Abstract
Background
Mother-to-child transmission (MTCT) of syphilis and HIV continue to be important yet preventable causes of perinatal and infant morbidity and mortality.
Objectives
To systematically review, critically appraise and perform a meta-analysis to evaluate the operational characteristics of dual rapid diagnostic tests (RDTs) for HIV/syphilis and evaluate whether they are cost effective, acceptable and easy to use.
Design
Systematic review and meta-analysis.
Data sources
We searched seven electronic bibliographic databases from 2012 to December 2016 with no language restrictions. Search keywords included HIV, syphilis and diagnosis.
Review methods
We included studies that evaluated the operational characteristics of dual HIV/syphilis RDTs. Outcomes included diagnostic test accuracy, cost effectiveness, ease of use and interpretation and acceptability. All studies were assessed against quality criteria and assessed for risk of bias.
Results
Of 1914 identified papers, 18 were included for the meta-analysis of diagnostic accuracy for HIV and syphilis. All diagnostic accuracy evaluation studies showed a very high sensitivity and specificity for HIV and a lower, yet adequate, sensitivity and specificity for syphilis, with some variation among types of test. Dual screening for HIV and syphilis was more cost effective than single rapid tests for HIV and syphilis and prevented more adverse pregnancy outcomes. Qualitative data suggested dual RDTs were highly acceptable to clients, who cited time to result, cost and the requirement of a single finger prick as important characteristics of dual RDTs.
Conclusion
The results of this systematic review and meta-analysis can be used by policy-makers and national programme managers who are considering implementing dual RDTs for HIV and syphilis.
INTRODUCTION
Approximately 1.5 million pregnant women annually are infected with HIV, and 900 000 are infected with syphilis.1,2 Mother-to-child transmission (MTCT) of HIV and syphilis remain significant causes of perinatal morbidity and mortality.3 HIV MTCT can occur during pregnancy, delivery or breastfeeding. Without any intervention, HIV MTCT rates vary between 20% and 35% in breastfed infants or 15% and 20% for non-breastfed infants.4 However, these MTCT rates for HIV can be reduced to less than 5% on provision of effective intervention.5 Untreated maternal syphilis results in in-utero infection, associated with significant adverse pregnancy outcomes, such as stillbirth, preterm and low birth weight, neonatal death and clinical syphilis infection in infants born alive.6 Systematic reviews indicate that in pregnant women with untreated syphilis, more than half of pregnancies result in these adverse outcomes,7 and that an even higher proportion of pregnancies are affected in women with primary or secondary syphilis infections.8 Prenatal syphilis screening followed by treatment with injectable penicillin early in pregnancy effectively treats the pregnant woman and prevents congenital syphilis. In addition, maternal syphilis has been shown to increase the risk of MTCT of HIV.9 The WHO launched a global initiative for elimination of congenital syphilis in 20078 and has also prioritised the elimination of mother to child transmission (EMTCT) of HIV.5 Additionally in 2014, WHO HIV and STI programmes in collaboration with other UN partners joined forces to validate countries for the EMTCT of HIV and syphilis using shared guidelines and processes.5,10 Several countries have now achieved validation of EMTCT for HIV and/or syphilis.11
Screening all pregnant women for syphilis and HIV at first antenatal care visit is recommended in nearly all countries of the world and is being scaled up rapidly in countries committed to EMTCT of HIV and syphilis.12,13 However, while the testing of pregnant women for HIV is relatively well resourced, syphilis-infected pregnant women often go undiagnosed and untreated. While many countries have antenatal syphilis screening policies, more than 350 000 adverse pregnancy outcomes occur annually due to untreated maternal syphilis, despite the low cost of testing and treatment.14 To meet current targets, calls have been made to accelerate the dual EMTCT of syphilis and HIV.15 Early diagnosis and treatment of both HIV and syphilis in pregnant women has been proven as an effective strategy in the prevention of both adverse outcomes of pregnancy and MTCT. Key populations, such as men who have sex with men (MSM), transgender people, injecting drug users and sex workers would also benefit from improved HIV and syphilis screening coverage,16–18 as described in key policy documents published by the WHO.19,20
In 2015, the SD BIOLINE HIV/Syphilis Duo Test (Standard Diagnostics, Korea) was accepted for the WHO list of prequalified in vitro diagnostics.21 Other rapid diagnostics tests (RDTs) are also available that can simultaneously test for antibodies to HIV and Treponema pallidum antigens, ensuring that both tests can be conducted in a single visit to a single health facility. Herein, we describe a systematic review and meta-analysis of published literature to evaluate the operational characteristics of currently available RDTs for HIV and syphilis, including diagnostic accuracy, cost-effectiveness, acceptability and ease of test interpretation.
METHODS
Eligibility criteria
We followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines.22 Studies were included that evaluated, in either laboratory or field settings, any commercially available RDT (that satisfies the specifications in the ASSURED criteria23,24) that simultaneously tests for HIV and syphilis on the same cartridge or device. Studies were included that involved any sexually active populations in any geographic location. The primary outcome was diagnostic test accuracy (ie, sensitivity, specificity, positive predictive value, negative predictive value) for both HIV and syphilis. Secondary outcomes included cost-effectiveness, usability, ease of test interpretation and acceptability. The types of studies that were eligible for inclusion were evaluation studies, cost-effectiveness analyses and usability and acceptability studies. For the meta-analysis of diagnostic accuracy, studies were included if an acceptable reference standard for both HIV and syphilis was used (HIV: either enzyme immunoassay (EIA), Western blot (WB) or two RDTs; syphilis: T. pallidum particle agglutination assay (TPPA) or T. pallidum haemagglutination assay (TPHA) with or without non-treponemal testing). Studies were excluded if HIV and syphilis diagnosis were not conducted on a dual RDT (ie, on the same cartridge/device). Studies were included regardless of sample size.
Search terms and strategy
We searched the following electronic bibliographic databases: Medline, Embase, KoreaMed, PAHO Library Catalogue, China National Knowledge Infrastructure, Russian Science Citation Index and J-stage. The search strategy included terms relating to HIV, syphilis and diagnosis (see online supplementary material). No language restrictions were used. Studies published between January 2012 and the December 2016 were sought. The searches were rerun immediately before the final analyses to check for recent relevant literature. Additional records were identified by searching bibliographies of relevant publications.
Data extraction
Titles and abstracts were checked for relevance. For the meta-analysis of diagnostic accuracy, the data extracted included study title, dates of enrolment, country, test(s) evaluated, laboratory or field evaluation (and if so, sample type used), the population studied and for laboratory evaluations, whether fresh or archived specimens were used. For both the HIV and syphilis diagnosis components of each study, the following information was either extracted or calculated using two by two tables: the number of participants/samples used, prevalence (%), reference standard test, number of true positives, false positives, false negatives and true negatives. Study investigators were contacted if further information was required.
Two reviewers (HDG and MMT) independently extracted data from the included studies. Disagreements were resolved by consensus or by consulting external advisors. The updated standards for the reporting of diagnostic accuracy studies (STARD) checklist25,26 was used to evaluate the methodology of included studies. To critically appraise the included evaluation studies, the quality assessment of diagnostic accuracy studies (QUADAS-2) checklist27 was used.
Data synthesis
Forest plots and summary receiver operating characteristic (SROC) curves were constructed using RevMan.28
RESULTS
Study characteristics
Among the 1914 records identified and screened (figure 1), we included 28 studies for the data synthesis, and 18 of these were also used in the meta-analysis. Two-by-two table data were not available for one study.29 Two studies included in the meta-analysis evaluated the performance of multiple tests. Diagnostic accuracy studies evaluated the performance of the SD BIOLINE HIV/Syphilis Duo Test, the MedMira Multiplo Rapid TP/HIV Antibody Test (MedMira, Canada) and the Chembio Dual Path Platform (DPP) HIV/Syphilis Assay (Chembio Diagnostic Systems, USA) (table s1). These studies were conducted in a range of WHO regions including Africa (South Africa, Kenya, Nigeria, Malawi, Ghana, Togo), South-East Asia (Nepal, Myanmar), the Western Pacific (China, Lao People’s Democratic Republic) and the Americas (Haiti, Peru, Mexico, USA). The populations studied were primarily key populations (such as sex workers, injection drug users (IDUs), transgender women, MSM and sexual health clinic attendees). Three studies evaluated the diagnostic accuracy of the test in antenatal care settings.
