Abstract
Background/Purpose:
Better disease activity and quality of life have been observed among patients with rheumatoid arthritis (RA) who drink alcohol. This association might be explained by reverse causality. We identified predictors of changes in alcohol use and evaluated independent associations between alcohol use and RA activity and mortality.
Methods
Participants in Forward, The National Databank for Rheumatic Diseases, were asked about alcohol use (any v. none), and disease activity was collected through the Patient Activity Scale-II (PAS-II) on semi-annual surveys. We identified factors associated with changes in alcohol use and determined associations between alcohol use and disease activity and mortality using linear and logistic regression models, Cox proportional hazards models, and marginal structural models.
Results
A total of 121,280 observations were studied among 16,762 unique participants. Discontinuation and initiation of alcohol were common among drinkers and abstainers (8.2% and 8.4% of observations, respectively). Greater discontinuation and less initiation were observed with greater disease activity, older age, female sex, non-white race, obesity, greater comorbidity, low quality of life, low educational level, low income, and work disability. While alcohol users had lower PAS-II [β: -0.15 (-0.18,-0.11) p<0.001], and a lower mortality [OR 0.87 (0.76,0.98) p=0.03] in traditional models, associations were not seen in marginal structural models.
Conclusions:
Higher disease activity, disability, comorbidity, and poor quality of life contribute to reductions in alcohol use. Active use and changes in use were not associated with disease activity or mortality when adjusting for confounding, suggesting no clear benefit of alcohol consumption in RA.
Keywords: alcohol, rheumatoid arthritis, disease activity
There is interest in identifying dietary and behavioral exposures that may contribute to the activity and severity of rheumatoid arthritis (RA). For example, several previous studies have demonstrated that moderate use of alcohol is associated with lower disease activity, superior quality of life and better functional status (1–7). Furthermore, in the general population, moderate alcohol consumption has been associated with reduced cardiovascular mortality (8, 9). Thus, providers may be tempted to encourage moderate alcohol consumption among patients with RA.
While alcohol use has been associated with lower RA disease activity and superior function in RA in cross-sectional studies, studies evaluating other, more long-term outcomes have been inconsistent. Some studies have found a reduced risk of radiographic damage and its progression among alcohol users, while others have found the opposite (5–7). A recent study also found that alcohol users were less likely to reach clinical remission (10).
A challenge for epidemiologic studies in this area is that alcohol use is not static over the lifetime, and its use has been associated with changes in health status and quality of life in other populations (11, 12). Poor and worsening health status, RA disease activity and severity, comorbidity, and quality of life may all result in reductions in use of alcohol and result in bias in epidemiologic studies due to reverse causality, though this has never been studied (13). In RA, the use of potentially hepatotoxic agents at higher doses may further influence these behaviors. If severely affected individuals are more likely to discontinue alcohol use over time, studies that consider alcohol use at a fixed point in time, perhaps at enrollment in a disease registry, are likely identify a protective association of alcohol use when one, in fact, does not exist.
We evaluated the longitudinal associations between patient-reported disease activity, disability, comorbidity, and quality of life on initiation and discontinuation of alcohol use over time in large registry of patients with RA and determined if changes in alcohol use were independently associated with subsequent changes in disease activity and mortality. Finally, we aimed to assess the risk of adverse outcomes from current alcohol use using statistical methods that more effectively account for time-varying confounding.
Methods
Study Setting
Patients were active participants in Forward, The National Databank for Rheumatic Diseases, between 1999 and 2016. Forward is a patient-based multi-disease, multi-purpose rheumatic disease registry and cohort study with patients enrolled from community-based rheumatology practices across the U.S. and followed-up with semi-annual detailed questionnaires (14). Key patient data is validated regularly using medical records. The registry has been described in detail elsewhere (14, 15). The study is approved by the Via Christi Hospitals Wichita, Inc. Institutional Review Board (IRB00001674). All participants provide signed informed consent.
Assessment of Alcohol Use in Follow-up
Participants in the registry are regularly asked about alcohol use. Between 2002 and 2007 patients were asked “Do you regularly drink alcoholic beverages?”. This question was modified and participants between 2007 and 2017 were asked “How often do you drink alcohol?”. Those that endorsed use of alcohol are subsequently asked to provide the average number of drinks per day. Participants were considered to have discontinued alcohol use if they answered “never” to either question but reported any amount of use on the prior survey. Participants were considered to have initiated use if they reported any consumption but reported no prior use on the prior survey.
We performed additional sensitivity analyses limiting our definition to moderate use only (observations with greater than moderate use were excluded). Moderate alcohol use was defined as ≤1 drink per day for women and ≤2 drinks per day for men among those who reported use (16).
Disease Activity Assessments
Disease activity was assessed using the PAS-II, a self-reported assessment of function, pain, and overall health, collected on each questionnaire (17, 18). Low, moderate, and high disease activity were defined as previously described (17). A clinically important change for PAS-II has not been defined. We defined an important change in disease activity as a change of greater than 1 unit (0.5 × standard deviation) (19). This is comparable to the 3.6/30 unit minimal clinically important change defined for the Routine Assessment of Patient Index Data (20).
