Abstract
Purpose
This study aimed to compare the efficacy of and patient satisfaction with the wide-awake local anesthesia with no tourniquet (WALANT) technique in open cubital and carpal tunnel release surgery.
Methods
From January 2016 to February 2017, 20 cubital tunnel syndrome (CuTS) patients were in a wide-awake (WA) group and 22 in a general (GA) anesthesia group in . Also, 20 carpal tunnel syndrome (CTS) patients were in a WA group, 22 in a local anesthesia (LA) group, and 20 in a GA group. Injection pain, perioperative pain, and postoperative pain were assessed using a 10-point pain VAS. In CuTS, functional outcome on the “quick” Disabilities of the Arm, Shoulder, and Hand questionnaire were evaluated. In CTS, subjective outcomes were assessed using the Korean version of the Michigan Hand Outcomes Questionnaire.
Results
Both CuTS and CTS showed significant postoperative pain reduction in group WA. In CuTS, group WA had less pain than group GA up to 48 hours after surgery (P<0.05). Supplemental opioid injections were used on hospitalization day by 12% of group WA and 35% of group GA. In CTS, the postoperative VAS scores in group WA were lower during the first 24 hours than groups LA and GA (P<0.05). Opioid injections were used on hospitalization day by 5% of WA, 18% of LA, and 32% of group GA. There was no difference in postoperative functional outcomes according to anesthesia method in CuTS or CTS.
Conclusion
Cubital and carpal tunnel surgery using the WALANT technique was comparable in function to other anesthesia methods and superior for pain. Immediate postoperative pain was much lower than other groups, which could reduce the use of opioids during hospitalization.
Keywords: cubital tunnel syndrome, carpal tunnel syndrome, wide-awake surgery, opioid
Introduction
Traditionally, open cubital and carpal tunnel release has been performed with general anesthesia (GA) and a tourniquet. This anesthetic method has the advantage of ensuring sufficient operation time and allowing surgery in the bloodless field through the tourniquet. However, disadvantages are that an elderly patient with underlying disease may have complications due to anesthesia and that it takes a lot of time and cost to prepare for anesthesia. The anesthetic method designed to compensate for the disadvantages of this conventional anesthetic method is the wide-awake local anesthesia with no tourniquet (WALANT) technique. WALANT has been increasingly used by hand surgeons due to its convenience, cost-effectiveness, and high level of patient satisfaction since it was introduced by Lalonde and Martin in 2007.1 WALANT allows for safe operations on older patients and prevents intraoperative bleeding without a tourniquet. The wide-awake technique had previously been used for tendon surgery, mainly by hand surgeons.2 Recently, it has become widely used for hand/wrist fractures and nerve-entrapment syndrome.3 This study compared patient satisfaction with pain relief and the efficacy of the WALANT technique versus conventional anesthesia with the arm tourniquet in open cubital and carpal tunnel release surgery.
Methods
This retrospective study's protocols were approved by the Committee on Medical Research Ethics of Chungbuk National University Hospital in accordance with the Declaration of Helsinki (2019-02-008). This single-center, open-label, case–control study was conducted from January 2016 to February 2017. A total of 55 cubital tunnel syndrome (CuTS) patients who underwent surgery at our center between 20–70 years were included. After written informed consent had been obtained, 42 patients were enrolled (exclusions: six did not consent, three had chronic renal failure, two had rheumatoid arthritis, and one each with previous surgery and trauma). Patients were divided into two groups: WALANT and GA. Twenty patients were in the wide-awake (WA) group without a tourniquet and 22 patients in the GA group with 250 mmHg tourniquet application. In CuTS, pain was observed up to 2 weeks and functional outcome observed up to 12 months postoperatively. A total of 80 carpal tunnel syndrome (CTS) patients who underwent surgery at our center aged 20–70 years were included. Finally, 62 patients were enrolled (exclusions: seven who did not consent, four with chronic renal failure, two with peripheral arterial disease, two with trauma history, one with previous surgery, and one each with rheumatoid arthritis and psychiatric disease). Patients were divided into three groups: WALANT, local anesthesia (LA), and GA. Twenty patients were in the WA group without a tourniquet, 22 patients in the LA group (10 mL 1% lidocaine alone as an LA agent around the incision under intravenous propofol sedation with a 250 mmHg tourniquet application), and 20 patients were in the GA group with a 250 mmHg tourniquet application. In CTS, pain was observed up to 2 weeks and functional outcome observed up to 6 months postoperatively.
