Abstract
Most of the microorganisms living in a symbiotic relationship in different animal body sites (microbiota) reside in the gastrointestinal tract (GIT). Several studies have shown that the microbiota is involved in host susceptibilities to pathogens. The fecal microbiota of domestic and wild suids was analyzed. Bacterial communities were determined from feces obtained from domestic pigs (Sus scrofa) raised under different conditions: specific-pathogen-free (SPF) pigs and domestic pigs from the same bred, and indigenous domestic pigs from a backyard farm in Kenya. Secondly, the fecal microbiota composition of the African swine fever (ASF) resistant warthogs (Phacochoerus africanus) from Africa and a European zoo was determined. African swine fever (ASF) is a devastating disease for domestic pigs. African animals showed the highest microbial diversity while the SPF pigs the lowest. Analysis of the core microbiota from warthogs (resistant to ASF) and pigs (susceptible to ASF) showed 45 shared OTUs, while 6 OTUs were exclusively present in resistant animals. These six OTUs were members of the Moraxellaceae family, Pseudomonadales order and Paludibacter, Anaeroplasma, Petrimonas, and Moraxella genera. Further characterization of these microbial communities should be performed to determine the potential involvement in ASF resistance.
Subject terms: Computational biology and bioinformatics, Microbial communities
Introduction
The characterization of the microbiota diversity inhabiting particular mucosal surfaces or other body sites has been an active topic of research in recent years, due to the involvement in many vital processes. In the gut, the high microbial diversity has the potential to provide metabolic activities that the host lacks. Specifically, the gut microbiota of mammals has been shown to confer health benefits to the host through the production of digestible food components, inhibition and prevention of colonization by pathogens, and development and maintenance of the host immune system1–3.
The microbiota composition from the pigs’ gastrointestinal tract (GIT) has been subject of many investigations. Piglets are first exposed to microbes at birth and they will eventually be colonized with different microbial populations by constant exposure to microbes4. Early gut colonization is critically for both morphological and immunological development of the GIT5. During growth, the microbiome changes exhibiting increased diversity, a relevant indicator of GIT health4. Once established, the microbiota ultimately reaches an equilibrated community composition6 that is essential in maintaining the health of the animal. However, under several pathological conditions or antimicrobial treatments, disruption of the microbiota may occur leading to a dysbiosis state. Moreover, there are other important factors that may impact the GIT microbiota composition such as diet6,7, age8,9 and the environment where animals are raised10. Intensive farming has led to pigs being mostly raised indoors, where they are exposed to a limited diversity of microorganisms. The extreme cases are germ-free laboratory animals, grown under conditions that totally restrict microbial exposure. These animals show physiological and behavioral abnormalities with impaired development of the immune system11. Given the complexity of breeding germ-free pigs, the vast majority of animal experiments are performed with specific pathogen-free (SPF) pigs, raised under a highly controlled environment free of certain potentially pathogenic microorganisms (bacteria, viruses and parasites). SPF animals have a different degree of maturation of the innate immune system when compared with the domestic counterparts12. This impaired immune system has been also associated with increased disease susceptibility, including African swine fever13.
African swine fever (ASF) is a devastating hemorrhagic disease of pigs caused by a large DNA virus with mortality rates near 100%14. ASF has been mainly confined to sub-Saharan Africa in a sylvatic cycle including wild suids and arthropod vectors together with domestic pigs. Recently, it has spread out of Africa and into the Russian Federation, China, and Europe, heightening awareness of threat to the global pig industry15. With no vaccines or treatment available for this disease, it is of urgent need to develop alternative means of control. With the exception of African wild pigs16,17 (warthogs and bushpigs), all pigs are susceptible to the infection with virulent strains of ASFV. It has been hypothesized that African domestic pigs may have genetic characteristics related to ASFV tolerance, but these genomic signatures have not been clearly detected18. SPF pigs, conventional domestic pigs and wild boars, succumb after experimental challenge with a highly virulent strain of ASFV, independently of their sex and age19. Additional evidences from our lab demonstrated that SPF pigs infected with intermediate doses of attenuated strains of the African swine fever virus, although safe for domestic pigs, resulted in a lethal outcome for 100% of the SPF animals13. The reasons behind this enhanced susceptibility of SPF pigs may be partially explained by a different and less diverse gut microbiota composition, since both SPF and domestic pigs are both from the same breed. The association between a more diverse and rich microbiota and a healthier state has been demonstrated not only for livestock20, but also for laboratory animals21 and humans22,23. African warthogs are known to be natural reservoir hosts of ASFV in the wild. Under experimental conditions, captive warthogs also demonstrated resistance to ASFV infection, showing no clinical signs of disease when infected with the same highly virulent isolates of ASFV that induce rapid, hemorrhagic death in domestic pigs24–26. Being aware that pigs (Sus scrofa) and warthogs (Phacochoerus africanus) belong to different species, and that genetic differences will most probably play key roles in differential susceptibility, we hypothesized that the warthog GIT microbiota could contribute to the ASFV resistance phenotype. The fecal microbial populations of these animal species have not been thoroughly studied27, hence the knowledge of the microorganisms inhabiting the gut of wild animals can provide insight into their potential role on resistance to diseases.
In this study we aimed to firstly, determine the microbial composition of specific-pathogen-free (SPF) pigs, which exhibit higher susceptibility to ASF28 in comparison with domestic pigs from a commercial farm. Additionally, the microbial communities from indigenous pigs from Africa were compared with both SPF and commercial pigs to analyze the environmental effect on this animal species (Sus scrofa). Secondly, our goal was to study the microbiota composition of ASF-resistant warthogs (Phacochoerus africanus). The fecal microbiota composition of both wild warthogs and animals raised in a confined space, from the Barcelona Zoo, were analyzed to shed light on the differences acquired within this species when raised under different environment conditions. Furthermore, the core microbiota of ASF-resistant warthogs was determined and compared with the microbiota of susceptible animals in order to investigate the microorganisms that might be involved in disease resistance.
Results
The fecal bacterial community in SPF and indigenous pigs from Africa
The microbiota composition was analyzed from fecal samples obtained from domestic adult pigs (Sus scrofa) raised under different conditions. Fecal samples from SPF pigs (n = 11) were obtained from pure Large-White pigs raised in ANSES biocontainment facility (French Agency for Food, Environmental and Occupational Health and Safety, Ploufragan, France), while samples from domestic (pure Large-White) commercial pigs (COM pigs, n = 9) were obtained from a PRRSV-free conventional farm from France with a high health status. The feed for both SPF and COM pigs was composed of cereals with addition of a mix of minerals and vitamins, free of anti-parasites or antibiotics. Fecal samples from African pigs (AFR pig) were obtained from a backyard farm in Kenya (n = 15).
All the fecal samples were subjected to DNA extraction and 16S rRNA gene sequencing. A total of 8,172,478 sequences were obtained from fecal samples from different animals sequenced individually. A median number of reads per sample obtained after processing was 208,098 (range 21,134–523,022) with a mean sequence length of 451 nt (Supplementary Table S1), corresponding to 88.5% of the total reads. Operational taxonomic units (OTUs) were identified by clustering sequences at 97% sequence homology.
SPF pig’s microbiota was composed of three main Phyla (>1% relative abundance), where Firmicutes (66.93%), Bacteroidetes (29.10%) and Proteobacteria (2.41%), represented 98.44% of the total composition (Fig. 1A; Table 1). Interestingly, Ruminococcaceae within the phylum Firmicutes, was the most relatively abundant family in SPF pig feces, representing 36.89% of all the sequences found at the family level, which was higher than in both AFR (20.41%) and COM pigs (21.42%). Within Firmicutes, Lachnospiraceae, represented 14.10% of the assigned families (Fig. 1B, Table 1). From Bacteroidetes, Prevotellaceae was the most relatively abundant (17.59%), followed by Bacteroidaceae (5.18%), and Porphyromonadaceae (3.45%). Notably, Rikenellaceae (2.73%), from Bacteroidetes, represented a unique family that was specific for SPF pigs. Remarkably, many families found in AFR and COM pigs, were completely absent in SPF pigs, these included Flavobacteriaceae, Lactobacillaceae, Planococcaceae, Sphingobacteriaceae, Spirocaetaceae, among others. The unassigned sequences represented only 12.40% of sequences at the family level.
Figure 1.