Other Assessments
Comorbidity burden was calculated using the Rheumatic Disease Comorbidity Index (RDCI), a validated quantitative measure of comorbid illness (21). Patient assessment of mental and physical quality of life was derived from the SF-36’s physical and mental component summary scores (22). Other potential confounding factors were assessed including demographics, smoking, work disability, RA disease duration, depression, heart disorders, lung diseases, psychiatric disease, gastrointestinal disorders (liver disease, ulcer), educational level (16 or more years), household income, marital status, and calendar date of the observation. Vital status was determined from the National Death Index and alternative family member contact.
Statistical Analysis
Predictors of Changes in Alcohol Use
In this analysis, the outcomes of interest were the report of cessation of drinking at the subsequent survey among observations where active drinking was reported. We also evaluated the report of initiation of drinking among abstainers. Multiple observations over time in a single participant were permitted to be included in these analyses and exposure was permitted to vary over time. We assessed associations between disease activity, health status, and subsequent changes in alcohol use by the time of the next survey. Population-averaged logistic regression models incorporating generalized estimating equations (GEE) with robust estimators were utilized to assess factors associated with the probability of any alcohol use at the time of the next questionnaire among observations where drinking was reported. Similar analyses were performed among abstainers. Partially-adjusted models included demographics, BMI, PAS-II scores, and RA therapies (methotrexate, prednisone, and biologic use). Fully-adjusted models further considered variables such as smoking, disease duration, comorbidity, quality of life, educational level, income, health satisfaction, marital status, and disability. Backward selection of variables with p<0.2 was performed on fully-adjusted models to generate final reduced models shown in data tables.
Associations Between Changes in Alcohol Use and Disease Activity and Mortality
Multivariable logistic regression models incorporating GEE were used to determine if reporting of discontinuation or initiation of alcohol since the prior survey was associated with a significant worsening or improvement in disease activity at time of the subsequent survey. A schema for the study design is shown in Supplementary Figure 1. Extended Cox proportional hazards models were also used to assess associations between discontinuation of alcohol use among those reporting use on the prior survey and subsequent mortality (separate analyses evaluated the risk of initiation of alcohol use among abstainers). These analyses were adjusted for factors that were identified in the aforementioned selection process to be associated with alcohol discontinuation or initiation.
Associations of Active Drinking with Disease Activity and Mortality
Exposures often vary over time in observational studies and this variation may be related to important changes in health. Standard approaches for adjustment of confounding may be biased when time-dependent confounders exist that are affected by a previous exposure. Marginal structural models offer an approach that can allow for improved adjustment for this type of confounding. We compared a marginal structural model approach to a more traditional multivariable modeling approach using GEE.
We used marginal structural models to evaluate associations between active drinking and PAS-II over time as well as the risk of mortality. These models use stabilized inverse probability weighting to balance the probability of being exposed to alcohol across drinkers and abstainers. Variables included in the models for propensity scores for drinking status and later censoring included all variables noted above including current values, values from the prior visit, values from the first non-missing observation as described previously (23). Time in follow-up was also adjusted for using cubic splines. For analyses evaluating relationships with disease activity, we excluded PAS-II, physical, and mental quality of life from models used to generate propensity scores as these variables capture similar constructs as the disease activity outcome. A complete list of variables included in propensity scores is provided in Supplementary Tables 1 and 2. Standardized differences were visualized prior to and after application of the inverse probability weights (Supplementary Figure 2).
In all analyses, registry observations that were missing data for alcohol use (primarily visits occurring prior to initiation of the alcohol use questions introduced in 2002; 34%) were not eligible for inclusion in these analyses. Analyses were performed using Stata 14.2 software (StataCorp, LP, College Station, TX).
Results
In 121,280 observations among 16,762 unique patients, at least some alcohol use was reported at 63,524 observations (53%). Among these, 9,327 reported more than moderate use (15%). The characteristics of participants that never used alcohol, never abstained from alcohol, or sometimes used alcohol in follow-up are shown in Table 1.
