Significance
The QS Agr system of the pathogen S. aureus is a social trait based on bacterial population density that orchestrates the expression of toxins crucial for virulence. Paradoxically, Agr-defective mutants are selected during infection. We studied the selection and fitness of agr mutants under infection-relevant conditions, such as antibiotic stress and hypoxia. Under aerobic growth, the Agr-controlled PSM toxins are produced. These are toxic for Staphylococci and select for agr mutants, which are nonproducers. Contrastingly, hypoxia favors QS maintenance and even allows hyperactivation of the system without imposing a fitness burden. We propose that changing oxygen environments encountered during infection not only alters the virulence potential, but also the course of microbial evolution of the S. aureus community.
Keywords: quorum sensing, oxidative stress, Agr, Staphylococcus aureus, PSMs
Abstract
Quorum sensing (QS) is the central mechanism by which social interactions within the bacterial community control bacterial behavior. QS-negative cells benefit by exploiting public goods produced by the QS-proficient population. Mechanisms to keep the balance between producers and nonproducers within the population are expected but have not been elucidated for peptide-based QS systems in gram-positive pathogens. The Agr system of Staphylococcus aureus comprises the secretion and sensing of an autoinducing peptide to activate its own expression via the response regulator AgrA as well as the expression of a regulatory RNAIII and psmα/psmß coding for phenol-soluble modulins (PSMs). Agr mutants can be monitored on blood agar due to their nonhemolytic phenotype. In vitro evolution and competition experiments show that they readily accumulate in a process that is accelerated by ciprofloxacin, while the wild type (WT) is retained in the population at low numbers. However, agr mutants possess a fitness advantage only under aerobic conditions. Under hypoxia, Agr activity is increased but without the expected fitness cost. The Agr-imposed oxygen-dependent fitness cost is not due to a metabolic burden but due to the reactive oxygen species (ROS)-inducing capacity of the PSMs and RNAIII-regulated factors. Thus, selection of mutants is dictated by the QS system itself. Under aerobic conditions, emergence of agr-negative mutants may provide the population with a fitness advantage while hypoxia favors QS maintenance and even affords increased toxin production. The oxygen-driven tuning of the Agr system might be of importance to provide the pathogen with capabilities crucial for disease progression.
Bacterial QS is based on the secretion of a diffusible signal molecule which, upon reaching a critical concentration, activates a cognate receptor leading to changes in gene expression. Receptor activation facilitates the density-dependent production of extracellular products. These molecules have been termed “public goods” because they can benefit neighboring cells including nonproducers in the population. The production of the QS signal as well as the production of the public goods is assumed costly for the producer (1). QS deficient mutants, often called “cheaters,” have been reported to evolve during infections caused by different bacteria (2–7). The social traits of QS have been extensively studied in proteobacteria that utilize mainly acyl-homoserine lactones as autoinducers and intracellular receptor molecules as transcriptional regulators. Laboratory models have shown that several mechanisms to control cheaters have evolved in these organisms (3, 8). In gram-positive species, such as S. aureus, short modified oligopeptides function as autoinducers, and histidine kinases function as receptors. However, QS systems in gram-positive pathogens have been sparsely studied from an evolutionary perspective (9–11).
