cautiously considered a protein exclusive to vertebrates.
Figure 2: Phylogenetic analysis of SV2 paralogs and SVOP.
Shown is a dendrogram illustrating the relatedness of SV2, SVOP and homologous proteins in invertebrates. To generate the dendrogram, Human SV2A, SV2B, SV2C and SVOP protein sequences were used in a series of NCBI BLASTP queries. The best BLASTP hit (lowest E-value) per paralog for each species was taken as a representative sequence. Protein sequences were aligned using the sequence alignment tool MUSCLE [136]. Sequence alignment was further refined using MAFFT under the Li-NSI option [137]. The phylogenetic tree was generated using the Maximum Likelihood method as implemented in ETE 3 [138]. Schematic drawings of paralogs were generated using Protter [135]. Protein sequences used for tree construction were: synaptic vesicle glycoprotein 2A [Homo sapiens] (NP_001315603); synaptic vesicle glycoprotein 2A [Mus musculus] (NP_071313); synaptic vesicle glycoprotein 2A [Rattus norvegicus] (NP_476558); synaptic vesicle glycoprotein 2A [Danio rerio] (XP_696434); synaptic vesicle glycoprotein 2A [Xenopus tropicalis] (XP_012825002); synaptic vesicle glycoprotein 2B [Homo sapiens] (NP_001309966); synaptic vesicle glycoprotein 2B [Mus musculus] (NP_001347503); synaptic vesicle glycoprotein 2B [Rattus norvegicus] (NP_476555); synaptic vesicle glycoprotein 2B [Danio rerio] (XP_005161575); synaptic vesicle glycoprotein 2B [Xenopus tropicalis] (XP_004912713); synaptic vesicle glycoprotein 2C [Homo sapiens] (NP_055794); synaptic vesicle glycoprotein 2C [Mus musculus] (NP_083486); synaptic vesicle glycoprotein 2C [Rattus norvegicus] (NP_113781); synaptic vesicle glycoprotein 2C [Danio rerio] (NP_001121811); synaptic vesicle glycoprotein 2C [Xenopus tropicalis] (XP_002934873); synaptic vesicle glycoprotein 2C isoform X1 [Strongylocentrotus purpuratus] (XP_003723431); synaptic vesicle glycoprotein 2C-like [Aplysia californica] (XP_012946265); synaptic vesicle 2-related protein isoform X2 [Strongylocentrotus purpuratus] (XP_003726207); synaptic vesicle 2-related protein isoform X3 [Xenopus tropicalis] (XP_002932106); synaptic vesicle 2-related protein isoform 1 [Homo sapiens] (NP_061181); synaptic vesicle 2-related protein isoform 1 [Mus musculus] (NP_081081); synaptic vesicle 2-related protein isoform 1 [Rattus norvegicus] (NP_599231); synaptic vesicle 2-related protein [Danio rerio] (XP_005165254); CG3168, isoform A [Drosophila melanogaster] (NP_572345); CG4324, isoform A [Drosophila melanogaster] (NP_611868); Dmel_CG31272 [Drosophila melanogaster] (NP_650013); svop-1 [Caenorhabditis elegans] (NP_498960.2).
While bona fide SV2 appears to be a relatively late development, divergence of the gene family occurred rapidly. There is a single SV2C-like protein in scallop (Lophotrochozoa, XP_021368892), whereas all three SV2 paralogs are present in cartilaginous fishes (Chondrichthyes) (Figure 2). The emergence of multiple paralogs likely provided differential regulation of SV2 expression or regulation, as has been proposed for the organic ion transporter family [32].
SV2 is essential for normal nervous system function.
The phenotypes associated with mutations in SV2 genes indicate that SV2 is required for normal synaptic function. Humans homozygous for a point mutation in the most widely expressed SV2 (SV2AR383Q) experience severe epilepsy and cognitive impairment [33]. Rats homozygous for a different point mutation in SV2A (SV2A-L174Q) demonstrate reduced neurotransmission in GABAergic neurons, and are more susceptible to seizures [34, 35]. Although mice homozygous for SV2A gene disruption (SV2A−/−) appear normal at birth and demonstrate no difference in brain morphology or synapse number, they fall behind in growth and begin experiencing severe seizures by nine days after birth. Nearly all die before three weeks of age. Partial loss of SV2A (SV2A+/−) leads to elevated seizure frequency [36, 37]. The correlation between seizures and aberrant SV2A function likely reflects the fact that SV2A is the only SV2 paralog in most GABAergic neurons. Consistent with this, spontaneous inhibitory neurotransmission is reduced in the hippocampus of SV2A−/− mice leading to increased excitatory activity [38]. In contrast, SV2B knockout mice are viable [37, 39] with only mild neurotransmission deficits in the retina [40], at synapses in which SV2B is the primary isoform [41]. In the same vein, loss of SV2C affects neurotransmission from dopaminergic neurons that express primarily SV2C [25]. Mice lacking both SV2A and SV2B are phenotypically similar to SV2A knockouts, consistent with SV2A being the most widely expressed paralog and the only SV2 supporting fast inhibitory neurotransmission.
Overexpression of SV2 also impacts synaptic function. Dissociated neurons engineered to express high levels of SV2A have neurotransmission deficits nearly identical to those seen in neurons lacking SV2 [28]. Likewise, overexpression of SV2A or SV2C in insulin secreting endocrine cells resulted in reduced glucose-induced secretion of insulin [42]. The requirement for optimal levels of SV2 suggests it acts as part of a multi-factor system in which multiple effectors converge (and compete) at a locus regulating vesicle fusion.
SV2 regulates the ability of calcium to induce vesicle fusion in quiescent synapses.
Neurons and endocrine cells in which SV2 is knocked out or down demonstrate reduced vesicle fusion in response to increased calcium [25, 36, 39, 40, 42–44]. The decrease is not due to effects on the basic machinery of vesicle fusion because loss of SV2 does not alter the frequency of spontaneous, action potential-independent transmitter release [36, 39, 43]. Likewise, the mechanics of calcium regulated vesicle fusion remain intact, as demonstrated by the normal kinetics and calcium dependence of regulated vesicle fusion that remains in SV2-deficient cells [39, 43, 44].
Analyses of SV2 knockout mice have eliminated other potential actions of SV2. For example, loss of SV2 does not reduce the number of synaptic or secretory vesicles, indicating that SV2 is not required for vesicle biogenesis nor is it an essential structural protein [36, 37, 39, 44]. Likewise, the number of vesicles “docked” at the active zone in presynaptic terminals is normal in neurons lacking SV2 [39, 44], ruling out a requirement for SV2 in vesicle recruitment to or retention at the membrane. Lastly, loss of SV2 does not alter the amplitude of spontaneous miniature neurotransmission events indicating that it is not needed for adequate filling of neurotransmitters into vesicles [36, 37, 39, 43]. Thus, SV2 is not a component of the basic machinery of membrane trafficking. Rather, it provides an essential regulatory action unique to calcium-regulated exocytosis.
Loss of SV2 results in fewer release-competent vesicles [39, 43]. This is reflected in a decrease in the “readily releasable pool” of vesicles [39, 44] and a decrease in the proportion of preassembled fusion protein (SNARE) complexes [44]. Thus, SV2 appears to facilitate the progression to a release-competent state after vesicle docking at the plasma membrane.
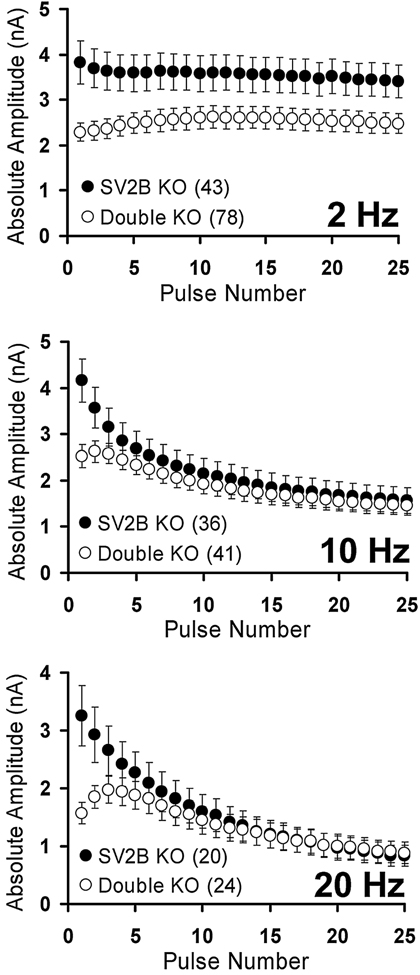
The neurotransmission deficits observed in the absence of SV2 have several intriguing features. Most striking is that reduced neurotransmission is observed only in quiescent or slowly firing synapses. At frequencies of 10 Hz or higher, synaptic responses in SV2-deficient neurons become equal to wild-type responses by the 10th response (Figure 3). Furthermore, the mutant phenotype can be transiently reversed by sustained high frequency stimulation whereas a lower frequency train does not [43]. Transient rescue does not occur if vesicle fusion is induced with hypertonic sucrose, which induces calcium-independent fusion of vesicles [39]. Thus, high concentrations of calcium relieve the requirement for SV2 action. Together these findings suggest that the molecular events that produce high frequency, sustained exocytosis, differ from those operating in quiescent or slowing firing synapses – and that SV2’s role in facilitating exocytosis is limited to the latter. Modulating the initiation of synaptic activity is especially important in neural networks where initial spikes provide the most salient information [45]. This makes SV2 action especially important in organisms with complicated nervous systems, consistent with its later emergence in evolution.
