Abstract
Non-thermal atmospheric pressure plasma has been widely used for preclinical studies in areas such as wound healing, blood coagulation, and cancer therapy. We previously developed plasma-activated medium (PAM) and plasma-activated Ringer’s lactate solutions (PAL) for cancer treatments. Many in vitro and in vivo experiments demonstrated that both PAM and PAL exhibit anti-tumor effects in several types of cancer cells such as ovarian, gastric, and pancreatic cancer cells as well as glioblastoma cells. However, interestingly, PAM induces more intracellular reactive oxygen species in glioblastoma cells than PAL. To investigate the differences in intracellular molecular mechanisms of the effects of PAM and PAL in glioblastoma cells, we measured gene expression levels of antioxidant genes such as CAT, SOD2, and GPX1. Microarray and quantitative real-time PCR analyses revealed that PAM elevated stress-inducible genes that induce apoptosis such as GADD45α signaling molecules. PAL suppressed genes downstream of the survival and proliferation signaling network such as YAP/TEAD signaling molecules. These data reveal that PAM and PAL induce apoptosis in glioblastoma cells by different intracellular molecular mechanisms.
Subject terms: Electrical and electronic engineering, Cancer
Introduction
Non-thermal atmospheric pressure plasma is a partially ionized gas that consists of electrons, ions, radicals, and photons, and has been recently used for medical applications1–10. Many researchers have developed non-thermal plasma sources and found dramatic effects on sterilization11–15, wound healing16–20, blood coagulation21–23, and cancer treatment24–30. Non-thermal plasma is widely believed to induce oxidative stress in cells and tissues by producing reactive oxygen species (ROS) and reactive nitrogen species. However, interactions between plasma and biological systems are complex, and the details re dated31–33.
Plasma-activated solutions have been widely developed with various plasma sources and various liquids34–36. Thus, plasma-activated solutions have become more and more important as an option for cancer treatment. We previously developed non-thermal atmospheric pressure plasma with high electron density and applied this plasma for cancer treatments26,37. We showed that plasma-irradiated medium, which we called plasma-activated medium (PAM), exhibits anti-tumor effects against glioblastoma38,39, ovarian40,41, gastric42, pancreatic43, and lung cancer cells44. We also demonstrated that PAM induces apoptosis in glioblastoma cells by downregulating survival and proliferation signaling networks such as the Phosphoinositide 3-kinase (PI3K)/AKT signal transduction pathway38,39. We further developed plasma-activated Ringer’s lactate solution (PAL) for cancer treatments, and showed that PAL also induces apoptosis in glioblastoma cells45. However, the intracellular molecular mechanisms of cell death by each plasma-activated solution remain to be elucidated.
In this study, we compared the intracellular molecular mechanisms of cell death between PAM-treated and PAL-treated glioblastoma cells. Both PAM and PAL downregulated phospho-AKT. However, microarray analyses and quantitative real-time PCR analyses revealed differences in downstream signaling networks that are influenced by PAM and PAL. PAM upregulated gene expression of stress-inducible signaling pathways such as Growth arrest and DNA-damage-inducible protein (GADD45α) signaling to induce apoptosis. PAL downregulated gene expression of downstream signals of the survival and proliferation signaling network such as Yes-associated protein (YAP)/Transcriptional enhancer associated domain (TEAD) signaling to induce apoptosis. These results are consistent with the results that PAM induced more intracellular ROS than PAL.
Results
Both PAM and PAL downregulated phospho-AKT in glioblastoma cells
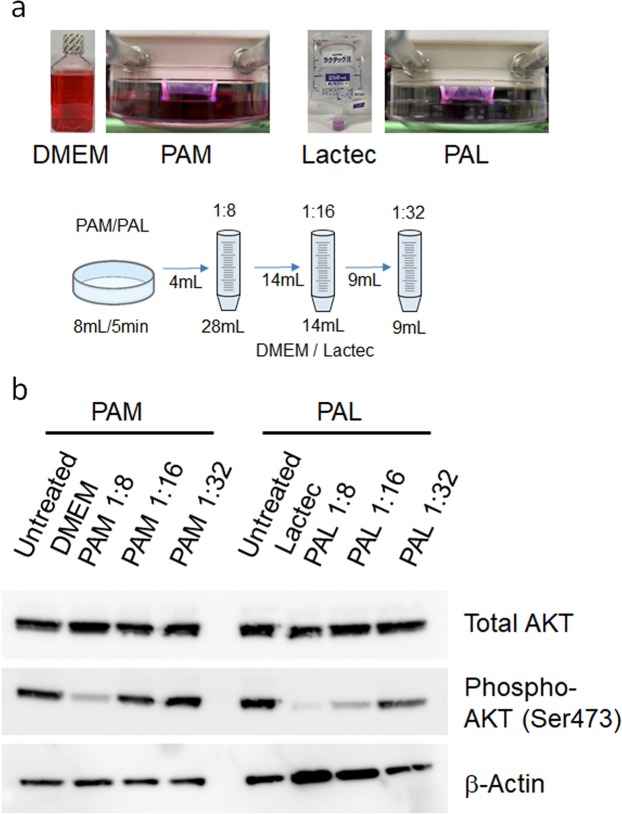
To produce PAM and PAL, 8 mL culture medium (Dulbecco’s Modified Eagle Medium; DMEM) or Ringer’s lactate solution (Lactec) was treated with plasma (the distance between the plasma source and the samples: L = 3 mm, 2.0 standard liters/min (slm)) for 5 min, as described previously45. PAM and PAL were diluted 8, 16, and 32 times with culture medium or Lactec, respectively, as shown in Fig. 1a. We previously reported that PAM induces apoptosis in glioblastoma cells by downregulating survival and proliferation signaling pathways including the PI3K-AKT signaling pathway38,39. To investigate whether PAL also affects the PI3K-AKT signaling pathway, we performed western blotting of both PAM- and PAL-treated glioblastoma cells (Fig. 1b). A range of 8-fold, 16-fold, and 32-fold dilutions of PAL downregulated phosphorylated AKT, whereas 8-fold and 16-fold dilutions of PAM downregulated phosphorylated AKT. These results suggest that PAL has a stronger effect on the PI3K-AKT signaling pathway than PAM.
Figure 1.
Both PAM and PAL downregulated phospho-AKT in glioblastoma cells. (a) Preparation of PAM and PAL and the experimental workflow. DMEM or Lactec in a 60-mm dish was treated with plasma, and PAM and PAL were diluted 8, 16, and 32 times with culture medium and Lactec, respectively. (b) Western blotting of total AKT and phosphorylated AKT (at Ser473) was performed on U251SP cells. β-actin was used as a loading control.