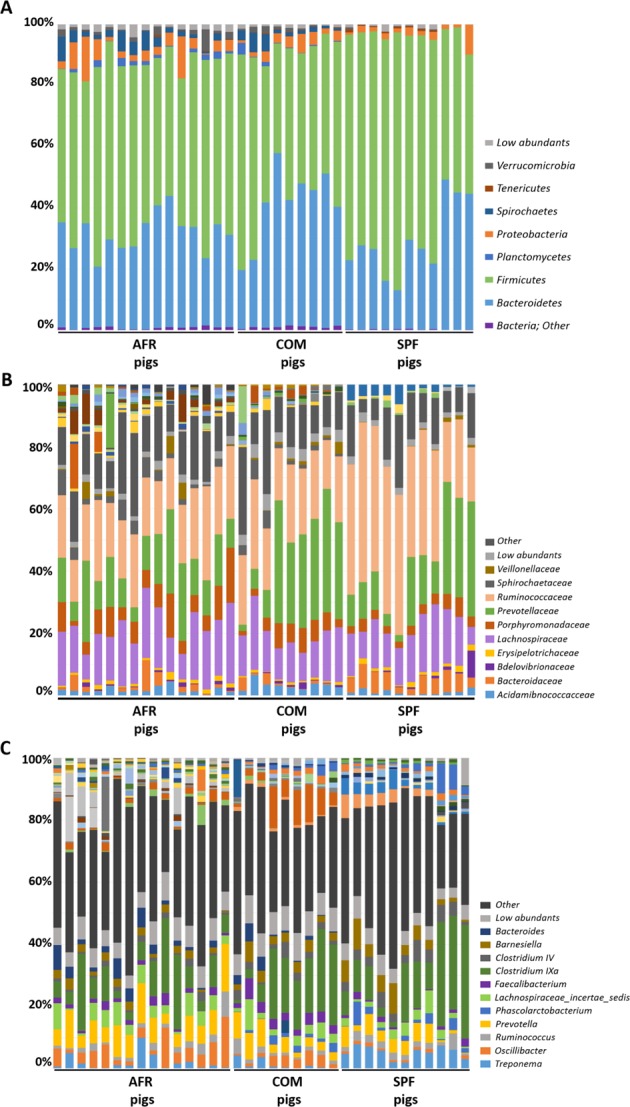
GIT microbiota composition of domestic pigs (sus scrofa) obtained from Africa (AFR pig), from a conventional commercial farm (COM pig) and from SPF facilities (SPF pig). The mean relative abundance (%) of OTUs found in feces is presented. Each graph represents the OTUs at different taxonomical levels: phylum (A), family (B), genus (C). Only the ten most relatively abundant OTUs are shown in the legend.
Table 1.
Relative abundance of OTUs found the fecal microbiota of the different animals analyzed throughout this study.
| Genera | Relative abundance (%) | P value* | ||||
|---|---|---|---|---|---|---|
| SPA Warthog | AFR Warthog | AFR pig | COM pig | SPF pig | ||
| Family | Relative abundance (%) | P value* | ||||
| SPA Warthog | AFR Warthog | AFR pig | COM pig | SPF pig | ||
| Genera | Relative abundance (%) | P value* | ||||
| SPA Warthog | AFR Warthog | AFR pig | COM pig | SPF pig | ||
| Bacteroidetes | 23.96 | 36.85 | 31.77 | 40.11 | 29.10 | <0.05 |
| Firmicutes | 60.06 | 51.95 | 54.71 | 50.65 | 66.93 | <0.01 |
| Proteobacteria | 6.94 | 3.18 | 5.44 | 3.12 | 2.41 | <0.05 |
| Spirochaetes | 4.16 | 2.64 | 3.56 | 2.47 | 0.00 | <0.001 |
| Tenericutes | 2.20 | 0.26 | 0.09 | 0.13 | 0.36 | <0.001 |
| Verrucomicrobia | 0.22 | 2.04 | 1.08 | 0.90 | 0.18 | <0.001 |
| Acidaminococcaceae | 0 | 5.06 | 1.79 | 3.28 | 1.07 | <0.001 |
| Anaeroplasmataceae | 1.18 | 0 | 0 | 0 | 0 | <0.05 |
| Bacillaceae 1 | 0 | 0.24 | 1.41 | 0 | 0 | <0.001 |
| Bacteroidaceae | 2.84 | 0 | 2.03 | 1.2 | 5.18 | <0.001 |
| Bdellovibrionaceae | 1.92 | 0.58 | 0.49 | 0.57 | 1.15 | <0.01 |
| Enterobacteriaceae | 0 | 0 | 2.83 | 0 | 0 | <0.001 |
| Erysipelotrichaceae | 1.58 | 2.11 | 1.11 | 0.61 | 1.24 | <0.001 |
| Flavobacteriaceae | 0.72 | 1.64 | 1.16 | 0.91 | 0 | <0.001 |
| Lachnospiraceae | 16.56 | 19.93 | 16.73 | 14.13 | 14.1 | <0.05 |
| Lactobacillaceae | 0 | 0 | 1.21 | 0.5 | 0 | <0.001 |
| Marinilabiaceae | 0 | 1.61 | 0.39 | 0.7 | 0 | <0.001 |
| Peptostreptococcaceae | 1.84 | 0.56 | 0.26 | 0 | 0.32 | <0.05 |
| Planctomycetaceae | 0 | 0.95 | 1.09 | 0.73 | 0 | <0.001 |
| Planococcaceae | 0 | 0.41 | 0.51 | 1.36 | 0 | <0.005 |
| Porphyromonadaceae | 4.78 | 2.63 | 7.63 | 5.07 | 3.45 | 0.54 |
| Prevotellaceae | 9.68 | 14.61 | 14.25 | 24.04 | 17.59 | <0.001 |
| Rhodospirillaceae | 1.8 | 0 | 0 | 0 | 0 | <0.001 |
| Rikenellaceae | 0 | 0 | 0 | 0 | 2.74 | <0.001 |
| Ruminococcaceae | 21.72 | 13.88 | 20.41 | 21.42 | 36.89 | <0.001 |
| Sphingobacteriaceae | 0 | 0 | 0.45 | 1.51 | 0 | <0.001 |
| Spirochaetaceae | 4.16 | 2.64 | 3.06 | 2.44 | 0 | <0.001 |
| Subdivision5_genera_incertae_sedis | 0 | 2.02 | 0.45 | 0.6 | 0 | <0.001 |
| Veillonellaceae | 0 | 0.79 | 1.37 | 1.01 | 0.43 | 0.63 |
| Verrucomicrobiaceae | 0 | 0 | 1.03 | 0 | 0 | <0.001 |
| Acetanaerobacterium | 0.42 | 0 | 0 | 0.97 | 2.68 | <0.001 |
| Akkermansia | 0 | 0 | 1.02 | 0.23 | 0 | <0.001 |
| Alistipes | 0 | 0 | 0 | 0 | 2.73 | <0.001 |
| Alloprevotella | 0 | 0.81 | 1.51 | 6.72 | 0 | <0.001 |
| Anaeroplasma | 1.18 | 0 | 0 | 0 | 0 | <0.001 |
| Bacillus | 0 | 0.24 | 1.41 | 0 | 0 | <0.001 |
| Bacteroides | 2.84 | 0 | 2 | 1.2 | 5.18 | <0.001 |
| Barnesiella | 1.18 | 1.28 | 5.31 | 2.6 | 0.78 | <0.001 |
| Clostridium IV | 2.06 | 0 | 2.08 | 1.62 | 2.22 | <0.001 |
| Clostridium XI | 1.84 | 0.56 | 0.26 | 0 | 0.32 | <0.05 |
| Clostridium XlVa | 6.42 | 7.01 | 7.81 | 4.89 | 3.79 | 0.1 |
| Escherichia/Shigella | 0 | 0 | 2.71 | 0 | 0 | <0.001 |
| Faecalibacterium | 0 | 1.31 | 0 | 1.4 | 2.07 | <0.05 |
| Lachnospiracea_incertae_sedis | 2.24 | 1.93 | 4.3 | 3.71 | 2.72 | <0.1 |
| Lactobacillus | 0 | 0 | 1.2 | 0.49 | 0 | <0.001 |
| Oscillibacter | 0 | 0.96 | 2.17 | 3.29 | 4.64 | <0.001 |
| Paludibacter | 1.56 | 0 | 0.67 | 0.35 | 0 | <0.001 |
| Paraprevotella | 1.68 | 3.03 | 0.71 | 1.12 | 1.2 | <0.001 |
| Phascolarctobacterium | 0 | 4.92 | 1.66 | 3.25 | 1.05 | <0.001 |
| Prevotella | 7.34 | 10.08 | 9.89 | 15.12 | 16.03 | 0.58 |
| Roseburia | 1.4 | 3.54 | 0.53 | 1.12 | 2.54 | <0.01 |
| Ruminococcus | 4.34 | 1.9 | 1.33 | 2.32 | 3.84 | <0.001 |
| Rummeliibacillus | 0 | 0 | 0.11 | 1.32 | 0 | 0.57 |
| Saccharofermentans | 0 | 1.25 | 0 | 0 | 0 | <0.001 |
| Sphaerochaeta | 1.08 | 0 | 0 | 0.56 | 0 | <0.001 |
| Subdivision5_genera_incertae_sedis | 0 | 2.02 | 0.45 | 0.6 | 0 | <0.001 |
| Treponema | 3.08 | 2.36 | 2.9 | 1.89 | 0 | <0.001 |
| Vampirovibrio | 1.92 | 0.59 | 0.49 | 0.57 | 1.15 | <0.05 |
*P values were obtained perfoming Kruskal-Wallis test, P value < 0.05 means at least one of the mean abundances between the groups is different.
Six main phyla were found in AFR pigs feces, where Firmicutes (55.64%) and Bacteroidetes (31.14%) were the most relatively abundant representing 86.78% of the total composition (Fig. 1A, Table 1). Proteobacteria (4.87%) was the third most abundant phylum followed by Spirochaetes (3.06%), Verrucomicrobia (1.53%) and Planctomycetes (1.09%). At the phylum level, unassigned sequences (designated as ‘other’) represented less than 1% of the total sequences. Among the families identified, Ruminococaceae family (from Firmicutes) was the most relatively abundant, as in SPF pigs, representing 20.41% of all the assigned families (Fig. 1B). Also within the phylum Firmicutes, Lachnospiraceae was highly abundant representing 14.13%. Prevotellaceae was the most abundant within the Bacteroidetes phylum for AFR pigs, with 14.25% of relative abundance. Remarkably, within this phylum, the Porphyromonadaceae family was found in high abundance in AFR pigs (7.62%). Curiously, the family Verrucomicrobiaceae, was only found in these pigs. The percentage of unassigned sequences at family level was 14.76%.