Table 1:
Baseline characteristics (first non-missing data) of participants who never reported alcohol use through follow-up, never reported abstaining from alcohol, and those who sometimes reported drinking alcohol.
| Never Drank Alcohol (N=6706) |
Never Abstained from Alcohol (N=6703) |
Sometimes Drank Alcohol (N=3,353) |
p | |
|---|---|---|---|---|
| Age, yrs | 60.2 (13.1) | 56.5 (13.5) | 57.6 (13.3) | <0.001 |
| Male, N (%) | 1,141 (17%) | 1,544 (23%) | 615 (18%) | <0.001 |
| White, N (%) | 6,252 (93%) | 6,398 (95%) | 3,092 (92%) | <0.001 |
| BMI, kg/m2 | 29.2 (7.4) | 27.8 (6.2) | 28.9 (7.1) | <0.001 |
| Disease Duration, yrs | 11.8 (5.5, 22.3) | 10.6 (4.8, 20.3) | 10.3 (5.1, 20.1) | <0.001 |
| Smoking, N (%) | 360 (5%) | 375 (6%) | 227 (7%) | 0.01 |
| Patient Activity Scale-II | 4.1 (2.2) | 3.14 (2.0) | 3.50 (2.1) | <0.001 |
| Methotrexate, N (%) | 3,415 (53%) | 3,083 (49%) | 1,498 (46%) | <0.001 |
| Prednisone, N (%) | 2,210 (34%) | 1,855 (29%) | 1,051 (32%) | <0.001 |
| Biologic Use, N (%) | 2,097 (33%) | 2,373 (37%) | 1,107 (34%) | <0.001 |
| Mental QOL | 47.4 (12.3) | 49.0 (11.3) | 48.9 (11.5) | <0.001 |
| Physical QOL | 33.9 (10.7) | 39.3 (10.8) | 36.7 (10.7) | <0.001 |
| Work Disabled, N (%) | 1,335 (20%) | 649 (10%) | 478 (14%) | <0.001 |
| Education >16 yrs, N (%) | 1,732 (26%) | 2,742 (41%) | 1,201 (36%) | <0.001 |
| Mean Income* | 35K (15K, 65K) | 55K (35K, 95K) | 45K (25K, 75K) | <0.001 |
Values presented are mean (SD) or median (IQR) unless otherwise noted.
In U.S. Dollars
Abbreviations: BMI=Body Mass Index; QOL=Quality of Life
Discontinuation of Alcohol Use Among Active Drinkers
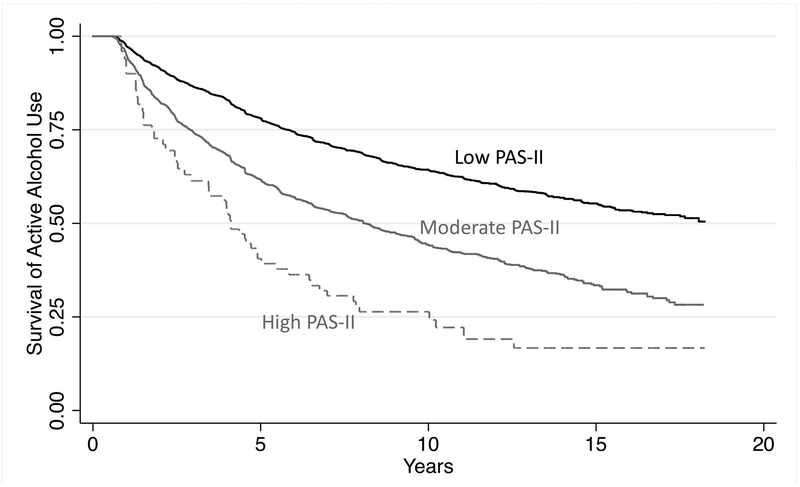
Discontinuation of alcohol was common among drinkers (4,285 events in 52,345 eligible observations; 8.2%). Figure 1 shows the predicted time-to-discontinuation of alcohol use by disease activity category adjusting for age, sex, race, and BMI. High disease activity was associated with a substantially shorter time to discontinuation of alcohol use [HR: 2.40 (1.81, 3.17) p<0.001]. Greater PAS-II was associated with a greater likelihood of discontinuing alcohol use by the next survey after adjusting for age, sex, race, BMI, and RA therapies. Compared to participants with low PAS-II, those with a moderate or high PAS-II had a substantially higher odds of alcohol discontinuation [OR 1.36 (1.27, 1.44) p<0.001; OR: 1.85 (1.37, 2.51) p<0.001, respectively]. Fully-adjusted models including comorbidity, work disability, and physical and mental quality of life, completely attenuated this association (Table 2). The most important confounders were mental and physical quality of life suggesting that the effect of disease activity on the behavior is almost entirely explained by its association with quality of life.
Figure 1: Predicted time-to-discontinuation of alcohol use among individuals who remain in different disease activity groups over long-term follow-up adjusting for age, sex, race, BMI, and RA therapies.
*PAS-II is evaluated as time varying- participants can contribute follow-up time to multiple disease activity categories over time.
