Abstract
The correct differentiation of oligodendrocyte precursor cells (OPCs) is essential for the myelination and remyelination processes in the central nervous system. Determining the regulatory mechanism is fundamental to the treatment of demyelinating diseases. By analyzing the RNA sequencing data of different neural cells, we found that cyclin-dependent kinase 18 (CDK18) is exclusively expressed in oligodendrocytes. In vivo studies showed that the expression level of CDK18 gradually increased along with myelin formation during development and in the remyelination phase in a lysophosphatidylcholine-induced demyelination model, and was distinctively highly expressed in oligodendrocytes. In vitro overexpression and interference experiments revealed that CDK18 directly promotes the differentiation of OPCs, without affecting their proliferation or apoptosis. Mechanistically, CDK18 activated the RAS/mitogen-activated protein kinase kinase 1/extracellular signal-regulated kinase pathway, thus promoting OPC differentiation. The results of the present study suggest that CDK18 is a promising cell-type specific target to treat demyelinating disease.
Electronic supplementary material
The online version of this article (10.1007/s12264-019-00376-7) contains supplementary material, which is available to authorized users.
Keywords: OPC, Oligodendrocyte, Myelination, CDK18, ERK
Introduction
Myelin is a concentric membrane structure surrounding neuronal axons that plays a pivotal role in insulation, nutrition, and action potential conduction. In the central nervous system (CNS), myelin is formed by the cytomembrane of oligodendrocytes, which originate from oligodendrocyte precursor cells (OPCs). The correct differentiation of OPCs is crucial for the genesis of oligodendrocytes and for myelin formation. OPCs originate from the ventral ventricular zone of the embryonic spinal cord, spread along the blood vessels to the whole CNS, and then gradually differentiate into oligodendrocytes. In adults, OPCs still exist in large numbers and are uniformly distributed throughout the CNS, undertaking the responsibility for new myelin formation and natural oligodendrocyte replacement [1, 2]. In neurological diseases, demyelination is an important pathological feature, not only in classic demyelinating diseases like multiple sclerosis, but also, for example, in spinal cord injury, schizophrenia, and depression [3, 4]. An in-depth study of OPC differentiation would be of great value to determine the mechanism of myelination and to find new therapeutic targets for neurological diseases affecting myelin.
Currently, three signaling pathways are known to be involved in the regulation of OPC differentiation, namely the Wnt (wingless/integrated)/β-catenin pathway, the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT serine/threonine kinase AKT/mechanistic target of rapamycin kinase (mTOR) pathway, and the RAS/mitogen-activated protein kinase kinase 1 (MEK)/extracellular signal-regulated kinase (ERK) pathway. The Wnt/β-catenin pathway negatively regulates the differentiation of OPCs by binding to T-cell-specific transcription factor/lymphoid enhancer binding factor family members. The PI3K/AKT/mTOR and RAS/MEK/ERK pathways positively regulate OPC differentiation [5–7]. At the transcription factor level, many transcription factors are involved in the regulation of myelination, such as the Olig family, the D and E subgroup of the SRY-box family, zinc finger protein 24 (also known as ZFP191), survival of motor neuron protein interacting protein 1, and zinc finger protein 488 of the zinc finger protein family; Hes family basic helix-loop-helix (bHLH) transcription factor 1 (HES1) and HES5 of the bHLH family; and inhibitor of DNA binding 2 (ID2) and ID4 of the helix-loop-helix family [8]. These studies have partially determined the differentiation mechanism of OPCs; however, transcription factors are difficult to manipulate, and the PI3K/AKT/mTOR and RAS/MEK/ERK pathways are widely distributed in various nerve cells. None of them is proper target for drugs promoting the specific differentiation of OPCs [5, 7]. There is still a need to find new targets that are exclusively expressed in oligodendrocyte lineage cells and are easy to manipulate.
Cyclin-dependent kinases (CDKs) are a class of kinases that contain cyclin-binding domains. More than 20 members have been discovered, and most of them are highly correlated with cell proliferation and differentiation processes [9–12]. Studies with CDKs have mainly focused on tumors [13–16]. Various CDKs are associated with the occurrence, metastasis, and prognosis of tumors, and drugs targeting CDKs have been developed [17, 18]. In the CNS, CDKs also participate in certain processes: notably, CDK5 promotes OPC differentiation, while CDK14 inhibits it, and both of them affect the PI3K/AKT/mTOR signaling pathway [19, 20]. However, CDK5 and CDK14 are widely expressed in a variety of neural cells with no cell-type specificity. By analyzing the RNA sequencing data of different neural cells [21], we found that CDK18 is specifically expressed in newly-formed and myelinating oligodendrocytes, and correlates highly with the differentiation of OPCs. Thus, CDK18 is likely to be involved in the regulation of OPC differentiation. CDK18 is a newly-discovered member of the CDK family, about which there have been few functional studies. Its known functions include regulation of tau phosphorylation, and maintenance of chromosome stability [22, 23]. The role of CDK18 in OPC differentiation has not been studied.
Materials and Methods
Animal Experiments
The animal experiments were carried out in adherence to the National Institutes of Health Guidelines on the Use of Laboratory Animals and approved by the Second Military Medical University Committee on Animal Care. Serial sections of the spinal cord and corpus callosum of rats were obtained, and 3–5 sections were randomly selected from each tissue for subsequent experiments. Focal demyelination in the dorsal spinal cord was induced by L-a-lysophosphatidylcholine (LPC; lysolecithin; Sigma-Aldrich, St. Louis, MO) as previously described [36, 37]. Briefly, 1 μL of 1% LPC in 0.9% NaCl was microinjected into the dorsal or ventral column at T11–T12 in the spinal cord of Sprague-Dawley rats (10 weeks–12 weeks) using a micromanipulator and a glass tip attached to a syringe (Hamilton, NE, Switzerland), following a laminectomy. The day of LPC injection was designated as day 0 (0 dpi). Control mice were injected with 1 μL of saline.