The most relatively prevalent genus found in AFR pigs was Prevotella from Prevotellaceae (Bacteroidetes) comprising 9.89% of all the assigned genera (Fig. 1C, Table 1), although in lower abundance than in SPF (16.03%) or COM pigs (15.12%). Within the Ruminococcaceae family, Oscillibacter was the most relatively abundant in all groups (2.17%, 3.29% and 4.64% in AFR, COM and SPF pigs, respectively), however many differences in composition were found depending on the group. For instance, the feces from SPF animals showed a higher relative abundance of Ruminococcus (3.84%), when compared to COM (2.32%) or AFR (1.33%) pigs. Interestingly, some genera found in SPF pig microbiota, were completely absent or undetected in AFR pigs, such as Faecalibacterium and Acetanaerobacterium. Among the genera specific for each group, we also identified Alistipes in SPF pigs, while Bacillus and Escherichia/Shigella were specific for AFR pigs. No genus, with relative abundance higher than 1%, was specific of COM pigs. Moreover, several genera were found more relatively abundant in SPF pigs than in AFR pigs, including Bacteroides (5.18%) and Roseburia (2.54%). Conversely, other genera were found less abundant than in AFR pigs such as Clostridium XIVa (3.79%), Lachnospiracea incertae sedis (2.72%) and Barnesiella (0.78%) or were even undetected, such as the potentially pathogenic Treponema and Alloprevotella. Noteworthy, many genera present in AFR pigs were completely absent or in low abundance in SPF pig feces (Table 1, Fig. 1C). Unfortunately, a significant percentage of the sequences could not be confidently assigned to specific genus (36.59%, 37.15% and 34.26% for AFR, SPF and COM pigs, respectively) and additional analyses should be done to better characterize them.
The fecal bacterial community in warthogs (Phacochoerus africanus)
African warthog (AFR warthog) feces were obtained by Kenyan wildlife service (KWS) through visual identification of the animals and collected just after deposition (n = 14). Moreover, samples from warthogs (n = 5) born and raised in the Barcelona zoo from Spain (SPA warthogs) were also included in the analysis. The raw reads obtained were 13,295,340 for this group.
The warthog fecal microbiota was comprised of five main phyla (>1% relative abundance) both in wild (AFR warthogs) and captive animals (SPA warthogs). Firmicutes and Bacteroidetes were the most abundant phyla, representing 88.80% of the total microbiota composition (Fig. 2A, Table 1) in AFR warthogs, and 84.02% in SPA warthogs. Proteobacteria was the third most abundant phylum in both groups, although with significant differences in its relative abundance (6.94% SPA and 3.18% AFR warthogs). The relative abundance of the Spirochaetes phylum was almost doubled in the SPA warthogs (4.16%) in comparison with African animals (2.64%). The fifth phylum was different depending on the group, being Tenericutes (2.20%) for SPA warthogs and Verrucomicrobia (2.04%) for AFR warthogs.
Figure 2.
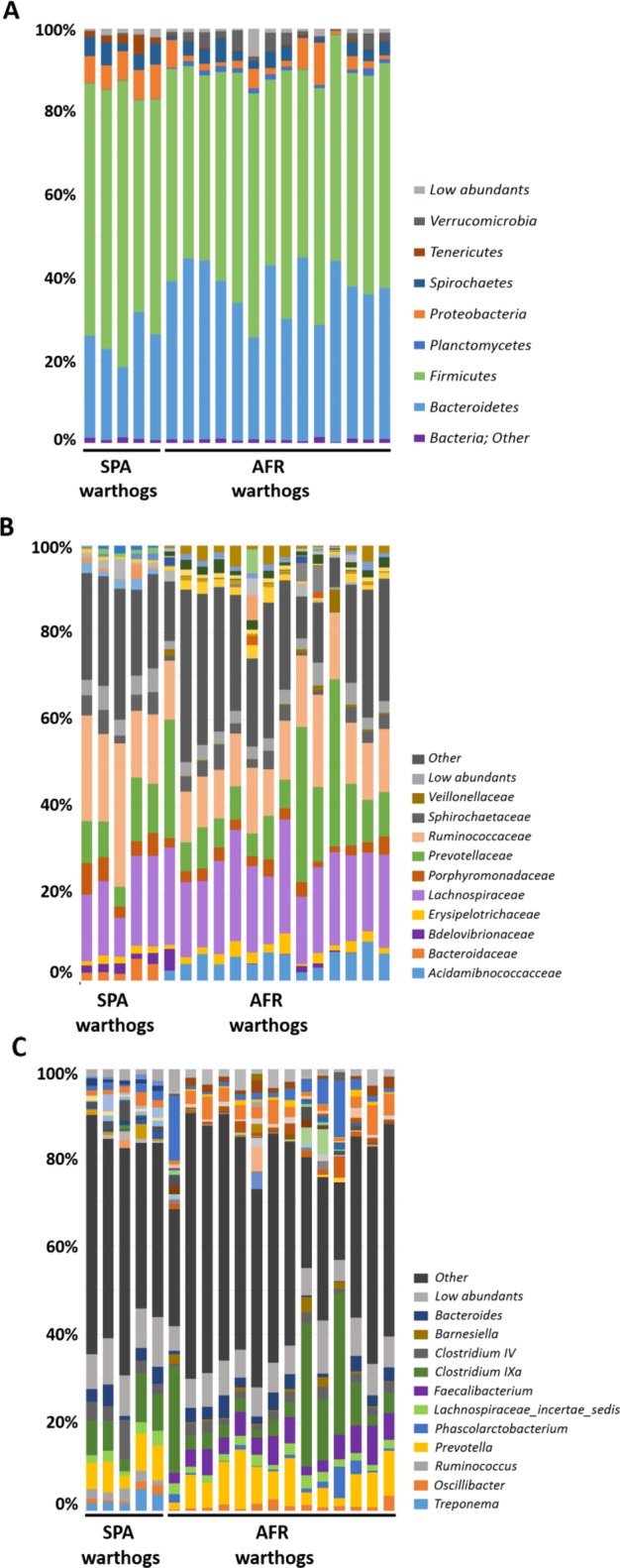
GIT microbiota composition of warthogs (Phacochoerus africanus) coming from Africa (AFR warthog) and from captive animals from Barcelona (Spain) zoo (SPA warthog). The mean relative abundance (%) of OTUs found in feces is presented. Each graph represents the OTUs at different taxonomical levels: phylum (A), family (B), genus (C). Only the ten most relatively abundant OTUs are shown in the legend.
Within Firmicutes, the Lachnospiraceae family was the most abundant in AFR warthogs, representing 19.93% of all the families found, while it represented 16.56% for SPA warthogs (Fig. 2B). The most abundant family found in SPA warthogs was the Ruminococcaceae family from Firmicutes with 21.72%, which represented only 13.88% of all the OTUs assigned at family level in AFR warthogs. Prevotellaceae was the most abundant family within the Bacteroidetes phylum for AFR warthogs, with 14.61% of relative abundance, while it represented 9.68% in SPA warthogs. Within this phylum, the Porphyromonadaceae family was more abundant in captive warthogs than in wild animals from Africa (7.62% and 2.63% for SPA and AFR warthogs, respectively). Many families present in AFR warthogs were absent or in low abundance in SPA warthogs, such as Acidaminococcaceae (5.06%, AFR warthogs), Marinilabiaceae (1.61%, AFR warthogs), and Subdivision5 genera incertae sedis (2.02%, AFR warthogs); while others showed the opposite tendency, such as Anaeroplasmataceae (1.18%, SPA warthogs), Bacteroidaceae (2.84%, SPA warthogs) Peptostreptococcaceae (1.84%, SPA warthogs) and Rhodospirillaceae (1.8%, SPA warthogs). The percentage of unassigned sequences at family level was similar for both groups, being 24.22% for AFR warthogs and 24.62% for SPA warthogs.
The genera (Fig. 2C, Table 1) exhibiting the highest relative abundance were the same in both groups, with Prevotella as the most relatively abundant (10.08% in AFR and 7.34% in SPA warthogs) and Clostridium IVa, as the second most relatively abundant in both cases (7.01% for AFR and 6.42% in SPA warthogs). Interestingly, Phascolarctobacterium, the third most abundant genus in AFR warthogs (4.92%), was not detected in captive animals (SPA). Some abundant genera within the AFR warthog fecal population were in lower abundance in SPA warthog feces, such as Faecalibacterium (1.31%) and Saccharofermentans (1.25%). By contrast, several genera found in SPA warthogs were absent in their African counterparts, e.g. Anaeroplasma (1.18%), Bacteroides (2.84%), Clostridium IV (2.06%), Paludibacter (1.56%), Ruminococcus (4.34%) and Sphaerochaeta (1.08%).
Unfortunately, a significant percentage of the sequences could not be confidently assigned to a specific genus (43.04% and 45.5% for AFR and SPA warthogs respectively) and require further characterization.