PAM induced more intracellular ROS than PAL
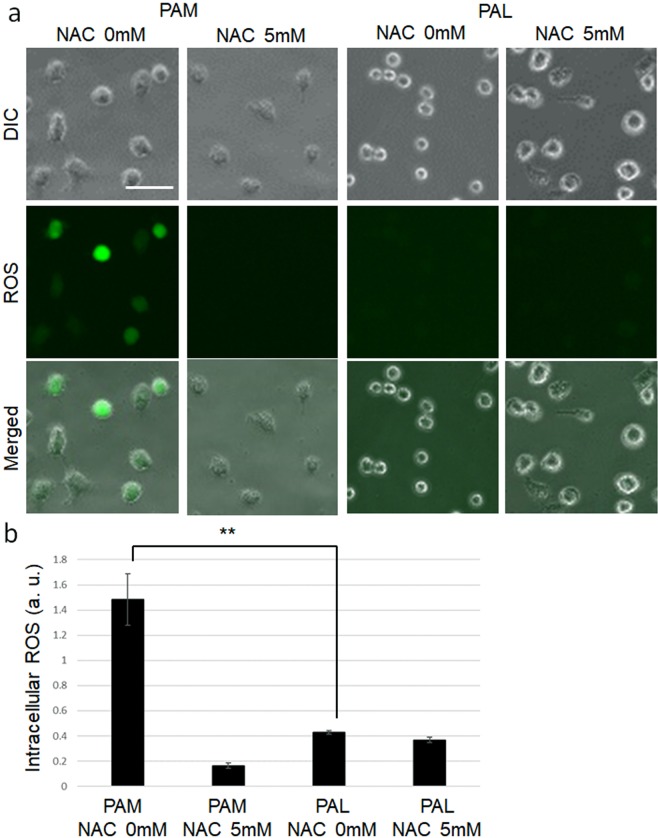
Non-thermal plasma generally induces intracellular ROS in cells. To investigate the extent to which PAM and PAL induced intracellular ROS, we measured the fluorescent intensity of the CM-H2DCFDA reagent, which detects many varieties of intracellular ROS, in single cells using a fluorescence microscopy (Fig. 2a,b). To compare the intracellular ROS levels, 16-fold dilutions of PAM and PAL were used. Intracellular ROS levels in PAM-treated glioblastoma cells were significantly higher than ROS in PAL-treated glioblastoma cells. Pretreatment with 5 mM N-acetyl cysteine (NAC), a ROS scavenger, decreased intracellular ROS in PAM-treated glioblastoma cells.
Figure 2.
PAM- and PAL-treated glioblastoma cells with and without NAC. (a) Intracellular ROS generated in response to PAM and PAL. Image of U251SP cells. Scale bar represents 50 μm. DIC, differential interference contrast. (b) Intracellular ROS levels were evaluated by measuring fluorescent intensity of the CM-H2DCFDA reagent. More than 50 cells were measured. Data are the mean ± SEM. **P < 0.01 versus control.
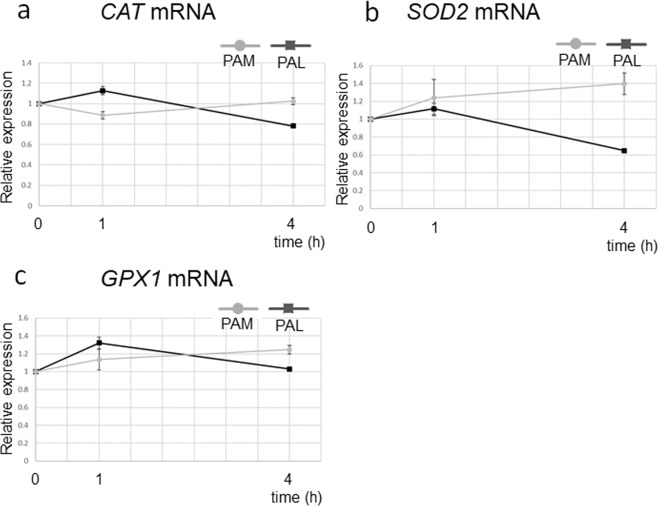
To investigate gene expression of anti-oxidant genes, we performed quantitative real-time PCR (qRT-PCR) in PAM-treated and PAL-treated glioblastoma cells 1 and 4 h after PAM/PAL treatment (Fig. 3). Gene expression of the representative anti-oxidant genes, Catalase (CAT), Superoxide dismutase (SOD2), and Glutathione peroxidase (GPX1), was examined. Surprisingly, expression of these anti-oxidant genes was not elevated by PAM or PAL treatments. These results suggest that PAM/PAL induce other anti-oxidant genes or PAM and/or PAL induces cell death by other mechanisms.
Figure 3.
Anti-oxidant gene expression was not elevated in PAM- or PAL-treated glioblastoma cells. Relative mRNA expression of CAT (a), SOD2 (b), and GPX1 (c) was calculated using qRT-PCR.