The QS Agr system present in the Staphylococci is well studied on the molecular level (12–15). The system is composed of AgrA, AgrB, AgrC, and AgrD encoded by the agrBDCA operon and the divergently transcribed RNAIII molecule (depicted in Fig. 1C). The autoinducing peptide (AIP) is encoded by agrD. Extracellular AIP results in autophosphorylation of the histidine kinase AgrC and phosphotransfer to the response regulator AgrA. Phosphorylated AgrA triggers transcription of its own operon (agrBDCA) as well as the divergently transcribed regulatory RNAIII. Many extracellular virulence factors, such as hemolysins are under the control of RNAIII (15). Of note, RNAIII also encodes a small peptide, Hld (16, 17). The response regulator AgrA also binds to the promoters of the psm operons, psmα and psmβ coding for the PSMs; PSMα1–4 and PSMβ1–2, respectively (depicted in Fig. 1C) (18). Hld and PSMs are a family of amphipathic α-helical peptides that have multiple roles in staphylococcal pathogenesis and contribute a large extent to the pathogenic success of virulent staphylococci (19). They are cytotoxic, stimulate inflammatory responses, and contribute to biofilm dissemination (20). However, PSMs and Hld may also interfere with bacteria. Hld and proteolytically processed derivatives of PSMα1 and PSMα2 possess antimicrobial activity against Streptococccus pyogenes (21, 22). They also interact with the producer’s own membrane and promote the release of membrane vesicles from the cytoplasmic membrane via an increase in membrane fluidity (23, 24). Recently, PSMs were also shown to reduce persister formation (25, 26). The antibacterial effect of PSMs is further supported by the necessity for the producer to protect itself from PSMs by the specific PSM transporter (Pmt) export system (27).
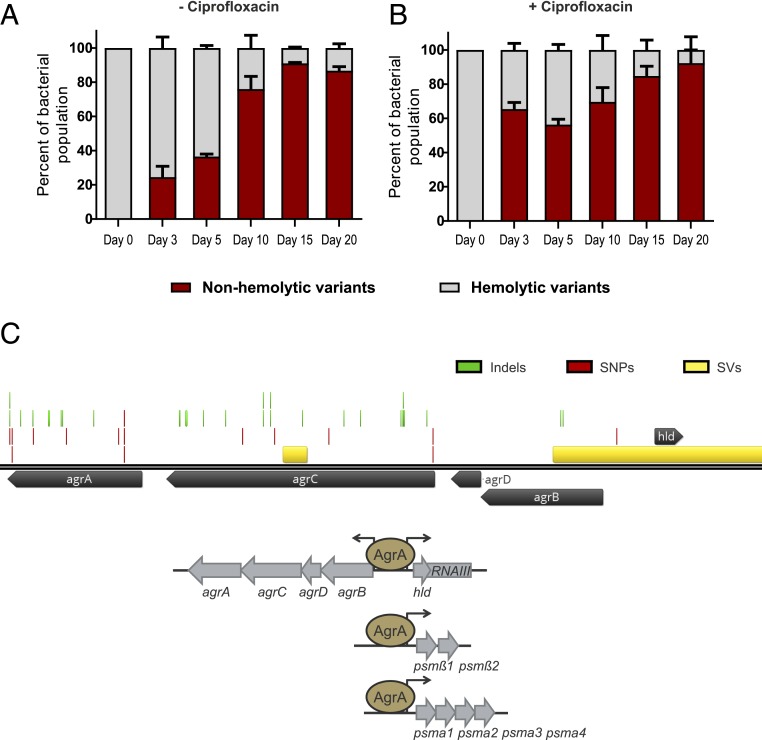
Fig. 1.
Evolution of agr mutants in vitro. Long-term evolution of HG001 (A) without and (B) with sub-minimum inhibitory concentration (MIC) of ciprofloxacin (0.125 µg/mL). Percentage of nonhemolytic and hemolytic subpopulations was determined based on colony phenotype on blood agar. (C) Representation of the mutations occurring within the agr locus after evolution in an aerobic environment. Mutations comprise single residue insertions or deletions (indels), single nucleotide polymorphisms (SNPs), or larger deletions, i.e., structural variants (SVs). The agr locus comprises the agrBDCA operon and the divergently transcribed RNAIII molecule which also encodes δ-hemolysin (Hld). The autoinducing peptide (AIP) is encoded by agrD. Accumulation of extracellular AIP results in autophosphorylation of the histidine kinase AgrC and phosphotransfer to the response regulator AgrA. Phosphorylated AgrA triggers transcription of its own operon (agrBDCA) as well as RNAIII. AgrA also binds to the promoters of the psm operons, psmα and psmβ coding for the PSMs; PSMα1–4 and PSMβ1–2, respectively.