Figure 1.
PRISMA flow diagram showing the number of records initially identified and that were subsequently excluded or included in the meta-analysis on the performance and operational characteristics of dual point-of-care tests for HIV and syphilis. PRISMA, preferred reporting items for systematic reviews and meta-analysis.
Included and excluded studies
The 18 diagnostic accuracy studies that were included in the meta-analysis for diagnostic accuracy are detailed in table 1. The median sample sizes were 415 and 450 for HIV and syphilis, respectively, with the range for each falling between 150 and 10 000.30–47
Table 1.
Characteristics and results of studies evaluating the diagnostic test accuracy of dual HIV/syphilis RDTs that were included in the meta-analysis according to order of date published
| HIV | Syphilis | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author and year | Location | Population | Study setting (lab or field) | Index test | Sample | Reference test | TP | FP | FN | TN | Sens (%) | Spec (%) | Prev (%) | Reference test | TP | FP | FN | TN | Sens (%) | Spec (%) | Prev (%) |
| Ondondo30 2013 | Kenya | HIV serodiscordant couples | Lab | SD BIOLINE HIV/Syphilis Duo Test | Serum | 2 RDTs, confirmed by 2 EIAs | 345 | 0 | 1 | 352 | 99.7 | 100.0 | 49.6 | RPR, confirmed by TPHA | 85 | 0 | 0 | 559 | 100.0 | 100.0 | 12.2 |
| Chiappe31 2013 | Peru | Archived specimens | Lab | SD BIOLINE HIV/Syphilis Duo Test | Serum | EIA and WB | 91 | 0 | 0 | 571 | 100.0 | 100.0 | 13.7 | RPR, confirmed by TPPA | 198 | 2 | 0 | 465 | 100.0 | 99.6 | 29.8 |
| Hess32 2014 | California, USA | STD clinic attendees, IDUs, MSM | Field | Chembio DPP HIV/Syphilis Assay (reverse order) |
Whole blood | EIA | 44 | 2 | 2 | 606 | 95.7 | 99.7 | 6.7 | TPPA | 37 | 3 | 41 | 576 | 47.4 | 99.5 | 10.6 |
| Humphries33 2014 | California, USA | Archived specimens | Lab | SD BIOLINE HIV/Syphilis Duo Test | Serum | EIA and WB | 94 | 0 | 2 | 53 | 97.9 | 100.0 | 64.0 | TPPA | 80 | 0 | 6 | 64 | 93.0 | 100.0 | 57.3 |
| Humphries33 2014 | California, USA | Archived specimens | Lab | Chembio DPP HIV/Syphilis Assay | Serum | EIA and WB | 94 | 1 | 2 | 52 | 97.9 | 98.1 | 64.0 | TPPA | 82 | 0 | 4 | 64 | 95.3 | 100.0 | 57.3 |
| Humphries33 2014 | California, USA | Archived specimens | Lab | MedMira Multiplo Rapid TP/HIV Antibody Test |
Serum | ElA and WB | 94 | 3 | 2 | 49 | 97.9 | 94.2 | 64.0 | TPPA | 81 | 2 | 5 | 62 | 94.1 | 96.9 | 57.3 |
| Omoding34 2014 | Uganda | Pregnant women | Lab | SD BIOLINE HIV/Syphilis Duo Test | Serum | 3 RDTs | 16 | 1 | 0 | 203 | 100.0 | 99.5 | 7.3 | TPHA | 19 | 0 | 0 | 201 | 100.0 | 100.0 | 8.6 |
| Bristow35 2014 | Ghana, Mexico, Lao People’s Democratic Republic, Togo, Kenya and Myanmar | Archived specimens from STI clinic attendees | Lab | SD BIOLINE HIV/Syphilis Duo Test | Serum | EIA, WB and RDTs | 1123 | 4 | 1 | 1208 | 99.9 | 99.7 | 48.1 | TPPA/TPHA/EIA | 609 | 4 | 2 | 1444 | 99.7 | 99.7 | 29.7 |
| Dagnra36 2014 | Togo | Key populations | Lab | SD BIOLINE HIV/Syphilis Duo Test | Serum | EIA | 107 | 0 | 0 | 203 | 100.0 | 100.0 | 34.5 | / | / | / | / | / | / | / | / |
| Bristow37 2015 | Peru | MSM and transgender women | Lab | MedMira Multiplo Rapid TP/HIV Antibody Test | Serum | EIA and WB | 74 | 10 | 0 | 114 | 100.0 | 91.9 | 37.4 | TPPA | 104 | 6 | 6 | 77 | 94.6 | 92.8 | 57.0 |
| Yin38 2015 | Nanjing, China, Zaria, Nigeria and Ibadan, Nigeria | Archived samples | Lab | SD BIOLINE HIV/Syphilis Duo Test | Serum | EIA | 721 | 8 | 7 | 778 | 99.0 | 99.0 | 48.1 | TPPA or TPHA | 710 | 7 | 25 | 772 | 96.6 | 99.1 | 48.5 |
| Yin38 2015 | Nanjing, China, Zaria, Nigeria and Ibadan, Nigeria | Archived samples | Lab | MedMira Multiplo Rapid TP/HIV Antibody Test | Serum | EIA | 724 | 13 | 4 | 773 | 99.5 | 98.3 | 48.1 | TPPA or TPHA | 692 | 22 | 43 | 757 | 94.2 | 97.2 | 48.5 |
| Yin38 2015 | Nanjing, China, Zaria, Nigeria and Ibadan, Nigeria | Archived samples | Lab | Chembio DPP HIV/Syphilis Assay | Serum | EIA | 725 | 17 | 3 | 769 | 99.6 | 97.9 | 48.1 | TPPA or TPHA | 713 | 3 | 22 | 776 | 97.0 | 99.6 | 48.5 |
| Shimelis39 2015 | Ethiopia | STI clinic attendees | Lab | SD BIOLINE HIV/Syphilis Duo Test | Serum | RDTs and EIA | 200 | 1 | 0 | 199 | 100 | 99.5 | 50.0 | TPHA | 83 | 4 | 2 | 96 | 97.6 | 96.0 | 45.9 |
| Leon40 2016 | Peru | MSM and transgender women | Lab | Chembio DPP HIV/Syphilis Assay | Serum | EIA and WB | 151 | 4 | 0 | 295 | 100.0 | 98.7 | 33.6 | TPPA | 142 | 0 | 8 | 300 | 94.7 | 100.0 | 33.3 |
| Bristow41 2016 | Peru | MSM and transgender women | Field | SD BIOLINE HIV/Syphilis Duo Test | Whole blood | EIA and WB | 104 | 2 | 1 | 308 | 99.1 | 99.4 | 25.3 | TPPA | 149 | 3 | 18 | 243 | 89.2 | 98.8 | 40.4 |
| Bristow42 2016 | Haiti | STI clinic attendees | Field | SD BIOLINE HIV/Syphilis Duo Test | Whole blood | RDTs | 128 | 5 | 1 | 164 | 99.2 | 97.0 | 43.3 | TPHA and ELISA | 109 | 17 | 4 | 168 | 96.5 | 90.8 | 37.9 |
| Bristow43 2016 | Peru | Sex workers, MSM and transgender women | Field | MedMira Multiplo Rapid TP/HIV Antibody Test | Whole blood | EIA and WB | 15 | 0 | 1 | 159 | 93.8 | 100.0 | 7.8 | TPPA | 17 | 0 | 4 | 153 | 81.0 | 100.0 | 10.2 |
| Shakya44 2016 | Nepal | Pregnant women | Lab | SD BIOLINE HIV/Syphilis Duo Test | Serum | 3 RDTs | 19 | 0 | 0 | 9981 | 100.0 | 100.0 | 0.2 | RPR, confirmed by TPHA | 42 | 13 | 2 | 9943 | 95.5 | 99.9 | 0.4 |
| Black45 2016 | South Africa | Female sex workers | Field | SD BIOLINE HIV/Syphilis Duo Test | Whole blood | EIA | 185 | 0 | 2 | 62 | 98.8 | 100.0 | 75.1 | TPPA and RPR (titre ≥1:8) | 34 | 4 | 17 | 194 | 66.7 | 98.0 | 20.5 |
| Bowen46 2016 | Malawi | Pregnant women | Field | Chembio DPP HIV/Syphilis Assay | Whole blood | RDT | / | / | / | / | / | / | / | TPPA | 55 | 6 | 25 | 1702 | 68.8 | 99.6 | 4.5 |
| Kalou47 2016 | Georgia, USA | Archived specimens | Lab | Chembio DPP HIV/Syphilis Assay | Serum | EIA and WB | 426 | 9 | 1 | 554 | 98.8 | 100.0 | 43.1 | TPPA & EIA (Trep Sure) | 639 | 2 | 8 | 341 | 98.8 | 99.4 | 65.4 |
EIA, enzyme immunoassay; FP, false positive; FN = false negative; IDU, injection drug user; MSM, men who have sex with men; Prev, prevalence; RDT, rapid diagnostic test; RPR, rapid plasma regain; Sens, sensitivity; Spec, specificity; TN, true negative; TP, true positive; TPHA, Treponema pallidum haemagglutination assay; TPPA, T. pallidum particle agglutination assay; WB, Western blot.