Table 2:
Adjusted associations between disease activity and other factors and discontinuation of alcohol use among active drinkers.
| Odds of Discontinuation Among Active Drinkers (N=7,817; Obs=51,073) |
Odds of Initiation Among Abstainers (N=7,768; Obs=48,413) |
|||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age <50 | Reference | Reference | ||
| Age 50–60 | 1.01 (0.90, 1.12) | 0.90 | 0.85 (0.77, 0.94) | 0.002 |
| Age 60–70 | 1.04 (0.93, 1.16) | 0.13 | 0.76 (0.68, 0.85) | <0.001 |
| Age 70–80 | 1.12 (0.98, 1.27) | 0.002 | 0.66 (0.58, 0.74) | <0.001 |
| Age >80 | 1.27 (1.08, 1.51) | 0.005 | 0.72 (0.60, 0.85) | <0.001 |
| Male | 0.72 (0.64, 0.81) | <0.001 | 1.18 (1.04, 1.34) | 0.01 |
| White | 0.73 (0.62, 0.87) | <0.001 | 1.10 0.92, 1.31) | 0.30 |
| BMI | ||||
| Low | 1.03 (0.71, 1.50) | 0.86 | 0.94 (0.64, 1.36) | 0.73 |
| Normal | Reference | reference | ||
| Overweight | 1.04 (0.93, 1.17) | 0.47 | 0.99 (0.88, 1.11) | 0.84 |
| Obese | 1.37 (1.22, 1.54) | <0.001 | 1.05 (0.94, 1.18) | 0.38 |
| Methotrexate | 1.05 (0.97, 1.13) | 0.21 | 0.90 (0.84, 0.97) | 0.004 |
| Prednisone | 1.06 (0.98, 1.14) | 0.16 | 0.95 (0.88, 1.02) | 0.16 |
| Any Biologic | 0.94 (0.87, 1.01) | 0.08 | 1.06 (0.99, 1.14) | 0.12 |
| PAS-II | ||||
| Remission | Reference | Reference | ||
| Low | 1.07 (1.00, 1.15) | 0.06 | 0.97 (0.91, 1.04) | 0.43 |
| Mod-High | 1.22 (0.90, 1.66) | 0.19 | 0.95 (0.77, 1.18) | 0.66 |
| Work Disability | 1.18 (1.06, 1.32) | 0.003 | 0.91 (0.83, 1.00) | 0.001 |
| RDCI | 1.02 (1.00, 1.04) | 0.02 | -- | -- |
| Liver Disease | 1.23 (0.99, 1.53) | 0.07 | -- | -- |
| Heart Disease | -- | -- | 0.90 (0.81, 0.99) | 0.06 |
| Diabetes | -- | -- | 0.83 (0.74, 0.93) | 0.002 |
| SF-36 PCS | 0.99 (0.98, 0.99) | <0.001 | 1.01 (1.01, 1.01) | <0.001 |
| SF-36 MCS | 0.99 (0.99,1.00) | 0.001 | -- | -- |
| Education ≥16 yrs | 0.84 (0.76, 0.93) | 0.001 | 1.19 (1.07, 1.31) | 0.001 |
| Income (Ref 0–25K) | -- | -- | ||
| 35–55K | 0.84 (0.77, 0.92) | <0.001 | 1.16 (1.08, 1.26) | <0.001 |
| 65–150K | 0.72 (0.65, 0.79) | <0.001 | 1.37 (1.24, 1.51) | <0.001 |
Also tested but not associated and not included in final models: Odds of discontinuation: disease duration, smoking, marital status, depression, psychiatric disease, malignancy, heart disorders, lung disease, and gastrointestinal disorders. Odds of initiation: disease duration, smoking, marital status, health satisfaction, depression, psychiatric disease, malignancy, lung disease, liver disease, and gastrointestinal disorders.
Abbreviations: Obs=Observations; OR= Odds Ratio; CI= Confidence Interval; BMI= Body Mass Index; PAS-II= Patient Activity Scale-II; RDCI= Rheumatic Disease Comorbidity Index; PCS= Physical Component Summary; MCS= Mental Component Summary
Factors that were independently associated with greater odds of discontinuing alcohol use included older age, obesity, greater comorbidity, and work disability. Factors associated with a lower odds of discontinuing alcohol use included male sex, white race, greater physical and mental quality of life, higher educational level, and greater household income (Table 2).
Sensitivity analyses limited to moderate alcohol use only were similar to the primary analyses and are shown in Supplementary Table 3.
Initiation of Alcohol Use Among Abstainers
Initiation of alcohol use was common among abstainers (4,258 events in 46,364 eligible observations; 8.4%) and was less likely to occur among those with moderate or high PAS-II scores compared to those with low PAS-II scores [OR 0.83 (0.79, 0.88) p<0.001; OR: 0.74 (0.61, 0.89) p=0.002, respectively] after adjusting for age, sex, race, BMI, and RA treatments. As with discontinuation of alcohol, the association between disease activity and alcohol initiation was fully attenuated and not significant in adjusted models that included disability, physical quality of life, educational level, and household income [OR: 0.95 (0.77, 1.18) p=0.66] (Table 2). The most important confounders were work disability and physical quality of life. Older age, work disability, and use of methotrexate were each associated with a lower odds of initiating alcohol use while male sex, superior physical quality of life, higher educational level, and greater household income were each associated with a greater odds of initiating use (Table 2).
Sensitivity analyses limited to moderate use only were similar to the primary analyses (Supplementary Table 4).
