Abstract
Background:
C-reactive protein (CRP) and lipoprotein (a) (Lp[a]) play essential roles in cardiovascular disease incidence. This study aimed to review the association between the intake of conjugated linoleic acid (CLA) in the form of dietary supplement or enriched food with different treatment durations and the levels of Lp(a) and CRP in human studies.
Methods:
All the articles published in Cochrane Library, ProQuest, Scopus, and Google Scholar from November 2014 to October 2015 were searched and the clinical trials on the effects of CLA on Lp(a) and CRP levels were assessed. Of the 2249 articles initially retrieved, 21 eligible randomized clinical trials were enrolled in this systematic review. The publication dates of the eligible articles ranged from 2005 to 2013. The mean difference and the standard deviation of changes in CRP and Lp(a) levels in intervention and control groups were used as effect-size measures for meta-analysis. The obtained data from the eligible randomized controlled trials were meta-analyzed using Stata, version 13.
Results:
The intake of CLA as a dietary supplement led to a significant increase in CRP levels (standardized mean difference [SMD]=0.41, 95% CI: 0.28 to 0.54; P=0.001). Subgroup analysis based on the duration of CLA consumption showed that CLA consumption more than 24 weeks resulted in a significant increase in the levels of CRP (SMD=0.52, 95% CI: 0.36 to 0.68; P=0.001) and Lp(a) (SMD=0.24, 95% CI: 0.01 to 0.47; P=0.04).
Conclusion:
The current systematic review and meta-analysis showed that the long-term consumption of CLA increases the levels of CRP and Lp(a).
Keywords: Conjugated linoleic acid , C-reactive protein , Lipoprotein (a) , Meta-analysis
What’s Known
Conjugated linoleic acid (CLA), prescribed as a safe nutraceutical, has shown efficacy against obesity, carcinoma, diabetes mellitus, and cardiovascular disease and boosts the immune system.
What’s New
However, we found that CLA administered as a supplement increases C-reactive protein as a risk factor for cardiovascular disease.
Introduction
Recent studies have shown that functional foods have the potential to positively affect and contribute to public health. Conjugated linoleic acid (CLA), a fatty acid with 2 isomers, namely cis-9, trans-11 (c9,t11) and trans-10, cis-12 (t10,c12), is considered a functional food1 and is produced through the hydrogenation of vegetable oils in industry, by bacteria residing in the rumen of animals, or via bioconversion in the mammary gland of ruminants.2 The isomers of CLA have demonstrated anti-obesity characteristics.3 Furthermore, according to the latest studies on animals, CLA has proven to be advantageous both for the prevention of carcinoma, diabetes mellitus, and cardiovascular disease (CVD) and for the furtherance of body fat decrement, lean body mass increment, and the immune system.4 Research on the possible adverse effects of the long-term consumption of CLA remains to be done. CLA belongs to a subgroup of fatty acids entitled “trans fatty acids (TFAs)”5 and randomized controlled trials (RCTs) have indicated that TFAs are detrimental to CVD risk factors.6
C-reactive protein (CRP) and lipoprotein (a) (Lp[a]) as nontraditional risk factors have been known to play essential roles in CVD incidence.7,8 A review of previous work on this subject reveals that there are serious discrepancies and controversies regarding the association between CLA consumption and these CVD risk factors.9-11 Some of these investigations have indicated a significant increment in Lp(a) and CRP levels following CLA consumption.12
Since the RCTs on the effects of CLA consumption on the levels of CRP and Lp(a) have yielded discrepant findings, a systematic review and meta-analysis is necessary to settle the controversies and offer more reliable findings, especially given that commercial CLA is used as a safe dietary supplement and can be easily purchased everywhere.13 Accordingly, in the present study, we aimed to systematically review the association between CLA consumption in 2 distinct forms of dietary supplement and enriched food, with their respective different treatment durations, and the levels of Lp(a) and CRP in human studies.
Methods
Literature Search
A thorough search was conducted on all the articles published from November 2014 to October 2015 in Cochrane Library, ProQuest, Scopus, and Google Scholar. Additional records were identified through other sources, namely PubMed, Science Direct, and Ovid. The keywords consisted of “trans-10, cis-12-conjugated linoleic acid”, “cis-9, trans-11-conjugated linoleic acid”, “CLA fatty acid”, “CLA”, “conjugated linoleic acid”, “trans fatty acid”, “TFA”, “C-reactive protein”, “CRP”, “lipoprotein (a)”, and “Lp(a)” with the medical subject heading [MeSH]. No restrictions were applied with regard to age, gender, and language during the search. The clinical trials investigating the effects of CLA (in the form of either dietary supplement or enriched food) on CRP or Lp(a) levels in healthy adults were objectively and meticulously assessed. The criteria for the inclusion of studies in the meta-analysis were RCT design, research on humans, healthy adult populations, intervention by using CLA dietary supplements or CLA enrichment in foods, and determination of serum CRP or Lp(a) levels as the outcome variable. Animal studies, studies with any design other than CRT design, studies on unhealthy or non-adult individuals, studies on the combined effects of CLA along with other factors such as creatine monohydrate, and studies on sport were excluded. Also excluded were studies examining the effects of CLA on other outcomes or studies lacking sufficient data (e.g., those without control groups or baseline information). As the first step, the titles and abstracts of the articles were examined and relevant papers were selected. Thereafter, the papers were screened, sorted according to their titles and abstracts, and carefully reviewed.
Data Extraction
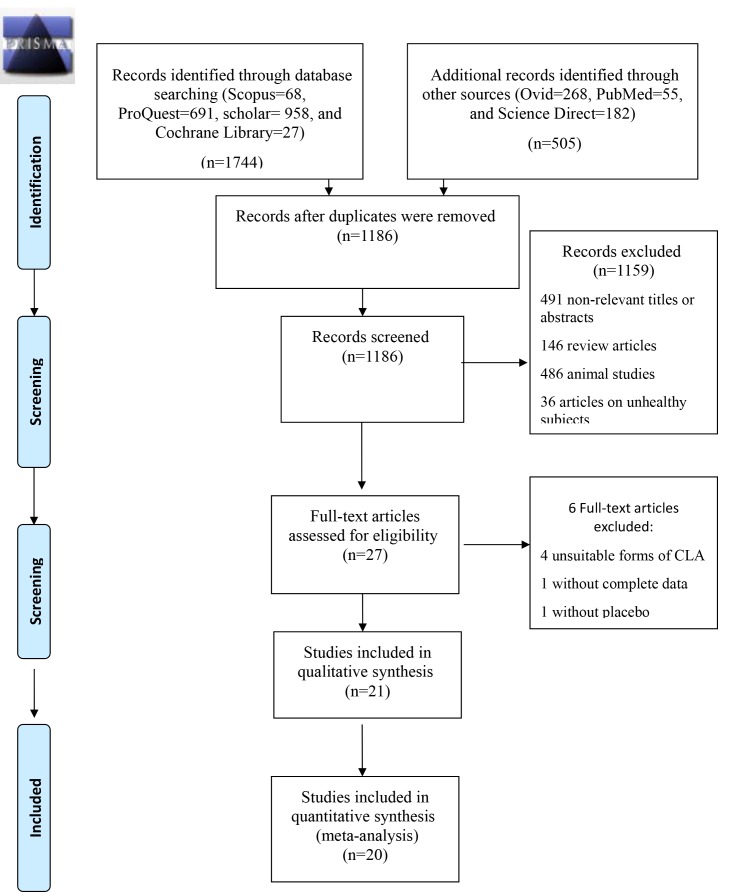
The data of 27 articles which investigated the effects of CLA intake in either dietary supplement form or enriched food on Lp(a) and CRP in healthy adult populations were entered in the meta-analysis. However, the data of the following RCTs were not extracted: a study without complete data for analysis,14 a study without a placebo group,15 and 4 studies with unsuitable forms of CLA.16-19Tables 1 and 2, respectively, provide summaries of the RCTs on the association between the intake of CLA in either dietary supplement form or enriched food and CVD risk factors in human studies.2,11-12,14, 17-36 All the information regarding the excluded RCTs is presented in figure 1. The mean differences and standard deviations (SDs) for changes in Lp(a) and CRP levels before and after the intervention for each of the intervention groups and control groups were extracted. In addition, the mean differences and SDs reported for the changes in Lp(a) and CRP levels within each group of intervention and control were extracted.