Injection technique in WA group
The anesthetic solution was injected a minimum of 30 minutes before surgery to allow the epinephrine to take optimal effect and provide an adequately bloodless field.4 Patient status was monitored by measuring oxygen saturation, heart rate, electrocardiography, and blood pressure until the end of the operation after LA.
Open cubital tunnel release (OCuTR)
Lalonde recommended the use of 10 mL buffered 1% lidocaine in hand surgery.5 In order to make an injection over a wide range around the elbow, we injected 20 mL buffered 0.5% lidocaine with 1:200,000 epinephrine subcutaneously in the most proximal part of the incision, followed by 20 mL in the middle of the incision, and then 20 mL at the end of the incision.
Open carpal tunnel release (OCTR)
We injected 10 mL buffered 1% lidocaine with 1:100,000 epinephrine just ulnar side to the palmaris longus tendon at the proximal injection point (2 mL injected just subcutaneously in the fat, 8 mL for the median nerve block). After the initial 10 mL, we came back to the subcutaneous plane with the needle tip and slowly infiltrated 10 mL proximally to distally in an antegrade direction down the palm between the skin and the superficial palmar fascia.
Surgical intervention
The surgery was performed by a single surgeon. The operative procedure was based on standard technique (OCuTR and OCTR) and identical in all patients.
OCuTR
After an incision of about 7 cm, we released from the proximal arcade of Struthers to the distal two heads of the flexor carpi ulnaris (Figure 1). However, anterior transposition of the ulnar nerve was performed only in patients with subluxation. Postoperatively, immobilization with a long arm splint for 1 week was performed for each patient.
Figure 1.
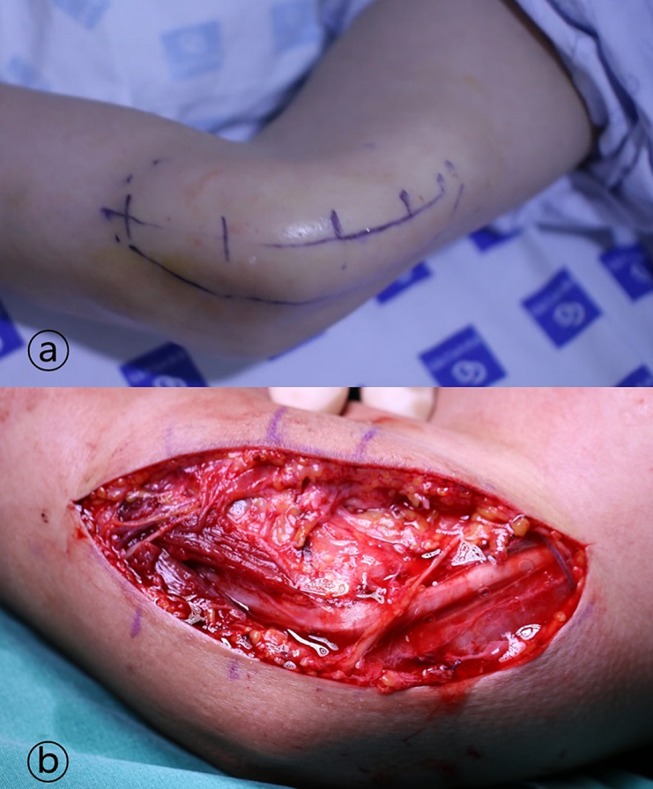
The anesthetic solution was injected a minimum of 30 minutes before surgery.