Figure 3: Loss of SV2 decreases synaptic transmission in quiescent and slowly firing synapses.
In neurons lacking both SV2A and SV2B (Double KO), responses to action-potential inducing stimuli are reduced only during slow stimulation or in the initial responses to higher frequency stimulation. During high frequency stimulation, responses are normal. Data are from dissociated autaptic hippocampal neurons cultured from SV2B (SV2B KO) and SV2A/B knockout (Double KO) mice. Note that responses from SV2B KO are identical to wild type and they are used in place of wild-type to allow comparison of neurons from littermate animals [39].
Is SV2 a multi-functional protein?
Initial hypotheses of SV2 function were based on its structure. Given its membership in MFS transporter superfamily, significant attention has been focused on identifying a transport substrate. Yet despite the clear structural similarities between SV2 and solute transporters, no transport activity has yet been identified. Early speculation that it acted as a general neurotransmitter transporter [3] was not supported by the subsequent identification of specific vesicular transporters for each type of neurotransmitter. The related conjecture that SV2 functions as an accessory transporter for specific transporters was belied by the normal quantal size of neurotransmission in neurons from SV2 KO mice [39]. More recently, SV2A was reported to function as a galactose transporter when expressed in yeast [46]. However, SV2 is not likely to transport galactose in neurons, as there is no measurable galactose uptake by purified synaptic vesicles (R. Edwards, personal communication). A possibility yet to be explored is that SV2 transports hydrated ions or sugars that have no functional role themselves, but rather influence vesicular contents by altering the effective concentration of vesicle contents and/or affecting vesicular fusion properties by impacting membrane turgidity. Either of these actions might explain the intriguing observation that, in solution, synaptic vesicles appear to swell when filling with glutamate and that swelling is absent in vesicles lacking the major SV2 paralog SV2A [47].
The other striking structural feature of SV2 is its large, highly glycosylated lumenal domain between TM 7 and 8. This region distinguishes SV2s from MFS transporter proteins. The three asparagines in the domain are extensively modified with keratan sulfate moieties [13]. This feature suggested that SV2 might provide a gel matrix to the inside of synaptic vesicles that would facilitate the concentration and release of transmitters [48]. However, the normal quantal size of neurotransmission in neurons lacking SV2 [36, 39, 43] indicates that it is not necessary for adequate vesicle filling.
In the absence of evidence demonstrating these functions suggested by SV2’s structure, studies turned to analyses of synaptic function in SV2 mutant mice and biochemical analyses of its molecular interactions. These suggest SV2 may have multiple functional roles in the presynaptic terminal.
SV2 regulates the stability and trafficking of the calcium sensor protein synaptotagmin.
SV2 is part of a protein complex that includes the synaptic vesicle protein synaptotagmin [49], the primary calcium sensor in regulated secretion [50]. Via two calcium and lipid binding motifs termed C2 domains, synaptotagmin engages with assembled fusion (SNARE) complexes [51], binds to lipids in the plasma membrane [52–55], interacts with the SNARE chaperone complexin [56], and engages clathrin adaptor proteins during endocytosis [57, 58]. Loss of synaptotagmin abolishes synchronized transmitter release in response to action potentials [59, 60].
The interaction of SV2 with synaptotagmin is regulated by calcium. All three SV2 proteins demonstrate calcium-inhibited binding to synaptotagmin [19, 41]. In addition to a binding site present in all three paralogs, SV2A and SV2C have a synaptotagmin-binding site in their cytoplasmic amino terminal domains, which are longer than the amino terminus of SV2B [19]. SV2 binding is mediated by basic residues in the second C2 (C2B) domain of synaptotagmin [49]. This “basic pocket” also mediates synaptotagmin’s interaction with phospholipids [61, 62], clathrin adaptors [63, 64], other synaptotagmins [65], and complexin [56], suggesting that these molecules either compete for binding to synaptotagmin or bind synaptotagmin sequentially as vesicles progress towards fusion with the plasma membrane and through endocytosis.
The SV2-synaptotagmin interaction regulates synaptotagmin expression levels and trafficking to synaptic vesicles. Brains from SV2A/B knockout mice have half the synaptotagmin as brains from wild type mice [66]. Similarly, loss of SV2B, which is the primary SV2 in retina, results in a significant loss of synaptotagmin in retinal synaptic layers [41]. While the total levels of synaptotagmin are reduced by ~50% of SV2A/B knockout mice, the amount in synaptic vesicles is reduced by ~85%, indicating that SV2 has an additional effect on the trafficking of synaptotagmin to synaptic vesicles. In contrast to its effects on synaptotagmin, SV2 does not affect the expression or trafficking of the vesicle proteins VAMP, VGLUT-1, or synaptophysin [66]. Thus, SV2 is not a general endocytosis factor, but rather one specific to synaptotagmin.
SV2A contains tyrosine-based endocytosis motifs (YXXØ) in its cytoplasmic domains that interact with the clathrin adaptor complex AP2 [66]. Peptides containing these sequences stimulate the interaction of AP2 with synaptotagmin [20]. The first motif in SV2A is located in the cytoplasmic N terminus and shared with SV2C while the second motif is at the end of the cytoplasmic loop before the 7th transmembrane domain (shared with SV2B). These motifs play a crucial role in SV2 trafficking and function. A mutation in the first motif (Y46A) rendered SV2A unable to support synaptic function or normal retrieval of synaptotagmin. A point mutation in the second endocytic motif, Y443A, disrupted SV2A trafficking to synapses [66].
The importance of SV2’s role in endocytosis is supported by the observation that it is regulated. The interaction of SV2 and synaptotagmin is regulated by phosphorylation. A conserved threonine in the amino terminus of all SV2s (Thr84 in SV2A) is a substrate for casein kinase 1. Phosphorylation of SV2A at this site increases binding to synaptotagmin [67], and is required for synaptotagmin retrieval [68]. An analogous threonine is conserved in all mammalian SV2 paralogs. Thus, SV2 contributes to the regulation of presynaptic function regulating synaptotagmin internalization.
The specificity of SV2’s effect on synaptotagmin trafficking is consistent with synaptic vesicle proteins being internalized as complexes via specialized adaptor proteins [69]. Synaptotagmin is an interesting case because it has two specialized adaptor proteins, SV2 and stonin2. Loss of either SV2 or stonin2 disrupts normal recycling of synaptotagmin [66, 70]. Loss of both SV2A/B and stonin2 has an additive effect. Furthermore, stonin2 levels increase in the absence of SV2 [71], suggesting their expression is coordinated to maintain synaptotagmin trafficking.
What emerges from analyses of neurotransmission in the context of gene disruption or mutation is that SV2 contributes to the priming of vesicles by regulating the protein interactions, and thus function of, the calcium sensor synaptotagmin.
Structure-function analyses reveal synaptotagmin-independent actions of SV2
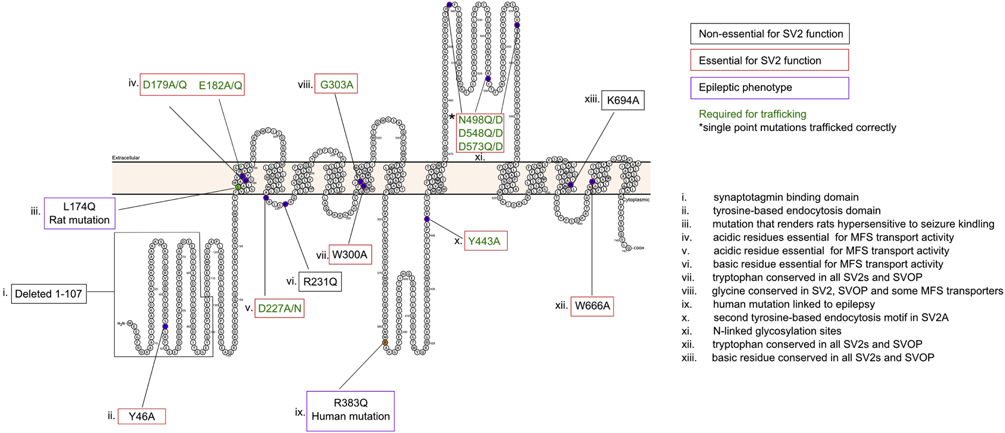
Acute expression of SV2 in neurons from SV2A/B knockout mice re-establishes normal evoked response amplitudes and short-term plasticity indicating SV2 plays an acute (as opposed to developmental) role in neurotransmission. Because acute expression is sufficient to rescue loss of SV2, mutational analysis could be used to test the role of putative functional domains in the action of SV2. To date, studies have focused on the signature motifs of MFS transport function, motifs implicated in endocytosis, and residues conserved between SV2 paralogs across species (Figure 4). These studies revealed that SV2 folding and trafficking is highly sensitive to mutation [17, 18, 43]. Of fourteen point mutations in SV2, seven disrupted trafficking to synaptic terminals. Multi-residue mutations in SV2 also disrupted trafficking. The exception was deletion of the first 107 amino acids of SV2A [43], a surprising finding given that this region contains an endocytosis motif [66] and a phosphorylation site [68] shown to be essential for SV2 function. This domain also contains an accessory synaptotagmin-binding site unique to SV2A and SV2C [19]. The ability of the N-terminal deletion mutant to support SV2 function suggests a hierarchy of function in which the more recently evolved longer amino termini of SV2A and SV2C predominate over the action of other functional domains.