Cell Cultures
Primary cortical OPCs were prepared according to previous reports [38, 39]. Mixed cortical glial cell cultures were generated from neonatal rat cerebra and cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum for 10 days at 37°C with 5% CO2. The medium was changed every 3 days. Ten days later, the flasks were shaken for 1 h on an orbital shaker (180 rpm) to remove microglia, followed by an additional 16 h at 200 rpm with fresh medium at 37°C. The purified OPCs were collected from mixed glia by leaving the cell suspension to adhere in uncoated Petri dishes. Then they were seeded at 5,000 cells/cm2 to 50,000 cells/cm2 on coverslips coated with poly-D-lysine one day before experiments. To examine differentiation, OPCs were cultured in Neurobasal medium supplemented with 2% B27. To expand the OPCs and keep them undifferentiated, the culture medium was supplemented with 30% B104 conditioned medium [40].
Lentivirus Transduction and Electroporation
For lentivirus transduction, the target sequence of the CDK18 siRNA was GCCCAAACCACTCACCCGAAT, which was ligated into the GV493 plasmid (GeneChem, Shanghai, China). The CDK18-overexpressing virus constructed by the GV492 plasmid was also from GeneChem (Shanghai, China). The titer of concentrated viral particles was 5×108–1 × 109 transducing units/ml. Lentiviral particles were added to cultured oligodendrocytes at a multiplicity of infection of 3 and the supernatant was changed 12 h after infection.
Immunocytofluorescence Staining
Cells on the coverslips were fixed with 4% paraformaldehyde (PFA) for 15 min followed by three washes in PBS at room temperature. Then cells were incubated with primary antibodies for overnight at 4°C as follows: mouse anti-MBP (1:50, MAB382, Chemicon), chicken anti-GFP (1:300, Millipore, Billerica, MA, USA), mouse anti-Caspase3 (1:100, AB3623, Millipore, Billerica, MA). Cells then were incubated in FITC- or TRITC-conjugated secondary antibodies (1:100, Jackson ImmunoResearch, West Grove, PA, USA) containing Hoechst for 2 h at room temperature. Cells are counted in at least 10 randomly selected fields from one coverslip and at least 3 coverslips for each group are counted.
Immunohistofluorescence Staining
Animals were anesthetized and perfused with 4% (PFA) via transcardiac perfusion. Tissues were embedded at − 20°C. Frozen tissues samples of corpus callosum, spinal cords and LPC lesions of rats were prepared into 14 um cryostat sections for further histological analysis. Cryostat coronal sections were boiled for 15 min in 10 mmol/L citrate buffer (pH 6.0) at 95°C before being permeabilized with 0.5% Triton for 15 min, then placed in blocking solution, and incubated overnight at 4°C with primary antibodies, including goat anti-PDGFα (1:50, AF1062, R&D systems, Minneapolis, MN, USA), mouse anti-CC1(1:100, MABC200, Millipore, Billerica, MA), rabbit anti-CDK18 (1:100, Invitrogen, Carlsbad, CA, USA), mouse anti-oligodendrocyte transcription factor 1 (Olig1; 1:300, MAB5540, Millipore, Billerica, MA), mouse anti-Neuronal Nuclei (1:50, MAB377, Millipore, Billerica, MA), goat anti-Ionized Calcium-binding Adapter Molecule 1 (1:50, ab5076, Abcam, Cambridge, UK), mouse anti-Glial Fibrillary Acidic Protein (1:50, G3893, Sigma, St. Louis, MO). Tissues then were incubated in FITC- or TRITC-conjugated secondary antibodies (1:100, Jackson ImmunoResearch, West Grove, PA) containing Hoechst for 2 h at room temperature. The samples were examined by confocal microscopy (Leica, Buffalo Grove, IL, USA) to take pictures. Three spinal cord sections were selected for each sample, and 3–5 rats were tested in each experimental group.
RNA Isolation and qPCR Analysis
Cells were lysed with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and total RNA was extracted according to the manufacturer’s instructions. After removal of the residual DNA by the DNase I treatment, cDNA was synthesized using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific Fermentas, Logan, UT, USA). The mRNA level was then detected using real-time quantitative PCR (qPCR). It was performed with the SYBR Green Real-time PCR Master Mix (TOYOBO, Shanghai, China). Gene expression was normalized to a standard housekeeping gene GAPDH using the ΔΔCT method. PCRs were performed under the following conditions: 95°C for 3 min followed by 45 cycles at 95°C for 10 s, 65°C for 30 s, and 72°C for 10 s, and 72°C for 10 min. The primers used for CDK18 were F: CAAACCACTCACCCGAATGTCC R: CACGAGGTTCTCTGTCAACTTGC .The primers used for GAPDH were F: CCATCAACGACCCCTTCATT R: ATTCTCAGCCTTGACTGTGC. The primers used for MBP were F: CTTGTTAATCCGTTCTAATTCCG, R: TTCTGGAAGTTTCGTCCCT.
Western Blotting Analysis. Western blotting is done in accordance with standard protocols. Primary antibodies include: mouse anti-CDK18(1:200, Crus, sc-393262, Santa, Dallas, TX, USA) and mouse anti-MBP(1:500, MAB382, Chemicon, Temecula, CA, USA), horseradish peroxidase (HRP)-conjugated anti-gapdh (1:10000, Kangcheng Biotechnology, SH, China) were incubated overnight at 4°C. HRP-conjugated secondary antibodies (1:3000, Kangcheng Biotechnology, Shanghai, China) were incubated for 2 h. The protein bands were analyzed and quantified using 422 Image Lab analysis (Bio-Rad, Hercules, CA, USA).
BrdU Incorporation
To detect the proliferation of OPCs, BrdU (5-Bromo-2-deoxyUridine, 10 μmol/L, Sigma-Aldrich, St. Louis, MO) was added to the medium for 6 h before the cells were fixed in 4% (PFA). The cells on coverslips were then exposed to 0.3% Triton for 10 min, then incubated in 2 N HCl for 30 min and incubated in 0.1 mol/L borate buffer (pH 8.0) for 20 min. The cells were then blocked in PBS containing 5% goat serum for 1 h at room temperature and incubated overnight with anti-BrdU antibody (1:100) and anti-GFP antibody (1:300, Millipore, Billerica, MA). The cells were then incubated with FITC- or TRITC-conjugated secondary antibodies (1:100, Jackson ImmunoResearch, West Grove, PA) for 2 h at room temperature. The percentage of BrdU+ cells versus GFP+ cells was calculated.