PAM promoted stress-related gene expression that induced apoptosis
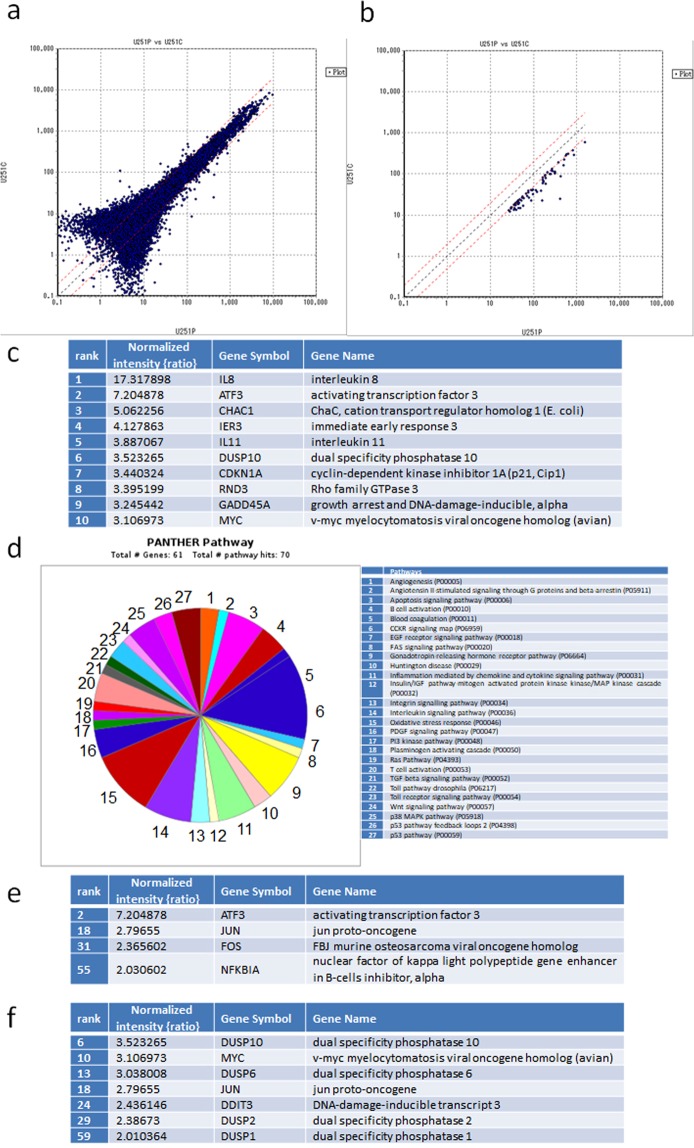
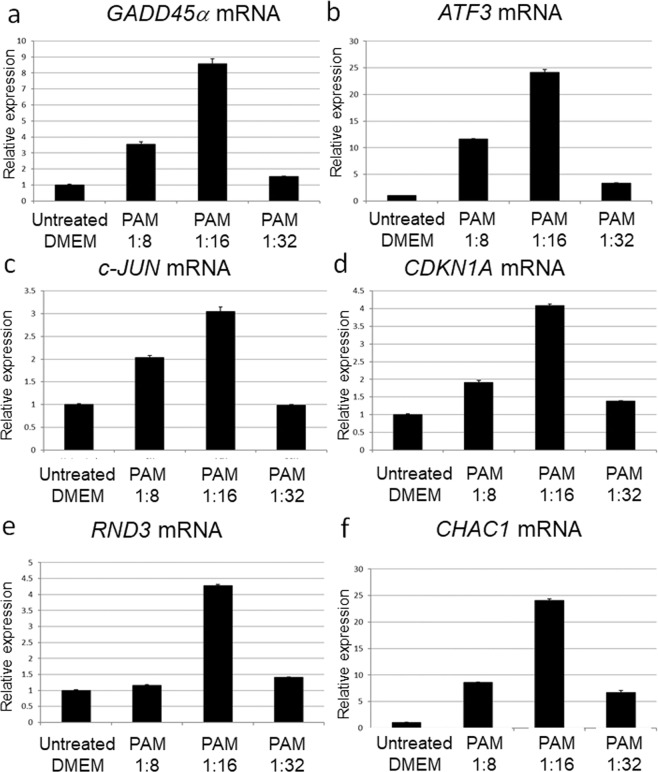
To investigate the gene transcription networks that are activated in PAM-treated glioblastoma cells, we performed microarray-based gene expression profiling in these cells (Fig. 4). Sixty-one genes were upregulated more than 2-fold by PAM treatment (Fig. 4a,b, Table S1). The top 10 stress-related genes that induced apoptosis included Activating transcription factor 3 (ATF3, rank2), Cyclin-dependent kinase inhibitor 1A (CDKN1A, also known as p21, rank7), and GADD45α (rank9) (Fig. 4c). ATF3 and c-JUN act downstream of GADD45α to mediate the stress-related pathway in glioblastoma cells46. Consistent with this, c-JUN was also upregulated by PAM (rank18, Table S1). CDKN1A also interacts with GADD45α to mediate tumor suppressor activity47. To validate these results, GADD45α, ATF3, c-JUN, and CDKN1A expression levels were determined with qRT-PCR (Fig. 5a–d). Glioblastoma cells were treated with 8-fold, 16-fold, and 32-fold dilutions of PAM for 2 h, and gene expression levels were measured 4 h after PAM treatment. The expression levels of these genes were correlated with each other, and the 16-fold dilution of PAM elevated these genes to the highest level. Rho family GTPase (RND3, also known as RhoE), Cation transport regulator-like protein (CHAC1), and Immediate early response (IER3, also known as IEX1), which also induce apoptosis in glioblastoma cells48–50, were ranked in the top 10 (Fig. 4c). qRT-PCR analyses showed that RND3 and CHAC1 were significantly upregulated by 16-fold dilution of PAM (Fig. 5e,f).
Figure 4.
Microarray analysis revealed gene transcription networks that are activated in PAM-treated glioblastoma cells. (a) Gene expression profiling of PAM-treated glioblastoma cells (U251P) and untreated medium-treated glioblastoma cells (U251C) was performed using DNA microarrays. (b) Genes upregulated more than 2-fold in PAM-treated glioblastoma cells compared with medium-treated glioblastoma cells were selected. The cut-off value of gene expression levels of medium-treated glioblastoma cells was set at 10. (c) The top 10 genes upregulated in PAM-treated glioblastoma cells were ranked. (d) GO analyses using Panther software. We identified 61 genes that were upregulated more than 2-fold by PAM; these genes were categorized into GO terms of pathways. (e) Four genes that were categorized in the apoptosis signaling pathway. (f) Seven genes that were categorized in the oxidative stress pathway.
Figure 5.
Stress-related genes that induce apoptosis were elevated in PAM-treated glioblastoma cells. Relative mRNA expression of GADD45α (a), ATF3 (b), c-JUN (c), CDKN1A (d), RND3 (e), and CHAC1 (f) was calculated using qRT-PCR.
We performed gene ontology (GO) analysis of the 61 upregulated genes (Fig. 4d). Four genes (ATF3, JUN, FOS, and NFKBIA) were categorized into the term apoptosis pathway (Fig. 4e), and seven genes (DUSP10, MYC, DUSP6, JUN, DDIT3, DUSP2, and DUSP1) were categorized into the term oxidative stress pathway (Fig. 4f). These results suggest that PAM upregulated Dual-specificity phosphatase (DUSP) family genes to inhibit mitogen-activated protein kinases (MAPKs) through feedback regulation of MAPKs → AP-1 (c-FOS and c-JUN) → DUSP → MAPKs.
DNA damage-inducible transcript 3 (DDIT3), which is also known as C/EBP homologous protein (CHOP), is a pro-apoptotic transcription factor induced by oxidative stress, amino acid deprivation, hypoxia, and endoplasmic reticulum stress51. Consistent with the results in Fig. 3, anti-oxidant genes such as CAT, SOD2, and GPX1 were not ranked among genes that were upregulated more than 2-fold in microarray analysis (Table S1).