Figure 4: Mutational analyses of SV2A.
Shown is a schematic of SV2A indicating residues that have been subjected to mutation. Each mutation is numbered and the feature of the corresponding residue is described in the key at the right. The effect of each mutation is indicated by the color of its outline. A black outline indicates mutants that were expressed and appropriately trafficked to synapses and restored normal neurotransmission, thus indicating that the residue is not essential to SV2 function. A red box indicates a mutation that trafficked to the synapse but did not restore normal neurotransmission, and thus is essential for SV2 actin at the synapse. A green box indicates a mutation that produced a protein that did not traffic to presynaptic terminals, indicating it is likely essential for proper SV2 folding. A purple box indicates a mutation linked to epilepsy. The illustration was generated using Protter [135].
With one exception, mutation of residues implicated in MFS transporter activity resulted in improper protein trafficking, consistent with their playing a role in protein folding. The exception was R231, a basic reside in the loop between the second and third transmembrane domains that is a component of the MFS signature [5]. SV2A-R231Q was trafficked to synapses when expressed in neurons from SV2A/B knockouts and restored normal synaptic depression [18], suggesting that the protein is functional. This may indicate that SV2 is not a transporter, or alternatively, that previous mutational studies of MFS transporter structure-function interpreted conflated effects on protein folding/trafficking with effects on function.
Mutations of conserved residues unique to SV2 proteins were less likely to produce proteins incapable of trafficking to synapses. SV2A-K694A was trafficked to synapses and fully functional [43], indicating that, although conserved, the residue is not essential for SV2 function. In contrast, mutation of tryptophans in the 5th or 10th transmembrane domains (SV2A-W300A and SV2A-W666A) impaired the ability of SV2A to rescue synaptic function [18]. Hydrophobic residues in these membrane domains are crucial to the action of the glucose transporter GLUT-1 [72] and to human organic anion transporter activity [73]. Interestingly, even though SV2A-W300A and SV2A-W666A did not restore normal neurotransmission, they did support expression and trafficking of synaptotagmin. Thus, it appears SV2 has a function that is independent of synaptotagmin, supporting the interpretation that it performs multiple functions at the presynaptic terminal.
SV2 affects calcium homeostasis in the presynaptic terminal
The fact that loss of SV2 specifically affects calcium-stimulated exocytosis raised questions about SV2’s role in regulating calcium at the presynaptic terminal. Indeed, several features of SV2-deficient synapses suggest that SV2 impacts cytoplasmic calcium. First was the observation that loading presynaptic terminals with the slow calcium buffer EGTA-AM restored short-term depression, an indicator of high synaptic release probability [37]. The effects were most pronounced at lower stimulus frequencies, consistent with EGTA-AM reversing calcium accumulation during stimulation [43]. This interpretation is supported by direct measures of cytoplasmic calcium in retinal bipolar neurons from SV2B knockout mice. Loss of SV2B, the primary SV2 paralog in these neurons, produced elevated resting calcium at the presynaptic terminal and greater calcium accumulation during stimulus trains [74]. As in hippocampal neurons lacking SV2A/B, this was associated with both decreased initial evoked release and greater cumulative vesicle exocytosis. In addition, these studies revealed an effect on membrane recovery following stimulation, which was slower in neurons lacking SV2B. Establishing higher cytoplasmic calcium in wild-type neurons by perfusion with calibrated calcium buffers produced the increased cumulative release and slowed endocytosis, but did not reproduce lower initial responses to stimulation observed in the mutant cells. This suggests that only a part of the SV2 mutant phenotype is due to increased cytoplasmic calcium.
Elevated presynaptic calcium in the absence of SV2 is consistent with SV2 transporting calcium into vesicles, as initially proposed by Janz et al [37]. Yet direct measures of calcium uptake by purified synaptic vesicles revealed no effect of loss of SV2 on calcium uptake ([42] and A. Schivell, R. Bartlett, S. Bajjalieh unpublished observations). Thus, the relationship between SV2 and calcium is not straightforward.
SV2 binds adenine nucleotides
Prior to the identification of the vesicular ATP transporter, VNUT (Slc17a9), [75, 76], the broadly released co-transmitter ATP was an obvious candidate SV2 transport substrate. ATP binds to and regulates several MFS transporters [77–79], suggesting that even if SV2 was not an ATP transporter, ATP might impact its possible transport function.
When assayed in vitro, all three SV2 paralogs bind nucleotides, with highest affinity for the adenine nucleotides ATP and NAD [80]. Mapping studies revealed two cytoplasm-facing regions in SV2A that are required for nucleotide binding; one that precedes the first transmembrane domain and one that precedes the seventh transmembrane domain. Interestingly, the mutation in human SV2A linked to epilepsy is in the second of these two regions [33]. However, SV2-dependent NAD/ATP binding was not associated with nucleotide transport. Therefore, nucleotide binding to SV2 is most likely to regulate its function rather than serve as the first step in ATP transport. This could be by regulating transport of a non-nucleotide substrate or promoting a conformation that favors or disfavors binding to synaptotagmin or other proteins. Alternatively, by binding adenine nucleotides SV2 may buffer NAD, a cofactor essential to several enzymatic steps in ATP production in both glycolysis and oxidative phosphorylation. The synaptic vesicle cycle is an energy-intensive process and thus regulation of ATP production could serve as a way to regulate synaptic function, especially during high frequency firing. Interestingly, a number of glycolytic enzymes are reported to associate with synaptic vesicles [81, 82]. Thus, SV2 may act as an NAD buffer, which could provide coordinated regulation between energy production and the synaptic vesicle cycle.
Changes in SV2 expression linked to disease
Changes in the expression of many synaptic proteins have been associated with disorders of the nervous system. The challenge is to determine which of these contribute to disease etiology or progression and which offer as targets for therapeutic intervention. At the cellular level, both loss and overexpression of SV2 produce synaptic dysfunction, suggesting that changes in SV2 expression or function might underlie disorders of the nervous system. Both directed studies and non-biased screens for associations between SV2 and diseases have produced a number of connections. As discussed below, altered SV2 expression has been linked to multiple neurodegenerative conditions including epilepsy, Alzheimer’s Disease, Parkinson’s Disease, Spinal Muscle Atrophy, and Huntington’s. Changes in SV2B expression are also implicated in protein trafficking important to kidney function. In each case, the SV2 paralog implicated appears to reflect the neuronal populations affected in the disease. Thus the association of different SV2 paralogs with different diseases reflects difference in expression rather than differences in paralog function. Further work is needed to determine whether functional differences also contribute to SV2’s role in each disease.
Epilepsy
The severe seizure phenotype associated with a point mutation in human SV2A [33] is evidence that it plays an important role in modulating nervous system excitability. Because SV2A is the primary SV2 paralog in the majority of GABAergic neurons, reductions in SV2A expression would be predicted to correlate with epilepsy and potentially contribute to its etiology. Indeed, decreased SV2 expression in the hippocampus was observed in immunolabeling and immunoblot analyses of post-mortem brain tissue from patients with temporal lobe epilepsy and sclerosis of the hippocampus. Inducing status epilepticus in rodents led to similar decreases before the onset of sclerosis, suggesting the decrease precedes the loss of synapses. As expression of the synaptic vesicle protein synaptophysin was unchanged, the effect appeared specific to SV2A [83].
