Autophagy plays an important role in the development and pathogenesis of various diseases. It can be induced by a variety of events such as hypoxia, nutrient-starvation, and mechanical damage. Many neurological disorders such Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease, cerebral ischemia, and acute spinal cord injury (ASCI), are closely related to autophagy. However, therapeutic strategies to manipulate autophagy have not yet been fully deciphered due to the limited knowledge of the molecular mechanisms underlying autophagy in these disorders.
ASCI is a severe condition characterized by major disability and poor prognosis. Due to the fact that pathological processes of secondary injury in ASCI usually last for several days or even months, the study and treatment of this disease have mainly focused on reducing the progression. Animal studies have shown that rat models with different degrees of contusion in the lower thoracic spinal cord have dramatically different prognoses, indicating that the proportion of residual spinal cord in transverse section is positively associated with the recovery of spontaneous movement of the hindlimb. Ganz et al. implanted a 3D constructs with embedded oral mucosa-derived cells to rebuild the connectivity in a spinal transaction model, and showed that even partial restoration of connectivity with small-amplitude motor evoked potentials can elicit substantial functional recovery in an animal [1]. Therefore, it is clinically critical to find an efficient way to restore the connectivity of more neurons in the spinal cord for the treatment of secondary injury.
From this insight, we review here the pros and cons of autophagy in acute spinal cord injury. We also introduce state-of-the-art techniques resolving the detection and cell localization of autophagy. Moreover, the signaling pathways responsible for initiating autophagy are discussed, in part to provide a deeper understanding of the mechanism and treatment of ASCI.
Autophagic Cell Death and Apoptosis
Autophagic cell death is characterized by many autophagic vacuoles formed with an intact nucleus, while apoptosis presents with chromatin condensation, DNA degradation, and apoptotic body formation, which can be morphologically identified with immunofluorescence or electron microscopy. The relationship between autophagy and apoptosis is complicated and has yet to be elucidated. First, autophagy can inhibit the occurrence of apoptosis by processing damaged organelles such as mitochondria, thus suppressing the activation of apoptosis. Second, there is a complex molecular network of signals between autophagy and apoptosis, such as bcl-2 (B-cell lymphoma 2), p53, and PTEN (phosphatase and tensin homologue). These factors have been shown to play an anti-apoptotic role in cells undergoing autophagy. Moreover, some autophagic proteins such as beclin-1 and atg-5 (autophagy-related 5), are cleaved by caspases or calpains, and such cleavage further inhibits autophagy and enhances apoptosis. Of course, the balance between autophagy and apoptosis signals is determined by internal or external stress, and eventually decide the survival or death of a cell.
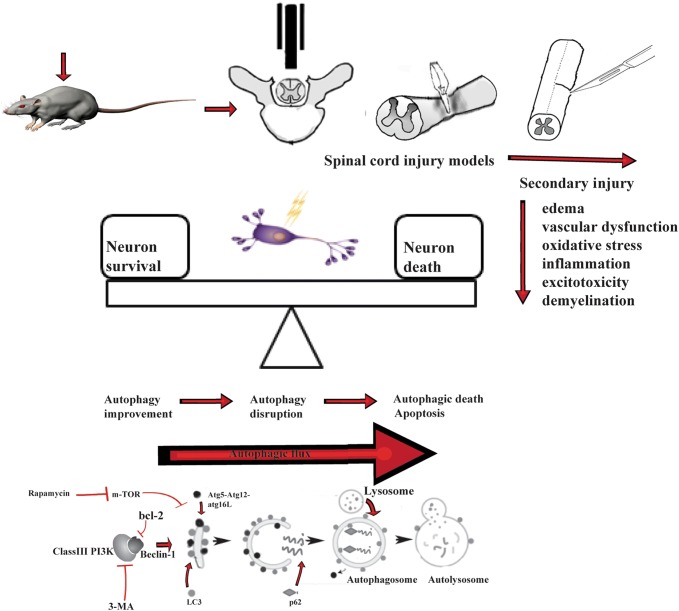
Three different forms of autophagy have been elucidated in recent years: macroautophagy, microautophagy, and chaperone-mediated autophagy. In this review, we mainly focus on the involvement of macroautophagy in ASCI as it is the most studied so far. Macroautophagy is initiated by the ATG (autophagy-related) protein which forms a double membrane structure known as an autophagosome to package damaged organelles or unused proteins. Afterwards, the autophagosome fuses with a lysosome, forming the autolysosome, in which the sequestered contents are degraded by acidic lysosomal hydrolase (Fig.1).