Table 1.
Eligible randomized controlled trials on supplementation with the CLA isomeric mixture with different proportions
| Reference | Population | Age (mean±SD) | Duration (wk) | CLA Dose and Form | Isomers | Placebo Dose and Form | Results |
|---|---|---|---|---|---|---|---|
| Mullen and colleagues,2 2007 | 30 healthy men | 49.4±1.8 | 8 | 2.2 g/d of CLA | (50:50) c9,t11 t10,c12 | 2.2 g/d of placebo | CLA supplementation significantly changed CRP levels. |
| Gaullier and colleagues,11 2004 | 180 men and women: healthy overweight | 45.83±10.3 | 48 | 3·4 g/d of CLA-TAG 3·6 g/d of CLA-NEFA | (50:50) c9,t11 t10,c12 | 4.5 g/d of olive oil | CLA supplementation increased the level of Lp(a). |
| Gaullier and colleagues,12 2007 | 118 men and women: healthy overweight or obese | 47.25± 9.6 | 24 | 3·4 g/d of CLA-TAG | (37.5:38) c9,t11 t10,c12 | 4·5 g/d of olive oil | Six months’ CLA supplementation marginally but significantly increased Lp(a) in both the CLA and placebo groups. CRP levels were significantly increased in the CLA group compared with the placebo group. |
| Tholstrup and colleagues,14 2008 | 75 healthy postmenopausal women | 60.16±4.46 | 16 | 4·6 g/d of CLA-mix | (41·17:39·90:1·79) c9,t11-t10,c12- other CLA | 5·5 g/d of olive oil | CRP was significantly higher in women supplemented with the CLA mixture than in those supplemented with CLA milk and the placebo. |
| 5·1 g/d of CLA-TAG (85·03:7·11:0·47) | c9,t11-t10,c12- other CLA | ||||||
| Ormsbee and colleagues,17 2014 | 22 men and women: inactive healthy overweight or obese | 35±3.45 | 8 | 1 g of fat, 99 mg of caffeine, 1510 of mg/d blend of: (green tea, CLA, and BCAA) | soybean oil | CLA supplementation had no effect on hs-CRP concentrations. | |
| Tarnopolsky and colleagues,18 2007 | 39 community-dwelling older adults | 71.1±5 | 24 | 5 g/d of CrM + 6 g/d of CLA-mix | (50:50) c9,t11 t10,c12 | 7 g/d of dextrose + 6 g/d of safflower oil | CrM+CLA supplementation had no significant effect on CRP levels. |
| Yonei and colleagues,19 2007 | 17 men and 18 women | 48.3±6.6 | 8 | 700 mg/d of CLA + 200 mg/d of L-carnitine | (33.1 : 33.9 ) c9, t11 t10, c12 | placebo | A small increase in serum levels of CRP was noted in the study group. |
| Watras and colleagues,20 2007 | 40 men and women: healthy overweight | 33±7.5 | 24 | 3·2 g/d of CLA-mix | (39·2:38·5) c9,t11 t10,c12 | 4 g/d of safflower oil | Within the CLA group, the CRP level was increased. |
| Steck and colleagues,21 2007 | 48 men and women: healthy obese | 34·50±4·85 | 12 | 3·2 g/d of CLA-TAG 6·4 g/d of CLA-TAG | (50:50) c9,t11 t10,c12 | 8 g/d of safflower oil | CLA increased the CRP level, although the absolute mean values remained within the normal limits. |
| Joseph and colleagues,22 2011 | 27 overweight hyperlipidemic men | 18-60 (range) | 8 | 3.5 g/d of CLA-mix 3.5 g/d of c9,t11 | (50:50) c9,t11 t10,c12 | 3.5 g/d of safflower oil | CRP was not significantly different in men supplemented with the CLA mixture than in those supplemented with c9, t11 or the placebo. |
| Pfeuffer and colleagues,23 2011 | 85 men: healthy overweight or obese | 45-68 (range) | 4 | 3·4 g/d of CLA-TAG | (50:50) c9,t11 t10,c12 | 4·5 g/d of safflower oil | No significant treatment-dependent changes in Lp(a) and hs-CRP were noted. |
| Gaullier and colleagues,24 2005 | 134 men and women: healthy overweight | 46.26±9.96 | 96 | 3·4 g/d of CLA-TAG | (50:50) c9,t11 t10,c12 | 3·4 g/d of placebo | Within-group comparisons showed that Lp(a) levels were increased significantly. |
| 3·4 g/d of CLA-NEFA | (50:50) c9,t11 t10,c12 | ||||||
| Blankson and colleagues,25 2000 | 60 men and women: healthy overweight or obese | 44.35±12.95 | 12 | 1·7 g/d of CLA-TAG 3·4 g/d of CLA-TAG 5·1 g/d of CLA-TAG 6·8 g/d of CLA-TAG | (50:50) c9,t11 t10,c12 | 9 g/d of olive oil | Changes in Lp(a) were not significantly different from the baseline values. |
| Berven and colleagues,26 2000 | 60 men and women: healthy overweight or obese | 47·05±3·9 | 12 | 3·4 g/d of CLA-TAG | (50:50) c9,t11 t10,c12 | 4·5 g/d of olive oil | Blood Lp(a) remained unchanged during the study. |
| Kim and colleagues,27 2008 | 51 women: healthy overweight Korean women | 28·24±20·3 | 12 | 2·25 g/d of CLA-NEFA | (37·95:38·84:0·96:1·35) c9,t11-CLA-t10, c12-CLA-c9,c11- CLA-t9, t11-CLA | 3 g/d of olive oil | Lp(a) changes between the treatment groups had no significant difference and the values were in the normal range. |
| 2·25 g/d of CLA-TAG | (37·83:38·55:0·98:1·86) c9,t11-CLA-t10, c12-CLA-c9,c11- CLA-t9,t11-CLA | ||||||
| Smedman and colleagues,28 2001 | 27 men and 26 women | 45.5±11.45 | 12 | 4·2 g/d of CLA | (50:50) c9,t11 t10,c12 | 4.2 g/d of olive oil | CLA supplementation increased levels of CRP. |
| Taylor and colleagues,29 2006 | 40 men: healthy overweight or obese | 46±7 | 12 | 4.5 g/d of CLA-mix | (35:36) c9,t11-t10,c12 (1-2%) c9,c11-c10,c12 (1·5%) t9,t11- t10,t11 (<1%) t8,c10-c11,t13 | 4.5 g/d of olive oil | There was no change in the estimated CRP concentration. |
CLA: Conjugated linoleic acid; CRP: C-reactive protein; Lp(a): Lipoprotein (a); CrM: Creatine monohydrate
Table 2.