Luxol Fast Blue Staining
Frozen tissues samples of LPC lesions were later cut at 14 μm on a cryostat for further Luxol fast blue staining, and the central region of the dorsal cord was examined. The sections were incubated in 100% ethanol for 5 min and in 0.1% Luxol fast blue overnight (14 h–16 h) at 58°C, followed by decoloring differentiation with 0.05% lithium carbonate and 70% ethanol.
Statistical Analysis
The two-tailed Student’s t-test was applied for statistical comparison of 2 groups, and a one-way ANOVA with Tukey’s post hoc test was used for multiple groups. The data are presented as the mean ± SEM, which generally represents biological replicates. A value of P < 0.05 was considered statistically significant.
Results
CDK18 is Highly Expressed in Oligodendrocytes
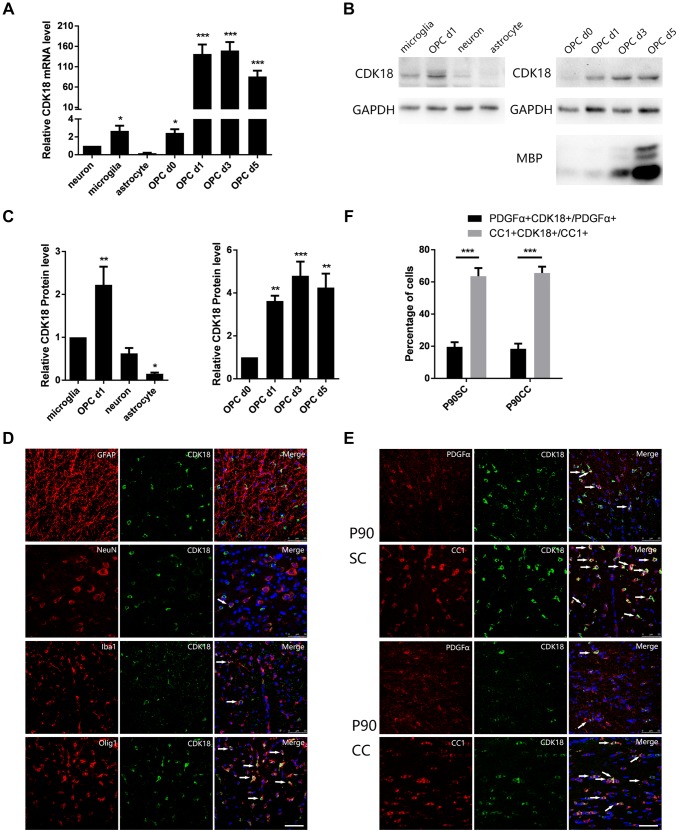
To analyze the expression pattern of CDK18 in the CNS, we first collected different rat neural cells and oligodendrocyte lineage cells at different stages of differentiation for primary culture in vitro. Quantitative real-time polymerase chain reaction (qPCR) showed that Cdk18 was rarely expressed in neurons, astrocytes, or microglia. In oligodendrocyte lineage cells, the mRNA expression level increased gradually with differentiation and Cdk18 was highly expressed in mature oligodendrocytes (Fig. 1A). Western blotting analysis gave results similar qPCR (Fig. 1B, C). Furthermore, immunofluorescence staining of adult rat spinal cord sections showed no co-localization of Cdk18 with the astrocyte marker Glial Fibrillary Acidic Protein, and a small proportion co-localized with the neuron marker Neuronal Nuclei or the microglia marker Ionized Calcium-binding Adapter Molecule 1. By contrast, extensive co-localization with the oligodendrocyte lineage cell marker Olig1 was observed (Fig. 1D). Finally, we analyzed the co-labeling of Cdk18 with the OPC marker platelet-derived growth factor receptor alpha (PDGFRα) and the oligodendrocyte marker CC1 using immunofluorescence staining of spinal cord and corpus callosum sections from adult rats. Cdk18 was identified in OPCs and oligodendrocytes; however, the proportion of co-labeled cells was much higher among oligodendrocytes (Fig. 1E, F). These results indicated that the expression level of Cdk18 gradually increases as OPCs differentiate, and is exclusively and highly expressed in oligodendrocytes.
Fig. 1.
Expression of CDK18 in several cell types in the CNS. A Quantitative PCR assays of the mRNA levels of Cdk18 during OPC differentiation and in other CNS cell types in vitro. B, C Western blots (B) and analysis (C) of CDK18 expression during OPC differentiation and in other CNS cell types in vitro. D Expression of CDK18 (green) in glial cells and neurons in the spinal cord of P60 rats detected by immunohistochemistry (arrows, cells showing co-localization; scale bar, 50 μm). E Expression of CDK18 (green) in PDGFα-positive (red) and CC1-positive (red) cells in the spinal cord and callosum of P90 rats detected by immunohistochemistry (arrows, cells showing co-localization; scale bar, 50 μm). F Quantitative analysis of the percentage of CDK18+PDGFα+ and CDK18+CC1+ double-positive cells among PDGFα+ or CC1+ cells. Values are the mean ± SEM; *P < 0.05, ** P < 0.01, ***P < 0001, one-way ANOVA with Tukey’s post hoc test; n = 4/group.