Additional research suggests a that changes in SV2A expression in either direction can be associated with epilepsy. In contrast to epilepsy-linked decreases in SV2A, electrically- or chemically-induced kindled seizures led to increased SV2A expression in the hippocampus of rats [84–86]. In one study, increased SV2A was accompanied by increased assembly of vesicle fusion (SNARE) complexes and decreased levels of the SNARE dissociation enzyme N-ethlymaleimide-sensitive factor (NSF) [87], suggesting that kindling induces global changes in synaptic protein expression. In another study, kindling induced a selective increase in SV2A expression that was limited to the hilar region of the dentate gyrus of the hippocampus, a structure that contains inhibitory GABAergic interneurons that control excitability in the hippocampus. This was hypothesized to produce a protective increase in GABAergic neurotransmission [86]. Given that increased SV2A can produce synaptic deficits in isolated neurons [28], it is not clear if increased SV2A expression would increase in GABAergic neurotransmission or decrease it, i.e. whether increased SV2A would protect against hyperexcitability or contribute to it. At this time, the most parsimonious conclusion of the combined research is that precise control of SV2A expression is required to prevent aberrant excitability associated seizures.
Alzheimer’s disease
Accumulating evidence suggests that the cognitive decline associated with Alzheimer’s disease is presaged by subclinical hyperexcitability in the hippocampus [88]. Due to the link between SV2 and epilepsy, it becomes a candidate protein in the search for causes and treatment of the disorder. A hallmark of Alzheimer’s disease is aberrant processing of Amyloid Precursor Protein (APP) and overproduction of the peptide termed Aβ, which is hypothesized to be toxic to neurons. SV2A has been linked to APP processing, suggesting it may also play a role in the etiology of Alzheimer’s Disease by modulating the production of Aβ. APP processing is aided by the multi-functional adaptor/scaffold protein FE65 which acts both in the nucleus and cytoplasm. An unbiased screen for human brain proteins that bind to FE65 revealed an association with SV2A. Co-expression of SV2A with FE65 in fibroblasts resulted in less FE65 in the nucleus than in cells expressing only FE65 [89]. Thus FE65 binding to SV2A may contribute to localizing FE65 to synaptic terminals where it can coordinate the proteolytic processing of APP.
SV2B has also been linked to the effects of increased Aβ. Treatment of neuroblastoma cells with Aβ induced the expression of an SV2B mRNA with an extended 3’ untranslated region (UTR) rich in AUUUA sequences. These sequences are linked to inhibitory cis regulatory control of mRNA stability and translation efficiency [90], suggesting increased Aβ leads to reduced levels of SV2B. Supporting this conclusion are studies of protein expression in cell models of Alzheimer’s disease. Mutations in the APP processing enzyme Presenillin1 linked to increased production of Aβ are the basis of one form of familial-based AD. Neurons derived from human embryonic stem cells engineered to overexpress mutant Presenilin1 produce higher levels of Aβ and demonstrate reduced spontaneous neurotransmission. Analyses of presynaptic protein expression revealed a selective decrease of SV2B and the synaptic vesicle GTPase RAB3A. Application of the Presenillin-directed drugs, BMS-708163 and Nilotinib restored normal APP processing, normal neurotransmission rates, and normal expression levels SV2B and RAB3A [91]. While this finding suggests that decreased SV2B expression contributes to a cellular Alzheimer’s phenotype, another study found that mice homozygous for loss of SV2B were protected from cognitive impairment induced by injection of Aβ oligomers into the brain [92]. Taken together, these findings suggest that reduced SV2B expression may be a protective response to excess Aβ production.
Spinal muscular atrophy
Spinal muscular atrophy, a disease characterized by muscle weakness and paralysis, is caused by mutations in survival motor neuron protein 1 (SMN1) [93], a protein linked to mRNA splicing [94]. Using a SMNΔ7 mouse model of the disease to explore effects on synaptic protein expression, Tejero et al. found reduced levels of SV2B in neuromuscular synapses in affected mice. The expression of synaptotagmins 1 and 2 were also reduced. In contrast, the synaptic proteins syntaxin1B and synaptotagmin 7 were expressed at normal levels [95]. This finding suggests the expression of SV2 paralogs and their associated synaptotagmins are co-regulated and the disease process affects a function mediated by the concerted action of both proteins.
Parkinson’s Disease
Parkinson’s disease is caused by impaired neurotransmission from dopaminergic neurons in the substantia nigra to neurons of the striatum. Epidemiological data suggesting that smoking is protective against Parkinson’s were the basis for a genome-wide genetic interaction study to identify genes that underlie nicotine’s effect. The gene encoding an SV2C homolog emerged as a promising candidate [96]. SV2C is the primary SV2 paralog in dopaminergic neurons and is required for normal levels of dopamine neurotransmission. Homozygous disruption of the SV2C gene in mice results in reduced dopamine release in the striatum, motor deficits, and loss of nicotine’s effects on dopamine release. Examination of post mortem tissue from humans and mouse models of Parkinson’s revealed decreased SV2C expression in striatal structures associated with Parkinson’s disease [25]. Furthermore, SV2C interacts with synuclein, a protein implicated in the etiology of Parkinson’s [97]. The increasingly clear link between aberrant SV2C action and Parkinson’s suggests it will be a promising candidate target in the development of new therapies to treat the disorder or slow its progression.
Huntington’s Disease
Huntington’s disease results from an expansion of the poly-glutamine region in the multi-functional scaffolding protein huntingtin. The mutation results in loss of inhibitory neurons in the basal ganglia, a cluster of nuclei that includes the striatum. In addition to the aberrant motor control that initially defined the disease, Huntington’s is also characterized by cognitive deficits that precede motor symptoms and are hypothesized to involve the hippocampus in addition to the basal ganglia. Changes in the expression of multiple presynaptic proteins are correlated with Huntington’s. Analyses of mouse and cellular models of Huntington’s revealed a specific and progressive loss in SV2C mRNA and protein expression. The loss was particularly pronounced in the striatum, substantia nigra, dentate gyrus and CA3 region of the hippocampus [98]. The clear association of reduced SV2C in Huntington’s disease suggests it could be a reliable marker of disease progression and may even provide a therapeutic target in slowing the progression of symptoms.
SV2 as a therapeutic target
Targeting SV2A in the treatment of epilepsy - levetiracetam
Levetiracetam is the first member of a new class of anti-epileptic medication that now includes Brivaracetam, Seletracetam and Padsevonil [99–101]. Originally approved in the US as an adjunct therapy for partial and generalized epilepsy, the use of levetiracetam is being expanded to treat a broad range of nervous system disorders linked to nervous system hyperexcitability. Its novel pharmacology in standard animal models of epilepsy was consistent with a unique receptor, which was subsequently found to be SV2A [102, 103].
SV2A is both necessary and sufficient for levetiracetam binding [103], and multiple observations suggest that it is the site of levetiracetam action. First, SV2A hypomorphs (SV2A+/− mice) are less responsive to levetiracetam’s anti-seizure effects [104]. Importantly, children homozygous for a mutation in SV2A present with non-levetiracetam responsive epilepsy [33]. Furthermore, the effectiveness of levetiracetam-related compounds in controlling seizures is directly correlated to affinity for SV2A [105]. Finally, a residue crucial to SV2 function, W666 [18] is also necessary for levetiracetam binding [106]. Despite the strong link to SV2, levetiracetam has also been reported to inhibit calcium channel function both at the plasma membrane [107, 108] and at IP3 gated channels on internal membranes [109]. Given the link between SV2 and cytoplasmic calcium concentrations [110], these findings are intriguing. Currently, however, there is no known functional link between SV2 and ion channel trafficking or function. Thus, modulating the action of SV2A is the most obvious proximal mechanism of levetiracetam action.
When applied to hippocampal slice preparations, levetiracetam decreases both excitatory and inhibitory synaptic transmission. Further studies suggest that it decreases synaptic output by decreasing the readily releasable pool of synaptic vesicles [111] due to faster manifestation of supply rate depression [112] and increased short term depression [113].
A unique feature of levetiracetam action is a requirement for synaptic activity for the effect to manifest. This is attributed to the need for SV2A exposure at the plasma membrane to allow drug binding [111, 114, 115]. This interpretation is supported by the observation that triggering intense synaptic vesicle turnover after drug exposure disrupts, or “washes out” the effect of levetiracetam [113]. While vesicle-mediated entry is not consistent with the fact that levetiracetam is miscible in both aqueous and organic solvents and thus expected to easily diffuse across membranes [116], the ability to “unload” the drug may indicate that it acts via the lumenal face of SV2A. Yet, intra-vesicular action is not required if levetiracetam acts upstream of vesicle priming, affecting transmission only after primed vesicles are used. This interpretation is consistent with levetiracetam’s induction of synaptic depression, which relies on vesicle retrieval and re-priming. It is also consistent with SV2’s role in synaptotagmin trafficking to synaptic vesicles [66] and with the observation that it restores normal levels of synaptotagmin expression in neurons engineered to overexpress SV2A [28]. Thus the role of SV2 in modulating synaptotagmin expression and trafficking is a potential site of levetiracetam action.