Fig. 1.
Schematics of acute spinal cord injury.
Autophagy in ASCI
Experimental Model of ASCI
ASCI models commonly use rats, including Sprague-Dawley and Wistar rats. The main damage segment focuses on T9–T11 of the spinal cord, where the body surface location is easy to identify. Three ASCI models are commonly described: the impact, compression, and hemisection models [2]. The impact model is the most used, in which different impact strengths and different degrees of ASCI can be induced. The compression model can also set different strengths of impact, but usually the strength is relatively fixed. In this model, an aneurysm clip is widely used to induce both dorsal and ventral spinal injury. The hemisection model can be used to compare the injured and healthy parts both at the histological and molecular levels. Studies have indicated that the hemisection model can also be used to investigate the influence of ASCI on axonal degeneration [2]. It should be noted that the features of autophagy are closely related to the ASCI model used, since the role of autophagy may be divergent in different ASCI models. In the impact/contusion model with a 10-g rod dropping from a height of 2.5 cm, many studies have confirmed that autophagic death increases neuronal loss, and inhibition of autophagic activity has a neuroprotective effect [3]. While in a compression model with a 15-g force for 1 min, the increase of autophagic activity after spinal injury has a neuroprotective effect [4], in a compression model with a 30-g force for 1 min or 20-g force for 2 min, the disruption by excessive autophagic activity gradually dominates and leads to neuron loss [5]. The state of autophagic activity after spinal injury may be closely related to the degree of injury, and further verification of this relationship between autophagy and injury under uniform conditions is needed.
Apart from the animal models, primary spinal cord neurons acutely dissected from animals have been introduced to study the mechanism of autophagy in ASCI. Among these cell models, a scratch model has been established to separately assess the effect of mechanical damage on autophagy. Although in vivo background interference can be avoided in ASCI cell models, their experimental applications are not particularly wide, mainly because neurons from the spinal cord are difficult to culture and differences in vivo and in vitro (Fig.1).
Detection and Distribution of Autophagy
Detection of autophagy in spinal cord injury includes the expression of biomarkers and morphological observations. LC3, Beclin-1, and P62 are commonly used for autophagy detection. LC3 and Beclin-1 are thought to play important roles in the process of double membrane formation [6]. P62 can be used to assay the activation of autophagic flux. The distribution of LC3, co-localization of activated caspase-3, and changes in expression levels of bcl-2 and Bax have been assessed to investigate the potential roles of autophagy and apoptosis [5]. On the other hand, morphological methods such as transmission electron microscopy allow direct observation of the formation of autophagosomes [7]. Autophagy occurs in neurons, and glia can be detected by immunofluorescence with double-staining, which can be combinatorically identified by the autophagy marker LC3 or Beclin-1 and cell-specific antigens (such as the neuron-specific antigen MAP-II or NeuN, the microcyte-specific antigen CD-11b, the oligodendrocyte-specific antigens CC-1 or Olig-2, and the astrocyte-specific antigen GFAP).