Cdk18 Expression Increases During Myelination
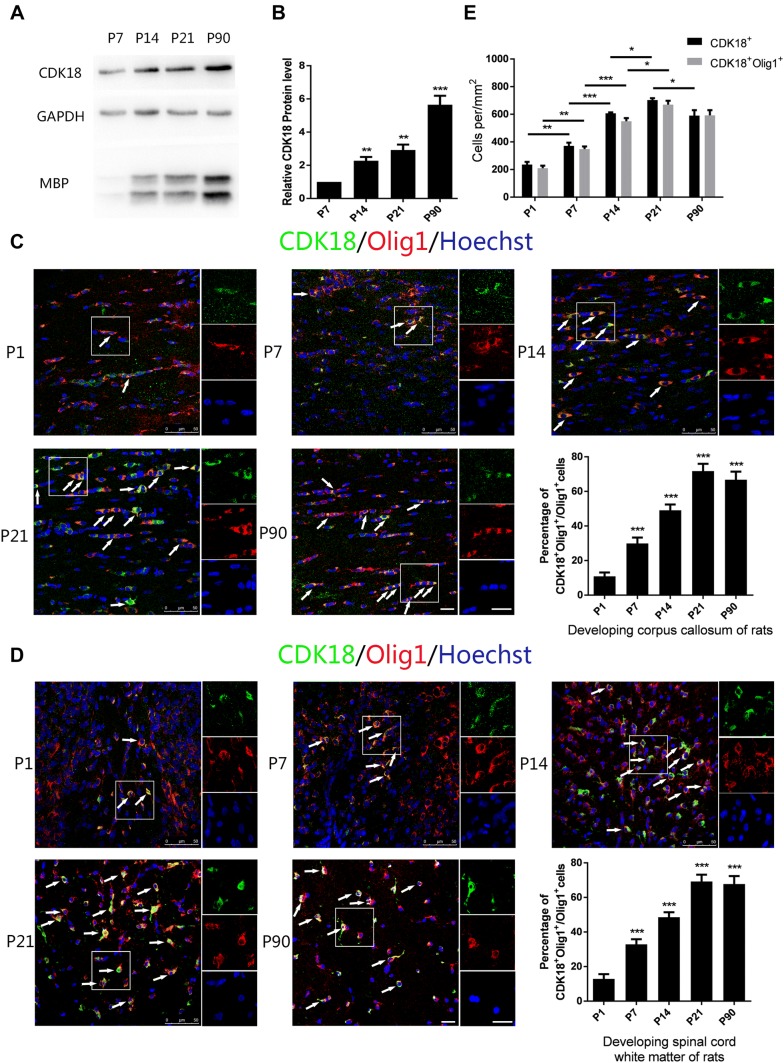
Next, we explored the relationship between CDK18 and myelination in vivo. The process of myelination in rats generally starts from postnatal day 7 (P7) and is basically completed by P60. We performed western blotting analysis using the corpus callosum from rats at P7, P14, P21, and P90, and found that the level of CDK18 increased as development progressed (Fig. 2A, B). At the same time, the corpus callosum and spinal cord of P1, P7, P14, P21, and P90 rats were isolated and analyzed using immunofluorescence staining. The results showed that the proportion of CDK18 and Olig1 double-positive cells among Olig1-positive cells increased as development progressed, both in the corpus callosum and spinal cord, and the trend became stable after P21 (Fig. 2C, D). This result also indicated that the higher protein level of CDK18 at P90 than at P21 might have been caused by further differentiation and maturation of oligodendrocytes with increased expression of CDK18 in single cells. In addition, we calculated the density of CDK18 and Olig1 double-positive cells and CDK18 single-positive cells in the white matter of the rat spinal cord, and found that the numbers of both types of cells increased along with the myelination process and became stable after P21 (Fig. 2E). The numbers of the two types of cells were similar, which was consistent with the previous results showing that CDK18 is predominantly expressed in oligodendrocytes. These results indicated that CDK18 expression gradually increases during myelin formation in the CNS, consistent with the expression pattern during OPC differentiation in vitro.
Fig. 2.
CDK18 is dynamically expressed during development. A, B Western blots (A) and analysis (B) of CDK18 expression in the corpus callosum from P7, P14, P21, and P90 rats. C, D Immunohistochemistry and quantitative analysis of CDK18 expression (green) in Olig1-positive cells (red) in the corpus callosum (C) and white matter of spinal cord (D) from P1, P7, P14, P21, and P90 rats (arrows, cells with co-localization; scale bars, 20 μm). E Quantitative analysis of the density of CDK18 + Olig1 + double-positive and CDK18 + cells throughout postnatal development in spinal cord white matter. Results are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA with Tukey’s post hoc test; n = 4/group.
CDK18 Levels are Upregulated During Remyelination in an LPC-induced Demyelination Model
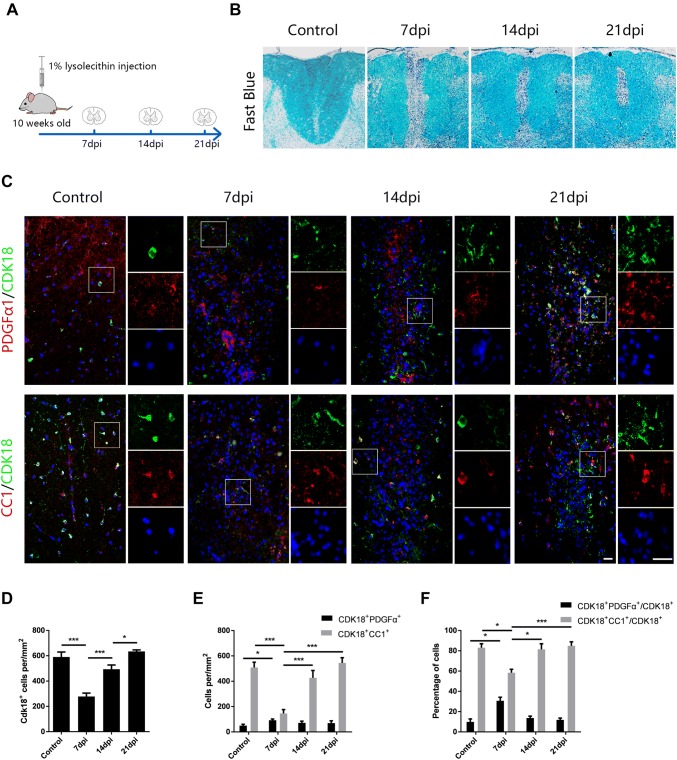
The expression of CDK18 is closely associated with the developmental differentiation of OPCs, so we sought to determine the relationship between CDK18 expression and the demyelination and remyelination process under pathological conditions. For this purpose, we constructed an LPC-induced demyelination model in the spinal cord of rats (Fig. 3A). This model reached peak demyelination on day 7 after LPC injection (7 dpi), showing clear remyelination markers at 14 dpi, and complete myelin repair at 21 dpi (Fig. 3B). Using immunofluorescence staining to assess the CDK18 expression in oligodendrocyte lineage cells at 7, 14, and 21 dpi, we found that the number of CDK18+ cells in the demyelinating lesions decreased at first, then increased, and almost returned to the control level at 21 dpi (Fig. 3C, D). This showed a close correlation between CDK18 expression and the demyelination and remyelination processes. Further statistical analysis showed that the density of CDK18 and CC1 double-positive cells was significantly reduced at 7 dpi, partially recovered at 14 dpi, and became close to the control group at 21 dpi, consistent with the myelin loss and recovery processes. The number of CDK18 and PDGFRα double-positive cells slightly increased at 7 dpi, which might have been caused by OPC proliferation, and returned to a level consistent with the control group at 14 and 21 dpi (Fig. 3E). In addition, CDK18 was expressed at a higher level in mature CC1+ oligodendrocytes during remyelination, while there were fewer co-labeled PDGFRα+OPCs (Fig. 3F). These results indicated that the expression of CDK18 is closely associated with the processes of myelin injury and regeneration, and increases during remyelination.