Diversity analysis
The diversity in the fecal microbiota of each group was estimated and compared. Rarefaction at maximum depth was done to measure evenly the richness within each group of animals. The mean observed species at this depth was of 2,396 for AFR warthogs, 2,218 for AFR pigs, 1,667 for SPA warthogs 2,183 for COM pigs and 1,764 for SPF pigs. In all samples, the plateau was reached at the maximum depth, meaning that the sampling procedure was adequate.
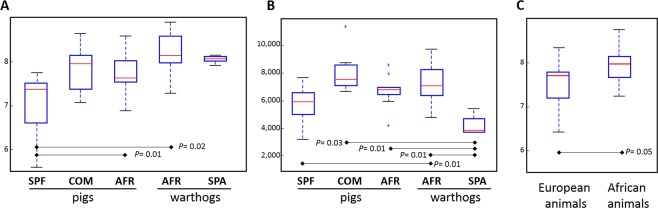
Analysis of alpha diversity metrics, using Shannon’s index showed the lowest diversity in the fecal microbiota of SPF pigs, which was statistically significant when compared with AFR warthogs and AFR pigs (non-parametric test 999 permutations, P < 0.05, Fig. 3A). Remarkably, the microbiota of AFR warthogs resembled the microbial diversity and the richness estimated through the Chao index (Fig. 3B), found in the domestic pigs (AFR pigs). Worth to mention, is the observation that SPA warthogs showed the same diversity in their feces as the wild counterparts (Fig. 3A), but the least richness together with SPF pigs when compared with the rest (Fig. 3B, Supplementary Table S2). The feces from African wild and domestic animals grouped together were the most diverse (Shannon P value = 0.05) when compared against all other groups (Fig. 3C).
Figure 3.
Alpha diversity on rarefied fecal samples from pigs (AFR, COM and SPF) and warthogs (AFR and SPA). Alpha diversity was compared between groups by measuring the Shannon-Wiener’s metrics (A). Species richness was computed through Chao1 index (B). Shannon-Wiener’s metrics were also calculated for African animals grouped together (AFR pigs and AFR warthogs) against European animals (SPA warthogs, COM and SPF pigs (C). Dotted lines represent the standard deviation Outliers are indicated with plus signs on the plot.
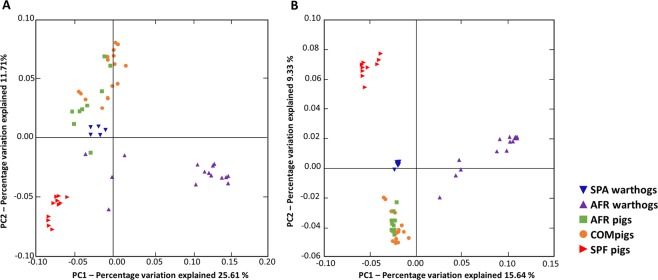
To understand the differences across the fecal microbiota of different samples, the beta diversity was estimated. Weighted and unweighted UniFrac phylogenetic distances were used to generate the beta diversity distance matrices and calculate the degree of differentiation among the samples. Samples were grouped to test the factors leading to clustering and principal coordinate analysis was done on each group. Resampling was performed repeatedly on a subset of the available data for each sample evenly (jackknifing) to measure the robustness of individual clusters (Fig. 4). Analysis of similarities was performed with a nonparametric statistical test (ANOSIM) and R was estimated based on the difference mean ranks between groups, where R = 0 indicates completely random grouping. The mean distances between all the groups were calculated and showed to be statistically different in both weighted (Fig. 4A; R = 0.6785, P = 0.001) and unweighted analyses (Fig. 4B; R = 0.9034, P = 0.001), showing both a quantitatively and qualitatively different composition of the microbiota according to the groups. Distance-based redundancy analysis (db-RDA) was performed to explore the clustering of the samples in an ordination plot when they were divided in two groups: resistance warthogs (SPA and AFR) or susceptible pigs (SPF, COM and AFR) animals (Supplementary Fig. S3). The db-RDA diagram depicting the community structure revealed a cluster of warthogs apart from pigs, where the greatest amount of variation was explained by the explanatory variable ‘resistance-to-ASF’ (P < 0.05).
Figure 4.
Principal Coordinate Analysis plots (jackknifed) representing beta diversity on rarefied samples. Beta diversity of fecal samples from pigs (AFR, COM and SPF) and warthogs (AFR and SPA) was computed through weighted (A) and unweighted (B) UniFrac analyses.
Description of the ASF resistant and susceptible core microbiota
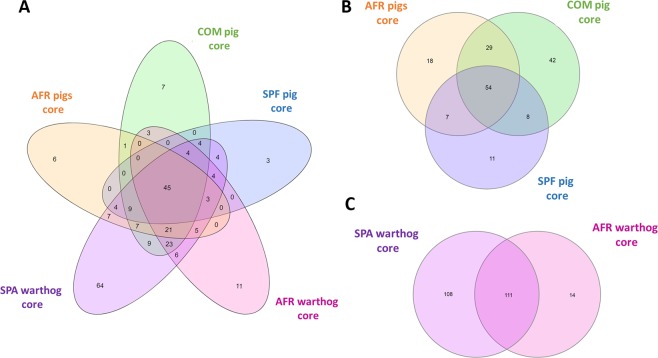
Many differences were detected in the fecal microbiota composition of the different groups analyzed throughout this study. However, one of the main objectives of this study was to identify particular OTUs differentially present in feces of ASF-resistant animals in comparison with susceptible ones. Since some bacteria found in feces could be in transit through the animal, but not representative of a stable bacterial community, a core analysis was made. The core was defined as those OTUs present in all the samples from either warthogs (AFR and SPA warthogs, resistant core) or pigs (COM and SPF pigs, susceptible core) with the aim of uncovering the main differences of permanent inhabitants of the gut from animals with different susceptibilities to ASF.
The Venn diagrams showing the number of OTUs found for each core are depicted together in Fig. 5A. Additionally, subgrouping comparisons are shown in Fig. 5B,C. The SPF pig core exhibited the lowest number with 81 OTUs, while the AFR pig core was composed of 109 OTUs and the COM pig core comprised 136 OTUs, as depicted in Fig. 5B. The susceptible core, composed of 54 OTUs, was considered as the intersection between the OTUs present in COM, SPF and AFR pig cores. The resistant core, defined as the OTUs present in all the samples from both AFR and SPA warthogs, consisted of 111 OTUs, as shown in Fig. 5C. The resistant and susceptible cores shared 45 OTUs. From the 111 OTUs present in the resistant core, 93 OTUs are shared with COM pigs, 56 OTUs are present in the SPF core and 74 OTUs in the AFR pig core, leaving 6 OTUs exclusively within the resistant core (Table 2). Likewise, from the 54 OTUs of the susceptible core, 9 OTUs are shared with the SPA warthog core, while absent in the AFR warthog core. Moreover, there are 7 OTUs exclusively present in the COM pig core, and only 3 OTUs were exclusively present in the ASFV highly-susceptible core from SPF animals. The full list of OTUs from each of the five cores is presented in Supplementary Tables S4 and S5. The mean relative abundances of the 6 OTUs exclusively from the resistant core, are shown in Table 2.
Figure 5.
Venn diagram showing the number of shared OTUs among the core communities of all groups of wild and domestic pigs.
Table 2.
Mean relative abundance (%) of the OTUs exclusively found in the core microbial composition of feces from AFSV resistant animals.