One study was identified that evaluated the diagnostic accuracy of the INSTI Multiplex downward-flow immunoassay48 (also called the INSTI Multiplex HIV-1/HIV-2/Syphilis Antibody Test) (bioLytical Laboratories, Canada). Using 200 archived serum specimens from high-risk individuals in Peru, the results of this study suggested a high sensitivity (100%, 95% CI 95.9% to 100%) and specificity (95.5%, 95% CI 89.9% to 98.5%) for HIV diagnosis and a slightly lower sensitivity (87.4%, 95% CI 81.4% to 92.0%) but a higher specificity (97%, 95% CI 84.2% to 99.9%) for syphilis diagnosis. These results were not included in the meta-analysis because only one diagnostic accuracy evaluation study for this diagnostic test was identified. A study published by Leon et al40 evaluated visual interpretation of the Chembio DPP HIV/Syphilis Assay compared with the use of an electronic reader to interpret the test.40 The sensitivity and specificity for the HIV component of the Chembio DPP HIV/Syphilis Assay did not alter according to whether visual interpretation or electronic reader was used. The sensitivity of the syphilis component was similarly unaffected, but the specificity was slightly lower when the electronic reader was used (99.7%, 95% CI 98.2% to 100%) compared with visual interpretation (100%, 95% CI 98.8% to 100%), although this was not statistically significant. Results for test interpretation using the electronic reader were not included in the meta-analysis.
Hess et al32 studied the performance of the Chembio DPP HIV/Syphilis lateral flow assay in its original configuration (in which the liquid first flowed across the syphilis test line, followed by the HIV test line), and also in a revised or ‘reversed’ configuration (HIV followed by syphilis). This revised form of the test became the final approved model of the test. The Hess et al32 study reported two sets of sensitivities and specificities for the original order of the test and the reverse order. Only the results of the reverse order (which has since become the standard order for the test) were included in the meta-analysis. The study by Hess et al32 also assessed the performance of an integrated test for HIV, hepatitis C virus (HCV) and syphilis on a single diagnostic platform (Chembio DPP HIV-HCV-Syphilis Assay); but these results were not included in the meta-analysis because no other studies were identified that evaluated this particular RDT.
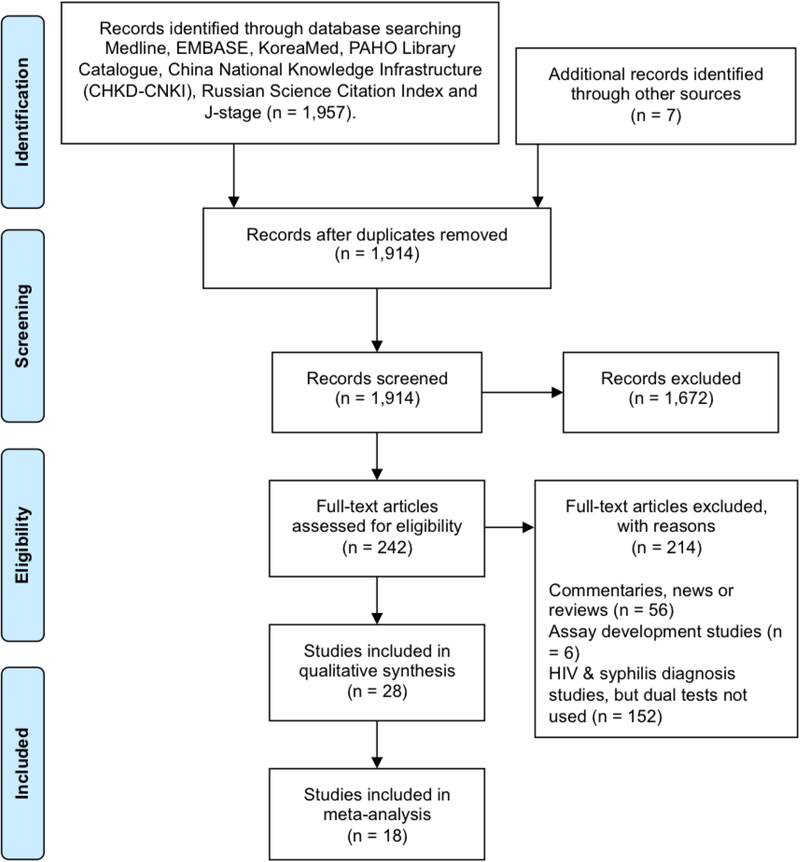
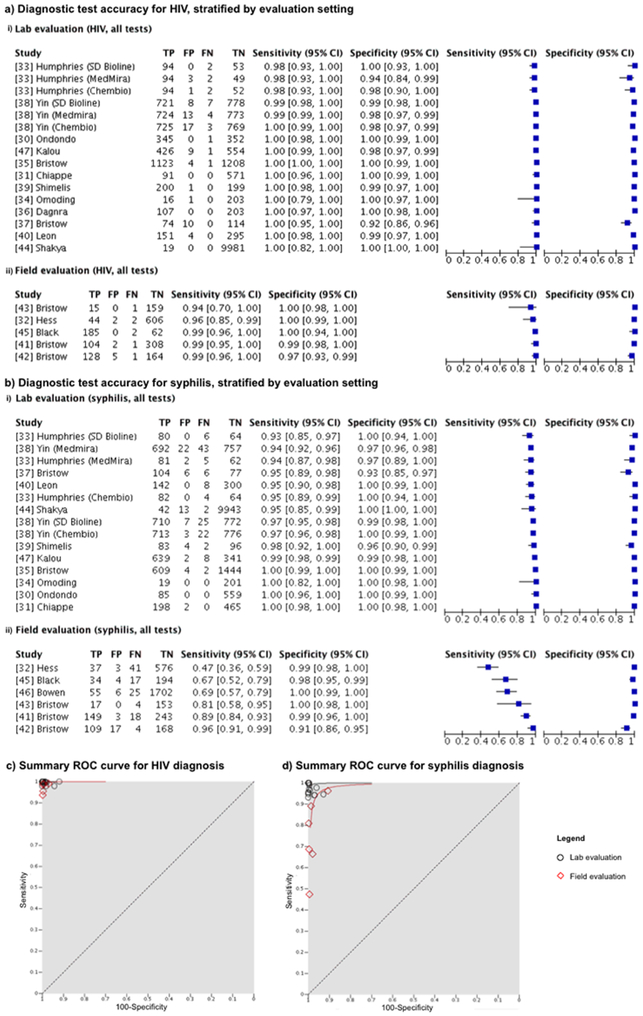
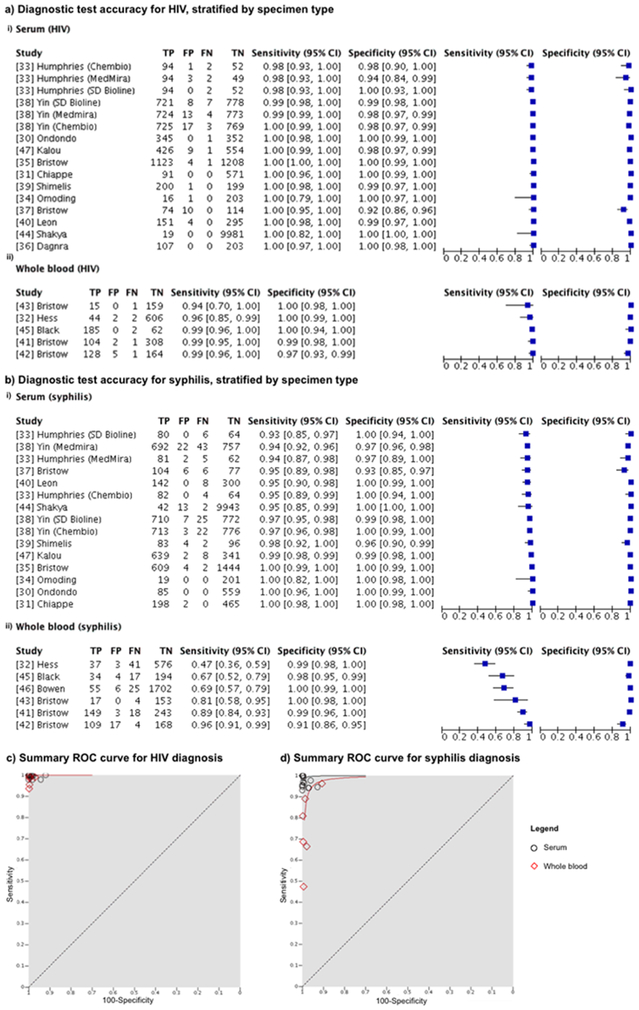
The meta-analysis stratification strategy is detailed in online supplementary figure 1. Tests were first stratified by manufacturer (figure 2), by evaluation setting (laboratory or field) (figure 3) and by specimen type used for evaluation (including serum versus whole blood, and archived versus fresh specimens) (figures 4 and 5).
Figure 2.
Meta-analysis of the diagnostic accuracy of dual HIV/syphilis RDTs, stratified according to manufacturer. Forest plots are sown for the diagnostic accuracy of (A) HIV and (B) syphilis diagnosis. Summary ROC curves are shown for the diagnosis of (C) HIV and (D) syphilis. RDTs, rapid diagnostic tests; ROC, receiver operating characteristic.
Figure 3.
Meta-analysis of the diagnostic accuracy of dual HIV/syphilis RDTs, stratified according to the setting in which the evaluation was conducted. Forest plots are shown for the diagnostic accuracy of (A) HIV and (B) syphilis diagnosis. Summary ROC curves are shown for (C) HIV and (D) syphilis diagnosis. RDTs, rapid diagnostic tests; ROC, receiver operating characteristic.
Figure 4.
Meta-analysis of the diagnostic accuracy of dual HIV/syphilis RDTs, stratified according to the specimen type (serum or whole blood) used in the evaluation studies. Forest plots are shown for the diagnostic accuracy of (A) HIV and (B) syphilis diagnosis. Summary ROC curves are shown for (C) HIV and (D) syphilis diagnosis. RDTs, rapid diagnostic tests; ROC, receiver operating characteristic.
