Abstract
Down syndrome (DS) is associated with significant perturbances in mitochondrial function. Here we tested the hypothesis that the suppression of mitochondrial electron transport in DS cells is due to high expression of cystathionine-β-synthase (CBS) and subsequent overproduction of the gaseous transmitter hydrogen sulfide (H2S). Fibroblasts from DS individuals showed higher CBS expression than control cells; CBS localization was both cytosolic and mitochondrial. DS cells produced significantly more H2S and polysulfide and exhibited a profound suppression of mitochondrial electron transport, oxygen consumption, and ATP generation. DS cells also exhibited slower proliferation rates. In DS cells, pharmacological inhibition of CBS activity with aminooxyacetate or siRNA-mediated silencing of CBS normalized cellular H2S levels, restored Complex IV activity, improved mitochondrial electron transport and ATP synthesis, and restored cell proliferation. Thus, CBS-derived H2S is responsible for the suppression of mitochondrial function in DS cells. When H2S overproduction is corrected, the tonic suppression of Complex IV is lifted, and mitochondrial electron transport is restored. CBS inhibition offers a potential approach for the pharmacological correction of DS-associated mitochondrial dysfunction.
Keywords: metabolism, mitochondria, bioenergetics, H2S
Down syndrome (DS)—trisomy of human chromosome 21 (HSA21)—remains the most common chromosomal disorder (1). It is associated with significant perturbances in many morphological and biochemical features. One of its biochemical hallmarks is mitochondrial dysfunction. Suppression of mitochondrial function—including inhibition of mitochondrial Complex IV—has been documented in cells from DS individuals (DSCs) (2). Complex IV is an essential electron transport component; its best-characterized inhibitors include cyanide (an irreversible inhibitor) and hydrogen sulfide (H2S) (a reversible inhibitor) (3).
H2S is an endogenous mammalian gaseous mediator, which regulates many cellular processes including mitochondrial function (4). Its effects are often bell shaped: At lower concentrations H2S stimulates cellular bioenergetics, while at toxicological concentrations H2S suppresses mitochondrial function via inhibition of Complex IV (4). Cystathionine-β-synthase (CBS)—located on HSA21—is a principal enzyme responsible for H2S production. Overexpression of CBS (5) and increased levels of the H2S metabolite thiosulfate (6) were previously reported in DS. We have now directly tested the hypothesis whether the excess H2S produced by CBS is responsible for the suppression of mitochondrial electron transport in DSCs.
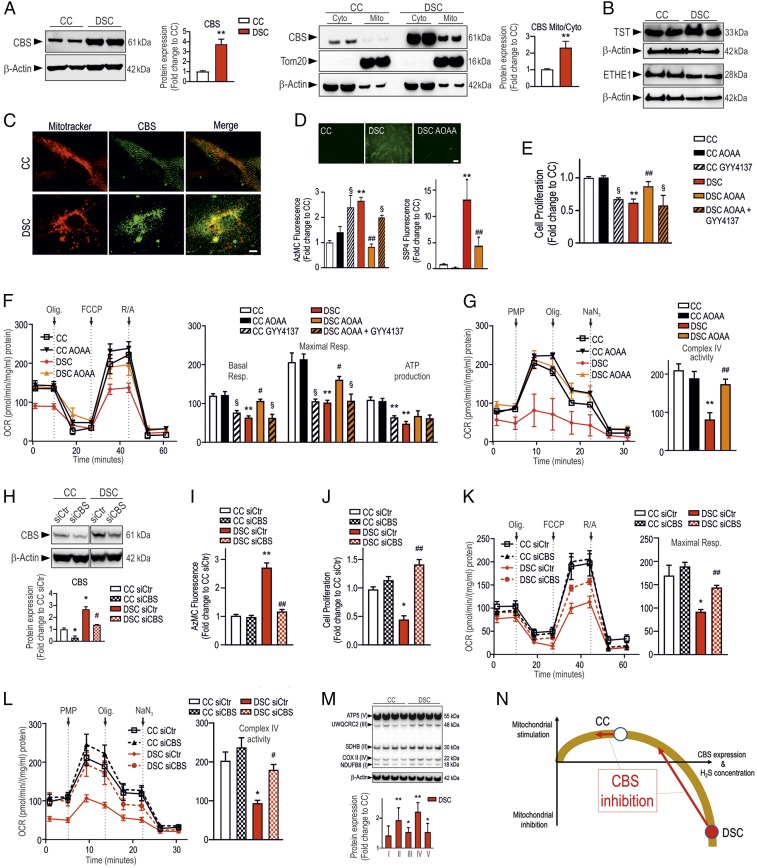
DSCs exhibited markedly higher CBS expression than the healthy control cells (CCs). CBS was both cytosolically and mitochondrially localized (Fig. 1 A–C). The expression of various H2S metabolizing enzymes was unaffected by DS (Fig. 1B). DSCs produced significantly more H2S—as well as reactive polysulfides—than CCs (Fig. 1D). The increased CBS expression in DSCs was associated with a profound suppression of cell proliferation, mitochondrial electron transport, oxygen consumption, Complex IV activity, and ATP generation (Fig. 1 E–G).