PAL suppressed survival- and proliferation-related gene expression
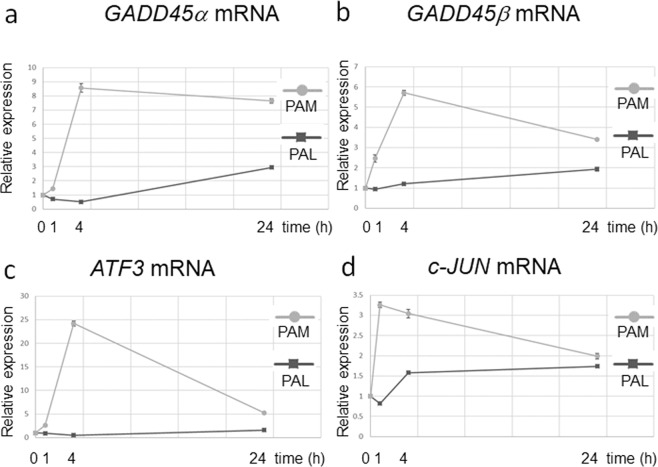
To elucidate the different intracellular molecular mechanisms of the effects of PAM and PAL on glioblastoma cells, we investigated the dynamics of gene expression of PAM-treated and PAL-treated glioblastoma cells. We performed qRT-PCR in both PAM-treated and PAL-treated glioblastoma cells 1, 4, and 24 h after PAM/PAL treatment (Fig. 6). PAM upregulated the stress-inducible gene, GADD45α, and other genes related to the stress-induction pathway, whereas PAL did not greatly upregulate GADD45α (Fig. 6a). PAM also upregulated GADD45β, but PAL had a minimal effect (Fig. 6b). These results are consistent with the observation that PAM induced more oxidative stress than PAL (Fig. 2). PAL did not upregulate ATF3 or c-JUN, which are downstream of GADD45α, (Fig. 6c,d). PAL downregulated the expression of c-JUN 1 h after PAL treatment.
Figure 6.
Differences in gene expression dynamics between PAM- and PAL-treated glioblastoma cells. Relative mRNA expression of GADD45α (a), GADD45β (b), ATF3 (c), and c-JUN (d) was calculated using qRT-PCR.
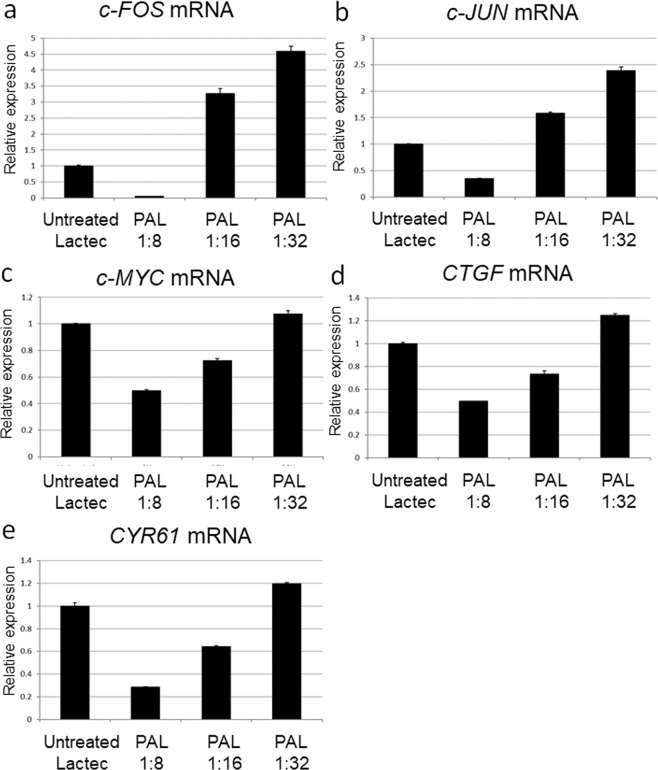
We reasoned that PAL induced apoptosis in glioblastoma cells by downregulating survival and proliferation signaling networks. Thus, we investigated the expression of genes that are downstream of the survival and proliferation signaling pathways (Fig. 7). Glioblastoma cells were treated with 8-fold, 16-fold, and 32-fold dilutions of PAL for 2 h, and gene expression levels were measured 4 h after PAL treatment. Components of the AP-1 complex, c-FOS and c-JUN, were downregulated by 8-fold dilution of PAL (Fig. 7a,b). Interestingly, genes that are downstream of YAP-TEAD signaling, including the proto-oncogene, c-MYC, Connective tissue growth factor (CTGF), and Cysteine-rich angiogenic inducer 61 (CYR61), were downregulated by 8-fold and 16-fold dilutions of PAL (Fig. 7c–e). These results suggest that PAL downregulated the survival and proliferation signaling networks, and are consistent with the results that PAL downregulated phospho-AKT (Fig. 1b).
Figure 7.
Genes downstream of the survival and proliferation signaling networks were downregulated in PAL-treated glioblastoma cells. Relative mRNA expression of c-FOS (a), c-JUN (b), c-MYC (c), CTGF (d), and CYR61 (e) was calculated using qRT-PCR.
Discussion
Non-thermal plasma is believed to provide therapeutic effects by controlling the redox balance of tissues and cells. Non-thermal plasma generally produces short-lifetime and long-lifetime reactive species through interactions between plasma and air, and finally induces intracellular ROS in cells due to direct plasma treatment. PAM also induces intracellular ROS in cells through interactions among plasma, air, and liquids. Plasma interacts with components in liquids, and the physiological effects depend on the components of the plasma-activated solutions. Indeed, PAL induced less intracellular ROS than PAM (Fig. 2). These results suggest that PAL induces cell death via redox-independent mechanisms compared with PAM.