Figure 5.
Meta-analysis of the diagnostic accuracy of dual HIV/syphilis RDTs, stratified according to the specimen type (archived, ie, frozen specimens or fresh specimens). Forest plots are shown for the diagnostic accuracy of (A) HIV and (B) syphilis diagnosis. Summary ROC curves are shown for (C) HIV and (D) syphilis diagnosis. FN, false negative; FP, false positive; RDTs, rapid diagnostic tests; ROC, receiver operating characteristic; TN, true negative; TP, true positive.
Diagnostic accuracy of HIV and syphilis by RDT manufacturer
HIV diagnostic performance by manufacturer
The diagnostic accuracy for HIV and syphilis of RDTs produced by three different manufacturers are detailed in figure 2. There were 12 studies that evaluated the SD BIOLINE HIV/Syphilis Duo Test, four studies that assessed the MedMira Multiplo Rapid TP/HIV Antibody Test and six that appraised the Chembio DPP HIV/Syphilis Assay, although one of these, reported by Bowen et al46 only reported the accuracy of syphilis diagnosis.
All but one of the evaluation studies reported a sensitivity of HIV diagnosis of at least 98%. This study, by Bristow et al43 reported a sensitivity of 94% for the MedMira Multiplo Rapid TP/HIV Antibody Test. The specificity values for HIV diagnosis ranged from 97% to 100% for the SD BIOLINE HIV/Syphilis Duo Test and 92% to 100% for the MedMira Multiplo Rapid TP/HIV Antibody Test. All of the specificity values for HIV diagnosis reported for the Chembio DPP HIV/Syphilis Assay were 100% (figure 2A).
The summary ROC curves for the three test manufacturers are presented in figure 2C, and D. Summary HIV ROC curve for the MedMira Multiplo Rapid TP/HIV Antibody test falls slightly below that of the curve for the SD BIOLINE HIV/Syphilis Duo Test and Chembio DPP HIV/Syphilis Assay, indicating that this test might have a lower diagnostic performance for HIV (figure 2C).
Syphilis diagnostic performance by manufacturer
For syphilis diagnosis, the reported sensitivities for the SD BIOLINE HIV/Syphilis Duo Test were all between 89% and 100%, except for one study published by Black et al45 which reported a sensitivity of 67%. The authors of this study noted that patients with a rapid plasma regain (RPR) titre of ≥1:4 (an indicator of active syphilis) were more likely to test positive using this RDT. The specificity values reported for syphilis diagnosis using SD BIOLINE HIV/Syphilis Duo Test ranged from 91% to 100% (figure 2B).
The ranges for sensitivity and specificity reported for the syphilis component of MedMira Multiplo Rapid TP/HIV Antibody Test were 81% to 95% and 93% to 100%, respectively.
Chembio DPP HIV/Syphilis Assay gave sensitivity ranges for syphilis diagnosis of 46% to 97%, although each evaluation study reported a specificity of 100%. Similar to the study by Black et al45 of the SD Bioline HIV/Syphilis Duo Test syphilis component, Bowen et al46 also reported in their study of the Chembio DPP HIV/Syphilis Assay that patients with a high RPR titre (≥1:4) were more likely to test positive for presence of treponemal antibody.46
The summary ROC curve in figure 2D shows that SD BIOLINE HIV/syphilis Duo Test gives the highest syphilis diagnostic accuracy, followed by the Chembio DPP HIV/Syphilis Assay and then the MedMira Multiplo Rapid TP/HIV Antibody Test. However, these differences are not statistically significant.
Diagnostic accuracy for HIV and syphilis in laboratory and field settings
Diagnostic accuracy results were also stratified according to whether the evaluation study was conducted in a laboratory or field setting (figure 3). Field evaluations were typically conducted in sexual health facilities,32,41,42,45 including one that actively recruited pregnant women.42 Another was conducted in antenatal settings46 and another at outreach sites for key populations.43 Results were combined, regardless of brand name or manufacturer.