Fig. 3.
Expression of CDK18 in oligodendrocyte lineage cells in LPC-induced demyelinating lesions. A Schematic of the LPC-induced spinal cord demyelination model. B Fast Blue staining of the demyelinating spinal cord at days 7, 14, and 21 after LPC injection. C Representative micrographs of immunostaining for CDK18 (green) and PDGFα or CC1 (red) in LPC-induced demyelinating lesions and in saline-injected control spinal cord. Right panels, high-magnification micrographs of the outlined regions (white boxes) showing co-localization of CDK18 with Hoechst and other OL lineage cell markers. Scale bars, 20 μm. D, E Quantitative analysis of the density of CDK18 + cells (D) and CDK18 + CC1 + OLs or CDK18 + PDGFα + OPCs (E) in saline-injected controls and LPC-injected demyelinating lesions. F Quantitative analysis of the percentage of CDK18 + PDGFα + and CDK18 + CC1 + double-positive cells among CDK18 + cells. Results are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA with Tukey’s post hoc test; n = 4/group.
CDK18 Promotes OPC Differentiation in Vitro
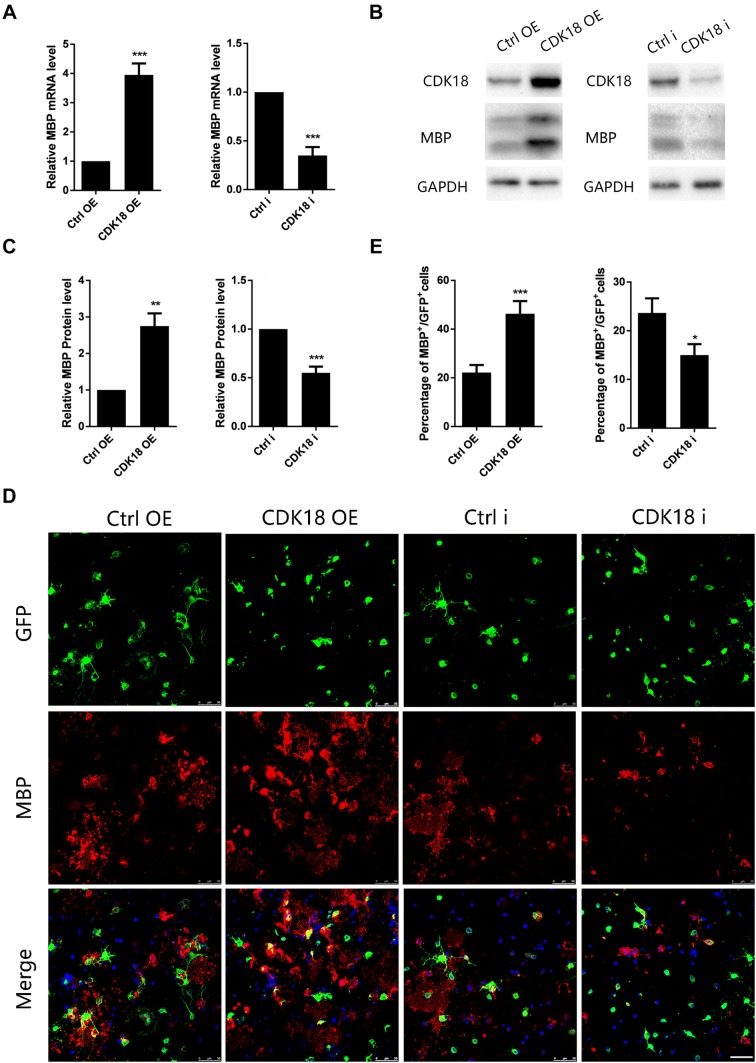
Does the increase in CDK18 expression affect the differentiation of OPCs? To answer this question, we constructed CDK18 overexpression and interfering lentiviruses (Fig. S1), transduced them into primary cultured rat OPCs, and examined their effects on OPC differentiation under differentiation culture conditions. The qPCR results showed that the mRNA level of myelin basic protein (MBP) was significantly increased at 48 h after transduction of the CDK18-overexpressing lentivirus, whereas transduction of the CDK18-interfering lentivirus significantly reduced MBP mRNA expression (Fig. 4A). Furthermore, we assessed the MBP protein level using western blotting at 72 h after transduction of the lentivirus, and the results were similar to those of qPCR (Fig. 4B, C). At the same time, we used immunofluorescence staining to calculate the proportion of MBP-positive cells at 72 h after transduction of lentiviruses. Overexpression of CDK18 significantly increased the proportion of MBP-positive cells, and CDK18 silencing had the opposite effect (Fig. 4D, E). In addition, we examined the effect of overexpression or interference with CDK18 on OPC proliferation and apoptosis using BrdU incorporation assays and caspase 3 immunofluorescence staining, and found no significant difference (Fig. S2). These results indicated that CDK18 promotes the differentiation of OPCs in vitro, but has no significant effect on proliferation and apoptosis.