| Out | Mean relative abundance (%) | Mean relative abundance Group (%) | P value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Domain | Phyla | Order | Class | Family | Genus | SPA warthog | AFR warthog | SPF pig | COM pig | AFR pig | Resistant warthogs | Susceptible pigs | |
| Bacteria | Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | Paludibacter | 1.555 | 0.287 | 0.000 | 0.362 | 0.658 | 0.921 | 0.340 | <0.05 |
| Bacteria | Tenericutes | Mollicutes | Anaeroplasmatales | Anaeroplasmataceae | Anaeroplasma | 1.191 | 0.194 | 0.241 | 0.095 | 0.089 | 0.692 | 0.141 | <0.05 |
| Bacteria | Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | Petrimonas | 0.382 | 0.090 | 0.000 | 0.000 | 0.002 | 0.236 | 0.001 | <0.05 |
| Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | 0.023 | 0.580 | 0.003 | 0.002 | 0.022 | 0.301 | 0.009 | <0.05 | ||
| Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.020 | 0.578 | 0.002 | 0.001 | 0.021 | 0.299 | 0.008 | <0.05 | |
| Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | Moraxella | 0.006 | 0.294 | 0.000 | 0.000 | 0.001 | 0.150 | 0.000 | <0.05 |
Functional prediction
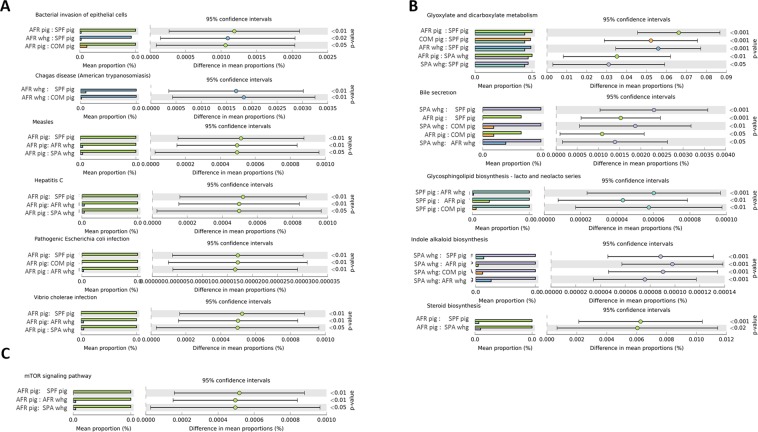
The community’s functional capabilities were predicted using PICRUSt (based on the 16rRNA gene data) to explore the function of gut bacteria and better characterize the potential role exerted by the microbiota of the different groups under study. Principal component analysis (PCA) was performed in order to evaluate the taxonomic profile differences among the microbial communities of each group (Fig. 6). When samples were analyzed according to the animal group they belonged (COM, SPF, AFR pigs or SPA, AFR warthogs) showed good clustering (Fig. 6A, PERMANOVA R2 = 0.2347, P = 0.001). However, the highest percentage of the variability was explained by grouping samples by the continent of origin, as depicted in Fig. 6B (PERMANOVA R2 = 0.4242, P = 0.001). Pathway analysis was conducted using Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways at three different levels. The level 1 KEGG pathways showed a predominant proportion of significant predicted functions linked to Metabolism pathways (48.5%) followed by Organismal Systems (13.2%) together with Human diseases (13.2%), and Environmental Processes (9.3%). Within these four most abundant pathways, we selected those pathways related to either infectious diseases, nutrition or immune response showing statistically significant differences. These active features at the level 2 KEGG pathway categories corresponded to Infectious Diseases, representing the 5.6% from the total of the active features, Signal Transduction (4.0%), Biosynthesis of Other Secondary Metabolites (5.3%), and Digestive System (2.6%). We focused on representative active pathways at the level 3 KEGG pathway categories that showed statistically significant differences within these active functions (Fig. 7). KEGG functional predictions related to infectious diseases showed significant differences between the AFR pigs or AFR warthogs when compared with the rest of the groups (Fig. 7A). Five metabolic pathways related to nutrition were found to be statistically different (Fig. 7B). KEGG gene families associated to glyoxylate and decarboxylate were significantly diminished in SPF pigs compared to all the other groups. Regarding the families associated with bile secretion, both samples from warthogs and AFR pigs were more highly represented than the rest. Moreover, those families related to glycosphingolipid biosynthesis had marked differences between SPF pigs compared with all other groups. The indole alkaloid biosynthesis pathway was higher in SPA warthogs, while the steroids biosynthesis was higher in AFR pigs when compared with SPF pig and SPA warthogs (Fig. 7B). Furthermore, the mTOR signaling pathway related to immune response (Fig. 7C) was higher in AFR pigs compared with both AFR and SPA warthogs, and SPF pigs.
Figure 6.
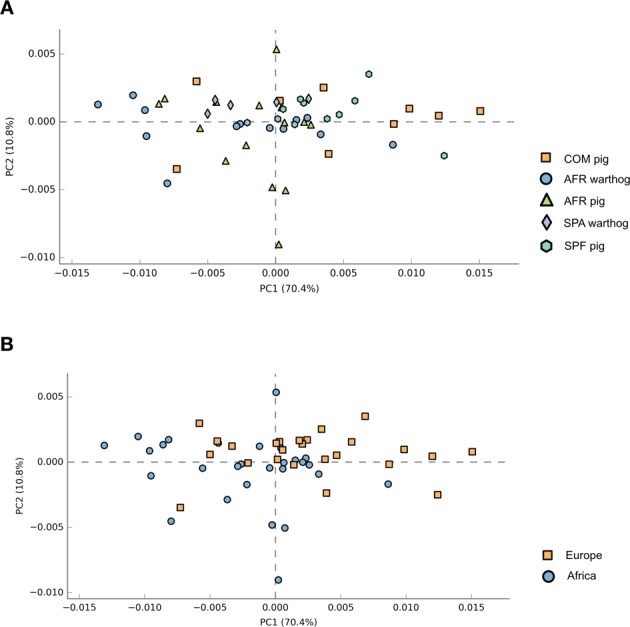
Principal component analysis illustrating distances between predicted sample pathway profiles. PC1 accounts for 70.4% and PC2 10.8% of the variation in the PICRUSt predicted pathways. Samples are colored by either the pig group they belonged to (A) or by continent of origin (B).
Figure 7.
Prediction of changed KEGG pathways using PICRUSt analysis. Selected pathways found to be significantly different among the different animal groups are depicted based on their relation to infection diseases (A), metabolic pathways (B) and immune response (C). Dot plots on the right show the differences in mean proportions between the two indicated groups using P values. All KEGG pathways that were significantly changed are detailed in Supplementary Table S6.
Discussion
Once a healthy gut microbial community has developed, it will be quite stable through life. The GIT microbiota is shaped as the diet becomes more complex and the immune-system matures leading to different digestibility capacities29. Many studies have demonstrated that factors, such as locality and habitat, are important in the microbial community structure, because variables like local flora and fauna, photoperiod, available food and climatic conditions may affect host microbiota30,31. The definition of a healthy microbiome is important since its maintenance is recognized as a major factor in animal health32, development33 and productivity34,35.
In this work, the microbiota of wild African warthogs and SPF pigs was deeply studied for the first time, unravelling many interesting differences when compared with both African domestic pigs and European warthogs raised in confined conditions in the zoo. Although there are many reports describing the GIT microbiota of domestic pigs, we decided to include this group in the analysis under the same experimental and in silico procedures for comparison. The variations found can be explained by genetic differences between animal species and/or the environmental conditions under which the animals were raised, including diet, antibiotic treatments or pathogen exposure. Therefore, when comparing groups where genetic differences are minimized, the environmental conditions, including diet, management and antibiotic treatments, become the main responsible of the differences in microbial diversity observed. AFR warthogs showed high diversity and richness, while their counterparts subjected to a completely different environment, SPA warthogs, showed lower richness but similar diversity. With many taxa missing from SPA warthogs, such as Acidaminococcaceae, Marinilabiaceae, Planctomycetaceae, Veillonaceae, some others were increased to relative abundances near to the values seen in COM pigs, probably related to similarity in the diets of these groups (e.g. Ruminococcaceae, Porphyromonadaceae, Bacteroidaceae). Importantly, the relative abundance of Prevotella was higher in wild warthogs when compared with the captives from the zoo, in agreement with a previous report27. Hence, the increase detected in other microbial families could be to counteract the loss of this beneficial genus, a hypothesis that need further confirmation.
Other important difference was revealed when analyzing SPF pig microbial composition. As expected, the microbiota diversity in SPF pigs exhibited a different scenario than in COM pigs. Firmicutes had the highest abundance in the microbiota within all the groups tested, suggesting that the reduction of pathogenic bacteria from other phyla, is probably fulfilled, at least in part, by this phylum. The same tendency was also observed in AFR warthogs, probably indicating that some bacteria within Firmicutes might be markers of a healthy gut microbiota. However, this hypothesis needs confirmation. Noteworthy, the family Porphyromonadaceae was observed in low amount in the SPF microbiota. This observation is potentially important given that the SPF microbiota was generally less diverse and rich. The lower relative abundance of this family therefore translates to drastic reduction in absolute numbers. This family should be investigated further, since the loss of members of the microbiota in SPF animals might be involved in the impaired functions or increased disease susceptibilities seen in this animal group13. Allistipes was found within the distinct genera found in SPF pigs. Interestingly, high abundances of this genus have been associated with autoimmune disorders36,37. SPF pigs lacked many other genera present in their commercial (non-SPF) counterparts, such as Treponema considered as the main genus driver of the enterotype-like previously defined for pigs38. Moreover, the lack of Treponema in this group might be associated with the high abundance of Ruminococcus in SPF pigs, since it is accepted that both Ruminococcus and Treponema are involved in the same cellulose and lignin degradation pathways39. The loss of Lactobacillus in the SPF pigs may be significant, as it is considered as a beneficial genus for pig gut health40. Moreover, the loss in richness and diversity is in agreement with the impaired immune system development41. Other interesting findings showed that potential pathogenic bacteria (for domestic pigs) are more often present in pigs in contrast to warthogs. For instance, the family Enterobacteriaceae, present only in pigs from Africa, are considered a major source of foodborne diseases in human42 and also cause different diseases in pigs43. The presence of this family is particular to the individual44 and it has proven to be absent from the core microbiota from the swine gut45.
When comparing AFR and COM pigs many aspects may be important. All the samples analyzed clustered together when analyzed using PCoA, meaning that many members of the fecal microbiota are common to these pigs, irrespective of the habitat they live (i.e. Lactobacillus). COM pigs showed higher richness, but this might represent an artifact of having higher numbers of unidentified OTUs in the AFR animals. This idea is supported by the fact that no frequently observed OTU (>1% relative abundance) was present in COM animals and absent in AFR pigs, while the opposite was observed in several cases; that is the case of Bacillaceae, Enterobaceriaceae and Peptostreptococcaceae. These three OTUs should be further studied as they might be involved in the increased resistance to ASFV infection of AFR pigs reported elsewhere46.