The cellular distribution patterns of autophagy biomarkers in different cells are diverse. Autophagic activity in the spinal cord grey matter is mainly concentrated in neurons, while autophagosomes accumulate mainly in microglia and oligodendrocytes in the white matter one day after spinal cord injury [6]. Tang et al. revealed that the number of LC3-positive neurons and astrocytes in the spinal cord is up-regulated with time [8]. Moreover, a recent study has shown that rapamycin inhibits the proliferation of astrocytes but improves neuron survival by phosphorylating AKT in both astrocytes and neurons [9]. Although existence of autophagic events in astrocytes remains to be demonstrated, these studies suggest that cells in the spinal cord experience a stressful environment after ASCI. The role and activities of autophagy in different cells may vary spatially and temporally. In fact, after spinal cord injury, ischemia, oxidative stress, and inflammation caused by the primary injury further expand the injured area, i.e., secondary injury, and the damage is often more severe closer to the secondary injury than the primary injury site [10], so autophagy may respond differently in different regions. The detection of autophagy in most research focuses on the region at the center of injury, so the spatial distribution still needs to be investigated in the same models. In terms of temporal regulation, since spinal ischemia, inflammation, and other reactions often last for days or even weeks, during this period, limitation or aggravation of injury also affect the expression of autophagy. This may be one of the reasons why some anti-inflammatory drugs lead to changes in autophagy. In terms of treatment, drugs should be used according to the autophagic response mechanism, whether it is the enhancement, interruption, or overexpression of autophagy.
Drug Intervention and Signal Pathway of ASCI
ASCI has been regarded as an intractable condition, lacking effective drugs. Even though methylprednisolone and gangliosides are widely used in the clinic, the curative rate is far from satisfactory. The use of drug intervention with autophagy in ASCI research is still at an early stage. Some drugs, such as methylprednisolone and valproic acid, have been shown to have protective action via regulating autophagic activity [11]. However, the role of autophagy in ASCI is still uncertain, so the results of drug intervention are ambiguous.
The principles of spinal cord injury therapy advocate early intervention (drugs and surgery) to minimize the loss of neurons in secondary injury [12]. Most of the research used drug intervention at an early stage to inhibit or promote autophagy based on the presence of autophagy in different models, In models with autophagic flux unblocked in the early stage, promoting autophagy can undoubtedly result in better neuron survival, while in those with autophagic flux interruption or excessive autophagy, the inhibition of autophagy often achieves better functional recovery. The role of autophagy in the recovery period from spinal injury has not been reported, and comparison of drug interventions is lacking. Some reports have pointed out that autophagic activity tends to reach a peak within a week and gradually drops to the control level one month after injury [8]; whether activation or inhibition of autophagy is beneficial to later functional recovery remains to be clarified.
To date, several signaling pathways have been suggested to participate in the autophagy after ASCI. Rapamycin, an autophagy agonist, inhibits the mTOR pathway and promotes the activation of autophagy. Zhang et al. showed that bFGF promotes the clearance of ubiquitinated proteins by stimulating autophagic activity, and this is mediated by the activation of PI3K/Akt/mTOR [13]. Another study demonstrated that metformin stimulates autophagic flux, which is suppressed by compound C, an AMPK inhibitor [14]. Moreover, Zhao et al. demonstrated that resveratrol has a neuroprotective effect by stimulating autophagy and inhibiting apoptosis via the SIRT1/AMPK pathway [15]. Nuclear factor kappa B (NF-κB) is a nuclear transcription factor that regulates the expression of multiple genes associated with the immune system, inflammation, the stress response, and apoptosis. Guo et al. revealed that granulocyte colony-stimulating factor induces the activation of autophagy via inhibition of the NF-κB signal pathway, resulting in reduced neural cell apoptosis and improved functional recovery after ASCI [16]. Xia et al. also showed that inhibition of the JAK-2/STAT3 signal pathway decreases the expression of LC3-II and other apoptotic proteins in the early stage of ASCI [17]. The mechanism for regulating autophagy is complicated, and may vary in different models and at different times. Besides, multiple disease-associated pathways have also been shown to be regulated by autophagy. Further studies on the spatial and temporal regulation of autophagy and associated pathways in ASCI need to be fully elucidated.