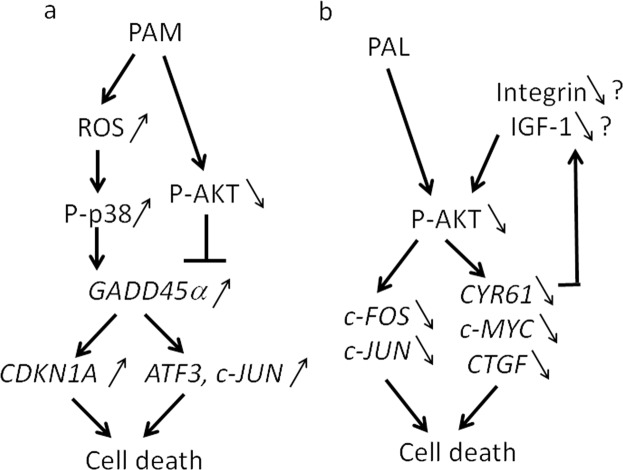
Our microarray and qRT-PCR analyses revealed various pathways that lead to apoptosis in PAM-treated glioblastoma cells. GADD45α, ATF3, c-JUN, and CDKN1A were consistently upregulated by PAM (Figs 4 and 5). GADD45 family members are stress-inducible genes, and various environmental and physiological stresses such as radiation, free radicals, and pro-apoptotic cytokines upregulate GADD4547,52. Cytokine production activates a GADD45α/p38 pathway that leads to increases in ATF3 and c-JUN transcription factor levels to induce apoptosis46. CDKN1A is also in the GADD45/p38 signaling pathway47. AKT inhibition induces GADD45α expression in soft tissue sarcoma cells53. Based on these results, we elucidated the intracellular molecular mechanisms that induce apoptosis in PAM-treated glioblastoma cells (Fig. 8a). RND3, which is a Rho GTPase, inhibits cell proliferation in glioblastoma cells by interfering with Rb inactivation48. Temozolomide, which is a chemotherapeutic drug for treatment of glioblastoma, highly upregulates CHAC1, and overexpression of CHAC1 significantly influences temozolomide-mediated apoptosis in glioblastoma49. Overexpression of IER3 sensitizes glioblastoma cells to γ-radiation-induced apoptosis50. These three genes (RND3, CHAC1, and IER3) were ranked in the top 10 genes that were upregulated by PAM. GO analyses revealed that PAM upregulated genes of the AP-1 complex (FOS, JUN) and DUSP genes (DUSP1, DUSP2, DUSP6, and DUSP10). DUSP family proteins are stress-induced enzymes that provide feedback inhibition of MAPKs54. These results suggest that PAM downregulates MAPK signaling by negative feedback through the MAPK → AP-1 → DUSP → MAPK pathway.
Figure 8.
Intracellular molecular mechanisms to explain the differences between PAM- and PAL-treated glioblastoma cells. Models of intracellular molecular mechanisms of cell death in PAM-treated (a) and PAL-treated glioblastoma cells (b) based on microarray and qRT-PCR.
Gene expression analyses also revealed differences in intracellular molecular mechanisms of cell death between PAM-treated and PAL-treated glioblastoma cells. Anti-oxidant genes such as CAT, SOD2, and GPX1 were not elevated in PAM- or PAL-treated glioblastoma cells (Fig. 3). Stress-inducible genes, such as GADD45α/β, ATF3, and c-JUN, which were remarkably upregulated in PAM-treated glioblastoma cells, were not upregulated by PAL (Fig. 6). On the other hand, genes downstream of the survival and proliferation signaling networks were downregulated by PAL (Fig. 7). In the U251SP glioblastoma cell line, AKT is constitutively active due to the loss of function of Phosphatase and tensin homologue deleted on chromosome ten (PTEN), and activated AKT protects cells from apoptosis55. Both PAM and PAL downregulated phospho-AKT in glioblastoma cells (Fig. 1b). The PI3K/AKT signaling pathway provides cell survival signals, in part, through activation of AP-1 transcription factors, which consist of c-FOS and c-JUN in glioblastoma cells56. The light-activated drug, Verteporfin, inhibits the growth of glioblastoma cells by downregulating YAP-TEAD-associated downstream signaling molecules such as c-MYC, CTGF, and CYR6157. CYR61 is overexpressed in glioblastoma and breast cancer cells and regulates proliferation through Integrin/Insulin-like growth factor 1 (IGF1)-AKT signaling pathways58,59. Based on these results, we constructed a schematic showing the putative intracellular molecular mechanisms that induce apoptosis in PAM- and PAL-treated glioblastoma cells (Fig. 8a, b respectively).
In this study, we found some differences in intracellular molecular mechanisms of cell death between PAM-treated and PAL-treated glioblastoma cells. Interestingly, PAM induced oxidative stress dependent cell death, and PAL induced oxidative stress independent cell death. These findings should be tested by in vivo studies. Based on our data, we can expect that we might use different plasma-activated solutions for cancers that are resistant to some plasma-activated solutions in the future.
Methods
Cell lines and culture
U251SP cells (human glioblastoma cell line, TP53 R273H mutation, PTEN E242fs mutation) derived at the Memorial Sloan-Kettering Cancer Institute (New York, NY)60 were grown in DMEM (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum and penicillin (100 U/mL)-streptomycin (100 μg/mL) in an atmosphere of 5% CO2 at 37 °C.
Preparation of PAM and PAL
The experimental setup to prepare PAM38 and PAL45 has been previously described. While argon gas was flowing, plasma in the main discharge region was excited by applying 10 kV from a 60-Hz commercial power supply to two electrodes 20 mm apart. The flow rate of argon gas was set at 2 slm, and the distance between the plasma source and the samples was fixed at L = 3 mm. Eight milliliters DMEM or Lactec in a 60-mm dish was treated with plasma (L = 3 mm, 2.0 slm), and then PAM and PAL were diluted 8, 16, and 32 times with culture medium and Lactec, respectively (Fig. 1). These PAM and PAL were immediately used for experiments after preparation. The cell viability of U251SP cell lines was measured to test the reproducibility of PAM and PAL as previously described38,45.
Western blot
Glioblastoma cells (approx. 300,000) were seeded in 3 mL medium in a six-well plate. On the following day, the medium of the cells in the six-well plate was replaced with 3 mL freshly prepared PAM or PAL. After 2 h, PAM and PAL were replaced with 3 mL culture medium. Two hours later, cells were collected, cell lysates were prepared, and western blotting was performed as previously described38. Western blotting for total AKT and phosphorylated AKT (at Ser473) was performed on U251SP cells. β-actin was used as a loading control.
Detection of intracellular ROS
U251SP cells (10,000) were seeded in an eight-well chamber slide in 200 μL culture medium. On the following day, the medium of the cells in the eight-well chamber slide was replaced with 200 μL CM-H2DCFDA (Life Technologies, Carlsbad, CA) (10 μM) in PBS with and without 5 mM NAC (Sigma-Aldrich). After 1 h, 200 μL CM-H2DCFDA with and without NAC in the cell culture chambers was replaced with freshly prepared 16 times diluted PAM or PAL. After 2 h, PAM and PAL were replaced with 200 μL culture medium. After 2 h, the cells were observed using a BZ9000 microscope (Keyence, Osaka, Japan).