Agr has been proven to be important for virulence in several animal models (5, 6, 7). Recently, Agr activity was also shown to be required for colonization of the human gastrointestinal tract (28). Many reports emphasize that Agr-dependent factors, such as α-hemolysin (29) and PSMs (19) are major virulence determinants. Correspondingly, the highly virulent community-acquired methicillin-resistant S. aureus (MRSA) strains, such as MW2 and USA300, are characterized by high Agr activity (30). However, several lineages of healthcare-acquired MRSA (haMRSA) strains and many clinical isolates were shown to have low or no Agr activity (5, 30). Agr-defective mutants also accumulate within the host, e.g., during chronic infections (31–33) or during persistent bacteremia (34–36) and were even linked to higher mortality in bacteremic patients (37). The use of certain antibiotics, such as fluoroquinolones seems to select for agr mutants not only in vitro (11, 38), but also during treatment (39). Of note, agr-positive and agr-negative strains often coexist in clinical samples (40, 41) or in animal models (42) suggesting that there are mechanisms at play keeping producer and cheater in balance. Thus, there is now compelling evidence for the Janus-faced role of Agr during different stages of colonization or infection. The selection pressures and mechanisms that shape the bacterial population; either tilting toward a net Agr function or dysfunction have not been defined. Here, we used an in vitro model to analyze the evolution and fixation of QS mutants in S. aureus populations under infection-relevant conditions, such as antibiotic stress and hypoxia. We find that agr mutants possess a fitness advantage only under aerobic conditions. Under hypoxia, Agr activity is increased but without a fitness cost. This Agr-imposed oxygen-dependent fitness cost is not due to a metabolic burden of QS but linked to oxidative stress.
Results
Evolution of agr Mutants In Vitro.
We set up an experimental system in which the selection of QS mutants can be monitored over time due to their characteristic nonhemolytic phenotype. We also employed ciprofloxacin, a known inducer of the SOS system to accelerate the mutation rate (38). Cultures were transferred daily for 20 d in the presence or absence of subinhibitory concentrations of ciprofloxacin. While nonhemolytic variants accumulated readily in both treated and untreated cultures, ciprofloxacin accelerated the accumulation of variants (Fig. 1 A and B). After only 3 transfers (Day 3), the nonhemolytic variant subpopulation accounted for ∼24% of the untreated cultures while in treated cultures, this subpopulation accounted for ∼65% of the total population. Interestingly, the WT population did not get completely outcompeted or lost from the population. Indeed, after 15 transfers, both subpopulations seemed to reach a “steady state” with about 10% of the WT maintained.
Nonhemolytic variants isolated from independent cultures (42 isolates in total) after 3, 18, or 20 transfers were resequenced. All nonhemolytic variants were found to contain mutations within the agr locus (Fig. 1C), mostly within agrA and agrC. Interestingly, with the exception of 1 isolate that bore a large deletion spanning RNAIII/hld and agrB, we did not detect SNPs or indels in other loci that influence hemolysis, i.e., hla, hld, psm, and sae. Thus, the Agr system imposes a strong fitness cost.
Fitness Advantage of agr Mutants.
We compared growth characteristics of the WT against various agr mutants (Fig. 2A). We selected 1 representative agr deficient isolate which bore a single point mutation in AgrAR165H without additional SNPs elsewhere in the genome. The point mutation lies in the C-terminal DNA-binding domain of AgrA and likely affects the ability to bind to target promoter regions. For comparison, we included an agr-deletion mutant in which the entire agr/RNAIII locus was deleted and a strain in which a single amino acid exchange in the histidine–kinase domain (AgrCR238H) rendered the enzyme constitutively active (43, 44).