Because SV2A is expressed ubiquitously, levetiracetam is thought to affect all neurotransmission. However, recent in vivo measurements of transmitter release revealed a levetiracetam-mediated selective increase in GABAergic transmission [117]. Furthermore, levetiracetam effects on inhibitory neurotransmission in hippocampal slice preparations require lower doses and manifest faster than its effects on excitatory neurotransmission [114]. A more potent effect on inhibitory transmission may result from the fact that most GABAergic neurons express only SV2A, and thus levetiracetam action is not diluted by the presence of SV2B or SV2C, which are not affected by levetiracetam. Determining whether SV2A-targeted drugs produce more pronounced effects on inhibitory vs excitatory neurotransmission in diseased neuronal circuits will provide crucial information in refining SV2-targeted therapies.
Levetiracetam blocks the development of seizures produced by electrical kindling. It also prevents the accompanying changes in gene expression that are thought to underlie the development epilepsy [118]. Kindling induces increased levels of SV2A, and with it, increased levels of preassembled vesicle fusion (SNARE) complexes. Both of these are prevented if levetiracetam is administered during kindling [84]. Levetiracetam has no effect on SV2 levels in non-kindled rats supporting the conclusion that levetiracetam effects manifest only under conditions of hyperexcitability or hypersynchronization. Nonetheless, in non-epileptic animals, chronic levetiracetam treatment led to down-regulation of several synaptic vesicle proteins in subsets of glutamatergic and GABAergic neurons, without affecting levels of SV2A or SV2B. In addition, chronic levetiracetam treatment led to increased levels of LRRK2, a kinase implicated in Parkinson’s disease known to regulate vesicular proteins trafficking and levels. Because there was no change in LRRK2 mRNA, these findings suggest that levetiracetam regulates protein levels both upstream and downstream of SV2A action by modulating protein interactions [119].
Targeting SV2A in the treatment of cognitive decline
The observation that cognitive deficits are often associated with aberrant neural activity is motivating testing of anti-epileptics as a potential prophylactic treatment [120]. In a recent small study of two human Alzheimer’s patients, asymptomatic seizures were observed in the hippocampus during sleep. Treatment with levetiracetam abated the seizures and slowed cognitive decline in one patient [88]. In mouse models of Alzheimer’s disease, levetiracetam reversed symptoms associated with the disorder. In a mouse line expressing a variant of Amyloid Precursor Protein that produces high levels of Aβ, acute and chronic levetiracetam treatment reduced brain hyperactivity but only chronic treatment improved performance on cognitive and memory tasks, suggesting levetiracetam remodels synaptic network activity to treat cognitive dysfunction [121]. Remarkably, the improvement was seen despite no reduction in the levels of Aβ. levetiracetam also reversed abnormal brain activity in mice expressing a human variant of the protein tau that is associated with Alzheimer’s [122]. In aged rats displaying mild cognitive deficits, low doses of levetiracetam improved performance in behavioral tests [123]. Together these findings suggest SV2 is a promising target in the treatment of brain disorders caused by abnormal network activity. Drugs currently approved for clinical use all target SV2A. Alternate compounds that target the other paralogs may prove useful in the treatment of other disorders. For example, compounds specific for SV2C could be effective in the treatment of Parkinson’s disease.
SV2 is a crucial component of the protein-lipid receptor for several botulinum toxins
Tetanus and botulinum (BoNT) neurotoxins comprise a group of bacterially secreted proteins that produce paralysis by blocking synaptic vesicle exocytosis. There are seven BoNT serotypes, denoted BoNT/A-G, that gain access to presynaptic terminals via a dual receptor consisting of the lumenal domain of a synaptic vesicle glycoprotein and membrane glycolipid [124]. BoNT/A is used to alleviate severe muscle spasms and also as a cosmetic treatment for facial lines.
SV2 proteins are the protein receptor for BoNT/A, E, D and tetanus toxins [125–127]. Although all SV2 paralogs support BoNT/A binding [126], the toxin has highest affinity for SV2C [128], the predominant paralog at neuromuscular junctions. The toxin binding site is in the large lumenal domain of SV2A, which is exposed to the extracellular space upon vesicle exocytosis. Because exposure of the receptor requires vesicle fusion, toxins are taken up in an activity dependent manner.
SV2 as a diagnostic tool
Tagging SV2A as a measure of synapse density
A hallmark prodromal feature of many neurodegenerative disorders is synapse loss. The fact that synapse loss could only be quantified post-mortem provided the impetus to develop a noninvasive technique to monitor synapse density in the early stages of a neurological disorder. As an obligate component of all synapses and the only synaptic vesicle protein for which a non-toxic drug exists, SV2 was a prime candidate for the development of positron emission tomography (PET) brain imaging tools. The first PET ligand developed for SV2A was 18F-UCB-H, and its specificity was determined by levetiracetam displacement [129]. With the goal of providing a better SV2A ligand, various 11C labeled compounds were developed that vary in their binding kinetics and affinity in the brain [130]. Of these, 11C-UCB-J [131, 132] and 11C-UCB-A [132, 133] have been verified as a valid measure of synapse density in living subjects, showing reduced binding in the hippocampus of Alzheimer’s patients.
SV2A is a marker for endocrine tumors
As treatments for different types of cancer become more specialized, determining the origins of different forms of metastatic cancer has become crucial to tailoring treatment. Because SV2 is expressed exclusively in neural and endocrine cells, it provides a precise marker for tumors of endocrine origin. The monoclonal antibody that originally defined SV2 [1] recognizes all three SV2 paralogs and labels all types of neuroendocrine tumors surveyed, including tumors from the gastrointestinal tract, pancreas, thyroid gland, parathyroid gland, and the adrenal gland [134]. The high specificity of the antibody and the uniqueness of SV2 expression to cells of neuroendocrine origin make anti-SV2 antibodies an optimal probe for identifying neuroendocrine tumors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Referenes
- [1].Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol 1985;100:1284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bajjalieh SM, Peterson K, Shinghal R, Scheller RH. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science 1992;257:1271–3. [DOI] [PubMed] [Google Scholar]
- [3].Feany MB, Lee S, Edwards RH, Buckley KM. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell 1992;70:861–7. [DOI] [PubMed] [Google Scholar]
- [4].Gingrich JA, Anderson PH, Tiberi M, Mestikawy S, Jorgensen PN, Fremeau RT, et al. Identification, characterization, and molecular cloning of a novel transporter-like protein localized to the central nervous system. FEBS 1992;312:115–22. [DOI] [PubMed] [Google Scholar]
- [5].Marger MD, Saier J, M. H. A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends in Biochemistry 1993;18:13–20. [DOI] [PubMed] [Google Scholar]
- [6].Reddy VS, Shlykov MA, Castillo R, Sun EI, Saier MH Jr., The major facilitator superfamily (MFS) revisited. The FEBS journal 2012;279:2022–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pao SS, Paulsen IT, Saier MH Jr., Major facilitator superfamily. Microbiol Mol Biol Rev 1998;62:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jacobsson JA, Haitina T, Lindblom J, Fredriksson R. Identification of six putative human transporters with structural similarity to the drug transporter SLC22 family. Genomics 2007;90:595–609. [DOI] [PubMed] [Google Scholar]
- [9].Pedersen BP, Kumar H, Waight AB, Risenmay AJ, Roe-Zurz Z, Chau BH, et al. Crystal structure of a eukaryotic phosphate transporter. Nature 2013;496:533–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Correa-Basurto J, Cuevas-Hernandez RI, Phillips-Farfan BV, Martinez-Archundia M, Romo-Mancillas A, Ramirez-Salinas GL, et al. Identification of the antiepileptic racetam binding site in the synaptic vesicle protein 2A by molecular dynamics and docking simulations. Frontiers in cellular neuroscience 2015;9:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang XC, Zhao Y, Heng J, Jiang D. Energy coupling mechanisms of MFS transporters. Protein science : a publication of the Protein Society 2015;24:1560–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lynch BA, Matagne A, Brannstrom A, von Euler A, Jansson M, Hauzenberger E, et al. Visualization of SV2A conformations in situ by the use of Protein Tomography. Biochem Biophys Res Commun 2008;375:491–5. [DOI] [PubMed] [Google Scholar]