We also found many differences between the two different animal species analyzed from Africa, AFR warthogs and AFR domestic pigs, which formed two distinct clusters according to PCoA (Fig. 4). Since both species share similar environment (African domestic pigs are free-ranging animals), the differences observed could probably reflect their genetic differences. Further studies should be needed to confirm this hypothesis. Although at phylum level there are no major differences between these two groups, when analyzing families many differences were present. For instance, Bacteroidaceae, a family that contains both commensal and pathogenic bacteria, was absent in AFR warthogs although present in African pigs (as in COM pigs). Among the bacteria belonging to this family, Bacteroides fragilis is a pathogen that has been associated with diarrheal disease in young pigs47, and seems to be colonizing specifically colonizing domestic Sus scrofa. The Prevotellaceae family, known to contain the most important primary fermenters48, is present in the feces from African warthogs and pigs at the same percentage, probably related to the digestion of complex carbohydrates derived from plants that this microbial network is involved in processing. The Ruminococcaceae family was present in lower abundance in AFR warthogs. The lower abundance of this family representing anoxic fermentative digestive tracts like rumen and large intestine of animals42 is, at least partially, due to the diminished abundance of the genus Ruminococcus. Another important genus that has been associated with many beneficial roles40, Lactobacillus, was detected only in domestic pigs, although in higher abundance in the African animals (AFR pigs) analyzed. Diet may also play a role in this finding, since African pigs are not cereal-fed to the same extent as commercial pigs, and many times are fed with household organic waste48. Unfortunately, the diet on this group was not available so the conclusions regarding the microbiota composition should be taken carefully due to potential impact of the different diet29 received in this group. Many of the similarities found when comparing AFR warthogs and AFR pigs are important features that unveil the complexity of community structure related to the environment. However, the similarities found between AFR and COM pigs that led to the clustering of these two groups together is perhaps surprising given the differences in environment. The absence of particular families in COM pigs should also be analyzed further. This might be related to the loss of a beneficial role in protection against pathogens in wild species.
Many different clades were identified throughout this study, however only a few predicted categories were found to be statistically different between the groups. Interestingly, when all the samples from Africa were grouped together and compared against all the European ones, two different clusters were created. This suggests that, although the microbial communities might be dissimilar between African animals, the overall functional pathways seem to be conserved. This is in agreement with what was reported in the Human Microbiome Project demonstrating stability of metabolic pathways despite variability of the underlying microbial taxa49. Based on large number of reports, it is now widely accepted that the commensal microbiota has a huge impact on the metabolism of the host11. Dysbiosis in the GIT has been linked to many alterations in immune responses and in disease development50. The fact that we found the mTOR signaling pathway more highly represented in AFR pigs deserves further study as this pathway is important in adaptive immunity and plays a crucial role in keeping the balance between T cell quiescence and activation51 which is essential for immune protection14. The link between the intestinal microbiome and hepatitis B virus infection52, HIV infection53 and severity of Malaria in Plasmodium infections54, has also been demonstrated. The differences among the infectious diseases pathways found in AFR pigs and warthogs when compared to the rest of the groups, suggest these gene families should be further analyzed in order to fully understand the differential disease susceptibility seen in these animals. Moreover, the differences in nutrition-related pathways might reflect the differences in the digestive system and can be useful for understanding the full structure of microbial community composition in these species. All these predictions about the microbiome function should be treated as hypotheses rather than conclusions and need validation through functional assays55.
The core analysis of the OTUs present in all the samples from ASFV resistant animals compared with the core elements present in susceptible animals, provided some remarkable findings. Bacterial commensal microbiota might be involved in the resistance to ASF disease, as it has been demonstrated for other viral infections in swine56,57. The OTUs found exclusively in resistant animals represent good candidates for testing experimentally. The fact that all the OTUs in the resistant core were occasionally present and in low abundance in animal feces, points out to the importance of characterizing the complete communities inhabiting the GIT. It cannot be ruled out that some more abundant species pertain to the resistant core, as the analysis does not include the unassigned OTUs, which, unfortunately, represent a high number in the warthog microbiota. Several core OTUs present in resistant animals appeared as potential candidates involved in the resistance towards ASF viral disease, and therefore require further investigation. For instance, probiotics from the Anaeroplasma genus have shown to improve both weight gain and feed intake, while reducing diarrhea in early-weaned piglets58. On the other hand, some of these exclusive genera have been associated with pathology. Within the vast diverse genus Moraxella, at least some species have been shown to be pathogenic, such as M. bovis59,60. Also, M. porci and M. plurianimalium that have been isolated from systemic lesions61,62. For other OTUs, there is controversial data indicating either beneficial or detrimental roles in the pig GIT, which may depend on the specific strain.
In this study, we described many relevant members from the microbiota composition of ASF-resistant animals that deserve further characterization. Nevertheless, it should be stated that, due to both the limitations of the technology used and the scarcity of data related to wild species available in the databases, the complete structure of the GIT microbiota has not been defined, since many taxa remained unidentified especially in warthogs. Moreover, the microbiome is the collection of all host-associated microorganisms including not only the bacterial and archaeal microbiome, but also the mycobiome (fungi), the virome (bacteriophages, eukaryotic viruses) and the meiofauna (unicellular protozoa and helminths)63,64, highlighting the importance of expanding future studies to uncover the entire microbiome. Further studies using metagenomics approaches will assist in the characterization of the full structure of the wild and domestic porcine GIT microbiota and unveil the potential novel functional pathways present in wild animals. It would potentially be of interest in the future to analyze the microbiota of African bush pigs (Potamochoerus larvatus) which also undergo asymptomatic infections with ASFV, to see if there are similarities with the warthogs, which are phylogenetically more distantly related to domestic pigs (Sus scrofa). To better define the optimal mixtures carrying out precise functions, we are currently characterizing bacterial isolates from warthog feces in greater depth. Meanwhile, we hypothesize, based on the success of fecal transplantation to treat infections65 in other species, that an exploration of this therapy as potential treatment for a wide variety of domestic pigs diseases may result in improved pathogen resistance.
Methods
Animals and sample collection
Fecal samples from 15 wild African warthogs (Phacochoerus africanus) were kindly collected by the Kenyan Wildlife Service (KWS) just after the observation of deposition and visual confirmation of the species of interest in Kenya (GPS coordinates −1.336498, 36.777597). Samples from twelve different African indigenous pigs (Sus scrofa) were collected from a specific backyard farm in Kenya (GPS coordinates −1.268028, 36.722050). SPF pig (Sus scrofa domesticus) feces were collected from the French Agency for Food, Environmental and Occupational Health and Safety, ANSES in Ploufragan, France. Fecal samples from 12 pure Large-White domestic pigs (Sus scrofa domesticus) from the same pen, were obtained from a high-health status commercial farm from France. Both groups of pigs were fed with same composition with the addition of a mix of minerals and vitamins and in the absence of antibiotics and anti-parasites. The food pellets of SPF pigs was additionally treated by heat at 140 °C and kept at 80 °C for 2 minutes, then the temperature was decreased to 15–20 °C. At the end of the process, the humidity was of 12–13%. Aiming to mimic the random collection of feces in the African fields, samples from males and females of different ages (post-weaned) were collected from both conventional and domestic pigs, taking into account that these are factors that do not influence in ASFV susceptibility. Feces from the five adult African warthogs (Phacochoerus africanus) born (same litter) and raised in the Barcelona zoo (Spain) were also included in the analysis. Captive warthogs from the zoo were fed with commercial cereal-based feed, but complemented with apples, potatoes and carrots. No antibiotic treatment was used at least three months prior to the collection of feces for any of the captive or domestic animals included in this study.
Samples from all the animals included in this study followed the sample collection procedure follow described. Feces were freshly obtained just after. A portion from the interior area of the fecal sample was collected with a sterile spoon and placed in a sterile tube until they were transported to the laboratory on ice. Upon arrival, 300 mg of fresh feces were stored at −80 °C for further analysis. According to European (Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes) and Spanish (Real Decreto 53/2013) normative, feces collection procedure did not require specific approval by an Ethical Committee (Chapter I, Article 1, 5 (f) of 2010/63/EU).
DNA extraction and 16S rRNA sequencing
Total DNA was extracted from 300 mg of resuspended feces in 900 µl of PBS using the Nucleospin Blood kit (Machinery Nagel). Purified DNA was eluted in a final volume of 50 μl of elution buffer (5 mM Tris, pH 8.5). The quality and quantity of genomic DNA was evaluated on a BioDrop DUO (BioDrop Ltd). The library preparation for sequencing was performed within 24 h after the DNA extraction, at Servei de Genomica, Autonomous University of Barcelona.
Sequencing and taxonomic analysis
Sequencing of the hypervariable region V3-V4 of 16S rRNA gene was preformed using Illumina pair-end 2 × 250 bp MiSeq platform following the manufacturer instructions (MS-102–2003 MiSeq® Reagent Kit v2, 500 cycle) under the same conditions reported previously66. Five different runs with individual barcoding and a maximum of 20 samples/run were used for this study. Each sequence was assigned to its original sample according to its barcode, which was removed from sequences before further processing.