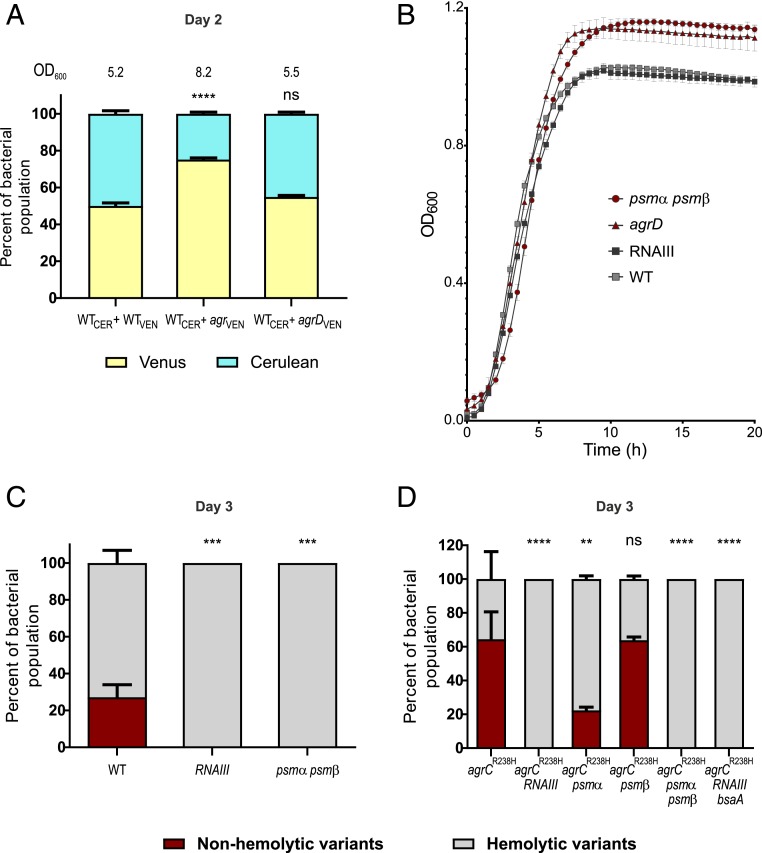
Fig. 2.
Fitness advantage of agr mutants. (A) Aerobic growth of HG001, agr, agrCR238H (constitutive agr), and agrAR165H in tryptic soy broth (TSB). Error bars represent SD (n = 3). (B) HG001 and agrCR238H evolved for 2 and 3 d with and without sub-MIC of ciprofloxacin (0.125 µg/mL). Percentage of nonhemolytic and hemolytic subpopulations was determined based on colony phenotype on blood agar. Error bars indicate SEM (n = 3). Statistical significance determined by two-way ANOVA with Tukey’s posttest. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001.
The agr mutants in which the Agr function had been abrogated had similar growth rates as the WT but differed in the final yield reached in the stationary phase. This is in line with the understanding that QS and thereby Agr is active only once the critical threshold of cell density is reached (SI Appendix, Fig. S1). Accordingly, constitutive expression independent of QS should already impose a fitness cost in the exponential phase. We could confirm that the growth of the constitutive agrCR238H mutant (doubling time 31.36 ± 0.2139 min, n = 3) is slightly retarded compared to WT (doubling time 29.18 ± 0.5663 min, n = 3; P < 0.05) (Fig. 2A).
We postulated that, in the constitutive agrCR238H derivative, Agr should impose a higher burden thereby accelerating the accumulation of agr mutants. agrCR238H cultures accumulated nonhemolytic variants after fewer transfers compared to the WT (Fig. 2B). After 3 transfers, the percentage of nonhemolytic variants that accumulated within the untreated agrCR238H population was comparable to that in ciprofloxacin-treated WT cultures and far exceeded that in untreated WT cultures. Interestingly, in contrast to the long-term experiment using the WT strain (Fig. 1 A and B), the Agr-functional ancestor was not maintained in the agrCR238H population as far as the limit of detection of the test system.
Fitness Cost Associated with the Various Nodes of the Agr System.
