Toll-like receptors (TLRs) are well-known as pattern-recognition receptors in the immune system for recognizing pathogen-associated and damage-associated molecular patterns [1]. TLRs play an essential role in the innate and adaptive immune responses. To date, 10 functional TLRs have been identified in humans (TLR1–TLR10) and 12 in mice (TLR1–TLR9 and TLR11–TLR13) [1]. TLRs are evolutionarily conserved type I transmembrane proteins and comprise an ectodomain characterized by leucine-rich repeats mediating the recognition of ligands, a transmembrane region, and cytosolic Toll-interleukin (IL)-1 receptor domains that activate the downstream signaling pathways [1]. Most types of TLRs form homodimers between themselves, but certain TLRs can also form heterodimers, such as TLR1/TLR2 and TLR2/TLR6. The adaptor protein MyD88 is required for the downstream signaling of most TLRs, but not for TLR3 signaling. The adaptor protein TRIF (TIR-domain-containing adapter-inducing interferon-β) is used by TLR3 and TLR4, culminating in the activation of nuclear factor kappa B (NFκB) and interferon regulatory factor 3. In the immune system, TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located in the plasma membrane to recognize bacterial membrane components, whereas TLR3, TLR7, TLR8, and TLR9 are predominantly located within intracellular endosomes to recognize nucleic acid-based ligands. Typical engagement of TLRs by immune cells initiates intracellular signaling pathways, leading to the synthesis and release of inflammatory cytokines and chemokines [1]. Mounting evidence has demonstrated that TLRs are also expressed in the central and peripheral nervous systems, in microglia, astrocytes, endothelial cells, and neurons. TLRs in the nervous system play pivotal roles in the pathogenesis of stroke, neurodegenerative diseases, multiple sclerosis, and chronic pain [2, 3]. Thus, targeting TLRs may be a promising strategy for treating many neurological disorders.
TLRs are positioned at the neuroimmune interface and are activated in neuronal and non-neuronal cells; they modulate nociceptive and pruriceptive signal processing by direct activation of primary sensory neurons and via the indirect production of pro-inflammatory mediators by non-neuronal cells [3, 4]. TLR2, TLR3, TLR4, TLR5, TLR7, and TLR9 are expressed by primary sensory neurons in the dorsal root ganglia (DRG) and play important roles in the generation of pain and itch [3, 5]. TLR2 and TLR4 mediate glial activation (astrocytes and microglia) in the spinal cord and contribute to the development of chronic pain and chronic itch [4, 6]. Interestingly, neuronal TLRs have distinct subcellular distributions. For example, neuronal TLR7 is located in the plasma membrane of DRG neurons, and couples with the ion channel transient receptor potential A1 to regulate neuronal excitability and pain [5]. However, the subcellular distribution, downstream signaling, and endogenous ligands of neuronal TLRs and their roles in chronic pain are still not well understood.
In a recently published study in the Journal of Experimental Medicine (December, 2018), Zhang and colleagues reported the striking finding that TLR8 and its endogenous ligand miR-21 in primary sensory neurons of the DRG contribute to neuropathic pain in mice [7]. Given that the Tlr7 and Tlr8 genes are highly homologous and are both located on the X chromosome, they generated a new strain of TLR8-deficient mice (Tlr8−/−) by targeting the Tlr8 gene with transcription activator-like effector nucleases to avoid impacting the nearby Tlr7 gene. They first found that the acute itch and basal pain responses were normal in Tlr8−/− mice, but nerve injury-induced mechanical allodynia and heat hyperalgesia following spinal nerve ligation (SNL) or paclitaxel were significantly reduced in Tlr8−/− mice. In sharp contrast, previous work has demonstrated that TLR7-deficient mice show reduced non-histaminergic itch but intact inflammatory and neuropathic pain [8]. Distribution analysis demonstrated that TLR8 is mainly expressed in small-to-medium-sized isolectin B4+ non-peptide neurons, and the percentage of TLR8-positive neurons and TLR8 expression are increased during the maintenance phase of neuropathic pain in mice. Thus, although both TLR8 and TLR7 are expressed by DRG neurons, and TLR8 is partially co-localized with TLR7, these results clearly indicate that neuronal TLR8 plays a role in the control of pain and itch sensation distinct from that of TLR7.
It is well-demonstrated that astrocytes and microglia are activated in the spinal cord after nerve injury, and this activation is correlated with neuropathic pain [4]. In Tlr8−/− mice, the reduced spinal activation of astrocytes and microglia after SNL is consistent with reduced neuropathic pain behaviors. As TLR8 is mainly expressed in small DRG neurons that give rise to unmyelinated (C-fibers) and thinly-myelinated axons (Aδ-fibers), and mediate noxious signal transmission to the spinal cord, it is likely that the decreased glial activation in Tlr8−/− mice is due to an indirect effect, such as reduced excitatory input from DRGs.