Microarray
Glioblastoma cells (300,000) were seeded in 3 mL medium in a six-well plate. On the following day, 4 mL culture medium in a 60 mm-dish was treated with plasma (L = 5 mm, 2.0 slm) and the medium of the cells in the six-well plate was replaced with 3 mL PAM. After 2 h, PAM was replaced with 3 mL culture medium. After 2 h, total RNA from PAM-treated cells was isolated using an RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol. RNA (1 μg) was labeled with Cy3 and then hybridized with CodeLink Human Whole Genome Bioarray (Applied Microarrays, Tempe, AZ) and scanned with a microarray scanner GenePix4000B (Olympus, Kyoto, Japan). Raw intensity measurements of all probe sets were background-corrected, normalized, and converted into expression measurements using the MicroArray Data Analysis Tool Version 3.2 (Filgen, Nagoya, Japan). GO analysis was performed using the PANTHER Classification System Resource 14.0 online software (http://pantherdb.org/).
qRT-PCR
Glioblastoma cells (300,000) were seeded in 3 mL medium in a six-well plate. On the following day, 8 mL culture medium or Lactec in a 60-mm dish was treated with plasma (L = 3 mm, 2.0 slm), and PAM and PAL were diluted 8, 16, and 32 times with culture medium and Lactec, respectively. The medium of the cells in the six-well plate was replaced with 3 mL PAM or PAL. After 2 h, PAM and PAL were replaced with 3 mL culture medium. One, four, and twenty-four hours after PAM or PAL treatment, RNA from PAM- and PAL-treated cells was extracted using the RNeasy Mini Kit (QIAGEN) according to the manufacturer’s protocol. Reverse transcription was performed using the Omniscript RT Kit (QIAGEN) to synthesize cDNA. qRT-PCR was conducted using KOD SYBR qPCR Mix (TOYOBO, Osaka, Japan) and monitored in real-time using the LightCycler®480 PCR system (Roche Diagnostics, Rotkreuz, Switzerland). Relative mRNA expression was calculated using the 2−ΔΔCT method. Expression of all target genes was normalized to GAPDH as a reference. Primers used in this study are described in Table 1. All PCR analyses were performed in triplicate.
Table 1.
The sequences of primers used for qRT-PCR.
| Target gene | Sequence |
|---|---|
| CAT | F′: 5′- GGTCATGCATTTAATCAGGCAGAA -3′ |
| R′: 5′- TTGCTTGGGTCGAAGGCTATC -3′ | |
| SOD2 | F′: 5′- CCAAATCAGGATCCACTGCAA -3′ |
| R′: 5′- CAGCATAACGATCGTGGTTTACTT -3′ | |
| GPX1 | F′: 5′- CAGTTGCAGTGCTGCTGTCTC -3′ |
| R′: 5′- GCTGACACCCGGCACTTTATTAG -3′ | |
| GADD45α | F′: 5′- CTGCAGTTTGCAATATGACTTTGG -3′ |
| R′: 5′- GGGCTTTGCTGAGCACTTC -3′ | |
| GADD45β | F′: 5′- CGAGTCGGCCAAGTTGATGA -3′ |
| R′: 5′- ACCCGCACGATGTTGATGTC -3′ | |
| ATF3 | F′: 5′- ACCAGGATGCCCACCGTTAG -3′ |
| R′: 5′- GACAATGGTAGCCACGGTGAAG -3′ | |
| c-JUN | F′: 5′- ACCAAGAACTGCATGGACCTAACA -3′ |
| R′: 5′- GCTCAGCCTCGCTCTCACAA -3′ | |
| CDKN1A | F′: 5′- CATGTGGACCTGTCACTGTCTTGTA -3′ |
| R′: 5′- ATCTTCAAGGAGCGTCACCACAC -3′ | |
| RND3 | F′: 5′- TCATGGATCCTAATCAGAACGTGAA -3′ |
| R′: 5′- GAAGTGTCCCACAGGCTCAACTC -3′ | |
| CHAC1 | F′: 5′- GTTTCTGGCAGGGAGACACCTT -3′ |
| R′: 5′- ATCTTCAAGGAGCGTCACCACAC -3′ | |
| c-FOS | F′: 5′- TCTTACTACCACTCACCCGCAGAC -3′ |
| R′: 5′- GGAATGAAGTTGGCACTGGAGAC -3′ | |
| c-MYC | F′: 5′- CCTGGTGCTCCATGAGGAGA -3′ |
| R′: 5′- CAGTGGGCTGTGAGGAGGTTT -3′ | |
| CTGF | F′: 5′- CTTGCGAAGCTGACCTGGAA -3′ |
| R′: 5′- AAAGCTCAAACTTGATAGGCTTGGA -3′ | |
| CYR61 | F′: 5′- CCAAGCAGCTCAACGAGGA -3′ |
| R′: 5′- TGATGTTTACAGTTGGGCTGGAA -3′ | |
| GAPDH | F′: 5′- CGCTCTCTGCTCCTCCTGTTC -3′ |
| R′: 5′- ATCCGTTGACTCCGACCTTCAC -3′ |
Statistical analysis
All data are presented as the mean ± the standard error of the mean (SEM). The unpaired Student’s t-test (two-tailed) was used.
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Supplementary information
Acknowledgements
This work was partly supported by Grants-in-Aid for Scientific Research on Innovative Areas “Plasma Medical Innovation” (Grant Nos 24108002 and 24108008), a Grant-in-Aid for Specially Promoted Research (No. 19H05462) and a Grant-in-Aid for Scientific Research (C) (No. 18K03599) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author Contributions
H.T., M.M., S.T., F.K. and M.H. designed the research; H.T. and Y.K. conducted the experiments; H.T. wrote the main manuscript under the supervision of M.M., S.T., F.K. and M.H.; H.T. analyzed and interpreted data. All authors joined discussions regarding the final draft of the manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-50136-w.