Raw sequences were initially filtered to keep only the high-quality-reads (Q > 25). Paired-end joining was done by using fastq join67,68 under default values. Classification of taxonomic abundances was done as previously reported66 using the Quantitative insights into microbial ecology69 (QIIME) software package (version 1.9). Sequences were clustered into OTUs at 97% similarity using UCLUST algorithm70 and the Greengenes database (version 13.8)71. Chimeric detection and removal was done with USEARCH 6.170,72 against the ChimeraSlayer reference database73. Taxonomic assignment was done with Naïve Bayes classification against RDP database74 (Release 11, 2016). For each taxon, the Kruskal Wallis test was perform to compare OTU frequencies in sample groups and to ascertain whether or not there are statistically significant differences between the OTU abundance in the different sample groups, P values were FDR-corrected for multiple hypotheses testing. Single rarefaction was done at 21,134 sequences/sample for alpha-diversity analysis. Alpha diversity and richness estimation through Shannon-Wiener and Chao1 indices respectively, were calculated on rarefied data using alpha_diversity.py script from QIIME. Alpha diversity between groups was compared through two-sample non-parametric t-tests (Monte Carlo method) at maximum depth in rarefied samples (with 999 permutations). UniFrac weighted and unweigthed distances were calculated to assess significant differences across samples75,76. Testing the significant differences between grouped samples was done with compare_categories.py script from QIIME75 using ANOSIM as the method77. The R statistic is calculated by ANOSIM, where an R value near +1 means that there is dissimilarity between the groups, while an R value near 0 indicates no significant dissimilarity between the groups. Samples were considered to be significantly different when the accompanying P value was < 0.05. Distance-based redundancy analysis was done to estimate the strength and statistical significance of sample groupings using Euclidean distance matrices.
Functional prediction of the microbiome were done using 16S rRNA gene sequence data using the platform NEPHELE (vs 2.2.2)78 which integrates the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States PICRUSt (vs 1.1.3)24. The closed reference OTU picking approach was done against Greengenes database (vs 13.8). Then, metagenome predicted functions were inferred using the Kyoto encyclopedia of Genes and Genomes (KEGG)79 database. Finally, the Statistical Analysis of Taxonomic and Functional Profiles software25 (STAMP vs 2.1.3) was used to both visualize functional prediction and test statistical hypothesis to analyze taxonomic and functional profiles. The analysis was done at level 3 using the unclassified data only for calculating sequencing profiles. Statistical tests to infer the biological relevance of features in the predicted metagenomic profiles between multiple groups were done with Kruskal Wallis80 followed by the Tukey-Kramer post hoc test. Effect sizes (eta-squared) and confidence intervals were also estimated to provide critical assessment of the biological relevance of test results incorporating the Bonferroni correction (p < 0.05 was considered statistically significant).
Supplementary information
Acknowledgements
This study was supported by the Ministry of Economy and Competitiveness (MINECO) from the Spanish Government (grant number AGL2016–78160-C2–1-R). The authors are also grateful to the Centres de Recerca de Catalunya (CERCA) Programme and Global Alliance for Research on African swine fever (GARA). The authors thank Frederic Paboeuf and Audrey Fougeroux for providing SPF and domestic pig samples.
Author Contributions
F.C.F. analyzed and interpreted the data, drafted the manuscript. M.B. made PICRUSt prediction analysis and statistical analysis in STAMP. M.J.N. participated in the sampling and provided with technical support. A.L. made DNA extractions in Africa. R.B. coordinated sample collection from wild animals in Africa and made input to the manuscript. C.O. and N.G. were involved in the field sampling and DNA preparation from warthogs in Kenya. M.-F.L.P. coordinated sample collection from SPF and COM pigs. V.A.D. coordinated sample collection from the Barcelona’s zoo. J.M. participated in sample collection and discussion of results. V.A. made contributions to the manuscript, discussed the results and microbiology analysis. F.R. conceived the study design and coordination, made contributions to the manuscript. All the authors reviewed and approved the final manuscript.
Data Availability
All the sequences included in this study are available at the NCBI database. SRA Accession number SUB4621742, BioProject PRJNA496331 and BioSamples SAMN10238771-824.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-49897-1.
References
- 1.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat. Immunol. 2013;14:676–684. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 3.Lallès J-P, Bosi P, Smidt H, Stokes CR. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007;66:260–268. doi: 10.1017/S0029665107005484. [DOI] [PubMed] [Google Scholar]
- 4.Isaacson R, Kim HB. The intestinal microbiome of the pig. Anim. Health Res. Rev. 2012;13:100–109. doi: 10.1017/S1466252312000084. [DOI] [PubMed] [Google Scholar]
- 5.Yeoman CJ, White BA. Gastrointestinal tract microbiota and probiotics in production animals. Annu. Rev. Anim. Biosci. 2014;2:469–486. doi: 10.1146/annurev-animal-022513-114149. [DOI] [PubMed] [Google Scholar]
- 6.Haenen D, et al. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J. Nutr. 2013;143:274–283. doi: 10.3945/jn.112.169672. [DOI] [PubMed] [Google Scholar]
- 7.Belanche A, et al. Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. J. Nutr. 2012;142:1684–1692. doi: 10.3945/jn.112.159574. [DOI] [PubMed] [Google Scholar]
- 8.Han GG, et al. Tracing of the fecal microbiota of commercial pigs at five growth stages from birth to shipment. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-017-17765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ke S, et al. Age-based dynamic changes of phylogenetic composition and interaction networks of health pig gut microbiome feeding in a uniformed condition. BMC Vet. Res. 2019;15:172. doi: 10.1186/s12917-019-1918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pluske JR, Turpin DL, Kim J-C. Gastrointestinal tract (gut) health in the young pig. Anim. Nutr. 2018;4:187–196. doi: 10.1016/j.aninu.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiebiger U, Bereswill S, Heimesaat MM. Dissecting the Interplay Between Intestinal Microbiota and Host Immunity in Health and Disease: Lessons Learned from Germfree and Gnotobiotic Animal Models. Eur. J. Microbiol. Immunol. 2016;6:253–271. doi: 10.1556/1886.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clapperton M, et al. Traits associated with innate and adaptive immunity in pigs: heritability and associations with performance under different health status conditions. Genet. Sel. Evol. GSE. 2009;41:54. doi: 10.1186/1297-9686-41-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacasta A, et al. Expression Library Immunization Can Confer Protection against Lethal Challenge with African Swine Fever Virus. J. Virol. 2014;88:13322–13332. doi: 10.1128/JVI.01893-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takamatsu H-H, et al. Cellular immunity in ASFV responses. Virus Res. 2013;173:110–121. doi: 10.1016/j.virusres.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 15.African swine fever: OIE - World Organisation for Animal Health. Available at, https://www.oie.int/en/animal-health-in-the-world/animal-diseases/african-swine-fever/.
- 16.Everett H, et al. Experimental infection of common warthogs (Phacochoerus africanus) and bushpigs (Potamochoerus larvatus) with classical swine fever virus. I: Susceptibility and transmission. Transbound. Emerg. Dis. 2011;58:128–134. doi: 10.1111/j.1865-1682.2011.01202.x. [DOI] [PubMed] [Google Scholar]
- 17.Oura CA, Powell PP, Anderson E, Parkhouse RM. The pathogenesis of African swine fever in the resistant bushpig. J. Gen. Virol. 1998;79(Pt 6):1439–1443. doi: 10.1099/0022-1317-79-6-1439. [DOI] [PubMed] [Google Scholar]
- 18.Mujibi FD, et al. Genetic diversity, breed composition and admixture of Kenyan domestic pigs. PLOS ONE. 2018;13:e0190080. doi: 10.1371/journal.pone.0190080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chenais E, et al. Epidemiological considerations on African swine fever in Europe 2014–2018. Porc. Health Manag. 2019;5:6. doi: 10.1186/s40813-018-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ober RA, et al. Increased microbiome diversity at the time of infection is associated with improved growth rates of pigs after co-infection with porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circovirus type 2 (PCV2) Vet. Microbiol. 2017;208:203–211. doi: 10.1016/j.vetmic.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 21.Rosshart SP, et al. Wild Mouse Gut Microbiota Promotes Host Fitness and Improves Disease Resistance. Cell. 2017;171:1015–1028.e13. doi: 10.1016/j.cell.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert JA, et al. Current understanding of the human microbiome. Nat. Med. 2018;24:392–400. doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson GR, Gainaru MD, Van Dellen AF. Experimental infection of warthos (Phacochoerus aethiopicus) with African swine fever virus. Onderstepoort J. Vet. Res. 1980;47:19–22. [PubMed] [Google Scholar]
- 25.Thomson GR. The epidemiology of African swine fever: the role of free-living hosts in Africa. Onderstepoort J. Vet. Res. 1985;52:201–209. [PubMed] [Google Scholar]
- 26.Anderson EC, Hutchings GH, Mukarati N, Wilkinson PJ. African swine fever virus infection of the bushpig (Potamochoerus porcus) and its significance in the epidemiology of the disease. Vet. Microbiol. 1998;62:1–15. doi: 10.1016/S0378-1135(98)00187-4. [DOI] [PubMed] [Google Scholar]