Notes: (A) Injection was performed considering an incision as long as possible. (B) After incision of about 7 cm, we released from proximal arcade of Struthers to distal two heads of flexor carpi ulnaris. Anterior transposition of ulnar nerve was performed only in patients with subluxation.
OCTR
After an incision of about 3 cm, we released the total transverse carpal ligament (Figure 2). Postoperatively, immobilization with a short arm splint for 1 week was performed for each patient.
Figure 2.
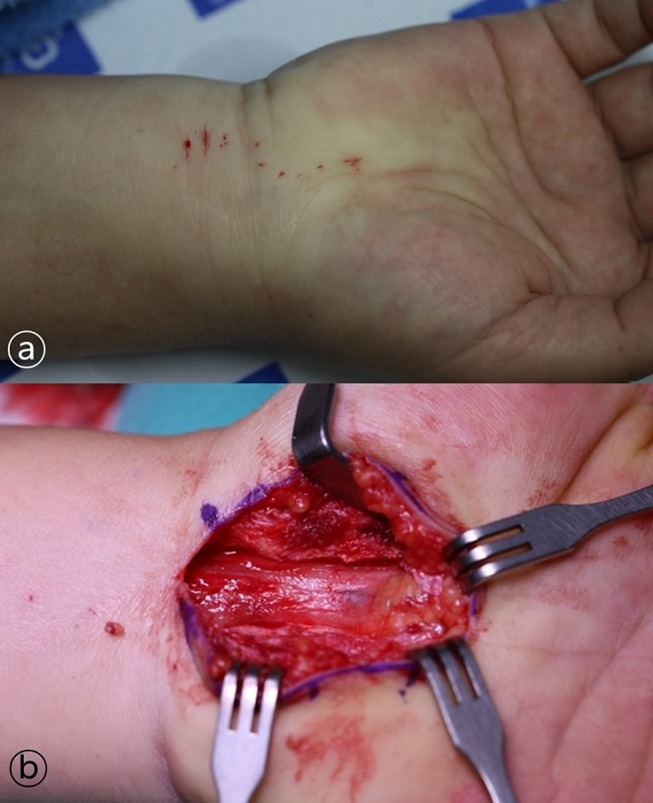
(A) Injection was performed considering an incision as long as possible. (B) After incision of about 3 cm, we released total transverse carpal ligament.
Postoperative pain control
After surgery, all patients were hospitalized for 1 day. For pain control, patients were given celecoxib 200 mg twice a day (usually for about 10 days after the operation), and during hospitalization uniform supplemental analgesic injections (tramadol 50 mg/mL intravenously) were administered upon patient request. The frequency of injections was recorded for each patient. Patients were instructed to record pain and adverse events occurring after discharge (postoperatively at 48 hours and 72 hours) and to come to the outpatient clinic 1 week and 2 weeks after the operation.
Patient discomfort and functional evaluation
Injection pain, perioperative pain, and postoperative pain were assessed using a 10-point pain VAS. VAS scores were measured in the ward up to 24 hours after surgery, at home at 48 and 72 hours, and at the follow-up visit 1 week after the operation. Patients knew that the surgeon could not see individual responses to questions and that results were aggregated by the third (blinded) person. The time to the procedure (skin incision to last suture) and any adverse events were also recorded. In OCuTR, functional outcome using the “quick” Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire6 was evaluated preoperatively and at 3 and 12 months postoperatively. Hand grip and key pinch were also measured using a dynamometer (Jamar pinch gauge; Asmow Engineering, Los Angeles, CA, USA). In OCTR, subjective outcome was assessed before surgery and at 6 months postoperatively by trained nurses using the Korean version of the Michigan Hand Outcomes Questionnaire (K-MHQ).7 Statistical analysis was performed using SPSS. Mann–Whitney U, χ2, Fisher’s exact, and ANOVA were used for analysis. P<0.05 was considered statistically significant.