Fig. 4.
CDK18 promotes OPC differentiation in vitro. A Quantitative PCR assays of mRNA levels of Mbp in purified OPCs transduced with overexpressing viruses (CDK18 OE) or interfering viruses (CDK18i) and control virus. B, C Western blots (B) and analysis (C) of CDK18 and MBP protein levels in purified OPCs transduced with CDK18 OE virus or CDK18i virus and control virus. D Representative images of anti-MBP immunocytofluorescence staining of OPCs transduced with CDK18 OE or CDK18i virus. Scale bar, 50 μm. E Quantification of MBP +/GFP + cells as described in D. The OPCs were all cultured in differentiation medium for 72 h. Results are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t-test; n = 4 independent experiments.
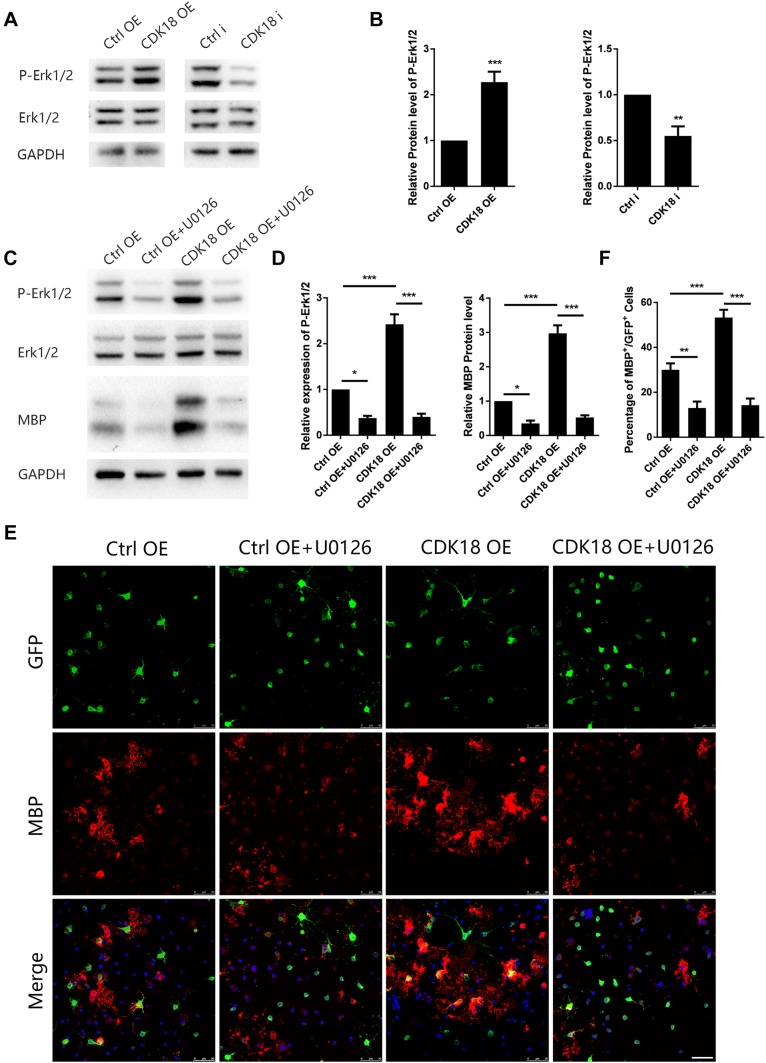
CDK18 Promotes OPC Differentiation by Regulating the RAS/MEK/ERK Signaling Pathway
A member of a kinase family, CDK18 is likely to affect signaling pathways involved in OPC differentiation, and the PI3K/AKT/mTOR and RAS/MEK/ERK pathway are the two most likely. So we next investigated the signaling pathway that might be responsible for the pro-differentiation effect of CDK18. Studies have shown that the above pathways are closely involved in the regulation of oligodendrocyte lineage cell differentiation. We therefore assessed the expression and phosphorylation levels of mTOR and ERK1/2 after CDK18 overexpression or interference. The phosphorylation level of ERK1/2 increased significantly after CDK18 overexpression, while interference had the opposite effect (Fig. 5A, B). The expression and phosphorylation of mTOR were unaffected by CDK18 (Fig. S3A, B). U0126, a specific inhibitor of MEK, blocked the effect of CDK18 overexpression on ERK1/2 phosphorylation, as well as preventing the increase in MBP expression (Fig. 5C, D). At the same time, we found that U0126 did not affect the protein level of CDK18 while blocking Erk phosphorylation in purified OPCs cultured in differentiation medium (Fig. S3C, D). This indicated that Erk1/2 is downstream of CDK18. Immunofluorescence staining also showed that the effect of CDK18 on the proportion of MBP-positive cells was completely blocked by U0126 (Fig. 5E, F). These results indicated that CDK18 regulates the RAS/MEK/ERK pathway, thus affecting OPC differentiation.
Fig. 5.
CDK18 promotes OPC differentiation through activating Erk1/2 signaling. A, B Western blots (A) and analysis (B) of P-Erk1/2 and Erk1/2 protein levels in purified OPCs transduced with CDK18 OE virus or CDK18i virus and control virus. C, D Representative western blots (C) and statistical analysis (D) of the effect of the Erk1/2 inhibitor U0126 (10 nmol/L) on the expression of P-Erk1/2, Erk1/2, and MBP in purified OPCs transduced with overexpressing virus (CDK18-OE) and control viruses. E Representative images showing the effect of U0126 (10 nmol/L) on the expression of MBP (red) among purified OPCs transduced with CDK18 OE and control virus (green) (scale bar, 50 µm). F Statistical analysis of the percentage of MBP +/GFP + cells in each group. The OPCs were all cultured in differentiation medium for 72 h. Results are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t-test in B one-way ANOVA with Tukey’s post hoc test in D, F; n = 3 independent experiments.