Eligible randomized controlled trials on foods enriched with CLA
| Reference | Population | Age (mean±SD) | Duration (wk) | CLA Dose and Form | Isomers | Placebo Dose and Form | Results |
|---|---|---|---|---|---|---|---|
| Desroches and colleagues,9 2005 | 16 men: healthy overweight or obese | 36.6±12.4 | 8 | butter- CLA (4.22 g/d of CLA) | (80:20) C9,t11/ other isomers | butter (0.38 g/d of CLA) | CRP levels did not change significantly between the 2 groups. |
| Raff and colleagues,30 2008 | 38 healthy young men | 25.9±3.9 | 5 | butter-CLA (4.6 g/d of CLA) | (39.4: 38.5) C9,t11 t10,c12 | butter (0.3 g/d of CLA) | The CRP concentration did not differ between the groups either at baseline or after the intervention. |
| Naumann and colleagues,31 2006 | 92 men and women: healthy overweight or obese with LDL phenotype B | 52.33±7.66 | 13 | drinkable dairy product-CLA (3 g/d of CLA) | (>80: <5) c9,t11 t10,c12 | drinkable dairy product (3 g/d of high-oleic-acid sunflower oil) | Plasma concentrations of CRP did not change differently between the groups. |
| drinkable dairy product -CLA (3 g/d of CLA) | (>80: <5 ) t10, c12 c9,t11 | ||||||
| Ramarkers and colleagues,32 2005 | 42 men and women: healthy moderately overweight or subjects with LDL-phenotype B | 55.6±6 | 13 | drinkable yogurt-CLA (3 g/d of c9,t11) | (>80:< 5 ) c9,t11 t10,c12 | drinkable yogurt (3 g/d of high-oleic acid sunflower oil) | CRP did not changed during CLA supplementation. |
| (3 g/d of t10,c12) | (>80:< 5 ) t10,c12 c9,t11 | ||||||
| López-Plaza and colleagues,33 2013 | 38 men and women: healthy overweight | 44±8 | 24 | skimmed milk-CLA (3 g/d of CLA) | (50: 50 ) c9,t11 t10,c12 | skimmed milk (3 g/d of olive oil) | CLA-enriched skimmed milk did not lead to a significant variation in CRP levels. |
| Penedo and colleagues,34 2013 | 29 healthy normal-weight young adults | 25.9±6.24 | 8 | butter-CLA (20 g/d of c9,t11 CLA) | c9,t11 CLA | low-fat dairy products (skimmed milk, fat-free yogurt and low-fat cheeses) | CLA-enriched butter had no effects on the serum levels of CRP. |
| Lopes and colleagues,35 2013 | 28 | 16 | milk-CLA (4 g/d of CLA) | milk (4 g/d of canola oil) | There was no significant difference between CRP levels obtained at months 0 and 4. | ||
| Smit and colleagues,36 2011 | 61 men and women: healthy | 31±14 | 3 | margarines and yogurt drinks -CLA (50 g/d of CLA) | (80:20) c9,t11 t10,c12 | margarines and yogurt drinks (50 g/d of high oleic acid sunflower oil) | There was no effect of c9, t11 CLA supplementation on CRP. |
CLA: Conjugated linoleic acid; CRP: C-reactive protein; LDL: Low-density lipoprotein; NEFA: Non esterified fatty acids
Figure1.
PRISMA flowchart shows the literature search and review.
Based on the consumption of CLA in either dietary supplement form or enriched food, 2 subgroups were defined. The 2 subgroups were meta-analyzed separately. Additionally, in order to account for and evaluate the effects of other factors such as the length of CLA dietary supplementation on CVD risk factors (CRP and Lp[a]), we devised 2 other subgroups. Furthermore, heterogeneity was diminished by determining and subsequently deleting the outlier studies based on the funnel plot. Therefore, 2 separate sets of data (with elimination and without elimination of the outlier studies) were included in the meta-analysis.
The process for study selection (i.e., screening and eligibility), method of data extraction (i.e., piloted forms, independently, and in duplicate), listing and defining the variables, and processes for data obtaining and confirmation were all selected and done in complete accordance and compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 checklist.
Risk of Bias Assessment
The qualitative Critical Appraisal Skills Program (CASP) for RCTs was used to assess the risk of bias in the studies included in the review. Based on this tool, 2 screening questions were first used to decide whether it was worth proceeding to the following 8 detailed questions: selection bias, allocation bias, confounding, blinding, data collection method, withdrawal and dropouts, statistical analysis, and intervention integrity. The detailed questions were used to make a qualitative decision as to whether or not to include the study in the review. In addition, the quantitative 5-Point Jadad Score was applied to assess the quality of the relevant studies based on randomization, concealment of the treatment allocation, blinding, completeness of follow-up, and use of intention to treat analysis. Each item was given a score with a possible score of 0 (lowest level of quality) to 5 (highest level of quality). Studies with higher scores were considered to possess higher qualities.
Statistical Analysis
All the outcomes were recorded as continuous variables, and pooled meta-analyses were completed for the studies which reported the same outcomes. The mean differences and SDs were combined with a Cohen random-effect model. The mean difference was calculated by subtracting the mean values before and after the intervention for the experimental and control groups. If it was not directly reported, the SD of the mean differences was calculated using,
where SDb=SD before, SDa=SD after, and r=correlation between the scores before and after the intervention. The correlation was estimated using,
where SDb, SDa, and SDc represent the SD for before, after, and change scores, respectively. The median correlation for change from the baseline among the trials included in the systematic reviews was 0.66 (interquartile range=0.40, 0.90) .11,21,24,30,37
It was found that a simple formula:
could be used to estimate the mean by using the values of the median (m) and the low end and high end of the range (a and b, respectively). Additionally, the variance can be estimated via the formula:
The I2 and χ2 tests were used to assess heterogeneity between the studies. All the meta-analyses were done with the Cohen random-effect analysis model. Forest plots were applied to illustrate the results of the meta-analyses as well as heterogeneity in the results. In addition, 95% confidence intervals (95% CIs) were calculated for the standardized mean difference (SMD), and all the results were reported at a significance level of 0.05. The presence of publication bias was explored by constructing a funnel plot in which the estimate of the reported effect size was plotted against a measure of precision. Because an inevitable degree of subjectivity exists in the interpretation of funnel plots, the corresponding Egger’s tests were used to reassuringly assess the potential existence of publication bias. All the statistical analyses were conducted with Stata, version 13 (College Station, TX, USA).