Results
OCuTR
There were no reoperations or readmissions in the groups or any complications. No significant differences were observed in patients’ demographic data between groups WA and GA, including age, sex, initial quick DASH score, or grip and pinch strength (Table 1). There were no statistically significant differences in operation time.
Table 1.
Patient demographics and preoperative functional evaluation in cubital tunnel syndrome
| Group WA (n=20) | Group GA (n=22) | P-value | |
|---|---|---|---|
| Age, years | 52.4±12.8 | 54.2±13.2 | 0.78 |
| Sex | |||
| Male | 15 | 16 | 0.68 |
| Female | 5 | 6 | |
| Initial “quick” DASH score | 48.5±13.2 | 49.2±14.8 | 0.42 |
| Initial grip strength (kg) | 26.8±8.8 | 27.2±9.2 | 0.64 |
| Initial pinch strength (kg) | 3.4±1.2 | 3.2±1.4 | 0.76 |
Abbreviations: WA, wide awake; GA, general anesthesia; DASH, Disabilities of Arm, Shoulder, and Hand.
Pain
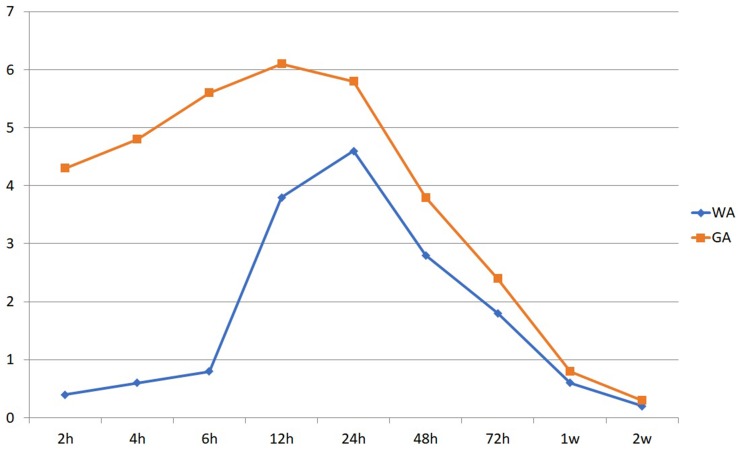
In group WA, mean VAS score for injection pain was 2.4±1.2 and perioperative pain 0.6±0.4. Compared with group GA, group WA had significantly less pain up to 48 hours after surgery (P<0.05). Postoperative VAS scores 2–12 hours in group WA were significantly lower than group GA (P<0.01, Figure 3). Supplemental tramadol injections were used on hospitalization day by 15% of group WA and 36% of group GA (P=0.003). There were no statistically significant differences after 48 hours postoperatively.
Figure 3.
Postoperative VAS scores in open cubital tunnel release.
Notes: Scores during 2–12 hours in group WA were significantly lower than group GA (P<0.01). There were no statistically significant differences at 48 hours postoperatively.
Functional outcome
Quick DASH scores and grip and pinch strength revealed significant postoperative improvement in each group (P<0.01), with no statistically significant differences between groups (WA versus GA) at 3 or 12 months after surgery (Table 2).
Table 2.
Comparison between groups WA and GA in cubital tunnel syndrome
| Preoperative | 3 months after surgery | 12 months after surgery | ||||
|---|---|---|---|---|---|---|
| WA | GA | WA | GA | WA | GA | |
| “Quick” DASH score | 48.5±13.2 | 49.2±14.8 | 33.4±12.2 | 32.8±12.8 | 17.8±10.8 | 17.6±11.0 |
| Grip strength (kg) | 26.8±8.8 | 27.2±9.2 | 28.6±10.2 | 28.4±10.8 | 32.8±9.6 | 33.2±9.8 |
| Pinch strength (kg) | 3.4±1.2 | 3.2±1.4 | 4.2±1.3 | 4.3±1.4 | 5.2±1.8 | 5.0±1.6 |
Abbreviations: WA, wide awake; GA, general anesthesia; DASH, Disabilities of Arm, Shoulder, and Hand.