- [13].Scranton TW, Iwata M, Carlson SS. The SV2 protein of synaptic vesicles is a keratan sulfate proteoglycan. J Neurochem 1993;61:29–44. [DOI] [PubMed] [Google Scholar]
- [14].Bajjalieh SM, Peterson K, Linial M, Scheller RH. Brain contains two forms of synaptic vesicle protein 2. Prodeedings of the National Academy of Sciences, USA 1993;90:2150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Janz R, Hofmann K, Sudhof TC. SVOP, an evolutionarily conserved synaptic vesicle protein, suggests novel transport functions of synaptic vesicles. J Neurosci 1998;15:9269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Janz R, Sudhof TC. SV2C is a synaptic vesicle protein with an unusually restricted localization: Anatomy of a synaptic vesicle protein family. Neurosci 1999;94:1279–90. [DOI] [PubMed] [Google Scholar]
- [17].Kwon SE, Chapman ER. Glycosylation is dispensable for sorting of synaptotagmin 1 but is critical for targeting of SV2 and synaptophysin to recycling synaptic vesicles. J Biol Chem 2012;287:35658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nowack A, Yao J, Custer KL, Bajjalieh SM. SV2 regulates neurotransmitter release via multiple mechanisms. Am J Physiol Cell Physiol 2010;299:C960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schivell AE, Mochida S, Kensel-Hammes P, Custer KL, Bajjalieh SM. SV2A and SV2C contain a unique synaptotagmin-binding site. Mol Cell Neurosci 2005;29:56–64. [DOI] [PubMed] [Google Scholar]
- [20].Haucke V, De Camilli P. AP-2 recruitment to synaptotamin stimulated by tyrosine-based endocytic motifs. Science 1999;285:1268–71. [DOI] [PubMed] [Google Scholar]
- [21].Bajjalieh SM, Franz G, Weimann JM, McConnell SK, Scheller RH. Differential expression of Synaptic Vesicle Protein 2 (SV2) isoforms. The Journal of Neuroscience 1994;14:5223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bragina L, Giovedi S, Barbaresi P, Benfenati F, Conti F. Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex: analysis of synaptogyrin, vesicle-associated membrane protein, and syntaxin. Neurosci 2010;165:934–43. [DOI] [PubMed] [Google Scholar]
- [23].Dardou D, Monlezun S, Foerch P, Courade JP, Cuvelier L, De Ryck M, et al. A role for Sv2c in basal ganglia functions. Brain Res 2013;1507:61–73. [DOI] [PubMed] [Google Scholar]
- [24].Gronborg M, Pavlos NJ, Brunk I, Chua JJ, Munster-Wandowski A, Riedel D, et al. Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein. J Neurosci 2010;30:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dunn AR, Stout KA, Ozawa M, Lohr KM, Hoffman CA, Bernstein AI, et al. Synaptic vesicle glycoprotein 2C (SV2C) modulates dopamine release and is disrupted in Parkinson disease. Proc Natl Acad Sci U S A 2017;114:E2253–E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Crevecoeur J, Foerch P, Doupagne M, Thielen C, Vandenplas C, Moonen G, et al. Expression of SV2 isoforms during rodent brain development. BMC neuroscience 2013;14:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mutch SA, Kensel-Hammes P, Gadd JC, Fujimoto BS, Allen RW, Schiro PG, et al. Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J Neurosci 2011;31:1461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nowack A, Malarkey EB, Yao J, Bleckert A, Hill J, Bajjalieh SM. Levetiracetam reverses synaptic deficits produced by overexpression of SV2A. PLoS One 2011;6:e29560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cohen JE, Lee PR, Chen S, Li W, Fields RD. MicroRNA regulation of homeostatic synaptic plasticity. Proc Natl Acad Sci U S A 2011;108:11650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim YM. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol 2007;177:265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 2008;452:234–8. [DOI] [PubMed] [Google Scholar]
- [32].Eraly SA, Hamilton BA, Nigam SK. Organic anion and cation transporters occur in pairs of similar and similarly expressed genes. Biochem Biophys Res Commun 2003;300:333–42. [DOI] [PubMed] [Google Scholar]
- [33].Serajee FJ, Huq AM. Homozygous Mutation in Synaptic Vesicle Glycoprotein 2A Gene Results in Intractable Epilepsy, Involuntary Movements, Microcephaly, and Developmental and Growth Retardation. Pediatric neurology 2015;52:642–6 e1. [DOI] [PubMed] [Google Scholar]
- [34].Tokudome K, Okumura T, Shimizu S, Mashimo T, Takizawa A, Serikawa T, et al. Synaptic vesicle glycoprotein 2A (SV2A) regulates kindling epileptogenesis via GABAergic neurotransmission. Scientific reports 2016;6:27420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tokudome K, Okumura T, Terada R, Shimizu S, Kunisawa N, Mashimo T, et al. A Missense Mutation of the Gene Encoding Synaptic Vesicle Glycoprotein 2A (SV2A) Confers Seizure Susceptibility by Disrupting Amygdalar Synaptic GABA Release. Frontiers in pharmacology 2016;7:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang H-Z, Peterson MR, et al. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proceedings of the National Academy of Science, USA 1999;96:115268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Janz R, Goda Y, Geppert M, Missler M, Sudhof TC. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron 1999;24:1003–16. [DOI] [PubMed] [Google Scholar]
- [38].Venkatesan K, Alix P, Marquet A, Doupagne M, Niespodziany I, Rogister B, et al. Altered balance between excitatory and inhibitory inputs onto CA1 pyramidal neurons from SV2A-deficient but not SV2B-deficient mice. Journal of Neuroscience Research 2012;90:2317–27. [DOI] [PubMed] [Google Scholar]
- [39].Custer KL, Austin NS, Sullivan JM, Bajjalieh SM. Synaptic vesicle protein 2 enhances release probability at quiescent synapses. J Neurosci 2006;26:1303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Morgans CW, Kensel-Hammes P, Hurley JB, Burton K, Idzerda R, McKnight GS, et al. Loss of the Synaptic Vesicle Protein SV2B results in reduced neurotransmission and altered synaptic vesicle protein expression in the retina. PLoS ONE 2009;4:e5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lazzell DR, Belizaire R, Thakur P, Sherry DM, Janz R. SV2B regulates synaptotagmin 1 by direct interaction. J Biol Chem 2004;279:52124–31. [DOI] [PubMed] [Google Scholar]
- [42].Iezzi M, Theander S, Janz R, Loze C, Wollheim CB. SV2A and SV2C are not vesicular Ca2+ transporters but control glucose-evoked granule recruitment. J Cell Sci 2005;118:5647–60. [DOI] [PubMed] [Google Scholar]
- [43].Chang WP, Sudhof TC. SV2 renders primed synaptic vesicles competent for Ca2+ -induced exocytosis. J Neurosci 2009;29:883–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xu T, Bajjalieh SM. SV2 modulates the size of the readily releasable pool of secretory vesicles. Nature Cell Bio 2001;3:691–8. [DOI] [PubMed] [Google Scholar]
- [45].Petersen RS, Diamond ME. Spatial-temporal distribution of whisker-evoked activity in rat somatosensory cortex and the coding of stimulus location. J Neurosci 2000;20:6135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Madeo M, Kovacs AD, Pearce DA. The human synaptic vesicle protein, SV2A, functions as a galactose transporter in Saccharomyces cerevisiae. J Biol Chem 2014;289:33066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Budzinski KL, Allen RW, Fujimoto BS, Kensel-Hammes P, Belnap DM, Bajjalieh SM, et al. Large structural change in isolated synaptic vesicles upon loading with neurotransmitter. Biophys J 2009;97:2577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Reigada D, Diez-Perez I, Gorostiza P, Verdaguer A, Gomez de Aranda I, Pineda O, et al. Control of neurotransmitter release by an internal gel matrix in synaptic vesicles. Proc Natl Acad Sci U S A 2003;100:3485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schivell AE, Batchelor RH, Bajjalieh SM. Isoform-specific, calcium-regulated interaction of the synaptic vesicle proteins SV2 and synaptotagmin. J Biol Chem 1996;271:27770–5. [DOI] [PubMed] [Google Scholar]
- [50].Sudhof TC. Calcium control of neurotransmitter release. Cold Spring Harbor perspectives in biology 2012;4:a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bhalla A, Chicka MC, Tucker WC, Chapman ER. Ca(2+)-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat Struct Mol Biol 2006;13:323–30. [DOI] [PubMed] [Google Scholar]
- [52].Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat Struct Mol Biol 2004;11:36–44. [DOI] [PubMed] [Google Scholar]