Pros and Cons of Autophagy in ASCI
Neuroprotective Role of Autophagy in ASCI
Initially, studies on neuroprotection mainly focused on the clinical treatment of brain trauma. However, researchers found that the early application of rapamycin has neuroprotective effects and improves the rate of postoperative functional recovery of the brain after injury. Further studies demonstrated that this protective effect is closely associated with the enhancement of autophagic activity [18]. Rapamycin was found to be efficient in increasing the expression of LC3 and Beclin1, and suppressing apoptosis, leading to the improvement of functional recovery [19]. Bai et al. suggested that disruption of autophagic flux with p62 could be enhanced by lysosomal dysfunction after ASCI, together with the facilitation of lysosomal biogenesis by Netrin-1 due to increasing TFEB expression via the AMPK/m-TOR signal pathway, thus improving functional recovery after ASCI [20].
Pathogenic Role of Autophagy in ASCI
Kanno et al. first demonstrated that autophagy participates the pathogenesis in hemisection injury models. Their research showed that both beclin-1 and LC-3 are expressed in neurons, astrocytes, and oligodendrocytes in animal models, while the nuclei in these cells are intact without fragments, indicating autophagic death of these cells [11]. The mechanism underlying autophagy-induced cell death in injury is that excessive autophagy may cause programmed cell death. However, whether autophagy directly contributes to cell death or has other cofactors needs further study. Interestingly, a recently study showed that chloroquine attenuates the degradation of ubiquitinated I-κBα via inhibition of autophagy, thereby blocking the translocation of NF-κB p65 and the expression of inflammatory factors.
Conclusions and Perspectives
At present, autophagy is increasingly studied in spinal cord injury. Although macroautophagy is the main topic, of course the role and possible mechanisms of chaperone-mediated autophagy and microautophagy in spinal cord injury cannot be neglected. Second, autophagy may have different responses in different cells, and the interaction between different cells, such as neurons-microglia, needs further study in the regulation of autophagy. Furthermore, the spatial and temporal expression of autophagy in spinal cord injury still needs further study, and the different patterns presented in different models need to be further clarified. Besides, the influence of the complications in organs or tissues after spinal cord injury should not be ignored. This also suggests that attention should be paid to changes in other organs as well as spinal cord injury, the treatment of which requires comprehensive consideration.
ASCI is considered to be a multifactorial condition involving ischemia/reperfusion, inflammation, oxidative stress, ionic disorder, neuronal excitotoxicity, apoptosis, necrosis, and autophagy. Although studies of animal models have suggested that some drugs improve motor functions after ASCI by inhibiting or activating autophagy, other regulatory mechanism and comprehensive treatment methods should also be taken into account for full understanding and treatment of spinal cord injury.
Acknowledgements
This insight was supported by the National Natural Science Foundation of China (81301047).
Contributor Information
Haodong Lin, Email: haodonglin@hotmail.com.
Jian Zhang, Email: zhang.jian@zs-hospital.sh.cn.
Zixian Chen, Email: chen.zixian@zs-hospital.sh.cn.
References
- 1.Ganz J, Shor E, Guo S, Sheinin A, Arie I, Michaelevski I, et al. Implantation of 3D constructs embedded with oral mucosa-derived cells induces functional recovery in rats with complete spinal cord transection. Front Neurosci. 2017;11:589. doi: 10.3389/fnins.2017.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheriyan T, Ryan DJ, Weinreb JH, Cheriyan J, Paul JC, Lafage V, et al. Spinal cord injury models: a review. Spinal Cord. 2014;52:588–595. doi: 10.1038/sc.2014.91. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Sarkar C, Dinizo M, Faden AI, Koh EY, Lipinski MM, et al. Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis. 2015;6:e1582. doi: 10.1038/cddis.2014.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou KL, Chen DH, Jin HM, Wu K, Wang XY, Xu HZ, et al. Effects of calcitriol on experimental spinal cord injury in rats. Spinal Cord. 2016;54:510. doi: 10.1038/sc.2015.217. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Liu F, Zhang L, Cao Y, Shao Y, Wang X, Jiang X, et al. Neuroserpin restores autophagy and promotes functional recovery after acute spinal cord injury in rats. Mol Med Rep. 2018;17:2957–2963. doi: 10.3892/mmr.2017.8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu D, Hao Z, Ren H, Wang G. Loss of VAPB Regulates Autophagy in a Beclin 1-Dependent Manner. Neurosci Bull. 2018;34:1037–1046. doi: 10.1007/s12264-018-0276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine. 2011;36:E1427. doi: 10.1097/BRS.0b013e3182028c3a. [DOI] [PubMed] [Google Scholar]
- 8.Tang P, Hou H, Zhang L, Lan X, Mao Z, Liu D, et al. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol. 2014;49:276–287. doi: 10.1007/s12035-013-8518-3. [DOI] [PubMed] [Google Scholar]
- 9.Goldshmit Y, Kanner S, Zacs M, Frisca F, Pinto AR, Currie PD, et al. Rapamycin increases neuronal survival, reduces inflammation and astrocyte proliferation after spinal cord injury. Mol Cell Neurosci. 2015;68:82–91. doi: 10.1016/j.mcn.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Saghazadeh A, Rezaei N. The role of timing in the treatment of spinal cord injury. Biomed Pharmacother. 2017;92:128–139. doi: 10.1016/j.biopha.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 11.Li HT, Zhao XZ, Zhang XR, Li G, Jia ZQ, Sun P, et al. Exendin-4 enhances motor function recovery via promotion of autophagy and inhibition of neuronal apoptosis after spinal cord injury in rats. Mol Neurobiol. 2016;53:4073–4082. doi: 10.1007/s12035-015-9327-7. [DOI] [PubMed] [Google Scholar]
- 12.Hachem LD, Ahuja CS, Fehlings MG. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J Spinal Cord Med. 2017;40(6):665–675. doi: 10.1080/10790268.2017.1329076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang HY, Wang ZG, Wu FZ, Kong XX, Yang J, Lin BB, et al. Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury. Mol Neurobiol. 2013;48:452–464. doi: 10.1007/s12035-013-8432-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, Xuan J, Zheng BB, Zhou YL, Lin Y, Wu YS, et al. Metformin Improves Functional Recovery After Spinal Cord Injury via Autophagy Flux Stimulation. Mol Neurobiol. 2017;54:1–15. doi: 10.1007/s12035-015-9635-y. [DOI] [PubMed] [Google Scholar]
- 15.Zhao H, Chen S, Gao K, Zhou Z, Wang C, Shen Z, et al. Resveratrol Protects Against Spinal Cord Injury by Activating Autophagy and Inhibiting Apoptosis Mediated by the SIRT1/AMPK Signaling Pathway. Neuroscience. 2017;348:241–251. doi: 10.1016/j.neuroscience.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y, Liu S, Zhang X, Wang L, Gao J, Han A, et al. G-CSF promotes autophagy and reduces neural tissue damage after spinal cord injury in mice. Lab Invest. 2015;95:1439. doi: 10.1038/labinvest.2015.120. [DOI] [PubMed] [Google Scholar]
- 17.Xia Y, Xia H, Chen D, Liao Z, Yan Y. Mechanisms of autophagy and apoptosis mediated by JAK2 signaling pathway after spinal cord injury of rats. Exp Thera Med. 2017;14:1589–1593. doi: 10.3892/etm.2017.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang YB, Li SX, Chen XP, Yang L, Zhang YG, Liu R, et al. Autophagy is activated and might protect neurons from degeneration after traumatic brain injury. Neurosci Bull. 2008;24:143–149. doi: 10.1007/s12264-008-1108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang ZY, Liu WG, Muharram A, Wu ZY, Lin JH. Neuroprotective effects of autophagy induced by rapamycin in rat acute spinal cord injury model. Neuroimmunomodulat. 2014;21:257–267. doi: 10.1159/000357382. [DOI] [PubMed] [Google Scholar]
- 20.Bai L, Mei X, Shen Z, Bi Y, Yuan Y, Guo Z, et al. Netrin-1 improves functional recovery through autophagy regulation by activating the AMPK/mTOR signaling pathway in rats with spinal cord injury. Sci Rep. 2017;7:42288. doi: 10.1038/srep42288. [DOI] [PMC free article] [PubMed] [Google Scholar]