The production of QS signaling molecules has been considered to be costly thereby imposing a metabolic burden (1, 45). To test whether this holds true for staphylococcal AIPs, we created a signal nonproducing agrD mutant. Such a mutant would be expected to have a fitness advantage relative to the WT. We performed competition experiments between agr mutants (full deletion or agrD) and the WT. Strains were labeled with the fluorescent protein Venus and competed at a 1:1 ratio with the WT labeled with the fluorescent protein Cerulean (Fig. 3A). After 2 subcultures, the percentage of WT and mutant subpopulations was assessed. As expected, in the pairing with the agr full deletion, the WT was outcompeted, and the culture reached higher bacterial density. However, the agrD mutant did not outcompete the WT. This shows that the agrD mutant was functionally complemented by the AIPs produced by the WT and that the synthesis of AIP itself does not exert a fitness burden. Venus-labeled WT in coculture with WT labeled with Cerulean remained in a stable 1:1 ratio and, thus, confirmed that the fitness traits of the competing strains were not influenced by either of the fluorescent proteins themselves.
Fig. 3.
Fitness cost associated with the various nodes of the Agr system. (A) HG001 bearing PsarA-Cerulean was competed for 2 d with either agr or agrD bearing PsarA-Venus, starting at a ratio of 1:1. The final bacterial yield in each culture (OD600) is indicated. Percentage of each bacterial subpopulation was measured based on fluorescence. Error bars indicate SEM (n = 3). Statistical significance determined by one-way ANOVA with Tukey’s posttest. Asterisks indicate statistical significance in comparison to the WT population (WTCER + WTVEN). (B) Aerobic growth of HG001, RNAIII, agrD, and psmα psmβ in TSB. Error bars represent SD (n = 3). (C) HG001, RNAIII, and psmα psmβ evolved for 3 d. Error bars indicate SEM (n = 5). Asterisks indicate statistical significance in comparison to the WT. (D) HG001 agrCR238H, agrCR238H RNAIII, agrCR238H psmα, agrCR238H psmβ, agrCR238H psmα psmβ, and agrCR238H RNAIII bsaA evolved for 3 d. Error bars indicate SEM (n = 3). Asterisks indicate statistical significance in comparison to HG001 agrCR238H. (C and D) Percentage of nonhemolytic and hemolytic subpopulations was determined based on colony phenotype on blood agar. Statistical significance determined by two-way ANOVA with Tukey’s posttest. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001.
Therefore, downstream consequences of Agr signaling must drive the selection of agr mutations. This might occur in possibly 2 ways: either through the production of the regulatory RNAIII, which is a key virulence determinant and controls the expression of several virulence and metabolic genes, or through the production of the cytolytic PSM peptides which are directly controlled by AgrA (18). To identify which specific node of the Agr system imposes the fitness disadvantage, we compared the growth of mutants bearing deletions of either RNAIII or psmα/psmβ genes. Surprisingly, the psm deletion was sufficient to confer the same growth advantage as the complete agr deletion (Figs. 2A and 3B). Deletion of RNAIII had only a slight effect on growth compared to WT. We next analyzed the accumulation of nonhemolytic variants in these backgrounds. Of note, mutations in RNAIII or psms result in an altered hemolytic phenotype but are still clearly distinguishable from nonhemolytic agr mutants. Interestingly, both deletions abrogated the accumulation of nonhemolytic variants in bacterial populations (Fig. 3C). This was confirmed in a constitutive agrCR238H background. No mutants were selectable in a psmα psmβ double mutant. psmα deletion alone was sufficient to significantly reduce the accumulation of nonhemolytic variants (Fig. 3D), indicating that PSMα is more active compared to PSMβ. In conclusion, the fitness advantage of an agr mutant can be attributed to the loss of PSMs and/or loss of RNAIII.
Agr Mutants Are Selected Only under Aerobic Growth Conditions.