Results
Of the 2249 articles initially identified, the titles and abstracts of all the articles were reviewed to identify and select appropriate articles for inclusion. A total of 27 eligible RCTs were selected for inclusion in the analysis. Next, the full text of each article was reviewed and the findings were re-evaluated. The flowchart depicting the literature search and review is provided in figure 1.
Of the eligible articles, 4 studies were excluded due to the use of non-eligible forms of CLA.16-19 One article14 lacked inclusive data, and another study15 did not use a placebo control. Twenty-one articles were left to be included in the meta-analysis. Out of all the studies evaluating the effects of CLA intake in the form of either dietary supplement or enriched food on CRP, 12,14,17-23, 31,32,39,40 the results of 7 studies indicated no significant change in CRP.2,17,18, 22,23,31,32 Out of all the studies included in the present review on the effects of the CLA isomeric mixture on Lp(a), with the exception of 2 investigations,11,24 none reported a significant change in Lp(a). The characteristics and main outcomes of the RCTs are depicted in Table 1 and Table 2.
CLA Intake in the Form of Dietary Supplement or Enriched Food and CRP
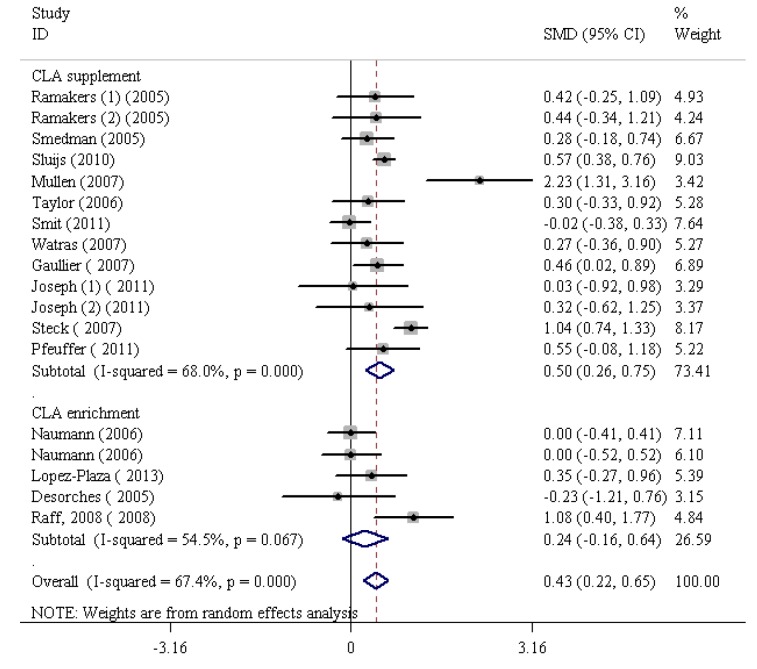
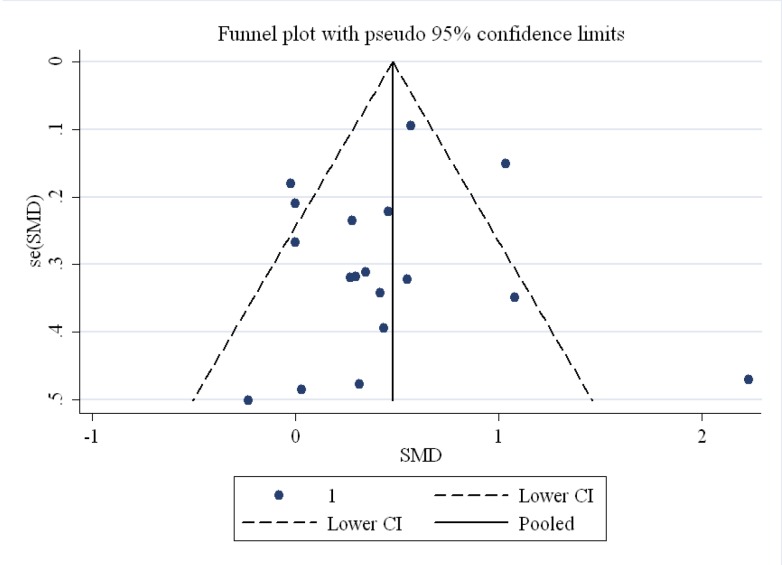
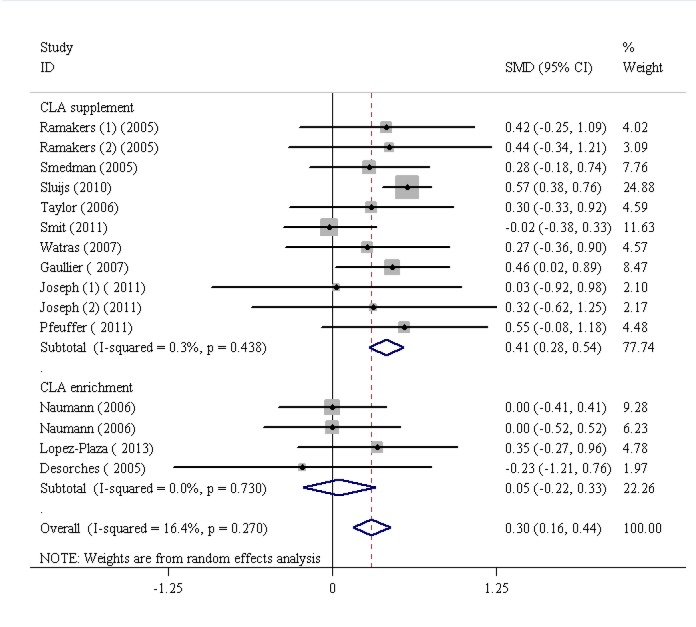
The findings of the human studies and the overall summary results for 15 RCTs are shown in figure 2. First, the analysis of the 15 RCTs demonstrated a significant increase in CRP (SMD=0.43, 95% CI: 0.22 to 0.65; P=0.001) with the highest heterogeneity (I2 [variation in the SMD attributable to heterogeneity]=67.4%, heterogeneity χ2=52.09 [df. =17]; P<0.001). Based on the funnel plot, the outlier studies2,21,30 were eliminated (figure 3; funnel plot) and consequently the heterogeneity was reduced to I2=16.4%. However, the results obtained following the elimination, confirmed that CLA intake significantly increased CRP levels (SMD=0.30, 95% CI: 0.16 to 0.44; P<0.001). Further, there was no significant heterogeneity between the studies (I2=16.4%, heterogeneity χ2=16.74 [df.=14]; P=0.27) (figure 4). The studies were categorized based on CLA sources. The subgroup analysis based on CLA sources showed that CLA dietary supplement led to a significant increase in CRP levels (SMD=0.41, 95% CI: 0.28 to 0.54; P=0.001). The subgroup analysis based on CLA-enriched foods showed that the consumption of these food items led to a nonsignificant increase in CRP levels (SMD=0.05, 95% CI:-0.22 to 0.33; P=0.7). The test for heterogeneity was not statistically significant for the CLA dietary supplement subgroup (I2=3%, heterogeneity χ2=10.03 [df.=10]; P=0.43) (figure 4) and nonsignificant for the CLA-enriched food subgroup (I2=0.00%, heterogeneity χ2=1.30 [df.=3]; P=0.73) (figure 4). Moreover, publication bias was found not to be significant (Egger’s test P=0.199).