Associations Between Changes in Alcohol Use, Disease Activity, and Mortality
An increase in disease activity was observed in 7,909/45,753 observations among previous drinkers (17.3%). Reporting of discontinuation of alcohol in the prior interval (compared to continued drinking) was not associated with worsening of disease activity in adjusted models (Table 3). In survival analyses, 615 deaths occurred among 8,114 ever drinkers. Discontinuation of alcohol was associated with a greater subsequent risk of death in models adjusting for age, sex, race, BMI, disease duration, and smoking status [HR: 1.58 (1.25, 2.00) p<0.001] (not shown). However, the association with mortality was attenuated and not significant in fully-adjusted models (Table 3).
Table 3.
(A) Associations between recent discontinuation/initiation of alcohol use in the prior period (versus no change in the behavior) and the risk of a change (>0.5 SD) in disease activity over the subsequent interval or the risk of subsequent death.
| (A) |
Increase in PAS-II Fully Adjusted (N=6,768; Obs=45,753) |
Risk of Death Fully Adjusted (N=7,808; P-Y=29,251) |
|||
| OR (95% CI) | p | HR (95% CI) | p | ||
|
Stopped Drinking (v. kept drinking) |
0.97 (0.89, 1.07) | 0.56 | 1.11 (0.85, 1.45) | 0.44 | |
|
Decrease in PAS-II Fully Adjusted (N=6,855, Obs=43,600) |
Risk of Death Fully Adjusted (N=7.708, P-Y=27,799) |
||||
| OR (95% CI) | p | HR (95% CI) | p | ||
|
Began Drinking (v. kept abstaining) |
0.98 (0.88, 1.08) | 0.65 | 0.84 (0.62, 1.16) | 0.30 | |
|
PAS-II Analyses (left): Adjusted for age, sex, race, enrollment BMI category, smoking, disease duration, PAS-II, methotrexate, prednisone, biologic therapy use, work disability, RDCI, liver disease, SF-36 PCS/MCS, work disability, household income, marital status, education level, health satisfaction, calendar year. Mortality Analyses (right): Adjusted for age, sex, race, enrollment BMI category, smoking, disease duration, PAS-II, methotrexate use, prednisone use, biologic therapy use, work disability, RDCI, diabetes, heart disorders, depression, high blood pressure, cancer, liver disease, psychiatric disease, SF-36 PCS/MCS, work disability, household income, education level, marital status, health satisfaction, and calendar date. | |||||
Abbreviations: Obs=Observations; CI= Confidence Interval; PAS-II= Patient Activity Scale 2; BMI= Body Mass Index; RDCI= Rheumatic Disease Comorbidity Index; QOL PCS= Physical Component Summary; MCS= Mental Component Summary
A subsequent improvement in disease activity occurred in 7,321/43,600 observations among previous abstainers (16.8%). Initiation of alcohol in the prior interval was not associated with an improvement in disease activity in fully-adjusted models (Table 3). There were 993 deaths among 8,129 eligible ever abstainers. Initiation of alcohol was associated with a reduced subsequent risk of death in models adjusting for age, sex, race, BMI, disease duration, and smoking status [HR: 0.72 (0.54, 0.95) p=0.02] (not shown). However, the association was attenuated and not significant in adjusted models (Table 3).
Associations of Active Drinking with Disease Activity and Mortality
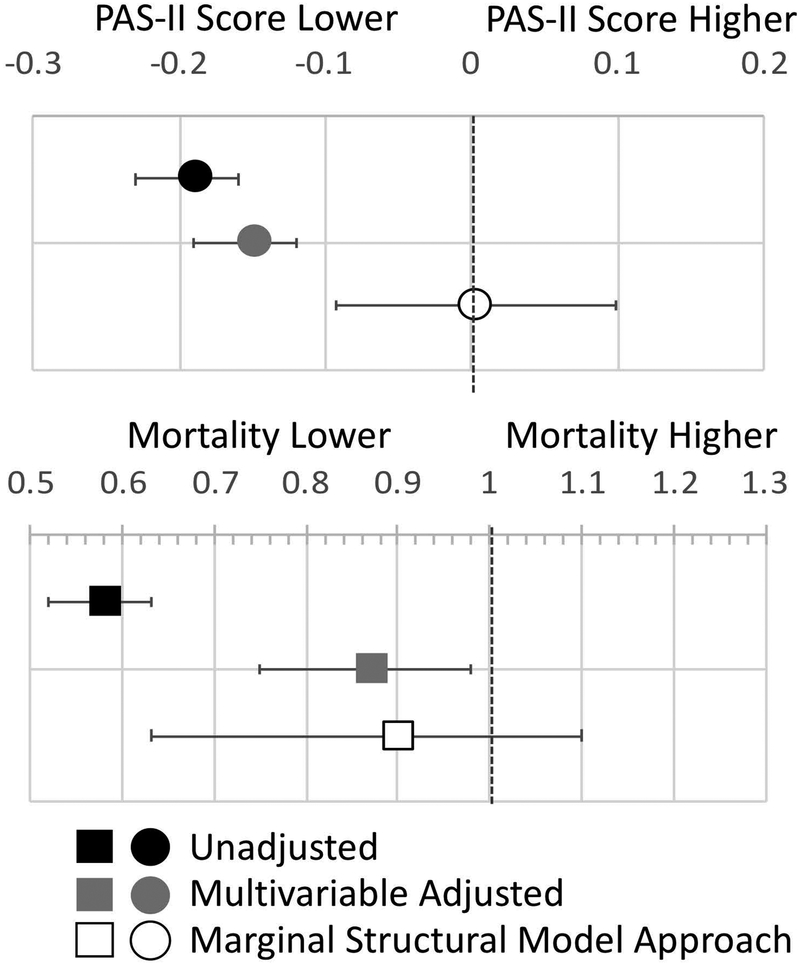
The reported use of alcohol was associated with lower PAS-II scores in unadjusted models incorporating GEE [β: -0.19 (-0.22, -0.15) p<0.001] (Figure 2, Supplementary Table 5). This was attenuated, although still significant, in multivariable models adjusting for a number of time-varying covariates [β: -0.15 (-0.18, -0.11) p<0.001]. A marginal structural modeling approach, aiming to balance the probability of being exposed to alcohol across drinkers and abstainers, demonstrated no significant difference in PAS-II scores among active drinkers [β: 0.002 (-0.094, 0.097) p=0.97].
Figure 2:
Association between alcohol use and disease activity (PAS-II, beta coefficient) and the risk of death (odds ratio) in: 1) unadjusted models (black), 2) pooled linear and logistic regression using traditional multivariable modeling (with GEE) (grey) and 3) marginal structural models that consider the propensity for current alcohol use based on current and prior covariates (white).