HIV diagnostic performance in lab and field settings
In laboratory settings, the sensitivity of HIV diagnosis ranged from 94% to 99%, and specificity from 92% to 100%. In field settings, reported HIV sensitivity values were between 96% and 99% for all but one of the field evaluations. The sensitivity of HIV diagnosis in the remaining study, published by Bristow et al43 was 94%. This study evaluated the MedMira Multiplo Rapid TP/HIV Antibody Test. The range for specificity of HIV diagnosis reported in field settings was 97% to 100%.
Syphilis diagnostic performance in lab and field settings
For syphilis diagnosis, reported sensitivities ranged from 93% to 100% in laboratory settings, whereas for field settings, they ranged from 47% to 96%. The sensitivity value of 47% for syphilis diagnosis was reported by Hess et al32 for the Chembio DPP HIV/Syphilis Assay. In this study, only 11% of positive TPPA cases were RPR reactive, suggesting that the majority of cases represented previously treated rather than active syphilis infection. The next two lowest sensitivity values were reported by Black et al45 (67%) and Bowen et al46 (69%) for the SD BIOLINE HIV/Syphilis Duo Test and the Chembio DPP HIV/Syphilis Assay, respectively. Both Black et al45 and Bowen et al46 also reported on results of TPPA+/RPR+ test results as a standard, distinguishing between RPR titres >1:4 and <1:4 as indicators of active (transmissible) syphilis infection and old or treated infections (less transmissible), respectively.6 Both found the syphilis component of the DPP had high sensitivity and specificity in TPPA-reactive samples with higher RPR titres.
Using TPPA positivity as the standard, specificity values for syphilis diagnosis for the Chembio DPP HIV/Syphilis Assay ranged from 93% to 100% and 91% to 100% for laboratory and field settings, respectively.
Diagnostic accuracy of HIV and syphilis by specimen type
It is possible that sample composition (ie, whole blood or serum) and storage can affect the diagnostic accuracy of RDTs. Long-term storage of frozen serum can affect the stability of proteins and other constituents of the sample.49 To investigate the diagnostic accuracy according to whether evaluation studies used serum or whole blood, and archived or fresh specimens, results were stratified according to specimen type (figure 4 and figure 5). Results were combined, regardless of brand name or manufacturer.
HIV diagnostic performance by specimen type
Reported sensitivities for HIV diagnosis were lower for studies that used whole blood (94% to 99%) compared with those that used serum (98% to 100%). However, studies using whole blood reported higher specificities (97% to 100%) than those using serum (92% to 100%) (figure 4A), leading to similar plotting of SROC curves for studies using serum and whole blood (figure 4C).
For studies using archived specimens, the sensitivity of HIV diagnosis ranged from 98% to 100%, and specificity from 94% to 100%. When fresh specimens were used, HIV diagnosis sensitivity values were between 94% and 100%, and specificity values were between 97% and 100% (figure 5A). Diagnostic accuracy for HIV was therefore minimally affected by specimen type.
Syphilis diagnostic performance by specimen type
Diagnostic accuracy for syphilis appears to be higher in studies that used serum rather than whole blood, with improved sensitivity ranges being reported (between 93% and 100% for studies that used serum, compared with 47% and 96% for studies that used whole blood) and specificities (93% to 100% for studies using serum compared with 91% to 100% for studies that used whole blood) (figure 4B).
Studies that used archived specimens reported syphilis sensitivities and specificities ranging from 93% to 100% and 97% to 100%, respectively. The diagnostic accuracy for syphilis was slightly poorer when fresh specimens were used, with sensitivities falling between 47% and 100% and specificities between 91% and 100% (figure 5B). This could reflect the fact that the evaluations using archived specimens were conducted in laboratory settings.
Secondary outcomes
Cost-effectiveness and impact on adverse pregnancy outcomes
A study by Bristow et al50 showed that dual HIV/syphilis RDTs are an efficacious means of reducing the number of adverse pregnancy outcomes compared with other screening algorithms. In this study, four screening algorithms were compared, including an HIV RDT only, dual HIV and syphilis rapid RDTs, single RDTs for both HIV and syphilis and finally, HIV and syphilis laboratory tests. Costs of prevention and care were calculated, showing that a dual HIV/syphilis rapid testing strategy was both the least costly (US$226.92 per pregnancy) and resulted in the fewest adverse pregnancy outcomes (15 370 per 100 000 pregnancies for dual HIV/syphilis testing compared with 15 820 for HIV rapid testing only, 15 779 for HIV rapid testing and syphilis laboratory testing and 15 778 for single, separate RDTs for HIV and syphilis).
Feasibility and acceptability
A qualitative study conducted among patients seeking STI, HIV testing or antenatal care in Haiti evaluated the importance of various factors for HIV and syphilis dual testing to patients.51 The majority of study participants cited cost as the most important factor, but also selected single finger prick sampling and time to result as important attributes for dual testing. Interestingly, pregnant women reported that they prioritised time to result over all other factors. In antenatal care (ANC) settings in Colombia, dual HIV/syphilis RDTs were shown to have similar acceptability values to patients compared with separate rapid tests for HIV and syphilis.52
From the service provider perspective, in both China and Nigeria, dual HIV/syphilis RDTs were found to be fairly easy or very easy to use and to interpret the results, as was reported by Yin et al.38 This study scored the SD BIOLINE HIV/Syphilis Duo Test, the Chembio DPP HIV/Syphilis Assay, and the MedMira Multiplo Rapid TP/HIV Antibody Test on a range of operational characteristics. The SD BIOLINE HIV/Syphilis Duo Test scored the highest out of the three, with significant advantages over other tests in clarity of kit instruction, ease of use, ease of interpretation of results and training time required.
Cost-effectiveness, ease of test interpretation and acceptability of individual rapid tests for simultaneous HIV and syphilis diagnosis
Limited data were available on the cost effectiveness, usability, ease of test interpretation and acceptability of single device dual tests for dual HIV/syphilis diagnosis. However, the following studies were identified, which provide data for these factors when syphilis and HIV were diagnosed at the same time, using individual RDTs.
A systematic review that evaluated the impact of introducing rapid syphilis testing (RST) in antenatal care settings on HIV and syphilis uptake and coverage showed that RST may increase both syphilis and HIV screening rates in antenatal care settings.53 Two studies cited by the review that supported this claim were conducted by Strasser et al54 in Uganda and Zambia, and by Fleming et al55 in ANC settings in rural Kenya. More recently, in Kampala, Uganda, the introduction of syphilis testing within integrated HIV-antenatal care settings was shown to be effective, feasible and successfully capitalised on programmes that have already been established and optimised for HIV care.56
The acceptability of simultaneous testing for HIV and syphilis using separate RDTs was investigated in key populations in Peru.57 The tests used were the SD BIOLINE HIV 1/2 3.0 and SD BIOLINE Syphilis 3.0 tests. Client perceptions and the feasibility of implementing simultaneous HIV and syphilis RDTs were evaluated. The proportion of clients tested who received timely results increased by 30.8% for HIV and 35.7% for syphilis in pregnant women. The RDTs for HIV and syphilis allowed for fewer hospital visits, less time spent waiting at the hospital and lower labour and resource costs for the hospital. All clients tested were either completely satisfied (52%) or satisfied (48%) with the process of simultaneous HIV/syphilis testing. Seventy-two per cent of study participants strongly agreed with the statement, ‘I liked the process of having the two tests taken at the same time’.
A study published by Owusu-Edusei et al58 simulated the cost-effectiveness of using separate laboratory-based diagnosis for HIV and syphilis in China. Their results suggested that incorporating syphilis screening into pre-existing antenatal HIV screening programmes was more cost-effective than HIV screening only even in very low prevalence settings, and that testing for both infections would prevent a larger number of adverse pregnancy outcomes.