Figure2.
Meta-analysis shows a significant increase in C-reactive protein following conjugated linoleic acid supplementation. The black squares show study-specific standardized differences (Std diff) in means, and the horizontal lines show 95% CIs. The area of the black squares is proportional to the specific-study weight in the overall meta-analysis. The center of the black diamonds indicates the pooled standardized difference in means, and their width represents the pooled 95% CI.
Figure3.
Funnel plot for the included studies on the effects of conjugated linoleic acid on C-reactive protein demonstrates outlier studies.
Figure4.
Meta-analysis shows a significant increase in C-reactive protein following conjugated linoleic acid supplementation after dropping the outlier studies. The black squares show study-specific standardized differences (Std diff) in means, and the horizontal lines show 95% CIs. The area of the black squares is proportional to the specific-study weight in the overall meta-analysis. The center of the black diamonds indicates the pooled standardized difference in means, and their width represents the pooled 95% CI.
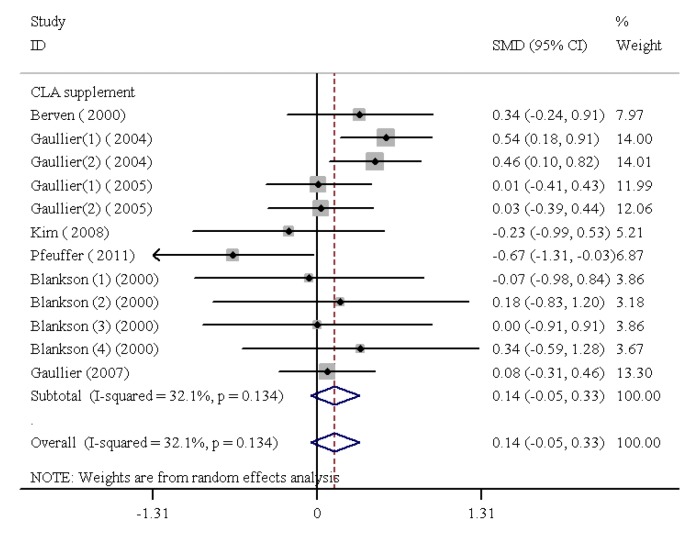
CLA Dietary Supplementation and Lp(a)
The findings of the human studies and the inclusive summary results for 8 RCTs are shown in figure 4. CLA dietary supplementation led to a nonsignificant change in Lp(a) levels (SMD=0.17, 95% CI: 0.02 to 0.32; P=0.15); the test result for heterogeneity was not statistically significant (I2=32.1%, heterogeneity χ2=16.21 [df.=11]; P=0.134) (figure 5). In addition, publication bias was found not to be weighty (Egger’s test P=0.446).
Figure5.
Meta-analysis shows no significant change in lipoprotein (a) following conjugated linoleic acid supplementation. The black squares show study-specific standardized differences (Std diff) in means, and the horizontal lines show 95% CIs. The area of the black squares is proportional to the specific-study weight in the overall meta-analysis. The center of the black diamonds indicates the pooled standardized difference in means, and their width represents the pooled 95% CI.
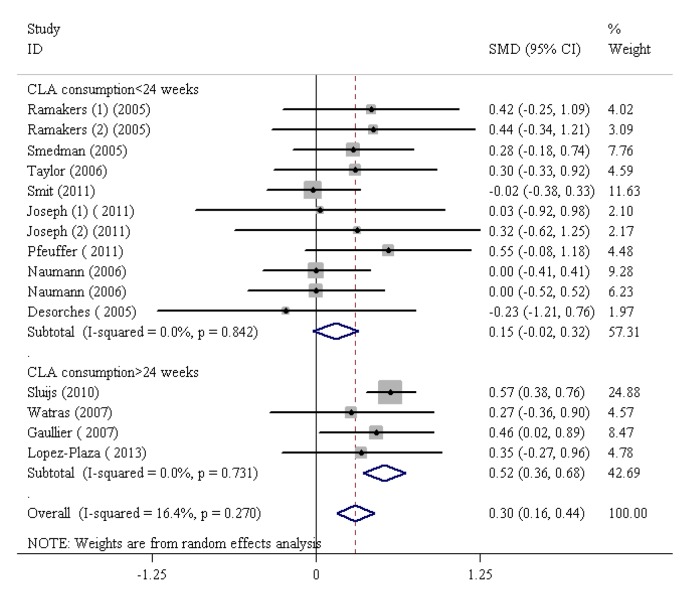
CLA Consumption Duration and CRP
The SMDs and 95% CIs for all the 12 studies on the effects of CLA, irrespective of its consumption in the form of dietary supplement or enriched food, on CRP are presented in figure 5. CLA consumption greatly increased CRP levels (SMD=0.30, 95% CI: 0.16 to 0.44; P=0.001). There was significant heterogeneity between the studies (I2=16.4%, heterogeneity χ2=16.74 [df.=14]; P<0.27) (figure 6). The studies were categorized based on the duration of CLA consumption. The subgroup analysis based on CLA consumption periods of less than 24 weeks indicated a nonsignificant rise in CRP levels (SMD=0.15, 95% CI: -0.02 to 0.32; P=0.08). The subgroup analysis based on the duration of CLA consumption demonstrated that CLA intake with regard to time intervals longer than 24 weeks led to a consequential increase in CRP levels (SMD=0.52, 95% CI: 0.36 to 0.68; P=0.001). The test for heterogeneity was not statistically notable for the subgroup with CLA consumption durations of more than 24 weeks (I2=00.0%, heterogeneity χ2=1.29 [df.=3]; P=0.73) and nonsignificant for CLA consumption durations of less than 24 weeks (I2=0.00%, heterogeneity χ2=5.67 [df.=10]; P=0.84) (figure 5). In addition, publication bias was found not to be considerable (Egger’s test P=0.199).
Figure6.
Meta-analysis shows a significant increase in C-reactive protein after conjugated linoleic acid supplementation for more than 24 weeks. The black squares show study-specific standardized differences (Std diff) in means, and the horizontal lines show 95% CIs. The area of the black squares is proportional to the specific-study weight in the overall meta-analysis. The center of the black diamonds indicates the pooled standardized difference in means, and their width represents the pooled 95% CI.