Discussion
Abnormal myelination and remyelination after demyelination disorders are related to the de-differentiation of OPCs. An in-depth study of the differentiation mechanism of OPCs is needed to reveal the pathogenesis of demyelinating diseases and to find new targets for their treatment. In the present study, we used RNA sequencing to demonstrate that CDK18 is highly expressed in oligodendrocytes, and confirmed its expression pattern in vitro and in vivo. Transduction of lentiviruses showed that CDK18 has a direct effect on the differentiation of OPCs. Our work started by seeking specifically highly expressed molecules in oligodendrocytes, and finally discovered new participants that affect the formation of the myelin sheath, providing a new strategy to screen for therapeutic targets for demyelinating diseases.
The CDK family has many members that function in a complex manner. Currently, it is mainly divided into two functional categories: cell cycle-related (such as CDK1, 4, and 5) and transcription-related (such as CDK7, 8, 9, 11, and 20). The former group affects cell cycle progression [24, 25], and the latter directly regulates the activity of the RNA polymerase II complex [26, 27]. According to evolutionary relationships, CDK18 is closely related to CDK5, which is widely expressed in a variety of cells and is mainly activated by binding to p35 or p39. These two activators are mainly expressed in terminally-differentiated cells, such as neurons, thereby restricting the role of CDK5 [28, 29]. No specific activator of CDK18 has been identified, and its mechanism of activation is still unclear, so CDK18 has not been classified. CDK18 is relatively highly expressed in the brain, spinal cord, and heart, showing stronger tissue specificity than CDK5. In the CNS, CDK18 is mainly expressed in differentiated oligodendrocytes, while other CDKs have no cell-type specificity. The unique expression pattern of CDK18 identifies it as a promising target for developing drugs to treat demyelinating diseases, and also suggests that its expression is specifically regulated.
Classical CDK molecules contain the “PSTAIRE” sequence; however, CDK16, CDK17, and CDK18 contain an analogous “PCTAIRE” sequence, and are thus defined as the PCTK subfamily [30]. PCTKs activate downstream pathways by binding to cyclins, but can also function in a cyclin-independent manner. In addition, some reports suggest that PCTKs behave differently to normal CDKs [31]. For example, the expression level of PCTK in testicular cells does not change during the cell cycle [32]; additional expression of CDKs in yeast with a Cds28 (equivalent to mammalian CDK1) deficiency can restore the cell cycle, but PCTKs cannot [33]. CDK5 and CDK14 affect the differentiation of OPCs via the PI3K/AKT/mTOR signaling pathway [20, 34]. However, in the present study we found that CDK18 had no effect on this pathway, but affected the RAS/MEK/ERK pathway to promote OPC differentiation. In addition, CDK18 had no effect on the proliferation or apoptosis of OPCs. Our results provide new evidence for the differences between PCTKs and classical CDKs; however, the reasons for this difference remain unknown.
By focusing on the major known pathways affecting OPC differentiation, we found that specifically inhibiting MEK in the RAS/MEK/ERK pathway completely blocked the effect of CDK18 on ERK phosphorylation and OPC differentiation. This suggested that the RAS/MEK/ERK pathway might be the main pathway by which CDK18 regulates the differentiation of OPCs. CDK18 also inhibits the focal adhesion kinase pathway and interacts with the RAD9 checkpoint clamp component, RAD17, and DNA topoisomerase II binding protein 1 [22, 35], but these functions are not related to OPC differentiation. In fact, there are few studies on the functions and mechanisms of action of CDK18. Our finding that CDK18 acts via the RAS/MEK/ERK pathway enriches CDK18 working models. The upstream effectors of CDK18 are also unclear, and although cyclin A2 activates CDK18, the kinase activity of CDK18 is not dependent on cyclin A2 [35]. Thus, the upstream regulation of CDK18 requires further research.
The results of the present study revealed the regulation of OPC differentiation by CDK18 and its main mechanism, which identifies CDK18 as a cell-specific target for the treatment of demyelinating diseases. Furthermore, as a kinase, CDK18 is relatively easy to manipulate. In future studies, strategies can be developed to exploit CDK18 as a target for the treatment of demyelinating diseases, thus moving a step closer to clinical application.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31700924, 31571066, 31771129, and 31871026) and the National Basic Research Development Program of China (2016YFA0100802).
Contributor Information
Cheng He, Email: chenghe@smmu.edu.cn.
Li Cao, Email: caoli@smmu.edu.cn.
References
- 1.Bergles DE, Richardson WD. Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol. 2015;8:a020453. doi: 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franklin RJM, Ffrench-Constant C. Regenerating CNS myelin - from mechanisms to experimental medicines. Nat Rev Neurosci. 2017;18:753–769. doi: 10.1038/nrn.2017.136. [DOI] [PubMed] [Google Scholar]
- 3.Nave KA, Werner HB. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- 4.Haroutunian V, Katsel P, Roussos P, Davis KL, Altshuler LL, Bartzokis G. Myelination, oligodendrocytes, and serious mental illness. Glia. 2014;62:1856–1877. doi: 10.1002/glia.22716. [DOI] [PubMed] [Google Scholar]
- 5.Guardiola-Diaz HM, Ishii A, Bansal R. Erk1/2 MAPK and mTOR signaling sequentially regulates progression through distinct stages of oligodendrocyte differentiation. Glia. 2012;60:476–486. doi: 10.1002/glia.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J Neurosci. 2009;29:6860–6870. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaesser JM, Fyffe-Maricich SL. Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp Neurol. 2016;283:501–511. doi: 10.1016/j.expneurol.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emery B, Lu QR. Transcriptional and Epigenetic Regulation of Oligodendrocyte Development and Myelination in the Central Nervous System. Cold Spring Harb Perspect Biol. 2015;7:a020461. doi: 10.1101/cshperspect.a020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santamaria D, Barriere C, Cerqueira A, Hunt S, Tardy C, Newton K, et al. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448:811–815. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- 10.Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118:493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Kollmann K, Heller G, Schneckenleithner C, Warsch W, Scheicher R, Ott RG, et al. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell. 2013;24:167–181. doi: 10.1016/j.ccr.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handschick K, Beuerlein K, Jurida L, Bartkuhn M, Muller H, Soelch J, et al. Cyclin-dependent kinase 6 is a chromatin-bound cofactor for NF-kappaB-dependent gene expression. Mol Cell. 2014;53:193–208. doi: 10.1016/j.molcel.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Osuga H, Osuga S, Wang F, Fetni R, Hogan MJ, Slack RS, et al. Cyclin-dependent kinases as a therapeutic target for stroke. Proc Natl Acad Sci U S A. 2000;97:10254–10259. doi: 10.1073/pnas.170144197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arif A. Extraneuronal activities and regulatory mechanisms of the atypical cyclin-dependent kinase Cdk5. Biochem Pharmacol. 2012;84:985–993. doi: 10.1016/j.bcp.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Firestein R, Bass AJ, Kim SY, Dunn IF, Silver SJ, Guney I, et al. CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature. 2008;455:547–551. doi: 10.1038/nature07179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iorns E, Turner NC, Elliott R, Syed N, Garrone O, Gasco M, et al. Identification of CDK10 as an important determinant of resistance to endocrine therapy for breast cancer. Cancer Cell. 2008;13:91–104. doi: 10.1016/j.ccr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32:825–837. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paternot S, Colleoni B, Bisteau X, Roger PP. The CDK4/CDK6 inhibitor PD0332991 paradoxically stabilizes activated cyclin D3-CDK4/6 complexes. Cell Cycle. 2014;13:2879–2888. doi: 10.4161/15384101.2014.946841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Wang H, Zhang J, Luo F, Herrup K, Bibb JA, et al. Cyclin dependent kinase 5 is required for the normal development of oligodendrocytes and myelin formation. Dev Biol. 2013;378:94–106. doi: 10.1016/j.ydbio.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang HJ, Wang L, Wang M, Ma SP, Cheng BF, Li ZC, et al. Serine/threonine-protein kinase PFTK1 modulates oligodendrocyte differentiation via PI3 K/AKT pathway. J Mol Neurosci. 2015;55:977–984. doi: 10.1007/s12031-014-0454-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barone G, Staples CJ, Ganesh A, Patterson KW, Bryne DP, Myers KN, et al. Human CDK18 promotes replication stress signaling and genome stability. Nucleic Acids Res. 2016;44:8772–8785. doi: 10.1093/nar/gkw615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda S, Kominato K, Koide-Yoshida S, Miyamoto K, Isshiki K, Tsuji A, et al. PCTAIRE kinase 3/cyclin-dependent kinase 18 is activated through association with cyclin A and/or phosphorylation by protein kinase A. J Biol Chem. 2014;289:18387–18400. doi: 10.1074/jbc.M113.542936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pozo K, Castro-Rivera E, Tan C, Plattner F, Schwach G, Siegl V, et al. The role of Cdk5 in neuroendocrine thyroid cancer. Cancer Cell. 2013;24:499–511. doi: 10.1016/j.ccr.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinds PW, Mittnacht S, Dulic V, Arnold A, Reed SI, Weinberg RA. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-C. [DOI] [PubMed] [Google Scholar]
- 26.Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140:3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- 27.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Asada A, Saito T, Hisanaga S. Phosphorylation of p35 and p39 by Cdk5 determines the subcellular location of the holokinase in a phosphorylation-site-specific manner. J Cell Sci. 2012;125:3421–3429. doi: 10.1242/jcs.100503. [DOI] [PubMed] [Google Scholar]
- 29.Cruz JC, Tsai LH. A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease. Curr Opin Neurobiol. 2004;14:390–394. doi: 10.1016/j.conb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Malumbres M, Harlow E, Hunt T, Hunter T, Lahti JM, Manning G, et al. Cyclin-dependent kinases: a family portrait. Nat Cell Biol. 2009;11:1275–1276. doi: 10.1038/ncb1109-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graeser R, Gannon J, Poon RY, Dubois T, Aitken A, Hunt T. Regulation of the CDK-related protein kinase PCTAIRE-1 and its possible role in neurite outgrowth in Neuro-2A cells. J Cell Sci. 2002;115:3479–3490. doi: 10.1242/jcs.115.17.3479. [DOI] [PubMed] [Google Scholar]
- 32.Whitfield ML, Sherlock G, Saldanha AJ, Murray JI, Ball CA, Alexander KE, et al. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell. 2002;13:1977–2000. doi: 10.1091/mbc.02-02-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, et al. A family of human cdc2-related protein kinases. EMBO J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyamoto Y, Yamauchi J, Tanoue A. Cdk5 phosphorylation of WAVE2 regulates oligodendrocyte precursor cell migration through nonreceptor tyrosine kinase Fyn. J Neurosci. 2008;28:8326–8337. doi: 10.1523/JNEUROSCI.1482-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuda S, Kawamoto K, Miyamoto K, Tsuji A, Yuasa K. PCTK3/CDK18 regulates cell migration and adhesion by negatively modulating FAK activity. Sci Rep. 2017;7:45545. doi: 10.1038/srep45545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun D, Yu Z, Fang X, Liu M, Pu Y, Shao Q, et al. LncRNA GAS5 inhibits microglial M2 polarization and exacerbates demyelination. EMBO Rep. 2017;18:1801–1816. doi: 10.15252/embr.201643668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo F, Burke K, Kantor C, Miller RH, Yang Y. Cyclin-dependent kinase 5 mediates adult OPC maturation and myelin repair through modulation of Akt and GsK-3beta signaling. J Neurosci. 2014;34:10415–10429. doi: 10.1523/JNEUROSCI.0710-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang X, Sun D, Wang Z, Yu Z, Liu W, Pu Y, et al. MiR-30a positively regulates the inflammatory response of microglia in experimental autoimmune encephalomyelitis. Neurosci Bull. 2017;33:603–615. doi: 10.1007/s12264-017-0153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, Shao Q, Li Z, Gonzalez GA, Lu F, Wang D, et al. Myt1L promotes differentiation of oligodendrocyte precursor cells and is necessary for remyelination after lysolecithin-induced demyelination. Neurosci Bull. 2018;34:247–260. doi: 10.1007/s12264-018-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Z, Watanabe M, Nishiyama A. Optimization of oligodendrocyte progenitor cell culture method for enhanced survival. J Neurosci Methods. 2005;149:50–56. doi: 10.1016/j.jneumeth.2005.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.