References
- 1.Laroussi M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process Polym. 2005;2:391–400. doi: 10.1002/ppap.200400078. [DOI] [Google Scholar]
- 2.Fridman G, et al. Applied plasma medicine. Plasma Process Polym. 2008;5:503–533. doi: 10.1002/ppap.200700154. [DOI] [Google Scholar]
- 3.Kong M G, Kroesen G, Morfill G, Nosenko T, Shimizu T, van Dijk J, Zimmermann J L. Plasma medicine: an introductory review. New Journal of Physics. 2009;11(11):115012. doi: 10.1088/1367-2630/11/11/115012. [DOI] [Google Scholar]
- 4.Weltmann KD, et al. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure Appl Chem. 2010;82:1223–1237. doi: 10.1351/Pac-Con-09-10-35. [DOI] [Google Scholar]
- 5.Morfill G E, Kong M G, Zimmermann J L. FOCUS ON PLASMA MEDICINE. New Journal of Physics. 2009;11(11):115011. doi: 10.1088/1367-2630/11/11/115011. [DOI] [Google Scholar]
- 6.Laroussi M. Low-Temperature Plasmas for. Medicine? Ieee T Plasma Sci. 2009;37:714–725. doi: 10.1109/Tps.2009.2017267. [DOI] [Google Scholar]
- 7.von Woedtke T, Reuter S, Masur K, Weltmann KD. Plasmas for medicine. Phys Rep. 2013;530:291–320. doi: 10.1016/j.physrep.2013.05.005. [DOI] [Google Scholar]
- 8.Weltmann KD, von Woedtke T. Campus PlasmaMed-From Basic Research to Clinical Proof. Ieee T Plasma Sci. 2011;39:1015–1025. doi: 10.1109/Tps.2011.2112674. [DOI] [Google Scholar]
- 9.Weltmann K.-D., von Woedtke Th. Basic requirements for plasma sources in medicine. The European Physical Journal Applied Physics. 2011;55(1):13807. doi: 10.1051/epjap/2011100452. [DOI] [Google Scholar]
- 10.Yousfi Mohammed, Merbahi Nofel, Pathak Atul, Eichwald Olivier. Low-temperature plasmas at atmospheric pressure: toward new pharmaceutical treatments in medicine. Fundamental & Clinical Pharmacology. 2013;28(2):123–135. doi: 10.1111/fcp.12018. [DOI] [PubMed] [Google Scholar]
- 11.Laroussi M. Nonthermal decontamination of biological media by atmospheric-pressure plasmas: review. analysis, and prospects. Ieee T Plasma Sci. 1992;30:1409–1415. doi: 10.1109/TPS.2002.804220. [DOI] [Google Scholar]
- 12.Iseki Sachiko, Ohta Takayuki, Aomatsu Akiyoshi, Ito Masafumi, Kano Hiroyuki, Higashijima Yasuhiro, Hori Masaru. Rapid inactivation of Penicillium digitatum spores using high-density nonequilibrium atmospheric pressure plasma. Applied Physics Letters. 2010;96(15):153704. doi: 10.1063/1.3399265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortsch M, et al. [H2O2 low temperature plasma sterilization. New possibilities for use with eye surgery instruments] Der Ophthalmologe: Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 1993;90:754–764. [PubMed] [Google Scholar]
- 14.Holler C, Martiny H, Christiansen B, Ruden H, Gundermann KO. The efficacy of low temperature plasma (LTP) sterilization, a new sterilization technique. Zentralblatt fur Hygiene und Umweltmedizin = International journal of hygiene and environmental medicine. 1993;194:380–391. [PubMed] [Google Scholar]
- 15.Graham GS, Mielnik TJ. Industrial low-temperature gas plasma sterilization. Medical device technology. 1997;8:28–30. [PubMed] [Google Scholar]
- 16.Awakowicz P, et al. Biological Stimulation of the Human Skin Applying Health-Promoting Light and Plasma Sources. Contrib Plasm Phys. 2009;49:641–647. doi: 10.1002/ctpp.200910068. [DOI] [Google Scholar]
- 17.Heinlin J, et al. Plasma medicine: possible applications in dermatology. J Dtsch Dermatol Ges. 2010;8:968–976. doi: 10.1111/j.1610-0387.2010.07495.x. [DOI] [PubMed] [Google Scholar]
- 18.Isbary G, et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Brit J Dermatol. 2010;163:78–82. doi: 10.1111/j.1365-2133.2010.09744.x. [DOI] [PubMed] [Google Scholar]
- 19.Mitra A, et al. Applications in plasma medicine: a SWOT approach. Compos Interface. 2012;19:231–238. doi: 10.1080/15685543.2012.700200. [DOI] [Google Scholar]
- 20.Masur K, et al. Human Skin Cell Activity Is Modulated by Cold Atmospheric Pressure Plasma. Wound Repair Regen. 2012;20:A102–A102. [Google Scholar]
- 21.Kalghatgi SU, et al. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. Ieee T Plasma Sci. 2007;35:1559–1566. doi: 10.1109/Tps.2007.905953. [DOI] [Google Scholar]
- 22.Ikehara S, et al. Plasma Blood Coagulation Without Involving the Activation of Platelets and Coagulation Factors. Plasma Process. Polym. 2015;12:1348–1353. doi: 10.1002/ppap.201500132. [DOI] [Google Scholar]
- 23.Miyamoto K, et al. Red blood cell coagulation induced by low-temperature plasma treatment. Archives of biochemistry and biophysics. 2016;605:95–01. doi: 10.1016/j.abb.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Kieft IE, Kurdi M, Stoffels E. Reattachment and apoptosis after plasma-needle treatment of cultured cells. Ieee T Plasma Sci. 2006;34:1331–1336. doi: 10.1109/Tps.2006.876511. [DOI] [Google Scholar]
- 25.Fridman G, et al. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem Plasma P. 2007;27:163–176. doi: 10.1007/s11090-007-9048-4. [DOI] [Google Scholar]
- 26.Iseki Sachiko, Nakamura Kae, Hayashi Moemi, Tanaka Hiromasa, Kondo Hiroki, Kajiyama Hiroaki, Kano Hiroyuki, Kikkawa Fumitaka, Hori Masaru. Selective killing of ovarian cancer cells through induction of apoptosis by nonequilibrium atmospheric pressure plasma. Applied Physics Letters. 2012;100(11):113702. doi: 10.1063/1.3694928. [DOI] [Google Scholar]
- 27.Vandamme M, et al. Antitumor Effect of Plasma Treatment on U87 Glioma Xenografts: Preliminary Results. Plasma Process Polym. 2010;7:264–273. doi: 10.1002/ppap.200900080. [DOI] [Google Scholar]
- 28.Keidar M, et al. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br J Cancer. 2011;105:1295–1301. doi: 10.1038/bjc.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlegel J, Köritzer J, Boxhammer V. Plasma in cancer treatment. Clinical Plasma. Medicine. 2013;1:2–7. [Google Scholar]
- 30.Kaushik Nagendra K., Uhm Hansup, Ha Choi Eun. Micronucleus formation induced by dielectric barrier discharge plasma exposure in brain cancer cells. Applied Physics Letters. 2012;100(8):084102. doi: 10.1063/1.3687172. [DOI] [Google Scholar]
- 31.Tanaka H, et al. Molecular mechanisms of non-thermal plasma-induced effects in cancer cells. Biological chemistry. 2018;400:87–91. doi: 10.1515/hsz-2018-0199. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka H, et al. New hopes for plasma-based cancer treatment. Plasma. 2018;1:150–155. doi: 10.3390/plasma1010014. [DOI] [Google Scholar]