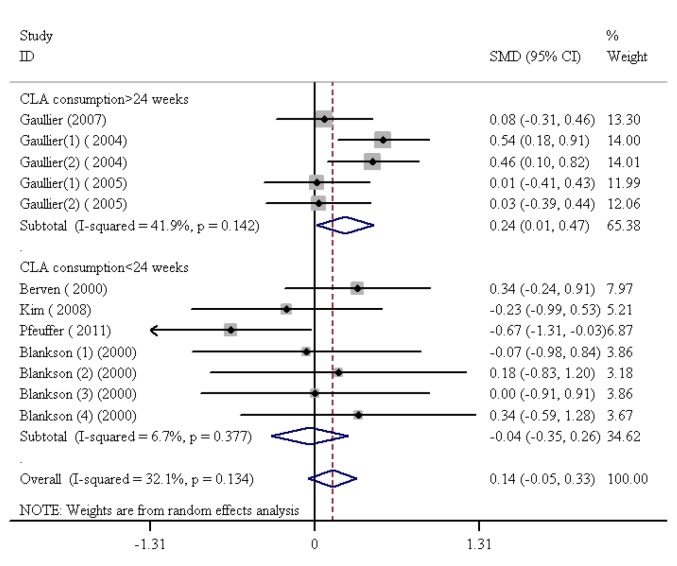
CLA Consumption Duration and Lp(a)
The SMDs and 95% CIs for all the 7 studies on the effects of CLA consumption in the form of dietary supplement on Lp(a) are outlined in figure 7. CLA consumption did not confer a significant boost to Lp(a) levels (SMD=0.14, 95% CI: -0.05 to 0.26; P=0.15). In addition, there was no significant heterogeneity among the studies (I2=32.1%, heterogeneity χ2=16.21 [df.=11]; P=0.13) (figure 7). The studies were categorized based on the duration of CLA consumption. Next, the subgroup analysis done on the basis of the duration of CLA consumption demonstrated that CLA supplementation for periods longer than 24 weeks led to a considerable increase in Lp(a) levels (SMD=0.24, 95% CI: 0.01 to 0.47; P=0.04). The test for heterogeneity was not statistically significant for the subgroups with CLA consumption for longer than 24 weeks (I2=41.9%, heterogeneity χ2=6.88 [df.=4]; P=0.14). On the other hand, the subgroup analysis based on CLA consumption periods of less than 24 weeks showed that the consumption of these food items resulted in a nonsignificant decrease in Lp(a) levels (SMD=-0.04, 95% CI: -0.35 to 0.26; P=0.37). Additionally, there was no noteworthy heterogeneity for subgroups with CLA consumption periods of less than 24 weeks (I2=6.7%, heterogeneity χ2=6.43 [df.=6]; P=0.37) (figure 7). Finally, publication bias was found not to be consequential (Egger’s test P=0.446).
Figure7.
Meta-analysis shows a significant increase in lipoprotein (a) after conjugated linoleic acid supplementation for more than 24 weeks. The black squares show study-specific standardized differences (Std diff) in means, and the horizontal lines show 95% CIs. The area of the black squares is proportional to the specific-study weight in the overall meta-analysis. The center of the black diamonds indicates the pooled standardized difference in means, and their width represents the pooled 95% CI.
Discussion
The present systematic review and meta-analysis of RCTs concluded that CLA intake and particularly CLA dietary supplements notably increased CRP levels. In accordance with the findings of the present meta- analysis, the results of several studies have asserted and justified the significant increases in CRP levels following CLA consumption, particularly in the form of commercial dietary supplements. 12,14,19-21, 39,40 Nonetheless, there are some RCTs in the existing literature reporting no considerable impact.7,18,22,23, 31,32 The findings of some studies have exhibited significant gains in CRP levels following t10,c12 CLA isomer supplementation.41,42 Moreover, the findings of some of the reviewed studies showed that the differences in the proportion of the isomers of CLA (c9,t11 and t10,c12) did not control the impact of CLA consumption on CRP. Numman and colleagues31 and Remark and colleagues32 examined 2 different proportions of 2 of the most abundant isomers of CLA (c9,t11 and t10,c12) on CRP in a group of participants and found no notable variations. We assume that other factors such as genetic predisposition, gender, and duration of CLA administration might account for and explain the incongruous findings having been reported in different studies. Abdelmagid and colleagues43 showed that the plasma concentration of CLA was influenced by stearoyl-CoA desaturase 1 (SCD1) genetic variations and hormonal status. It has been reported that the circulating CLA concentration is significantly higher in women than in men.43 It could be concluded that even a modest amount of t10,c12 CLA induces considerable inflammatory effects in humans. The duration of CLA administration might also explain the inconsistencies in findings and address the posited discrepancies. Our subgroup meta-analysis based on the duration of the intervention illustrated that CLA consumption for periods of 24 weeks or longer increased CRP levels.
The specific intricate mechanism which accounts for the impact of CLA consumption on CRP is still ambiguous and requires further research. Steck and colleagues21 reported that higher dosages of CLA dietary supplements might give rise to inflammatory markers in healthy obese adults. Consistently, CLA dietary supplementation in human subjects has increased the 15-keto-dihydro-PGF2a urine level, a marker of cyclooxygenase-catalyzed lipid peroxidation. It should be borne in mind that increases in CRP and 15-keto-dihydro-PGF2a are correlated.30 On the other hand, CLA has at least a trans double bond and belongs to the TFA group.44 The relationship between TFA and inflammation is clear and can be found elsewhere.45 CRP is an acute-phase protein produced by the liver up to 1000 times through inflammation. It is more sensitive to inflammatory cytokines than other acute-phase proteins.46 Therefore, a rise in CRP levels after an increase in CLA levels in circulation is expectable.
We observed no significant increase in Lp(a) concentration following CLA dietary supplementation in RCTs. However, when we categorized the studies based on the duration of CLA consumption, the subgroup analysis demonstrated that CLA supplementation for periods longer than 24 weeks led to a consequential increase in Lp(a) levels. The results obtained through our analysis proved to be in complete accordance with the findings of 5 other studies12,23,25-27 and in discordance with 2 others.11,24 The duration of CLA consumption can explain the discrepancy between the 2 groups of studies. Gaullier and colleagues,11,24 in 2004 and 2005, used various forms of CLA mixtures in healthy overweight subjects for 1 and 2 years, respectively, and concluded that CLA supplementation was able to increase the level of Lp(a). Nevertheless, in another study conducted in 2007, Gaullier and colleagues12 used CLA-TAG over a 6-month interval in healthy overweight and obese subjects and found no differences in Lp(a) levels between the CLA group and the placebo group. Similarly, other studies with durations of CLA supplementation for 4 weeks,23 12 weeks,25,26 and 24 weeks12 found no effect on Lp(a). In the studies conducted by Gaullier and colleagues, which took place over a period longer than 1 year, Lp(a) increment was accompanied by increases in leukocytes and thrombocytes, suggesting an inflammatory and immunological response to CLA supplementation. However, the precise process and functioning are still not fully clear and require further research.
In humans, no limits on safety parameters (duration, dosage, variety, etc.) have been reported with CLA consumption. Studies on overweight or obese adults, with different doses of CLA (i.e., 1.7, 3.4, 5.1, and 6.8 g/d) and durations (i.e., 4, 8, 12, 36, 48 weeks and more), have been conducted.28-30,33-36 A consensus should emerge on the usage of CLA and the upper limit for this functional food.
The major strength of the present study lies in its analysis of CLA dietary supplements and CLA-enriched foods in separation of one another. Moreover, consideration and examination of biases, with specific focus on publication bias, can be pointed out as another decisive and differentiating strength of the current study.
As for the potential limitations or shortcomings of the present study, we note that the current meta-analysis strives to reconcile the conflicting results previously reported and merely reflects the existing evidence. Furthermore, deficiencies which have their roots in the design, conduct, analysis, and interpretation of the research, along with the ability to identify sources of diversity across various types of studies have served as another source of limitation.