- [53].Bai J, Wang P, Chapman ER. C2A activates a cryptic Ca(2+)-triggered membrane penetration activity within the C2B domain of synaptotagmin I. Proc Natl Acad Sci U S A 2002;99:1665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schiavo G, Gu Q-M, Prestwich GD, Sollner TH, Rothman JE. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. PNAS, USA 1996;93:13327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Davletov BA, Sudhof TC. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J Biol Chem 1993;268:26386–90. [PubMed] [Google Scholar]
- [56].Zhou Q, Zhou P, Wang AL, Wu D, Zhao M, Sudhof TC, et al. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature 2017;548:420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Diril MK, Wienisch M, Jung N, Klingauf J, Haucke V. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev Cell 2006;10:233–44. [DOI] [PubMed] [Google Scholar]
- [58].Haucke V, Wenk MR, Chapman ER, Farsad K, De Camilli P. Dual interaction of synaptotagmin with mu2- and alpha-adaptin facilitates clathrin-coated pit nucleation. EMBO J 2000;19:6011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, et al. Synaptotagmin I: A major calcium sensor for transmitter release at a central synapse. Cell 1994;79:717–27. [DOI] [PubMed] [Google Scholar]
- [60].Nishiki T, Augustine GJ. Synaptotagmin I synchronizes transmitter release in mouse hippocampal neurons. J Neurosci 2004;24:6127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Perez-Lara A, Thapa A, Nyenhuis SB, Nyenhuis DA, Halder P, Tietzel M, et al. PtdInsP2 and PtdSer cooperate to trap synaptotagmin-1 to the plasma membrane in the presence of calcium. eLife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].van den Bogaart G, Meyenberg K, Diederichsen U, Jahn R. Phosphatidylinositol 4,5-bisphosphate increases Ca2+ affinity of synaptotagmin-1 by 40-fold. J Biol Chem 2012;287:16447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chapman ER, Desai RC, Davis AF, Tornehl CK. Delineation of the oligomerization, AP-2 binding, and synprint binding region of the C2B domain of synaptotagmin. J Biol Chem 1998;273:32966–72. [DOI] [PubMed] [Google Scholar]
- [64].Zhang JZ, Davletov BA, Sudhof TC, Anderson RGW. Synaptotagmin I is a high affinity receptor for clathrin AP-2: Implications for membrane recycling. Cell 1994;78:751–60. [DOI] [PubMed] [Google Scholar]
- [65].Chapman ER, An S, Edwardson JM, Jahn R. A novel function for the second C2 domain of synaptotagmin: Ca2+-triggered dimerization. J Biol Chem 1996;271:5844–9. [DOI] [PubMed] [Google Scholar]
- [66].Yao J, Nowack A, Kensel-Hammes P, Gardner RG, Bajjalieh SM. Cotrafficking of SV2 and synaptotagmin at the synapse. J Neurosci 2010;30:5569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pyle RA, Schivell AE, Hidaka H, Bajjalieh SM. Phosphorylation of synaptic vesicle protein 2 modulates binding to synaptotagmin. J Biol Chem 2000;275:17195–200. [DOI] [PubMed] [Google Scholar]
- [68].Zhang N, Gordon SL, Fritsch MJ, Esoof N, Campbell DG, Gourlay R, et al. Phosphorylation of synaptic vesicle protein 2A at Thr84 by casein kinase 1 family kinases controls the specific retrieval of synaptotagmin-1. The Journal of neuroscience : the official journal of the Society for Neuroscience 2015;35:2492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gordon SL, Cousin MA. The iTRAPs: Guardians of Synaptic Vesicle Cargo Retrieval During Endocytosis. Frontiers in synaptic neuroscience 2016;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kononenko NL, Diril MK, Puchkov D, Kintscher M, Koo SJ, Pfuhl G, et al. Compromised fidelity of endocytic synaptic vesicle protein sorting in the absence of stonin 2. Proc Natl Acad Sci U S A 2013;110:E526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kaempf N, Kochlamazashvili G, Puchkov D, Maritzen T, Bajjalieh SM, Kononenko NL, et al. Overlapping functions of stonin 2 and SV2 in sorting of the calcium sensor synaptotagmin 1 to synaptic vesicles. Proc Natl Acad Sci U S A 2015;112:7297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kasahara T, Kasahara M. Tryptophan 388 in putative transmembrane segment 10 of the rat glucose transporter Glut1 is essential for glucose transport. J Biol Chem 1998;273:29113–7. [DOI] [PubMed] [Google Scholar]
- [73].Perry JL, Dembla-Rajpal N, Hall LA, Pritchard JB. A three-dimensional model of human organic anion transporter 1: aromatic amino acids required for substrate transport. J Biol Chem 2006;281:38071–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wan QF, Zhou ZY, Thakur P, Vila A, Sherry DM, Janz R, et al. SV2 acts via presynaptic calcium to regulate neurotransmitter release. Neuron;66:884–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Estevez-Herrera J, Dominguez N, Pardo MR, Gonzalez-Santana A, Westhead EW, Borges R, et al. ATP: The crucial component of secretory vesicles. Proc Natl Acad Sci U S A 2016;113:E4098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A 2008;105:5683–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Carruthers A, Helgerson AL. The human erythrocyte sugar transporter is also a nucleotide binding protein. Biochemistry 1989;28:8337–46. [DOI] [PubMed] [Google Scholar]
- [78].Levine KB, Cloherty EK, Fidyk NJ, Carruthers A. Structural and physiologic determinants of human erythrocyte sugar transport regulation by adenosine triphosphate. Biochemistry 1998;37:12221–32. [DOI] [PubMed] [Google Scholar]
- [79].Levine KB, Cloherty EK, Hamill S, Carruthers A. Molecular determinants of sugar transport regulation by ATP. Biochemistry 2002;41:12629–38. [DOI] [PubMed] [Google Scholar]
- [80].Yao J, Bajjalieh SM. Synaptic vesicle protein 2 binds adenine nucleotides. J Biol Chem 2008;283:20628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Burre J, Beckhaus T, Corvey C, Karas M, Zimmermann H, Volknandt W. Synaptic vesicle proteins under conditions of rest and activation: analysis by 2-D difference gel electrophoresis. Electrophoresis 2006;27:3488–96. [DOI] [PubMed] [Google Scholar]
- [82].Morciano M, Burre J, Corvey C, Karas M, Zimmermann H, Volknandt W. Immunoisolation of two synaptic vesicle pools from synaptosomes: a proteomics analysis. J Neurochem 2005;95:1732–45. [DOI] [PubMed] [Google Scholar]
- [83].van Vliet EA, Aronica E, Redeker S, Boer K, Gorter JA. Decreased expression of synaptic vesicle protein 2A, the binding site for levetiracetam, during epileptogenesis and chronic epilepsy. Epilepsia 2008;50:422–33. [DOI] [PubMed] [Google Scholar]
- [84].Matveeva EA, Vanaman TC, Whiteheart SW, Slevin JT. Asymmetric accumulation of hippocampal 7S SNARE complexes occurs regardless of kindling paradigm. Epilepsy Res 2007;73:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Matveeva EA, Vanaman TC, Whiteheart SW, Slevin JT. Levetiracetam prevents kindling-induced asymmetric accumulation of hippocampal 7S SNARE complexes. Epilepsia 2008;49:1749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ohno Y, Ishihara S, Terada R, Kikuta M, Sofue N, Kawai Y, et al. Preferential increase in the hippocampal synaptic vesicle protein 2A (SV2A) by pentylenetetrazole kindling. Biochem Biophys Res Commun 2009;390:415–20. [DOI] [PubMed] [Google Scholar]
- [87].Matveeva EA, Whiteheart SW, Slevin JT. Accumulation of 7S SNARE complexes in hippocampal synaptosomes from chronically kindled rats. J Neurochem 2003;84:621–4. [DOI] [PubMed] [Google Scholar]
- [88].Lam AD, Deck G, Goldman A, Eskandar EN, Noebels J, Cole AJ. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nature medicine 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Nensa FM, Neumann MH, Schrotter A, Przyborski A, Mastalski T, Susdalzew S, et al. Amyloid beta a4 precursor protein-binding family B member 1 (FE65) interactomics revealed synaptic vesicle glycoprotein 2A (SV2A) and sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2) as new binding proteins in the human brain. Molecular & cellular proteomics : MCP 2014;13:475–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Heese K, Nagai Y, Sawada T. Identification of a new synaptic vesicle protein 2B mRNA transcript which is up-regulated in neurons by amyloid beta peptide fragment (1–42). Biochem Biophys Res Commun 2001;289:924–8. [DOI] [PubMed] [Google Scholar]
- [91].Nishioka H, Tooi N, Isobe T, Nakatsuji N, Aiba K. BMS-708163 and Nilotinib restore synaptic dysfunction in human embryonic stem cell-derived Alzheimer’s disease models. Scientific reports 2016;6:33427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Detrait E, Maurice T, Hanon E, Leclercq K, Lamberty Y. Lack of synaptic vesicle protein SV2B protects against amyloid-beta(2)(5)(−)(3)(5)-induced oxidative stress, cholinergic deficit and cognitive impairment in mice. Behavioural brain research 2014;271:277–85. [DOI] [PubMed] [Google Scholar]
- [93].Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995;80:155–65. [DOI] [PubMed] [Google Scholar]
- [94].Fischer U, Liu Q, Dreyfuss G. The SMN-SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell 1997;90:1023–9. [DOI] [PubMed] [Google Scholar]