- 33.Tanaka H, et al. State of the art in medical applications using non-thermal atmospheric pressure plasma. Rev. Mod. Plasma Phys. 2017;1(1):3. doi: 10.1007/s41614-017-0004-3. [DOI] [Google Scholar]
- 34.Yan Dayun, Sherman Jonathan H., Cheng Xiaoqian, Ratovitski Edward, Canady Jerome, Keidar Michael. Controlling plasma stimulated media in cancer treatment application. Applied Physics Letters. 2014;105(22):224101. doi: 10.1063/1.4902875. [DOI] [Google Scholar]
- 35.Mohades Soheila, Laroussi Mounir, Maruthamuthu Venkat. Moderate plasma activated media suppresses proliferation and migration of MDCK epithelial cells. Journal of Physics D: Applied Physics. 2017;50(18):185205. doi: 10.1088/1361-6463/aa678a. [DOI] [Google Scholar]
- 36.Yan, D. Y. et al. Stabilizing the cold plasma-stimulated medium by regulating medium’s composition. Sci Rep-Uk6, 26016, Artn 26016, 10.1038/Srep26016 (2016). [DOI] [PMC free article] [PubMed]
- 37.Iwasaki Masahiro, Inui Hirotoshi, Matsudaira Yuto, Kano Hiroyuki, Yoshida Naofumi, Ito Masafumi, Hori Masaru. Nonequilibrium atmospheric pressure plasma with ultrahigh electron density and high performance for glass surface cleaning. Applied Physics Letters. 2008;92(8):081503. doi: 10.1063/1.2885084. [DOI] [Google Scholar]
- 38.Tanaka H, et al. Plasma-Activated Medium Selectively Kills Glioblastoma Brain Tumor Cells by Down-Regulating a Survival Signaling Molecule, AKT Kinase. Plasma Medicine. 2013;1:265–277. doi: 10.1615/PlasmaMed.2012006275. [DOI] [Google Scholar]
- 39.Tanaka H, et al. Cell survival and proliferation signaling pathways are downregulated by plasma-activated medium in glioblastoma brain tumor cells. Plasma Medicine. 2014;2:207–220. doi: 10.1615/PlasmaMed.2013008267. [DOI] [Google Scholar]
- 40.Utsumi F, et al. Effect of Indirect Nonequilibrium Atmospheric Pressure Plasma on Anti-Proliferative Activity against Chronic Chemo-Resistant Ovarian Cancer Cells In Vitro and In Vivo. Plos One. 2013;8:e81576. doi: 10.1371/journal.pone.0081576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Utsumi F, et al. Selective cytotoxicity of indirect nonequilibrium atmospheric pressure plasma against ovarian clear-cell carcinoma. SpringerPlus. 2014;3:398. doi: 10.1186/2193-1801-3-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torii K, et al. Effectiveness of plasma treatment on gastric cancer cells. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2014;18:635–643. doi: 10.1007/s10120-014-0395-6. [DOI] [PubMed] [Google Scholar]
- 43.Hattori N, et al. Effectiveness of plasma treatment on pancreatic cancer cells. International journal of oncology. 2015;47:1655–1662. doi: 10.3892/ijo.2015.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adachi T, et al. Plasma-activated medium induces A549 cell injury via a spiral apoptotic cascade involving the mitochondrial-nuclear network. Free radical biology & medicine. 2014;79C:28–44. doi: 10.1016/j.freeradbiomed.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka H, et al. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci Rep. 2016;6:36282. doi: 10.1038/srep36282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jack GD, et al. Activated stress response pathways within multicellular aggregates utilize an autocrine component. Cellular signalling. 2007;19:772–781. doi: 10.1016/j.cellsig.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Liebermann DA, Hoffman B. Gadd45 in stress signaling. Journal of molecular signaling. 2008;3:15. doi: 10.1186/1750-2187-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poch E, et al. RhoE interferes with Rb inactivation and regulates the proliferation and survival of the U87 human glioblastoma cell line. Experimental cell research. 2007;313:719–731. doi: 10.1016/j.yexcr.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Chen PH, et al. The CHAC1-inhibited Notch3 pathway is involved in temozolomide-induced glioma cytotoxicity. Neuropharmacology. 2017;116:300–314. doi: 10.1016/j.neuropharm.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita K, Nakashima S, You F, Hayashi S, Iwama T. Overexpression of immediate early gene X-1 (IEX-1) enhances gamma-radiation-induced apoptosis of human glioma cell line, U87-MG. Neuropathology: official journal of the Japanese Society of Neuropathology. 2009;29:20–24. doi: 10.1111/j.1440-1789.2008.00932.x. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, et al. Transcription Factor C/EBP Homologous Protein in Health and Diseases. Frontiers in immunology. 2017;8:1612. doi: 10.3389/fimmu.2017.01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moskalev AA, et al. Gadd45 proteins: relevance to aging, longevity and age-related pathologies. Ageing research reviews. 2012;11:51–66. doi: 10.1016/j.arr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu QS, et al. Soft tissue sarcoma cells are highly sensitive to AKT blockade: a role for p53-independent up-regulation of GADD45 alpha. Cancer research. 2008;68:2895–2903. doi: 10.1158/0008-5472.CAN-07-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang CY, Tan TH. DUSPs, to MAP kinases and beyond. Cell &. bioscience. 2012;2:24. doi: 10.1186/2045-3701-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koul D. PTEN signaling pathways in glioblastoma. Cancer biology & therapy. 2008;7:1321–1325. doi: 10.4161/cbt.7.9.6954. [DOI] [PubMed] [Google Scholar]
- 56.Koul D, et al. PTEN down regulates AP-1 and targets c-fos in human glioma cells via PI3-kinase/Akt pathway. Molecular and cellular biochemistry. 2007;300:77–87. doi: 10.1007/s11010-006-9371-8. [DOI] [PubMed] [Google Scholar]
- 57.Al-Moujahed A, et al. Verteporfin inhibits growth of human glioma in vitro without light activation. Sci Rep. 2017;7:7602. doi: 10.1038/s41598-017-07632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie D, et al. Cyr61 is overexpressed in gliomas and involved in integrin-linked kinase-mediated Akt and beta-catenin-TCF/Lef signaling pathways. Cancer research. 2004;64:1987–1996. doi: 10.1158/0008-5472.CAN-03-0666. [DOI] [PubMed] [Google Scholar]
- 59.Sarkissyan S, et al. IGF-1 regulates Cyr61 induced breast cancer cell proliferation and invasion. Plos One. 2014;9:e103534. doi: 10.1371/journal.pone.0103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Natsume A, et al. IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer research. 2005;65:7573–7579. doi: 10.1158/0008-5472.CAN-05-0036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.