It is important to bear in mind, however, that in general, not only is meta-analyses unable to improve the quality of the original studies or address the variability of patient populations, but also they are incapable of enhancing the quality of the data and diminishing the potential for the underlying biases.
Conclusion
The current systematic review and meta-analysis of randomized placebo-controlled trials illustrated that CLA in the form of dietary supplement greatly affects some CVD risk factors such as CRP and Lp(a). Nonetheless, the length of intervention is a determining factor in the safety of the CLA consumer. We conclude that the long-term consumption of CLA increases CRP and Lp(a) levels.
Since CLA has long been widely present in various diets and also in commercial dietary supplements, long-term trials with different doses of CLA on various patient populations are deemed essential to attest the safety of commercial CLA dietary supplements, endorse their use, and ensure the absence of any adverse impact or danger to human health.
Acknowledgement
This study was supported in part by a grant from Bushehr University of Medical Sciences, Bushehr, Iran (DP/20/ 18/3/2316, 1/1/2015).
Conflict of Interest:None declared.
References
- 1.Lambert EV, Goedecke JH, Bluett K, Heggie K, Claassen A, Rae DE, et al. Conjugated linoleic acid versus high-oleic acid sunflower oil: effects on energy metabolism, glucose tolerance, blood lipids, appetite and body composition in regularly exercising individuals. Br J Nutr. 2007;97:1001–11. doi: 10.1017/S0007114507172822. [DOI] [PubMed] [Google Scholar]
- 2.Mullen A, Moloney F, Nugent AP, Doyle L, Cashman KD, Roche HM. Conjugated linoleic acid supplementation reduces peripheral blood mononuclear cell interleukin-2 production in healthy middle-aged males. J Nutr Biochem. 2007;18:658–66. doi: 10.1016/j.jnutbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Kloss R, Linscheid J, Johnson A, Lawson B, Edwards K, Linder T, et al. Effects of conjugated linoleic acid supplementation on blood lipids and adiposity of rats fed diets rich in saturated versus unsaturated fat. Pharmacol Res. 2005;51:503–7. doi: 10.1016/j.phrs.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Mougios V, Matsakas A, Petridou A, Ring S, Sagredos A, Melissopoulou A, et al. Effect of supplementation with conjugated linoleic acid on human serum lipids and body fat. J Nutr Biochem. 2001;12:585–94. doi: 10.1016/s0955-2863(01)00177-2. [DOI] [PubMed] [Google Scholar]
- 5.Rice BH, Kraft J, Destaillats F, Bauman DE, Lock AL. Ruminant-produced trans-fatty acids raise plasma HDL particle concentrations in intact and ovariectomized female Hartley guinea pigs. J Nutr. 2012;142:1679–83. doi: 10.3945/jn.112.160077. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motard-Belanger A, Charest A, Grenier G, Paquin P, Chouinard Y, Lemieux S, et al. Study of the effect of trans fatty acids from ruminants on blood lipids and other risk factors for cardiovascular disease. Am J Clin Nutr. 2008;87:593–9. doi: 10.1093/ajcn/87.3.593. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez C, Francisco G, Chacon P, Simo R. Lipoprotein(a) as a risk factor for cardiovascular mortality in type 2 diabetic patients: a 10-year follow-up study. Diabetes Care. 2005;28:931–3. doi: 10.2337/diacare.28.4.931. [DOI] [PubMed] [Google Scholar]
- 9.Desroches S, Chouinard PY, Galibois I, Corneau L, Delisle J, Lamarche B, et al. Lack of effect of dietary conjugated linoleic acids naturally incorporated into butter on the lipid profile and body composition of overweight and obese men. Am J Clin Nutr. 2005;82:309–19. doi: 10.1093/ajcn.82.2.309. [DOI] [PubMed] [Google Scholar]
- 10.Sluijs I, Plantinga Y, de Roos B, Mennen LI, Bots ML. Dietary supplementation with cis-9,trans-11 conjugated linoleic acid and aortic stiffness in overweight and obese adults. Am J Clin Nutr. 2010;91:175–83. doi: 10.3945/ajcn.2009.28192. [DOI] [PubMed] [Google Scholar]
- 11.Gaullier JM, Halse J, Hoye K, Kristiansen K, Fagertun H, Vik H, et al. Conjugated linoleic acid supplementation for 1 y reduces body fat mass in healthy overweight humans. Am J Clin Nutr. 2004;79:1118–25. doi: 10.1093/ajcn/79.6.1118. [DOI] [PubMed] [Google Scholar]
- 12.Gaullier JM, Halse J, Hoivik HO, Hoye K, Syvertsen C, Nurminiemi M, et al. Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Br J Nutr. 2007;97:550–60. doi: 10.1017/S0007114507381324. [DOI] [PubMed] [Google Scholar]
- 13.Whigham LD, Watras AC, Schoeller DA. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr. 2007;85:1203–11. doi: 10.1093/ajcn/85.5.1203. [DOI] [PubMed] [Google Scholar]
- 14.Tholstrup T, Raff M, Straarup EM, Lund P, Basu S, Bruun JM. An oil mixture with trans-10, cis-12 conjugated linoleic acid increases markers of inflammation and in vivo lipid peroxidation compared with cis-9, trans-11 conjugated linoleic acid in postmenopausal women. J Nutr. 2008;138:1445–51. doi: 10.1093/jn/138.8.1445. [DOI] [PubMed] [Google Scholar]
- 15.Tricon S, Burdge GC, Kew S, Banerjee T, Russell JJ, Grimble RF, et al. Effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on immune cell function in healthy humans. Am J Clin Nutr. 2004;80:1626–33. doi: 10.1093/ajcn/80.6.1626. [DOI] [PubMed] [Google Scholar]
- 16.Ahren B, Mari A, Fyfe CL, Tsofliou F, Sneddon AA, Wahle KW, et al. Effects of conjugated linoleic acid plus n-3 polyunsaturated fatty acids on insulin secretion and estimated insulin sensitivity in men. Eur J Clin Nutr. 2009;63:778–86. doi: 10.1038/ejcn.2008.45. [DOI] [PubMed] [Google Scholar]
- 17.Ormsbee MJ, Rawal SR, Baur DA, Kinsey AW, Elam ML, Spicer MT, et al. The effects of a multi-ingredient dietary supplement on body composition, adipokines, blood lipids, and metabolic health in overweight and obese men and women: a randomized controlled trial. J Int Soc Sports Nutr. 2014;11:37. doi: 10.1186/1550-2783-11-37. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarnopolsky M, Zimmer A, Paikin J, Safdar A, Aboud A, Pearce E, et al. Creatine monohydrate and conjugated linoleic acid improve strength and body composition following resistance exercise in older adults. PLoS One. 2007;2:e991. doi: 10.1371/journal.pone.0000991. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonei Y, Takahashi Y, Watanabe M, Yoshioka T. A double-blind, randomized controlled trial (RCT) of L-carnitine and conjugated linoleic acid-based health food with health claims. Anti-Aging Medicine. 2007;4:19–27. [Google Scholar]