Quality of studies
The STARD checklist was used to appraise the quality of reporting of studies included in the meta-analysis (see online supplementary file 1). Of the 30 items in the updated STARD checklist published by Bossuyt et al26 some items were universally well reported, such as the identification as a study of diagnostic accuracy (100%), scientific and clinical background (100%), index test and reference standard methods (100%), methods for estimating or comparing measures of diagnostic accuracy (94%) and implications for clinical practice (100%). However, other items were poorly reported, such as the rationale for choosing the reference standard where alternatives exist and whether clinical information and reference standard results were available to the performers of the index tests or if clinical information and index test results were available to assessors of the reference standard. In addition, few studies included a flow diagram of participants.
Quality of methodology was assessed using the QUADAS-2,27 and results are summarised in online supplementary table 3. Most studies either scored as low or unclear risk of bias where patient selection, index tests and study flow and timing were concerned. In particular, studies were reported as having an unclear risk of bias for the reference standard if they did not state that results of the reference standard were interpreted without knowledge of the results of the index tests. Little concern was identified regarding the applicability of patients, index tests and reference standards used in the studies.
DISCUSSION
The overall purpose of this study was to evaluate the literature relating to dual RDTs for HIV and syphilis, particularly with regards to their diagnostic accuracy, cost-effectiveness, feasibility, acceptability and ease of interpretation. This meta-analysis reviewed the results of 18 recently published studies on the performance characteristics of rapid dual HIV/syphilis tests when evaluated by manufacturer and performance in field versus laboratory settings. The diagnostic accuracy for HIV was found to vary minimally depending on test manufacturer, with SD BIOLINE HIV/Syphilis Duo Test and the Chembio DPP HIV/Syphilis Assay showing the highest diagnostic accuracy. Diagnostic accuracy for syphilis varied with manufacturer, with the SD BIOLINE HIV/Syphilis Duo Test being the most accurate. Performance of the test in the laboratory versus field setting did not result in a difference in the sensitivity or specificity for HIV but a poorer sensitivity was noted in two field-based studies for syphilis. Published literature on the cost-effectiveness and feasibility of dual RDTs for HIV and syphilis was limited but demonstrated encouraging results that, along with performance results, could be used to support the use of these tests for screening of populations at risk for HIV and syphilis.
The diagnostic accuracy for HIV of each of the three dual tests (SD BIOLINE HIV/Syphilis Duo Test, MedMira Multiplo Rapid TP/HIV Antibody Test and the ChemBio DPP HIV/Syphilis Assay) evaluated in the meta-analysis was consistently high (with all studies but one reporting sensitivities of over 98% and all but two reporting specificities of over 97%). The diagnostic accuracy for HIV was found to be slightly lower for the MedMira Multiplo Rapid TP/HIV Antibody Test, which gave sensitivities of between 94% and 100% and specificities between 98% and 100%. In comparison, the SD BIOLINE HIV/Syphilis Duo Test and Chembio DPP HIV/Syphilis Assay gave higher levels of sensitivities, with a range of 98% to 100% for each. Their reported specificities were also higher, with 97%–100% for the SD BIOLINE HIV/Syphilis Duo Test and 98%–100% for the Chembio DPP HIV/Syphilis Assay. This was also true for syphilis diagnostic accuracy, with the SD BIOLINE HIV/Syphilis Duo Test performing the best out of the three. It should be noted that single RDTs for syphilis have also shown sensitivities between 64% and 100% and reduced sensitivities for clinic-based evaluations compared with laboratory evaluations.54 A target product profile (TPP) for an ideal dual HIV/syphilis RDT was developed at the 1st Technical Consultation on Point-Of-Care Diagnostic Tests for Sexually Transmitted Infections convened by the WHO Reproductive Health Research Department in May 2004.59 This TPP set out the desired operational characteristics for a dual HIV/syphilis RDT and included minimal and optimal specifications for parameters such as sensitivity and specificity of HIV and syphilis diagnosis. The minimal sensitivity and specificity specifications were >98% and >98% for HIV, respectively, and >85% and >95%, respectively, for syphilis. The three RDTs that were included in the meta-analysis all fulfil each of these requirements, at least at the minimal level. Minimum standards for HIV RDTs have been suggested to be >99% for sensitivity and >98% specificity.60
Accuracy of HIV diagnosis was minimally affected by conducting the test in the laboratory compared with field settings. However, a reduction in diagnostic accuracy was seen in field settings for syphilis. This was particularly true for the Chembio DPP HIV/Syphilis Assay. Two studies identified improvements in sensitivity when RPR titre values were included to identify active syphilis.41–43,45,46 This suggests that despite lower overall reported sensitivities, these tests may preferentially detect active syphilis over old or treated syphilis, which is clinically advantageous. The SD Bioline HIV/Syphilis Test has received WHO prequalification21 and is ready for use according to country-established quality performance measures. Our results indicate that, although the syphilis performance component as assessed by these studies still meets the minimal criteria as specified for the TPP, efforts to ensure consistent, correct and repeated staff training and quality control measures should be undertaken at the field level to ensure appropriate use and interpretation of these tests.
The overall diagnostic accuracy for HIV was minimally affected according to whether serum or whole blood was used in the evaluation studies. However, studies that used serum reported a superior diagnostic performance for syphilis than those that used whole blood. Specimen type (archived versus fresh specimens) was shown to marginally affect diagnostic accuracy for syphilis but not for HIV. These findings mimic those of the lab versus field analysis as all of the archived specimens would have been tested in a laboratory setting. However, the findings of the archived versus fresh analysis demonstrate the good performance of the RDTs on archived specimens.
Evidence from qualitative studies gives a strong indication that dual testing for HIV and syphilis is acceptable to testing clients, feasible for implementation in a range of ANC and other programmes and cost-effective. In a modelling study, when compared with HIV testing alone using a RDT, two separate RDTs for HIV and syphilis, and separate laboratory testing for HIV and syphilis, dual RDTs for HIV and syphilis were shown to be the least costly and also prevented the largest number of adverse pregnancy outcomes.50 A study conducted in Haiti assessed the importance of a range of RDT characteristics for testing clients. The most important factors included cost, the requirement for a single finger prick sample and time to result.51 Published literature on simultaneous testing for HIV and syphilis using separate RDTs also provided evidence on the feasibility of implementation of incorporating syphilis testing into pre-existing HIV screening programmes, acceptability and cost-effectiveness.53,56–58 Unfortunately, less data were available in the literature concerning ease of dual RDT interpretation.
Our meta-analysis has some limitations. First, our search criteria may have missed some studies and the authors are aware of ongoing studies for which results are not yet available. Second, to date only a limited number of diagnostic accuracy evaluation studies have been published for each dual RDT. While the best diagnostic performance was observed for the SD BIOLINE HIV/Syphilis Duo Test, more evaluation studies were available for this test compared with the two others included in the meta-analysis. More evidence is required to inform our understanding of the performance of the MedMira Multiplo Rapid TP/HIV Antibody Test and Chembio DPP HIV/Syphilis Assay. In particular, more field evaluation studies are warranted, in order to assess the sensitivity of the syphilis component of the diagnostic tests under the conditions and using operators that are likely available in real world versus controlled, laboratory settings. Third, studies used a range of reference tests with which to evaluate the diagnostic accuracy of the dual RDTs. For example, some studies used treponemal tests (measuring ever exposure to T. pallidum, regardless of previous treatment) only, whereas others used both treponemal and non-treponemal tests (measuring active infection). Where archived specimens were used, studies did not mention when reference testing was carried out (ie, at the time of collection or at the same time as the index test), and this timing could affect the agreement between the results of reference and index test. Another limitation is that for the studies that used archived specimens, it was unclear what population these specimens were taken from and in what setting. Too few studies were available for a reliable performance comparison of the RDTs with use of treponemal and non-treponemal tests (n=4) versus treponemal only as reference standards for syphilis diagnosis. This analysis may be considered for future study. Finally, only three commercially available dual HIV/syphilis, RDTs were evaluated in this meta-analysis. However, other tests are available which we did not include in this analysis, such as the INSTI Multiplex HIV-1/HIV-2/Syphilis Antibody Test, and there are more in development, such as the mChip, a smartphone dongle that performs an ELISA on a small chip using microfluidics.61
Our results demonstrate excellent performance for the HIV component of the dual RDTs for HIV and syphilis and good performance for the syphilis component, similar to the performance of single syphilis RDTs.54,62 When considering performance, cost-effectiveness and feasibility, these tests should be prioritised for use in settings and among populations where HIV and syphilis screening are recommended, namely, antenatal care settings. As the screening recommendations for HIV and syphilis are similar in many respects, it is logical and practical to combine their diagnosis on a single cartridge.63 Dual RDTs for HIV and syphilis testing would allow same-day treatment for syphilis and immediate referral for HIV therapy, thus enhance the prevention of MTCT of HIV and syphilis.64 Dual RDTs for HIV and syphilis therefore represent an important measure in the elimination of MTCT of HIV and syphilis. This systematic review will inform the WHO process for developing diagnostic algorithms for the use of dual RDTs for HIV and syphilis diagnosis. In the interim, WHO has developed interim guidance for use and interpretation of these tests.65 Future work will be required to build a toolkit for programme and clinic managers, similar to the one that was established for rapid syphilis testing,66 which would provide guidance on planning, management and implementation of dual RDTs for HIV and syphilis.