In unadjusted pooled logistic regression models, active drinking was strongly associated with a lower risk of death [OR: 0.58 (0.53, 0.64) p<0.001]. In models adjusting for time-varying covariates, the association was attenuated, but still significant [OR: 0.87 (0.76, 0.99) p=0.03]. A marginal structural modeling approach did not demonstrate significant differences in mortality among active drinkers [OR: 0.90 (0.70, 1.17) p=0.44]. Sensitivity analyses limited to moderate use were similar (Supplementary Table 6).
Discussion
To our knowledge, this is the first study to identify factors associated with initiation and discontinuation of alcohol use in patients with RA over long-term follow-up. Higher disease activity, older age, comorbidity, disability, and poor physical/mental quality of life were associated with greater discontinuation of use among drinkers and a lower likelihood of initiating use among abstainers. Overall, these observations suggest that patients with RA are substantially less likely to use alcohol when their disease activity is high and their health and quality of life are poor. This study also found that active drinking, recent discontinuation of drinking, and recent initiation of drinking were not associated with disease activity or death in this population when considering the reasons for the changes in behavior.
Participants with high reported disease activity were more likely to discontinue and less likely to initiate alcohol use. This is important since prior studies have suggested protective effects of alcohol use on disease activity (1–7). The current study suggests that many individuals who were observed to not drink alcohol in these studies are likely to have discontinued (or to have never initiated) due to high disease activity and poor health. In other words, while it is true that alcohol use is associated with lower disease activity and better function, this association may be better explained by reverse causality as opposed to a biologically protective effect of drinking alcohol. This study emphasizes the importance of considering the potential for reverse causality when evaluating relationships between behaviors and RA disease activity in cross-sectional studies, particularly when the behaviors studied may be expected to change in association with poor health. The current study did not find an association between alcohol use and disease activity in marginal structural models that aim to balance confounding factors that vary with time. To our knowledge this is the first longitudinal study to use this approach to deal with this problem in this context.
We also identified strong associations between discontinuation of alcohol use and greater subsequent mortality among active users. While this might suggest that discontinuation of alcohol has adverse implications for health in RA, multivariable models suggest that these effects are largely dependent on current disease activity, disability, comorbidity and quality of life. Similarly, initiating alcohol appeared protective, but the effect was similarly confounded. This study is among the first to demonstrate relationships between discontinuation and initiation of alcohol use and long-term mortality in any population and supports the hypothesis that changes in this behavior might be related to mortality through non-causal mechanisms (24, 25).
Studies in the general population have observed lower risks of frailty and death among those who drink alcohol (16, 24, 26). However, reduced risks of death were not observed among younger individuals that drink alcohol, suggesting that changes in this behavior over time occurring with illness and aging as well as selection bias may result in residual confounding in these cohort studies (25, 27–30). Our results support the concept that bias, as opposed to a biologic benefit of alcohol, may represent the primary driver of these epidemiologic associations. This study did not have sufficient power to rule out a small beneficial effect of alcohol use on the risks of death.