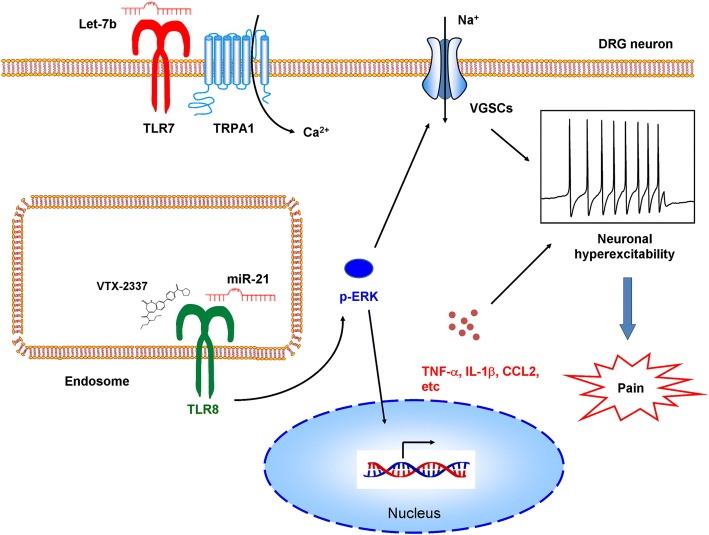
The canonical downstream signaling of intracellular TLRs (except for TLR3) induces MyD88-mediated NFκB activation and the subsequent induction of cytokines, and NF-κB activation has been used to monitor TLR function in immune cells [1]. However, the TLR8 agonist VTX-2337 does not activate NF-κB in the DRG cells that are reported to be involved in chronic pain. Interestingly, p-ERK expression, but not p38 and JNK, is markedly increased after intrathecal injection of VTX-2337. This group and others have demonstrated that p-ERK activation in the DRG plays a key role in pain after tissue inflammation or nerve injury. Intrathecal injection of a TLR8 agonist dose-dependently induces mechanical allodynia in both wild-type (WT) and MyD88−/− mice, indicating that TLR8 agonist-induced mechanical allodynia and ERK activation are not dependent on MyD88. Ma et al. reported that TLR8 activation causes inhibition of neurite outgrowth and induces apoptosis in cultured cortical neurons, and this also does not involve the canonical NF-κB signaling pathway [9]. TLR8 is not expressed in the membrane of DRG neurons, indicating that it cannot directly control plasma membrane excitability. Instead, TLR8 is expressed in the subcellular endoplasmic reticulum, endosomes, and lysosomes of DRG neurons, similar to its location in monocytes and macrophages. In contrast, TLR7 is expressed in the plasma membrane and is coupled with the transient receptor potential A1 (TRPA1) ion channel to control neuronal excitability [5]. Thus, these results suggest that the functions and downstream signaling of neuronal TLRs may be distinct from those in immune cells.
Pro-inflammation mediators are typical products of TLR activation in immune cells, and have been implicated in the pathogenesis of chronic pain [3]. It has been clearly demonstrated that the activation of ERK signaling increases TNF-α and IL-1β in DRG and trigeminal neurons. In addition, increasing evidence supports the view that ERK and inflammatory mediators enhance the excitability of DRG neurons by modulating voltage-gated Na+ channels. Thus, TLR8-mediated activation of ERK and the production of pro-inflammation mediators may form a positive feedback loop to induce neuronal hyperexcitability and contribute to the pathogenesis of neuropathic pain.
MicroRNAs (miRNAs) are small non-coding RNAs that are master regulators of gene expression by translational inhibition and mRNA degradation. MiRNAs in the nervous system are involved in the maladaptive plasticity mechanisms of chronic pain. Intriguingly, recent studies have emphasized the non-canonical functions of microRNAs in the regulation of pain. For instance, Ji et al. showed that extracellular miRNA-let-7b induces rapid inward currents and action potentials in DRG neurons, and intraplantar injection of let-7b elicits rapid spontaneous pain in a TLR7- and TRPA1-dependent manner [5]. These responses require the GUUGUGU motif, suggesting that interactions between miRNAs and TLR7 require specific structural motifs in the miRNAs. Ji et al. recently showed that extracellular miRNA-711 directly binds and activates TRPA1 to evoke acute and chronic itch in mice [10]. Functional analysis and computer simulations have revealed that miRNA-711 binds to the extracellular S5–S6 loop of TRPA1 through its core sequence GGGACCC [10]. Thus, these results indicate that miRNAs are able to regulate pain in an unconventional manner by modulating neuronal excitability via direct or indirect coupling to ion channels, distinct from the canonical roles of miRNAs in gene regulation.