Supplementary Material
Key messages.
In studies of dual HIV/syphilis tests on a single device, the accuracy of HIV diagnosis remained high regardless of test manufacturer or whether evaluations were conducted in laboratory or field settings.
The accuracy of syphilis testing was good (similar to single tests for syphilis), although not as high as HIV, in both laboratory and field settings and regardless of manufacturer.
Dual testing for HIV and syphilis has been shown to be more cost-effective and more effective at preventing adverse pregnancy outcomes than testing for HIV alone or using separate RDTs for HIV and syphilis.
Acknowledgements
The authors wish to acknowledge the helpful guidance of the WHO HIV diagnostics team.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The author(s) is(are) staff member(s) of the World Health Organization. The author(s) alone is(are) responsible for the views expressed in this publication and they do not necessarily represent the views, decisions or policies of the World Health Organization.
Provenance and peer review Commissioned; externally peer reviewed.
Trial registration number PROSPERO 2016:CRD42016049168.
Competing interests None declared.
REFERENCES
- 1.Wijesooriya NS, Rochat RW, Kamb ML, et al. Global burden of maternal and congenital syphilis in 2008 and 2012: a health systems modelling study. Lancet Glob Health 2016;4:e525–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. Global AIDS Update - 2016. 2016. www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016 (accessed 13 Oct 2016).
- 3.Mabey D, Peeling RW. Syphilis, still a major cause of infant mortality. Lancet Infect Dis 2011;11:654–5. [DOI] [PubMed] [Google Scholar]
- 4.Teasdale CA, Marais BJ, Abrams EJ. HIV: prevention of mother-to-child transmission. BMJ Clin Evid 2011;2011. [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Global guidance on Criteria and Processes for Validation: elimination of Mother-to-Child Transmission of HIV and Syphilis. 2014. http://apps.who.int/iris/bitstream/10665/112858/1/9789241505888_eng.pdf (accessed 21 Dec 2016).
- 6.Watson-Jones D, Changalucha J, Gumodoka B, et al. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. J Infect Dis 2002;186:940–7. [DOI] [PubMed] [Google Scholar]
- 7.Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ 2013;91:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. The global elimination of congenital Syphilis: rationale and strategy for Action. 2007. http://apps.who.int/iris/bitstream/10665/43782/1/9789241595858_eng.pdf (accessed 25 Jan 2017).
- 9.Mwapasa V, Rogerson SJ, Kwiek JJ, et al. Maternal syphilis infection is associated with increased risk of mother-to-child transmission of HIV in Malawi. AIDS 2006;20:1869–77. [DOI] [PubMed] [Google Scholar]
- 10.WHO. Global Health Sector Strategy on sexually transmitted infections 2016–2021: towards ending STIs. 2016. http://apps.who.int/iris/bitstream/10665/246296/1/WHO-RHR-16.09-eng.pdf?ua=1 (accessed 26 Jan 2017).
- 11.Ishikawa N, Newman L, Taylor M, et al. Elimination of mother-to-child transmission of HIV and syphilis in Cuba and Thailand. Bull World Health Organ 2016;94:787–787A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO-WPRO. Elimination of mother-to-child transmission of HIV and syphilis. www.wpro.who.int/hiv/topics/pmtct/en/ (accessed 27 Dec 2016).
- 13.PAHO. Elimination of Mother-to-Child Transmission of HIV and Syphilis in the Americas. 2015. www.iris.paho.org/xmlui/bitstream/handle/123456789/18372/9789275118702_eng.pdf?sequence=3&isAllowed=y (accessed 27 Dec 2016).
- 14.Newman L, Kamb M, Hawkes S, et al. Global estimates of syphilis in pregnancy and associated adverse outcomes: analysis of multinational antenatal surveillance data. PLoS Med 2013;10:e1001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiarie J, Mishra CK, Temmerman M, et al. Accelerating the dual elimination of mother-to-child transmission of syphilis and HIV: Why now? Int J Gynaecol Obstet 2015;130(Suppl 1):S1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suthar AB, Ford N, Bachanas PJ, et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Med 2013;10:e1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupte S, Daly C, Agarwal V, et al. Introduction of rapid tests for large-scale syphilis screening among female, male, and transgender sex workers in Mumbai, India. Sex Transm Dis 2011;38:1–502. [DOI] [PubMed] [Google Scholar]
- 18.Sabidó M, Benzaken AS, de-Andrade-Rodrigues EJ, et al. Rapid point-of-care diagnostic test for syphilis in high-risk populations, Manaus, Brazil. Emerg Infect Dis 2009;15:647–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. 2014. http://apps.who.int/iris/bitstream/10665/128048/1/9789241507431_eng.pdf?ua=1&ua=1 (accessed 26 Jan 2017). [PubMed]
- 20.WHO. Prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender people. Geneva: WHO 2011http://apps.who.int/iris/bitstream/10665/44619/1/9789241501750_eng.pdf?ua=1 (accessed 26 Jan 2017).
- 21.WHO. WHO prequalification of in vitro diagnostics programme PUBLIC REPORT. 2015. www.who.int/diagnostics_laboratory/evaluations/151028_final_report_0179-012-00_sd_bioline_hiv_syphilis2.pdf?ua=1 (Last accessed 10 Jan 2017).
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009;3:e123–30. [PMC free article] [PubMed] [Google Scholar]
- 23.Mabey D, Peeling RW, Ustianowski A, et al. Diagnostics for the developing world. Nat Rev Microbiol 2004;2:231–40. [DOI] [PubMed] [Google Scholar]
- 24.Mabey D, Peeling RW, Ballard R, et al. Prospective, multi-centre clinic-based evaluation of four rapid diagnostic tests for syphilis. Sex Transm Infect 2006;82(Suppl 5):v13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 2003;138:W1–12. [DOI] [PubMed] [Google Scholar]
- 26.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- 28.Review Manager(RevMan). [Computer program] Version 5.3.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2014.