Fig. 1.
CBS-derived H2S is responsible for the suppression of mitochondrial function in DSCs. DSCs exhibit markedly higher CBS expression—which is, in part, localized to the mitochondria—than healthy CCs, shown by (A) Western blotting and (C) confocal microscopy. (Scale bar: 5 µm.) (B) TST and ETHE1 expression was similar in DSCs and CCs. (D) DSCs contain increased amounts of intracellular H2S and polysulfide. Top photomicrographs: AzMC-based H2S live cell imaging. (Scale bar: 20 µm.) (E) DSCs exhibit reduced cell proliferation rate; this is normalized by AOAA (3 µM); treatment of CCs with the H2S donor GYY4137 (3 mM) phenocopies the inhibition of proliferation seen in DSCs. (F) DSCs exhibit low basal and maximal mitochondrial oxygen consumption rates (OCR); these parameters are improved by AOAA; exposure of CCs to GYY4137 phenocopies the metabolic suppression seen in DSCs. (G) Complex IV is blocked in DSCs but it is restored by AOAA. (H–L) SiRNA-mediated silencing of CBS in DSCs (H) suppresses H2S production (I), normalizes DSC proliferation (J), and enhances OCR (K) and Complex IV activity (L). (M) Increased expression of various mitochondrial proteins belonging to electron chain transport Complexes II, III, and IV in DSCs. (N) Bell-shaped cellular bioenergetic role of H2S in DSCs vs. CCs; effect of CBS inhibition. *P < 0.05 and **P < 0.01, significant difference between CCs and DSCs; #P < 0.05 and ##P < 0.01, significant effect of AOAA in DSCs; §P < 0.05, significant effect of GYY4137 in CCs or significant difference between AOAA and GYY4137+AOAA in DSCs. Data are shown as mean ± SEM of at least 3 experiments. One- and 2-way ANOVAs were performed, followed by a post hoc Bonferroni test.
Pharmacological inhibition of CBS with aminooxyacetate (AOAA) (3 µM) (4) normalized H2S production (Fig. 1D), restored Complex IV activity (Fig. 1G), improved mitochondrial electron transport (Fig. 1F), and restored DSC proliferation (Fig. 1E). The H2S-releasing molecule GYY4137 (4) phenocopied the effect of DS in CCs in terms of bioenergetics and proliferation and reversed the beneficial effects of AOAA in DSCs (Fig. 1 E–G). SiRNA-mediated silencing of CBS in DSCs recapitulated the effects of AOAA (Fig. 1 I–L).
The expression of electron transport chain proteins that are part of Complexes II, III, and IV was increased in DSCs (Fig. 1M), likely representing a compensatory response.
CBS, a key enzyme in the transsulfuration pathway, catalyzes the conversion of homocysteine into cystathionine (7). Lejeune (8) has already hypothesized in the 1990s that the overdosage of CBS may contribute to the metabolic alterations and overall clinical picture in DS. Both the footprint of excess CBS (low homocysteine) and the footprint of mitochondrial inhibition (accumulation of Krebs cycle intermediaries) have been demonstrated by metabolomic analysis of DS subjects (9). Moreover, increased urinary thiosulfate and circulating sulfhemoglobin levels have previously been reported in subjects with DS (6). The current report confirms and extends these findings: DSCs contain higher levels of H2S (as well as reactive polysulfides) than CCs.
The hypothesis that CBS-derived H2S may be responsible for the metabolic suppression in DS was originally put forward by Kamoun et al. in 2003 (6), but it has not been tested experimentally until now. Our data indicate that CBS-derived H2S is, indeed, responsible for the suppression of mitochondrial function in DSCs. When CBS activity or CBS expression is normalized, the H2S-mediated tonic suppression of Complex IV is lifted, and the cells regain their ability to perform mitochondrial oxidative phosphorylation (Fig. 1N). In contrast to DSCs, in CCs (which are not under the tonic suppressive effect of high H2S), CBS inhibition does not significantly affect proliferation or bioenergetics (Fig. 1N).