Overall, the findings in this study call into question prior evidence suggesting that moderate alcohol use provides a protective effect in patients with RA. However, the current study is limited in that it did not assess lifetime patterns of alcohol usage prior to enrollment. As in any study using a self-reported exposure, there may be inaccuracies in reporting of use, particularly among certain groups. Our study also did not explore the effect of alcohol across all different quantities of use and thus we cannot rule out a benefit at all levels of use. Furthermore, the nature of the registry does not provide the opportunity to assess other outcomes such as radiographic progression, sero-status, inflammatory markers, or other physician assessments. This study therefore cannot directly assess biologic relationships between the systemic inflammatory disease and alcohol use. However, it is likely that patient factors (pain, function, overall well-being) are most likely to influence and be influenced by patterns of alcohol consumption (30). Notably, it remains difficult to completely disentangle changes in use and changes in disease activity, even in this comprehensive longitudinal study and residual confounding may be present. Finally, regional and cultural differences in behaviors surrounding the use of alcohol may affect the generalizability of some of these observations. Despite these limitations, this study suggests that patients should not expect that a decision to alter their intake of alcohol would have an important impact on their RA disease activity. This study also illustrates a problem common to the observational studies that aim to study dietary or behavioral exposures that may vary in relationship to health status.
In summary, patient report of higher disease activity is associated with subsequent discontinuation of alcohol use and a lower likelihood of initiating use. These relationships are largely explained by comorbidity, disability, and poor physical and mental quality of life among those who report more active disease. In this study, active use and recent changes in alcohol use were not found to be associated with disease activity or death when considering confounding factors, suggesting no clear benefit of moderate alcohol consumption in RA.
Supplementary Material
Supplementary Figure 1: Schema for study design of analysis evaluating prior change in alcohol use and subsequent change in disease activity and risk of death.
Supplementary Figure 2: Standardized differences in key variables between drinkers and non-drinkers prior to and after weighting by the propensity score for mortality analyses.
Key Messages.
Patients with higher rheumatoid arthritis disease activity are more likely to discontinue the use of alcohol and less likely to initiate use.
Patients with greater comorbidity, disability, and poor physical and mental quality of life are less likely to use alcohol over time.
Active alcohol use and recent changes in use were not found to be associated with disease activity or death when considering some of the reasons contributing to the behavior.
This study refutes prior evidence suggesting a beneficial effect of alcohol in patients with rheumatoid arthritis.
Acknowledgements
Dr. Baker would like to acknowledge funding through a Veterans Affairs Clinical Science Research & Development Career Development Award (IK2 CX000955). The contents of this work do not represent the views of the Department of the Veterans Affairs or the United States Government.
Funding: Dr. Baker is funded by a Veterans Affairs Clinical Science Research & Development Career Development Award and Merit Award (IK2 CX000955, CX001703). Dr. Mikuls is funded by a Veterans Affairs Merit Award (CX000896) and a grant from NIH/NIGMS (U54GM115458). Dr. Mikuls and Michaud are funded by a grant from the Rheumatology Research Foundation.
Footnotes
Conflicts of Interest
JFB has received consulting fees from Bristol Myers Squibb (<$10,000).
References
- 1.Roseman C, Truedsson L, Kapetanovic MC. The effect of smoking and alcohol consumption on markers of systemic inflammation, immunoglobulin levels and immune response following pneumococcal vaccination in patients with arthritis. Arthritis Res Ther. 2012;14(4):R170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu B, Solomon DH, Costenbader KH, Keenan BT, Chibnik LB, Karlson EW. Alcohol consumption and markers of inflammation in women with preclinical rheumatoid arthritis. Arthritis Rheum. 2010;62(12):3554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman S, Symeonidou S, Andersson ML, Soderlin MK, group Bs. Alcohol consumption is associated with lower self-reported disease activity and better health-related quality of life in female rheumatoid arthritis patients in Sweden: data from BARFOT, a multicenter study on early RA. BMC Musculoskelet Disord. 2013;14:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu B, Rho YH, Cui J, Iannaccone CK, Frits ML, Karlson EW, et al. Associations of smoking and alcohol consumption with disease activity and functional status in rheumatoid arthritis. J Rheumatol. 2014;41(1):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxwell JR, Gowers IR, Moore DJ, Wilson AG. Alcohol consumption is inversely associated with risk and severity of rheumatoid arthritis. Rheumatology (Oxford). 2010;49(11):2140–6. [DOI] [PubMed] [Google Scholar]