- 29.Fakile Y, Hoover K, Delaney K, et al. 002.5 Evaluation of five rapid point-of-care tests for syphilis: two treponemal only, and three dual treponemal/hiv assays. Sex Transm Infect 2015;91(Suppl 2):A29. [Google Scholar]
- 30.Ondondo RO, Odoyo JB, Bukusi EA. O15.5 Performance Characteristics of SD Bio Line Rapid HIV-Syphilis Duo Test Kit For Simultaneous Detection of HIV and Syphilis Infections. Sex Transm Infect 2013;89(Suppl 1):A56. [Google Scholar]
- 31.Chiappe MA, Lopez-Torres L, Carcamo C, et al. P5.090 Evaluation of a Double Rapid Test For Syphilis and HIV: SD Bioline HIV/Syphilis Duo. Sex Transm Infect 2013;89(Suppl 1):A363. [Google Scholar]
- 32.Hess KL, Fisher DG, Reynolds GL. Sensitivity and specificity of point-of-care rapid combination syphilis-HIV-HCV tests. PLoS One 2014;9:e112190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humphries RM, Woo JS, Chung JH, et al. Laboratory evaluation of three rapid diagnostic tests for dual detection of HIV and Treponema pallidum antibodies. J Clin Microbiol 2014;52:4394–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omoding D, Katawera V, Siedner M, et al. Evaluation of the SD BIOLINE HIV/syphilis Duo assay at a rural health center in Southwestern Uganda. BMC Res Notes 2014;7:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bristow CC, Adu-Sarkodie Y, Ondondo RO, et al. Multisite Laboratory evaluation of a dual human immunodeficiency virus (HIV)/Syphilis Point-of-Care Rapid Test for simultaneous detection of HIV and Syphilis infection. Open Forum Infect Dis 2014;1:ofu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dagnra AY, Dossim S, Salou M, et al. Evaluation of 9 rapid diagnostic tests for screening HIV infection, in Lomé, Togo. Med Mal Infect 2014;44:525–9. [DOI] [PubMed] [Google Scholar]
- 37.Bristow CC, Leon SR, Ramos LB, et al. Laboratory evaluation of a dual rapid immunodiagnostic test for HIV and syphilis infection. J Clin Microbiol 2015;53:311–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin YP, Ngige E, Anyaike C, et al. Laboratory evaluation of three dual rapid diagnostic tests for HIV and syphilis in China and Nigeria. Int J Gynaecol Obstet 2015;130(Suppl 1):S22–6. [DOI] [PubMed] [Google Scholar]
- 39.Shimelis T, Tadesse E. The diagnostic performance evaluation of the SD BIOLINE HIV/syphilis duo rapid test in southern Ethiopia: a cross-sectional study. BMJ Open 2015;5:e007371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leon SR, Ramos LB, Vargas SK, et al. Laboratory evaluation of a Dual-Path Platform assay for rapid Point-of-Care HIV and Syphilis testing. J Clin Microbiol 2016;54:492–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bristow CC, Leon SR, Huang E, et al. Field evaluation of a dual rapid diagnostic test for HIV infection and syphilis in Lima, Peru. Sex Transm Infect 2016;92:182–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bristow CC, Severe L, Pape JW, et al. Dual rapid lateral flow immunoassay fingerstick wholeblood testing for syphilis and HIV infections is acceptable and accurate, Port-au-Prince, Haiti. BMC Infect Dis 2016;16:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bristow CC, Leon SR, Huang E, et al. Field evaluation of a dual rapid immunodiagnostic test for HIV and Syphilis infection in Peru. Sex Transm Dis 2016;43:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shakya G, Singh DR, Ojha HC, et al. Evaluation of SD Bioline HIV/syphilis duo rapid test kits in Nepal. BMC Infect Dis 2016;16:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Black V, Williams BG, Maseko V, et al. Field evaluation of Standard Diagnostics’ Bioline HIV/Syphilis Duo test among female sex workers in Johannesburg, South Africa. Sex Transm Infect 2016:495–8. [DOI] [PubMed] [Google Scholar]
- 46.Bowen V, Lupoli K, Chipungu G, et al. A bundle of health- syphilis test performance in the field evaluation of a novel dual HIV/syphilis rapid test - Malawi, 2014–2015. Sex Transm Infect 2015;43:S223. [Google Scholar]
- 47.Kalou MB, Castro A, Watson A, et al. Laboratory evaluation of the Chembio dual path platform HIV-Syphilis assay. Afr J Lab Med 2016;5:A433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herbst de Cortina S, Bristow CC, Vargas SK, et al. Laboratory evaluation of a Point-of-Care Downward-Flow assay for simultaneous detection of antibodies to Treponema pallidum and human immunodeficiency virus. J Clin Microbiol 2016;54:1922–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuhadar S, Koseoglu M, Atay A, et al. The effect of storage time and freeze-thaw cycles on the stability of serum samples. Biochem Med 2013;23:70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bristow CC, Larson E, Anderson LJ, et al. Cost-effectiveness of HIV and syphilis antenatal screening: a modelling study. Sex Transm Infect 2016;92:340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bristow CC, Lee SJ, Severe L, et al. Attributes of diagnostic tests to increase uptake of dual testing for syphilis and HIV in Port-au-Prince, Haiti. Int J STD AIDS 2017;28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaitán-Duarte HG, Newman L, Laverty M, et al. Comparative effectiveness of single and dual rapid diagnostic tests for syphilis and HIV in antenatal care services in Colombia. Rev Panam Salud Publica 2016;40:455–62. [PubMed] [Google Scholar]
- 53.Swartzendruber A, Steiner RJ, Adler MR, et al. Introduction of rapid syphilis testing in antenatal care: A systematic review of the impact on HIV and syphilis testing uptake and coverage. Int J Gynaecol Obstet 2015;130(Suppl 1):S15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strasser S, Bitarakwate E, Gill M, et al. Introduction of rapid syphilis testing within prevention of mother-to-child transmission of HIV programs in Uganda and Zambia: a field acceptability and feasibility study. J Acquir Immune Defic Syndr 2012;61:e40–6. [DOI] [PubMed] [Google Scholar]
- 55.Fleming E, Oremo J, O’Connor K, et al. The impact of integration of Rapid Syphilis Testing during Routine Antenatal Services in Rural Kenya. J Sex Transm Dis 2013;2013:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manabe YC, Namale G, Nalintya E, et al. Integration of antenatal syphilis screening in an urban HIV clinic: a feasibility study. BMC Infect Dis 2015:15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flores EC, Lluque ME, Chiappe M, et al. Operations research study to implement HIV and syphilis point-of-care tests and assess client perceptions in a marginalised area of Lima, peru. Int J STD AIDS 2015;26:723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Owusu-Edusei K, Tao G, Gift TL, et al. Cost-effectiveness of integrated routine offering of prenatal HIV and syphilis screening in China. Sex Transm Dis 2014;41:103–10. [DOI] [PubMed] [Google Scholar]
- 59.WHO. Point-Of-Care Diagnostic Tests (POCTs) for Sexually Transmitted Infections (STIs). 2014. www.who.int/RHRSTIPOCTs (accessed 10 Jan 2017).
- 60.WHO. HIV ASSAYS LABORATORY PERFORMANCE ANDOTHER OPERATIONAL CHARACTERISTICS. 2015. www.who.int/diagnostics_laboratory/evaluations/hiv/150819_hiv_assay_report18_final_version.pdf?ua=1 (accessed 2 Oct 2017).
- 61.Laksanasopin T, Guo TW, Nayak S, et al. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci Transl Med 2015;7:273re1. [DOI] [PubMed] [Google Scholar]
- 62.Rogozińska E, Kara-Newton L, Zamora JR, et al. On-site test to detect syphilis in pregnancy: a systematic review of test accuracy studies. BJOG 2017;124:734–41. [DOI] [PubMed] [Google Scholar]
- 63.Bristow CC, Klausner JD. Cuba: defeating mother-to-child transmission of syphilis. Lancet 2015;386:1533. [DOI] [PubMed] [Google Scholar]
- 64.Newman Owiredu M, Newman L, Nzomo T, et al. Elimination of mother-to-child transmission of HIV and syphilis: A dual approach in the African Region to improve quality of antenatal care and integrated disease control. Int J Gynaecol Obstet 2015;130(Suppl 1):S27–31. [DOI] [PubMed] [Google Scholar]
- 65.WHO. WHO Information note on the use of dual hiv/syphilis rapid diagnostic tests. 2017. http://apps.who.int/iris/bitstream/10665/252849/1/WHO-RHR-17.01-eng.pdf (accessed 2 Nov 2017).
- 66.LSHTM. Rapid Syphilis Test Toolkit: a Guide to Planning, Management and Implementation. 2011. www.lshtm.ac.uk/itd/crd/research/rapidsyphilistoolkit/ (accessed 20 Oct 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.