- 20.Watras AC, Buchholz AC, Close RN, Zhang Z, Schoeller DA. The role of conjugated linoleic acid in reducing body fat and preventing holiday weight gain. Int J Obes (Lond) 2007;31:481–7. doi: 10.1038/sj.ijo.0803437. [DOI] [PubMed] [Google Scholar]
- 21.Steck SE, Chalecki AM, Miller P, Conway J, Austin GL, Hardin JW, et al. Conjugated linoleic acid supplementation for twelve weeks increases lean body mass in obese humans. J Nutr. 2007;137:1188–93. doi: 10.1093/jn/137.5.1188. [DOI] [PubMed] [Google Scholar]
- 22.Joseph SV, Jacques H, Plourde M, Mitchell PL, McLeod RS, Jones PJ. Conjugated linoleic acid supplementation for 8 weeks does not affect body composition, lipid profile, or safety biomarkers in overweight, hyperlipidemic men. J Nutr. 2011;141:1286–91. doi: 10.3945/jn.110.135087. [DOI] [PubMed] [Google Scholar]
- 23.Pfeuffer M, Fielitz K, Laue C, Winkler P, Rubin D, Helwig U, et al. CLA does not impair endothelial function and decreases body weight as compared with safflower oil in overweight and obese male subjects. J Am Coll Nutr. 2011;30:19–28. doi: 10.1080/07315724.2011.10719940. [DOI] [PubMed] [Google Scholar]
- 24.Gaullier JM, Halse J, Hoye K, Kristiansen K, Fagertun H, Vik H, et al. Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr. 2005;135:778–84. doi: 10.1093/jn/135.4.778. [DOI] [PubMed] [Google Scholar]
- 25.Blankson H, Stakkestad JA, Fagertun H, Thom E, Wadstein J, Gudmundsen O. Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J Nutr. 2000;130:2943–8. doi: 10.1093/jn/130.12.2943. [DOI] [PubMed] [Google Scholar]
- 26.Berven G, Bye A, Hals O, Blankson H, Fagertun H, Thom E, et al. Safety of conjugated linoleic acid (CLA) in overweight or obese human volunteers. Eur J Lipid Sci Technol. 2000;102:455–62. [Google Scholar]
- 27.Kim JH, Kim OH, Ha YL, Kim JO. Supplementation of conjugated linoleic acid with γ-oryzanol for 12 weeks effectively reduces body fat in healthy overweight Korean women. Journal of Food Science and Nutrition. 2008;13:146–56. [Google Scholar]
- 28.Smedman A, Vessby B. Conjugated linoleic acid supplementation in humans--metabolic effects. Lipids. 2001;36:773–81. doi: 10.1007/s11745-001-0784-7. [DOI] [PubMed] [Google Scholar]
- 29.Taylor JS, Williams SR, Rhys R, James P, Frenneaux MP. Conjugated linoleic acid impairs endothelial function. Arterioscler Thromb Vasc Biol. 2006;26:307–12. doi: 10.1161/01.ATV.0000199679.40501.ac. [DOI] [PubMed] [Google Scholar]
- 30.Raff M, Tholstrup T, Toubro S, Bruun JM, Lund P, Straarup EM, et al. Conjugated linoleic acids reduce body fat in healthy postmenopausal women. J Nutr. 2009;139:1347–52. doi: 10.3945/jn.109.104471. [DOI] [PubMed] [Google Scholar]
- 31.Naumann E, Carpentier YA, Saebo A, Lassel TS, Chardigny JM, Sebedio JL, et al. Cis-9, trans- 11 and trans-10, cis-12 conjugated linoleic acid (CLA) do not affect the plasma lipoprotein profile in moderately overweight subjects with LDL phenotype B. Atherosclerosis. 2006;188:167–74. doi: 10.1016/j.atherosclerosis.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Ramakers JD, Plat J, Sebedio JL, Mensink RP. Effects of the individual isomers cis-9,trans-11 vs. trans-10,cis-12 of conjugated linoleic acid (CLA) on inflammation parameters in moderately overweight subjects with LDL-phenotype B. Lipids. 2005;40:909–18. doi: 10.1007/s11745-005-1451-8. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Plaza B, Bermejo LM, Koester Weber T, Parra P, Serra F, Hernandez M, et al. Effects of milk supplementation with conjugated linoleic acid on weight control and body composition in healthy overweight people. Nutr Hosp. 2013;28:2090–8. doi: 10.3305/nutrhosp.v28in06.7013. [DOI] [PubMed] [Google Scholar]
- 34.Penedo LA, Nunes JC, Gama MA, Leite PE, Quirico-Santos TF, Torres AG. Intake of butter naturally enriched with cis9,trans11 conjugated linoleic acid reduces systemic inflammatory mediators in healthy young adults. J Nutr Biochem. 2013;24:2144–51. doi: 10.1016/j.jnutbio.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Lopes DCF, Silvestre MPC, Silva VDM, Moreira TG, Garcia ES, Silva MR. Dietary supplementation of conjugated linoleic acid, added to a milk drink, in women. Asian Journal of Scientific Research. 2013;6:679. [Google Scholar]
- 36.Smit LA, Katan MB, Wanders AJ, Basu S, Brouwer IA. A high intake of trans fatty acids has little effect on markers of inflammation and oxidative stress in humans. J Nutr. 2011;141:1673–8. doi: 10.3945/jn.110.134668. [DOI] [PubMed] [Google Scholar]
- 37.Fu R, Vandermeer BW, Shamliyan TA, O’Neil ME, Yazdi F, Fox SH, et al. Handling Continuous Outcomes in Quantitative Synthesis. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ Methods for Effective Health Care. Maryland: Rockville (MD); 2008. [PubMed] [Google Scholar]
- 38.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smedman A, Basu S, Jovinge S, Fredrikson GN, Vessby B. Conjugated linoleic acid increased C-reactive protein in human subjects. Br J Nutr. 2005;94:791–5. doi: 10.1079/bjn20041419. [DOI] [PubMed] [Google Scholar]
- 40. National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 41.Riserus U, Basu S, Jovinge S, Fredrikson GN, Arnlov J, Vessby B. Supplementation with conjugated linoleic acid causes isomer-dependent oxidative stress and elevated C-reactive protein: a potential link to fatty acid-induced insulin resistance. Circulation. 2002;106:1925–9. doi: 10.1161/01.cir.0000033589.15413.48. [DOI] [PubMed] [Google Scholar]
- 42.Basu S, Smedman A, Vessby B. Conjugated linoleic acid induces lipid peroxidation in humans. FEBS Lett. 2000;468:33–6. doi: 10.1016/s0014-5793(00)01193-5. [DOI] [PubMed] [Google Scholar]
- 43.Abdelmagid SA, Clarke SE, Wong J, Roke K, Nielsen D, Badawi A, et al. Plasma concentration of cis9trans11 CLA in males and females is influenced by SCD1 genetic variations and hormonal contraceptives: a cross-sectional study. Nutr Metab (Lond) 2013;10:50. doi: 10.1186/1743-7075-10-50. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aydin R. Conjugated linoleic acid: chemical structure, sources and biological properties. Turk J Vet Anim Sci. 2005;29:189–95. [Google Scholar]
- 45.Mozaffarian D. Trans fatty acids - effects on systemic inflammation and endothelial function. Atheroscler Suppl. 2006;7:29–32. doi: 10.1016/j.atherosclerosissup.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]