- 6.Davis ML, Michaud K, Sayles H, Conn DL, Moreland LW, Bridges SL Jr., et al. Associations of alcohol use with radiographic disease progression in African Americans with recent-onset rheumatoid arthritis. J Rheumatol. 2013;40(9):1498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nissen MJ, Gabay C, Scherer A, Finckh A, Swiss Clinical Quality Management Project in Rheumatoid A. The effect of alcohol on radiographic progression in rheumatoid arthritis. Arthritis Rheum. 2010;62(5):1265–72. [DOI] [PubMed] [Google Scholar]
- 8.Byles J, Young A, Furuya H, Parkinson L. A drink to healthy aging: The association between older women’s use of alcohol and their health-related quality of life. J Am Geriatr Soc. 2006;54(9):1341–7. [DOI] [PubMed] [Google Scholar]
- 9.Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW Jr., et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337(24):1705–14. [DOI] [PubMed] [Google Scholar]
- 10.Bird P, Nicholls D, Barrett R, de Jager J, Griffiths H, Roberts L, et al. Longitudinal study of clinical prognostic factors in patients with early rheumatoid arthritis: the PREDICT study. Int J Rheum Dis. 2017;20(4):460–8. [DOI] [PubMed] [Google Scholar]
- 11.Eigenbrodt ML, Fuchs FD, Hutchinson RG, Paton CC, Goff DC Jr., Couper DJ. Health-associated changes in drinking: a period prevalence study of the Atherosclerosis Risk In Communities (ARIC) cohort (1987–1995). Prev Med. 2000;31(1):81–9. [DOI] [PubMed] [Google Scholar]
- 12.Ng Fat L, Cable N, Shelton N. Worsening of health and a cessation or reduction in alcohol consumption to special occasion drinking across three decades of the life course. Alcohol Clin Exp Res. 2015;39(1):166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frampton GK. Comment on: alcohol consumption is inversely associated with risk and severity of rheumatoid arthritis. Rheumatology (Oxford). 2011;50(2):423–4. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe F, Michaud K. A brief introduction to the National Data Bank for Rheumatic Diseases. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S168–71. [PubMed] [Google Scholar]
- 15.Michaud K The National Data Bank for Rheumatic Diseases (NDB). Clin Exp Rheumatol. 2016;34(5 Suppl 101):S100–S1. [PubMed] [Google Scholar]
- 16.Xi B, Veeranki SP, Zhao M, Ma C, Yan Y, Mi J. Relationship of Alcohol Consumption to All-Cause, Cardiovascular, and Cancer-Related Mortality in U.S. Adults. J Am Coll Cardiol. 2017;70(8):913–22. [DOI] [PubMed] [Google Scholar]
- 17.Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken). 2012;64(5):640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfe F, Michaud K, Pincus T. A composite disease activity scale for clinical practice, observational studies, and clinical trials: the patient activity scale (PAS/PAS-II). J Rheumatol. 2005;32(12):2410–5. [PubMed] [Google Scholar]
- 19.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–92. [DOI] [PubMed] [Google Scholar]
- 20.Pincus T, Hines P, Bergman MJ, Yazici Y, Rosenblatt LC, MacLean R. Proposed severity and response criteria for Routine Assessment of Patient Index Data (RAPID3): results for categories of disease activity and response criteria in abatacept clinical trials. J Rheumatol. 2011;38(12):2565–71. [DOI] [PubMed] [Google Scholar]
- 21.England BR, Sayles H, Mikuls TR, Johnson DS, Michaud K. Validation of the Rheumatic Disease Comorbidity Index. Arthritis Care Res (Hoboken). 2014. [DOI] [PubMed] [Google Scholar]
- 22.Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. [DOI] [PubMed] [Google Scholar]
- 24.Kojima G, Liljas A, Iliffe S, Jivraj S, Walters K. A systematic review and meta-analysis of prospective associations between alcohol consumption and incident frailty. Age Ageing. 2018;47(1):26–34. [DOI] [PubMed] [Google Scholar]
- 25.Naimi TS, Stockwell T, Saitz R, Chikritzhs T. Selection bias and relationships between alcohol consumption and mortality. Addiction. 2017;112(2):220–1. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, Stockwell T, Roemer A, Naimi T, Chikritzhs T. Alcohol Consumption and Mortality From Coronary Heart Disease: An Updated Meta-Analysis of Cohort Studies. J Stud Alcohol Drugs. 2017;78(3):375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stockwell T, Chikritzhs T. Commentary: another serious challenge to the hypothesis that moderate drinking is good for health? Int J Epidemiol. 2013;42(6):1792–4. [DOI] [PubMed] [Google Scholar]
- 28.Rehm J, Irving H, Ye Y, Kerr WC, Bond J, Greenfield TK. Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. Am J Epidemiol. 2008;168(8):866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng Fat L, Shelton N. Associations between self-reported illness and non-drinking in young adults. Addiction. 2012;107(9):1612–20. [DOI] [PubMed] [Google Scholar]
- 30.Liang W, Chikritzhs T. The association between alcohol exposure and self-reported health status: the effect of separating former and current drinkers. PLoS One. 2013;8(2):e55881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Schema for study design of analysis evaluating prior change in alcohol use and subsequent change in disease activity and risk of death.
Supplementary Figure 2: Standardized differences in key variables between drinkers and non-drinkers prior to and after weighting by the propensity score for mortality analyses.