H2S-mediated suppression of cellular bioenergetics provides a plausible mechanistic explanation for a host of characteristic biochemical and clinical features associated with DS, including the reduced tissue and whole-body O2 consumption and impaired metabolic fitness—characteristic features of DS (1)—since these processes are directly related to mitochondrial ATP production.
CBS overexpression was recently found to phenocopy the DS-like neurocognitive deficits in mice (10). It is conceivable that H2S overproduction and consequent inhibition of mitochondrial Complex IV explain some of the neurological and neurocognitive deficits associated with DS, because neurons heavily depend on ATP produced by oxidative phosphorylation.
Importantly, H2S-mediated inhibition of Complex IV is a reversible biochemical process. Therefore, follow-up studies should be conducted to determine whether pharmacological inhibition of CBS reverses some of the functional defects associated with DS in vivo. Several classes of CBS inhibitors exist that may be useful for such efforts—including AOAA (which has been in clinical trials in the 1970s), various AOAA prodrugs, natural-product CBS inhibitors (apigenin), and benserazide (a clinically approved drug that has a secondary pharmacological effect as a CBS inhibitor) (4).
Materials and Methods
Female dermal fibroblasts from control (Detroit 551; ATCC CCL-110) and DS (Detroit 539; ATCC CRL-84) subjects were obtained from LGC Standards and cultured in Advanced DMEM. CBS silencing was achieved by siCBS (SASI_Hs01_00214623) (Sigma). Proliferation was quantified by BrdU (11). H2S and polysulfide production were measured using 7-azido-4-methylcoumarin (AzMC) (12) and 3′,6′-di(O-thiosalicyl)fluorescein (SSP4) (13), respectively. Western blotting was performed using the iBlot 2 Dry Blotting System (11). Mitochondrial localization of CBS was performed by confocal microscopy with an anti-CBS antibody and MitoSOX Red (14) using a Leica TCS SP5 confocal microscope. Bioenergetic measurements were performed by extracellular flux analysis. Complex IV activity was measured in permeabilized cells using ascorbate and TMPD (15).
Acknowledgments
This work was supported by the Lejeune Foundation (Paris) (C.S.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Ruparelia A., Wiseman F., Sheppard O., Tybulewicz V. L., Fisher E. M., Down syndrome and the molecular pathogenesis resulting from trisomy of human chromosome 21. J. Biomed. Res. 24, 87–99 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince J., Jia S., Båve U., Annerén G., Oreland L., Mitochondrial enzyme deficiencies in Down’s syndrome. J. Neural Transm. Park. Dis. Dement. Sect. 8, 171–181 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Nicholls P., Marshall D. C., Cooper C. E., Wilson M. T., Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem. Soc. Trans. 41, 1312–1316 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Szabo C., Papapetropoulos A., International union of basic and clinical pharmacology. CII: Pharmacological modulation of H2S levels: H2S donors and H2S biosynthesis inhibitors. Pharmacol. Rev. 69, 497–564 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichinohe A., et al. , Cystathionine beta-synthase is enriched in the brains of Down’s patients. Biochem. Biophys. Res. Commun. 338, 1547–1550 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Kamoun P., Belardinelli M. C., Chabli A., Lallouchi K., Chadefaux-Vekemans B., Endogenous hydrogen sulfide overproduction in Down syndrome. Am. J. Med. Genet. A. 116A, 310–311 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Majtan T., et al. , Potential pharmacological chaperones for cystathionine beta-synthase-deficient homocystinuria. Handb. Exp. Pharmacol. 245, 345–383 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Lejeune J., Pathogenesis of mental deficiency in trisomy 21. Am. J. Med. Genet. Suppl. 7, 20–30 (1990). [DOI] [PubMed] [Google Scholar]
- 9.Caracausi M., et al. , Plasma and urinary metabolomic profiles of Down syndrome correlate with alteration of mitochondrial metabolism. Sci. Rep. 8, 2977 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marechal D., et al. , CBS overdosage is necessary and sufficient to induce cognitive phenotypes in mouse models of Down syndrome and interacts genetically with Dyrk1a. Hum. Mol. Genet. 28, 1561–1577 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Panagaki T., Michael M., Hölscher C., Liraglutide restores chronic ER stress, autophagy impairments and apoptotic signalling in SH-SY5Y cells. Sci. Rep. 7, 16158 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szczesny B., et al. , AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide 41, 120–130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bibli S. I., et al. , A selective and sensitive method for quantification of endogenous polysulfide production in biological samples. Redox Biol. 18, 295–304 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharyya S., et al. , Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS One 8, e79167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salabei J. K., Gibb A. A., Hill B. G., Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat. Protoc. 9, 421–438 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]