Zhang et al. provide another excellent example of an unconventional role of miRNA-21 (miR-21) in the regulation of neuropathic pain. TLR8 is known to recognize single-stranded RNA and miRNAs and this leads to the production of pro-inflammatory cytokines in immune cells. Zhang et al. focused on two microRNAs, miR-21 and miR-29a, as they have been reported to bind to TLR8 and ultimately lead to tumor growth and metastasis. Although miR-21 and TLR8 are only co-localized in small- and medium-diameter DRG neurons, miR-21 can be secreted from large neurons and be taken up by other neurons to reach endosomal TLR8 and thus activate TLR8 downstream signaling. After SNL surgery in mice, miR-21 dramatically increases more than miR-29a. In the injured DRGs, miR-21 expression is consistently increased. Intrathecal injection of miR-21 decreases the paw withdrawal threshold and increases p-ERK expression in the DRG in WT mice, but not in Tlr8−/− mice. Further, miR-21 increases the expression of TNF-α, IL-1β, IL-6, CCL2 [chemokine (C–C motif) ligand 2], and CXCL1 [chemokine (C–X–C motif) ligand 1] in WT mice, but not in Tlr8−/− mice. They further recorded changes in neuronal excitability in whole-mount DRGs after incubation with miR-21 and found that the number of action potentials markedly increases in the DRG neurons of WT mice, but not Tlr8−/− mice. Thus, the role of miR-21 in the regulation of pain, activation of ERK, production of inflammatory mediators, and neuronal excitability in DRGs requires TLR8.
Many important questions raised by this new study remain. For example, where does miR-21 come from? What is the core sequence required for the binding of miR-21 to TLR8? Are there any other endogenous ligands for TLR8 besides miR-21? What are the ion channel mechanisms underlying miRNA21-induced neuronal activation? Are subtypes of voltage-gated Na+ channels or TRP ion channels involved in this process? What is the role of non-neuronal TLR8 in the regulation of neuropathic pain, given that it is expressed by many non-neuronal cells? These issues warrant further investigation.
Chronic pain, including neuropathic, cancer-related, and inflammatory pain, is a major public health issue with an incidence of 20%–25% worldwide. Unfortunately, few patients with chronic pain obtain complete relief from the currently available analgesics. The major hurdle to designing new therapeutic strategies and pain-killers is the lack of understanding of the underlying mechanisms. This report demonstrated that TLR8 and its endogenous ligand miR-21, unlike TLR7, plays a pivotal role in the maintenance of neuropathic pain. Mechanistically, TLR8 acts through a non-canonical pathway dependent on ERK activation to produce inflammatory mediators, further increase neuronal excitability, and finally contribute to the neuropathic pain. Overall, Zhang et al. have identified a crucial role of miR-21 and its receptor TLR8 in the DRG in neuropathic pain (Fig. 1), which may serve as novel promising targets for the treatment of chronic pain.
Fig. 1.
Model for the roles of miR-21 and its receptor TLR8 in the pathogenesis of neuropathic pain.
Acknowledgements
We thank Prof. Yong-Jing Gao for critical reading of the manuscript. Tong Liu was supported by grants from the National Natural Science Foundation of China (81870874, 31371179, and 81300968) and the Natural Science Foundation of Jiangsu Province, China (BK20170004 and 2015-JY-029).
References
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2.Hanke ML, Kielian T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci (Lond) 2011;121:367–387. doi: 10.1042/CS20110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuda M. Modulation of pain and itch by spinal glia. Neurosci Bull. 2018;34:178–185. doi: 10.1007/s12264-017-0129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu XJ, et al. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron. 2014;82:47–54. doi: 10.1016/j.neuron.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu T, Han Q, Chen G, Huang Y, Zhao LX, Berta T, et al. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain. 2016;157:806–817. doi: 10.1097/j.pain.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang ZJ, Guo JS, Li SS, Wu XB, Cao DL, Jiang BC, et al. TLR8 and its endogenous ligand miR-21 contribute to neuropathic pain in murine DRG. J Exp Med. 2018;215:3019–3037. doi: 10.1084/jem.20180800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lu J, et al. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han Q, Liu D, Convertino M, Wang Z, Jiang C, Kim YH, et al. miRNA-711 binds and activates TRPA1 extracellularly to evoke acute and chronic pruritus. Neuron. 2018;99:449–463 e446. doi: 10.1016/j.neuron.2018.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]