OCTR
There were no reoperations, readmissions, or complications in the three groups. No significant differences were observed in patients’ demographic data among groups on age, sex, or K-MHQ scores (Table 3). There were no statistically significant differences in operation time.
Table 3.
Patient demographics and preoperative functional evaluation in carpal tunnel syndrome
| WA (n=20) | LA (n=22) | GA (n=20) | |
|---|---|---|---|
| Age, years | 60.4±13.2 | 61.8±12.5 | 62.4±14.2 |
| Sex | |||
| Male | 6 | 5 | 4 |
| Female | 14 | 17 | 16 |
| Preoperative K-MHQ score | |||
| Function | 44.2±18.2 | 45.8±17.8 | 43.6±18.8 |
| Activities of daily living | 52.6±18.8 | 53.8±18.2 | 55.6±17.8 |
| Work | 46.4±19.4 | 45.6±18.8 | 44.8±17.9 |
| Pain | 64.6±24.6 | 62.4±23.4 | 60.4±22.8 |
| Aesthetics | 68.8±25.8 | 66.8±24.6 | 68.2±24.2 |
| Satisfaction | 46.4±28.4 | 44.8±26.8 | 45.8±24.2 |
Abbreviations: K-MHQ, Korean version of Michigan Hand Outcomes Questionnaire; WA, wide awake; LA, local anesthesia; GA, general anesthesia.
Pain
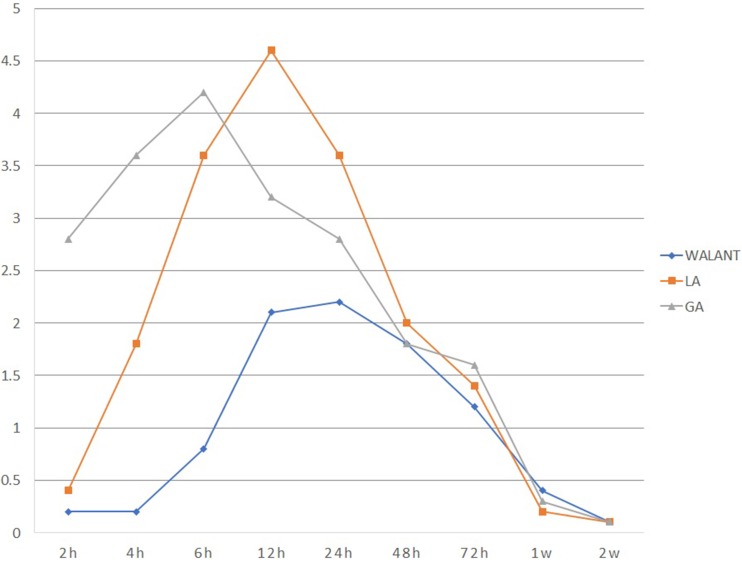
Group WA had significantly less pain at the time of injection compared with group LA (VAS — group WA 2.2±1.2, group LA 5.8±2.8; P=0.01). Postoperative VAS scores in group WA were significantly lower during the first 24 hours than groups LA and GA (P<0.05). Group WA showed significantly lower VAS scores during 6–12 hours than group LA and 2–12 hours than group GA (both P<0.01). There were no statistically significant differences after 24 hours postoperatively among the three groups (Figure 4). Supplemental tramadol injections were used on hospitalization day by 5% of group WA, 18% of group LA, and 30% of group GA (P=0.117).
Figure 4.
Postoperative VAS scores in open carpal tunnel syndrome.
Notes: Scores in group WA were significantly lower during the first 24 hours than groups LA and GA (P<0.05). Group WA had significantly lower scores during 6–12 hours than group LA and 2–12 hours than group GA (both P<0.01). There were no significant differences after 24 hours postoperatively among the groups.