- 27.McKenzie VJ, et al. The Effects of Captivity on the Mammalian Gut Microbiome. Integr. Comp. Biol. 2017;57:690–704. doi: 10.1093/icb/icx090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lacasta, A. et al. Live attenuated African swine fever viruses as ideal tools to dissect the mechanisms involved in viral pathogenesis and immune protection. Vet. Res. 46 (2015). [DOI] [PMC free article] [PubMed]
- 29.Kanengoni AT, et al. A comparison of faecal microbial populations of South African Windsnyer-type indigenous pigs (SAWIPs) and Large White × Landrace (LW × LR) crosses fed diets containing ensiled maize cobs. FEMS Microbiol. Lett. 2015;362:fnv100. doi: 10.1093/femsle/fnv100. [DOI] [PubMed] [Google Scholar]
- 30.Godoy-Vitorino F, et al. Differences in crop bacterial community structure between hoatzins from different geographical locations. Res. Microbiol. 2012;163:211–220. doi: 10.1016/j.resmic.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Hird, S. M., Carstens, B. C., Cardiff, S. W., Dittmann, D. L. & Brumfield, R. T. Sampling locality is more detectable than taxonomy or ecology in the gut microbiota of the brood-parasitic Brown-headed Cowbird (Molothrus ater). PeerJ2 (2014). [DOI] [PMC free article] [PubMed]
- 32.Holmes E, Li JV, Marchesi JR, Nicholson JK. Gut Microbiota Composition and Activity in Relation to Host Metabolic Phenotype and Disease Risk. Cell Metab. 2012;16:559–564. doi: 10.1016/j.cmet.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Sommer F, Bäckhed F. The gut microbiota — masters of host development and physiology. Nat. Rev. Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 34.Guan LL, Nkrumah JD, Basarab JA, Moore SS. Linkage of microbial ecology to phenotype: correlation of rumen microbial ecology to cattle’s feed efficiency. FEMS Microbiol. Lett. 2008;288:85–91. doi: 10.1111/j.1574-6968.2008.01343.x. [DOI] [PubMed] [Google Scholar]
- 35.McCormack UM, et al. Exploring a Possible Link between the Intestinal Microbiota and Feed Efficiency in Pigs. Appl Env. Microbiol. 2017;83:e00380–17. doi: 10.1128/AEM.00380-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown Christopher T., Davis-Richardson Austin G., Giongo Adriana, Gano Kelsey A., Crabb David B., Mukherjee Nabanita, Casella George, Drew Jennifer C., Ilonen Jorma, Knip Mikael, Hyöty Heikki, Veijola Riitta, Simell Tuula, Simell Olli, Neu Josef, Wasserfall Clive H., Schatz Desmond, Atkinson Mark A., Triplett Eric W. Gut Microbiome Metagenomics Analysis Suggests a Functional Model for the Development of Autoimmunity for Type 1 Diabetes. PLoS ONE. 2011;6(10):e25792. doi: 10.1371/journal.pone.0025792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Liao X, Sparks JB, Luo XM. Dynamics of Gut Microbiota in Autoimmune Lupus. Appl. Environ. Microbiol. 2014;80:7551–7560. doi: 10.1128/AEM.02676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramayo-Caldas Y, et al. Phylogenetic network analysis applied to pig gut microbiota identifies an ecosystem structure linked with growth traits. ISME J. 2016;10:2973–2977. doi: 10.1038/ismej.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niu, Q. et al. Dynamic Distribution of the Gut Microbiota and the Relationship with Apparent Crude Fiber Digestibility and Growth Stages in Pigs. Sci. Rep. 5 (2015). [DOI] [PMC free article] [PubMed]
- 40.Dowarah R, Verma AK, Agarwal N. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: A review. Anim. Nutr. 2017;3:1–6. doi: 10.1016/j.aninu.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 42.How Can We Define “Optimal Microbiota?”: A Comparative Review of Structure and Functions of Microbiota of Animals, Fish, and Plants in Agriculture. Front. Nutr.5, 90 (2018). [DOI] [PMC free article] [PubMed]
- 43.Guenther S, et al. Enterobacteriaceae populations during experimental Salmonella infection in pigs. Vet. Microbiol. 2010;142:352–360. doi: 10.1016/j.vetmic.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Schierack P, Walk N, Reiter K, Weyrauch KD, Wieler LH. Composition of intestinal Enterobacteriaceae populations of healthy domestic pigs. Microbiology. 2007;153:3830–3837. doi: 10.1099/mic.0.2007/010173-0. [DOI] [PubMed] [Google Scholar]
- 45.Holman, D. B., Brunelle, B. W., Trachsel, J. & Allen, H. K. Meta-analysis To Define a Core Microbiota in the Swine Gut. mSystems2 (2017). [DOI] [PMC free article] [PubMed]
- 46.Thomas LF, et al. Evidence for the presence of African swine fever virus in an endemic region of Western Kenya in the absence of any reported outbreak. BMC Vet. Res. 2016;12:192. doi: 10.1186/s12917-016-0830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myers LL, Shoop DS. Association of enterotoxigenic Bacteroides fragilis with diarrheal disease in young pigs. Am. J. Vet. Res. 1987;48:774–775. [PubMed] [Google Scholar]
- 48.Ushida K, Tsuchida S, Ogura Y, Toyoda A, Maruyama F. Domestication and cereal feeding developed domestic pig‐type intestinal microbiota in animals of suidae. Anim. Sci. J. 2018;87:835–41. doi: 10.1111/asj.12492. [DOI] [PubMed] [Google Scholar]
- 49.Peterson J, et al. The NIH Human Microbiome Project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Budden KF, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Zhang D, Liu X. mTOR signaling in T cell immunity and autoimmunity. Int. Rev. Immunol. 2015;34:50–66. doi: 10.3109/08830185.2014.933957. [DOI] [PubMed] [Google Scholar]
- 52.Chou H-H, et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc. Natl. Acad. Sci. USA. 2015;112:2175–2180. doi: 10.1073/pnas.1424775112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ling Z, et al. Alterations in the Fecal Microbiota of Patients with HIV-1 Infection: An Observational Study in A Chinese Population. Sci. Rep. 2016;6:30673. doi: 10.1038/srep30673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villarino NF, et al. Composition of the gut microbiota modulates the severity of malaria. Proc. Natl. Acad. Sci. USA. 2016;113:2235–2240. doi: 10.1073/pnas.1504887113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langille MGI. Exploring Linkages between Taxonomic and Functional Profiles of the Human Microbiome. mSystems. 2018;3:e00163–17. doi: 10.1128/mSystems.00163-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niederwerder MC, et al. Microbiome associations in pigs with the best and worst clinical outcomes following co-infection with porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circovirus type 2 (PCV2) Vet. Microbiol. 2016;188:1–11. doi: 10.1016/j.vetmic.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Huang, A. et al. Dynamic Change of Gut Microbiota During Porcine Epidemic Diarrhea Virus Infection in Suckling Piglets. Front. Microbiol. 10 (2019). [DOI] [PMC free article] [PubMed]
- 58.Xu J, et al. Yeast Probiotics Shape the Gut Microbiome and Improve the Health of Early-Weaned Piglets. Front. Microbiol. 2018;9:2011. doi: 10.3389/fmicb.2018.02011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lavin S, Lastras ME, Marco I, Cabaoes FX. Report of a Case of Bronchopneumonia Associated with Moraxella bovis Isolation in a Chamois (Rupicapra pyrenaica) J. Vet. Med. Ser. B. 2000;47:225–227. doi: 10.1046/j.1439-0450.2000.00332.x. [DOI] [PubMed] [Google Scholar]
- 60.Baptista PJHP. Infectious bovine keratoconjunctivitis: a review. Br Vet J. 1979;135:225–242. doi: 10.1016/S0007-1935(17)32882-8. [DOI] [PubMed] [Google Scholar]
- 61.Vela AI, et al. Moraxella porci sp. nov., isolated from pigs. Int J Syst Evol Microbiol. 2010;60:2446–50. doi: 10.1099/ijs.0.016626-0. [DOI] [PubMed] [Google Scholar]
- 62.Vela AI, et al. Moraxella pluranimalium sp. nov., isolated from animal specimens. Int J Syst Evol Microbiol. 2009;59:671–4. doi: 10.1099/ijs.0.006205-0. [DOI] [PubMed] [Google Scholar]
- 63.Stappenbeck TS, Virgin HW. Accounting for reciprocal host–microbiome interactions in experimental science. Nature. 2016;534:191–199. doi: 10.1038/nature18285. [DOI] [PubMed] [Google Scholar]
- 64.Norman JM, Handley SA, Virgin HW. Kingdom-Agnostic Metagenomics and the Importance of Complete Characterization of Enteric Microbial Communities. Gastroenterology. 2014;146:1459–1469. doi: 10.1053/j.gastro.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13:508–516. doi: 10.1038/nrgastro.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Correa-Fiz, F., Fraile, L. & Aragon, V. Piglet nasal microbiota at weaning may influence the development of Glässer’s disease during the rearing period. BMC Genomics17 (2016). [DOI] [PMC free article] [PubMed]
- 67.Aronesty E. Ea utils: Command-line tools for precessing biological sequencing data. (2011).
- 68.Aronesty E. TOBioJ: Comparison of sequencing Utility Programs. 10.2174/18750362013070100001 (2013)
- 69.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinforma. Oxf. Engl. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 71.DeSantis TZ, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinforma. Oxf. Engl. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haas BJ, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cole, J. R. et al. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 42 (2014). [DOI] [PMC free article] [PubMed]
- 75.Lozupone CA, Hamady M, Kelley ST, Knight R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007;73:1576–1585. doi: 10.1128/AEM.01996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okasnen, J. et al. Vegan: Community Ecology Package. R package version 2.3-0. 2015.
- 78.Office of Cyber Infrastructure and Computational Biology (OCICB), National Institute of Allergy and Infectious Diseases (NIAID). http://nephele.niaid.nih.gov (2016).
- 79.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kruskal WH, Wallis WA. Use of Ranks in One-Criterion Variance. Analysis. J. Am. Stat. Assoc. 1952;47:583. doi: 10.1080/01621459.1952.10483441. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the sequences included in this study are available at the NCBI database. SRA Accession number SUB4621742, BioProject PRJNA496331 and BioSamples SAMN10238771-824.