- [95].Tejero R, Lopez-Manzaneda M, Arumugam S, Tabares L. Synaptotagmin-2, and −1, linked to neurotransmission impairment and vulnerability in Spinal Muscular Atrophy. Human molecular genetics 2016;25:4703–16. [DOI] [PubMed] [Google Scholar]
- [96].Hill-Burns EM, Singh N, Ganguly P, Hamza TH, Montimurro J, Kay DM, et al. A genetic basis for the variable effect of smoking/nicotine on Parkinson’s disease. The pharmacogenomics journal 2013;13:530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Surmeier DJ. Determinants of dopaminergic neuron loss in Parkinson’s disease. The FEBS journal 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Peng C, Zhu G, Liu X, Li H. Mutant Huntingtin Causes a Selective Decrease in the Expression of Synaptic Vesicle Protein 2C. Neuroscience bulletin 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Klitgaard H, Matagne A, Nicolas JM, Gillard M, Lamberty Y, De Ryck M, et al. Brivaracetam: Rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment. Epilepsia 2016;57:538–48. [DOI] [PubMed] [Google Scholar]
- [100].Loscher W, Gillard M, Sands ZA, Kaminski RM, Klitgaard H. Synaptic Vesicle Glycoprotein 2A Ligands in the Treatment of Epilepsy and Beyond. CNS drugs 2016;30:1055–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Leclercq K, Matagne A, Klitgaard H, Kaminski RM. Protective effects of padsevonil in acute seizure models. American Epilepsy Society Annual Meeting Abstracts 2017;http://www.aesnet.org 1272. [Google Scholar]
- [102].Kaminski RM, Matagne A, Leclercq K, Gillard M, Michel P, Kenda B, et al. SV2A protein is a broad-spectrum anticonvulsant target: functional correlation between protein binding and seizure protection in models of both partial and generalized epilepsy. Neuropharmacology 2008;54:715–20. [DOI] [PubMed] [Google Scholar]
- [103].Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci U S A 2004;101:9861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kaminski RM, Gillard M, Leclercq K, Hanon E, Lorent G, Dassesse D, et al. Proepileptic phenotype of SV2A-deficient mice is associated with reduced anticonvulsant efficacy of levetiracetam. Epilepsia 2009;50:1729–40. [DOI] [PubMed] [Google Scholar]
- [105].Kaminski RM, Gillard M, Klitgaard H. Targeting SV2A for Discovery of Antiepileptic Drugs In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4th ed. Bethesda (MD)2012. [PubMed] [Google Scholar]
- [106].Shi J, Anderson D, Lynch BA, Castaigne JG, Foerch P, Lebon F. Combining modelling and mutagenesis studies of synaptic vesicle protein 2A to identify a series of residues involved in racetam binding. Biochemical Society transactions 2011;39:1341–7. [DOI] [PubMed] [Google Scholar]
- [107].Niespodziany I, Klitgaard H, Margineanu DG. Levetiracetam inhibits the high-voltage-activated Ca(2+) current in pyramidal neurones of rat hippocampal slices. Neurosci Lett 2001;306:5–8. [DOI] [PubMed] [Google Scholar]
- [108].Vogl C, Mochida S, Wolff C, Whalley BJ, Stephens GJ. The synaptic vesicle glycoprotein 2A ligand levetiracetam inhibits presynaptic Ca2+ channels through an intracellular pathway. Molecular pharmacology 2012;82:199–208. [DOI] [PubMed] [Google Scholar]
- [109].Cataldi M, Lariccia V, Secondo A, di Renzo G, Annunziato L. The antiepileptic drug levetiracetam decreases the inositol 1,4,5-trisphosphate-dependent [Ca2+]I increase induced by ATP and bradykinin in PC12 cells. J Pharmacol Exp Ther 2005;313:720–30. [DOI] [PubMed] [Google Scholar]
- [110].Wan Q-F, Zhou Z-Y, Thakur P, Vila A, Sherry DM, Janz R, et al. SV2 acts via presynaptic calcium to regulate neurotransmitter release. Neuron 2010;66:884–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Meehan AL, Yang X, McAdams BD, Yuan L, Rothman SM. A New Mechanism for Antiepileptic Drug Action: Vesicular Entry May Mediate the Effects of Levetiracetam. J Neurophysiol 2011;106:1227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Garcia-Perez E, Mahfooz K, Covita J, Zandueta A, Wesseling JF. Levetiracetam accelerates the onset of supply rate depression in synaptic vesicle trafficking. Epilepsia 2015;56:535–45. [DOI] [PubMed] [Google Scholar]
- [113].Yang X, Bognar J Jr., He T, Mohammed M, Niespodziany I, Wolff C, et al. Brivaracetam augments short-term depression and slows vesicle recycling. Epilepsia 2015;56:1899–909. [DOI] [PubMed] [Google Scholar]
- [114].Meehan AL, Yang X, Yuan LL, Rothman SM. Levetiracetam has an activity-dependent effect on inhibitory transmission. Epilepsia 2012;53:469–76. [DOI] [PubMed] [Google Scholar]
- [115].Yang XF, Rothman SM. Levetiracetam has a time- and stimulation-dependent effect on synaptic transmission. Seizure 2009;18:615–9. [DOI] [PubMed] [Google Scholar]
- [116].Physician’s Desk Reference. 61st ed: Thomson; 2007. [Google Scholar]
- [117].Luz Adriana PM, Blanca Alcira RM, Itzel Jatziri CG, Sergio Roberto ZH, Juan Luis CP, Karla Berenice SH, et al. Effect of levetiracetam on extracellular amino acid levels in the dorsal hippocampus of rats with temporal lobe epilepsy. Epilepsy Res 2018;140:111–9. [DOI] [PubMed] [Google Scholar]
- [118].Gu J, Lynch BA, Anderson D, Klitgaard H, Lu S, Elashoff M, et al. The antiepileptic drug levetiracetam selectively modifies kindling-induced alterations in gene expression in the temporal lobe of rats. Eur J Neurosci 2004;19:334–45. [DOI] [PubMed] [Google Scholar]
- [119].Marcotulli D, Fattorini G, Bragina L, Perugini J, Conti F. Levetiracetam Affects Differentially Presynaptic Proteins in Rat Cerebral Cortex. Frontiers in cellular neuroscience 2017;11:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 2012;74:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci U S A 2012;109:E2895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Das M, Maeda S, Hu B, Yu GQ, Guo W, Lopez I, et al. Neuronal levels and sequence of tau modulate the power of brain rhythms. Neurobiol Dis 2018;117:181–8. [DOI] [PubMed] [Google Scholar]
- [123].Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2010;35:1016–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Pellizzari R, Rossetto O, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philosophical transactions of the Royal Society of London Series B, Biological sciences 1999;354:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Yeh FL, Dong M, Yao J, Tepp WH, Lin G, Johnson EA, et al. SV2 mediates entry of tetanus neurotoxin into central neurons. PLoS pathogens 2010;6:e1001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science 2006;312:592–6. [DOI] [PubMed] [Google Scholar]
- [127].Dong M, Liu H, Tepp WH, Johnson EA, Janz R, Chapman ER. Glycosylated SV2A and SV2B mediate the entry of botulinum neurotoxin E into neurons. Mol Biol Cell 2008;19:5226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Mahrhold S, Rummel A, Bigalke H, Davletov B, Binz T. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett 2006;580:2011–4. [DOI] [PubMed] [Google Scholar]
- [129].Warnock GI, Aerts J, Bahri MA, Bretin F, Lemaire C, Giacomelli F, et al. Evaluation of 18F-UCB-H as a novel PET tracer for synaptic vesicle protein 2A in the brain. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2014;55:1336–41. [DOI] [PubMed] [Google Scholar]
- [130].Cai H, Mangner TJ, Muzik O, Wang MW, Chugani DC, Chugani HT. Radiosynthesis of (11)C-Levetiracetam: A Potential Marker for PET Imaging of SV2A Expression. ACS medicinal chemistry letters 2014;5:1152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin SF, Chen MK, et al. Imaging synaptic density in the living human brain. Science translational medicine 2016;8:348ra96. [DOI] [PubMed] [Google Scholar]
- [132].Chen MK, Mecca AP, Naganawa M, Finnema SJ, Toyonaga T, Lin SF, et al. Assessing Synaptic Density in Alzheimer Disease With Synaptic Vesicle Glycoprotein 2A Positron Emission Tomographic Imaging. JAMA neurology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Estrada S, Lubberink M, Thibblin A, Sprycha M, Buchanan T, Mestdagh N, et al. [(11)C]UCB-A, a novel PET tracer for synaptic vesicle protein 2A. Nuclear medicine and biology 2016;43:325–32. [DOI] [PubMed] [Google Scholar]
- [134].Portela-Gomes GM, Lukinius A, Grimelius L. Synaptic vesicle protein 2, A new neuroendocrine cell marker. The American journal of pathology 2000;157:1299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Omasits U, Ahrens CH, Muller S, Wollscheid B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 2014;30:884–6. [DOI] [PubMed] [Google Scholar]
- [136].Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic acids research 2004;32:1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 2013;30:772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Huerta-Cepas J, Serra F, Bork P. ETE 3: Reconstruction, Analysis, and Visualization of Phylogenomic Data. Mol Biol Evol 2016;33:1635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]