Functional outcome
All domains of the K-MHQ (function, activities of daily living, work, pain, aesthetics, and satisfaction) at 6 months postoperatively revealed significant postoperative improvement in each group (P<0.001) and no statistically significant differences among the three groups (Table 4).
Table 4.
Korean version of Michigan Hand Outcomes Questionnaire scores at 6 months postoperatively in carpal tunnel syndrome
| WA (n=20) | LA (n=22) | GA (n=20) | |
|---|---|---|---|
| Function | 68.4±18.6 | 66.8±19.8 | 67.8±18.2 |
| Activities of daily living | 78.8±16.4 | 75.6±18.8 | 79.6±17.6 |
| Work | 70.4±18.4 | 69.6±18.6 | 68.8±17.8 |
| Pain | 44.8±22.8 | 45.2±24.8 | 43.8±23.8 |
| Aesthetics | 56.6±24.6 | 56.6±22.8 | 58.2±23.6 |
| Satisfaction | 76.8±26.6 | 74.8±28.4 | 75.2±26.8 |
Abbreviations: WA, wide wake; LA, local anesthesia; GA, general anesthesia.
Discussion
In CTS, conventional OCTR is still considered the gold standard, although mini-OCTR and endoscopic CTR have been reported to be successful.8 In this study, we performed conventional OCTR only, in order to eliminate bias by surgical method. In CuTS, simple decompression alone without anterior transposition of the ulnar nerve has shown good results.9 Endoscopic CuTR techniques have recently been introduced.10 Anterior transposition of the ulnar nerve can be performed in ulnar nerve subluxation, severe disease, or revision surgery.11 In this study, all patients but two with ulnar nerve subluxation underwent in situ decompression. Diagnostic methods are also becoming more diverse, intraoperative examination using high-resolution ultrasonography being introduced in addition to the conventional methods (history-taking, physical examination, electrodiagnostic study, and magnetic resonance imaging).12
We wanted to investigate differences in postoperative pain in this study. Postoperative VAS scores in group WA were significantly lower during the immediate postoperative period. Tulipan et al reported that there was no difference in VAS scores at 2 weeks and 3 months after surgery when WA and sedation were compared as an anesthetic method of CTS.13 However, this study did not address immediate postoperative pain. Roberti et al performed a retrospective study of patients undergoing ulnar nerve transposition during CuTS surgery.14 In this study, when GA and LA were compared, VAS scores of the GA group were about twice those of the LA group 7 days postoperatively. Our concern, the difference in immediate postoperative pain, was reflected in the number of opioid injections during hospitalization. Intravenous opioid (tramadol) injections were used on hospitalization day by 15% of WA and 36% of GA in CuTS and by 5% of WA, 18% of LA, and 30% of GA in CTS. Fuzier et al reported that the rate of opioid use after carpal tunnel surgery was 39%.15 Recently, the use of opioids has caused serious social problems. In the US, 4% of adults are given the wrong opioids, and 33,000 die every year due to improper use of opioids.16 Such opioid abuse is increasing rapidly, and the US sees this as an opioid-epidemic crisis. Abuse of opioids can be reduced by reducing initial prescriptions.15 As shown in this study, carpal tunnel surgery through WALANT resulted in significantly lower use of opioids than other anesthesia methods, due to less postoperative pain. Therefore, WALANT is a good option for surgeons as one measure to solve the opioid crisis.
This study did not consider the economic effect of WALANT. It is well known that the WALANT technique is superior in terms of hospitalization and cost. Patients did not have randomized controls, due to economic factors in determining the method of anesthesia.In comparison of opioid usage due to differences in postoperative pain, we did not measure overuse of opioids through long-term follow-up among the three groups.
Conclusion
Cubital and carpal tunnel surgery using the WALANT technique was comparable in function compared to other anesthesia methods and superior for pain relief. Immediate postoperative pain was much lower than other groups, which could reduce the use of opioids during hospitalization.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lalonde D, Martin A. Tumescent local anesthesia for hand surgery: improved results, cost effectiveness, and wide-awake patient satisfaction. Arch Plast Surg. 2014;41(4):312–316. doi: 10.5999/aps.2014.41.4.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang JB. Wide-awake primary flexor tendon repair, tenolysis, and tendon transfer. Clin Orthop Surg. 2015;7(3):275–281. doi: 10.4055/cios.2015.7.3.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teo I, Lam W, Muthayya P, Steele K, Alexander S, Miller G. Patients’ perspective of wide-awake hand surgery–100 consecutive cases. J Hand Surg Eur Vol. 2013;38(9):992–999. doi: 10.1177/1753193412475241 [DOI] [PubMed] [Google Scholar]
- 4.McKee DE, Lalonde DH, Thoma A, Glennie DL, Hayward JE. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131(4):811–814. doi: 10.1097/PRS.0b013e3182818ced [DOI] [PubMed] [Google Scholar]
- 5.Lalonde DH. Latest advances in wide awake hand surgery. Hand Clin. 2019;35(1):1–6. doi: 10.1016/j.hcl.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 6.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602–608. doi: [DOI] [PubMed] [Google Scholar]
- 7.Roh YH, Yang BK, Noh JH, Baek GH, Song CH, Gong HS. Cross-cultural adaptation and validation of the Korean version of the Michigan hand questionnaire. J Hand Surg. 2011;36(9):1497–1503. doi: 10.1016/j.jhsa.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 8.Kim P-T, Lee H-J, Kim T-G, Jeon I-H. Current approaches for carpal tunnel syndrome. Clin Orthop Surg. 2014;6(3):253–257. doi: 10.4055/cios.2014.6.3.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies MA, Vonau M, Blum PW, Kwok BC, Matheson JM, Stening WA. Results of ulnar neuropathy at the elbow treated by decompression or anterior transposition. Aust N Z J Surg. 1991;61(12):929–934. doi: 10.1111/j.1445-2197.1991.tb00011.x [DOI] [PubMed] [Google Scholar]
- 10.Merolla G, Staffa G, Paladini P, Campi F, Porcellini G. Endoscopic approach to cubital tunnel syndrome. J Neurosurg Sci. 2008;52(3):93–98. [PubMed] [Google Scholar]
- 11.Staples JR, Calfee R. Cubital tunnel syndrome: current concepts. J Am Acad Orthop Surg. 2017;25(10):e215–e224. doi: 10.5435/JAAOS-D-15-00261 [DOI] [PubMed] [Google Scholar]
- 12.Cho C-H, Lee Y-H, Song K-S, Lee K-J, Lee S-W, Lee S-M. Accuracy of preoperative ultrasonography for cubital tunnel syndrome: a comparison with intraoperative findings. Clin Orthop Surg. 2018;10(3):352–357. doi: 10.4055/cios.2018.10.3.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tulipan JE, Kim N, Abboudi J, et al. Open carpal tunnel release outcomes: performed wide awake versus with sedation. J Hand Microsurg. 2017;9(2):74–79. doi: 10.1055/s-0037-1603200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberti del Vecchio PM, Christen T, Raffoul W, Erba P. Ulnar nerve transposition at the elbow under local anesthesia: a patient satisfaction study. J Reconstr Microsurg. 2015;31(3):187–190. doi: 10.1055/s-0034-1394159 [DOI] [PubMed] [Google Scholar]
- 15.Fuzier R, Serres I, Bourrel R, Palmaro A, Lapeyre-Mestre M. Analgesic drug prescription after carpal tunnel surgery: a pharmacoepidemiological study investigating postoperative pain. Reg Anesth Pain Med. 2018;43(1):19–24. doi: 10.1097/AAP.0000000000000685 [DOI] [PubMed] [Google Scholar]
- 16.Skolnick P. The opioid epidemic: crisis and solutions. Annu Rev Pharmacol Toxicol. 2018;58::143–159. doi: 10.1146/annurev-pharmtox-010617-052534 [DOI] [PubMed] [Google Scholar]