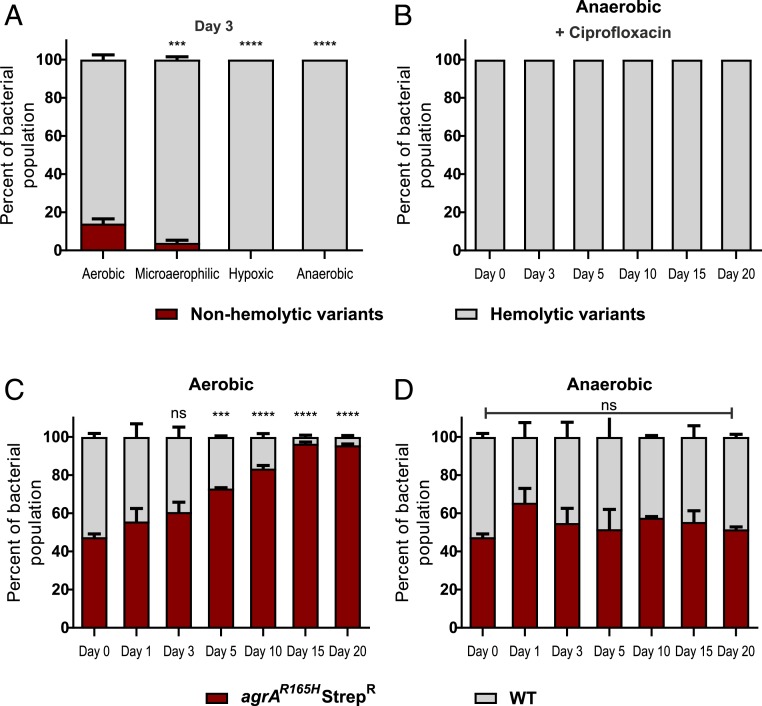
Different environmental cues are known to modulate Agr activity (14). Oxygen concentrations vary greatly across different tissues (46) affecting bacterial growth and signaling. There are conflicting results whether Agr is more (47) or less (48) active under hypoxia. We analyzed the Agr-associated fitness cost under different oxygen conditions (Fig. 4A). Surprisingly, agr mutants did not accumulate in cultures transferred under low oxygen conditions. With 5–15% oxygen (microaerophilic), the mutation frequency was significantly decreased compared to normoxic conditions. Under hypoxic or anaerobic growth, nonhemolytic variants were not selected. We next performed a long-term evolution experiment under anaerobic conditions. Even after 20 transfers with ciprofloxacin, no agr mutants were detectable (Fig. 4B). To ascertain these observations, we performed a competition experiment under aerobic and anaerobic conditions wherein the WT was competed with the streptomycin-resistant agrAR165H mutant starting at 1:1, 9:1 and 1:9 ratios (Fig. 4 C and D and SI Appendix, Fig. S3). Within 20 transfers, the agrAR165H mutant accumulated rapidly under aerobic growth conditions irrespective of the initial ratio. Competition of a psm mutant or a RNAIII mutant with the WT strain confirmed that both PSMs and RNAIII confer a fitness disadvantage over time (SI Appendix, Fig. S2). Under anaerobic growth, the relative starting ratios of the WT and agrAR165H mutants remained stable for 20 transfers (Fig. 4D). These results were unaltered in a competition experiment with the streptomycin-resistant WT and an unmarked agrAR165H mutant (SI Appendix, Fig. S3). Thus, both evolution and competition experiments show that Agr imposes a fitness burden only under aerobic growth.
Fig. 4.
Agr mutants are selected only under aerobic growth conditions. (A) HG001 evolved for 3 d under aerobic, microaerophilic, hypoxic, and anaerobic environments. Error bars indicate SEM (n = 3). Asterisks indicate statistical significance in comparison to the aerobic condition. (B) Long-term evolution of HG001 under anaerobic conditions with ciprofloxacin (C and D) HG001 was competed for 20 d with streptomycin-resistant agrAR165H, starting at a ratio of 1:1 under (C) aerobic or (D) anaerobic conditions. Percentage of each bacterial subpopulation was determined based on colony phenotype on blood agar. Error bars indicate SEM (n = 3). Asterisks indicate statistical significance in comparison to Day 0. Statistical significance determined by (A) two-way ANOVA (C and D) repeated measures two-way ANOVA with Tukey’s posttest. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001.
Increased Agr Activity in Low Oxygen Environments.
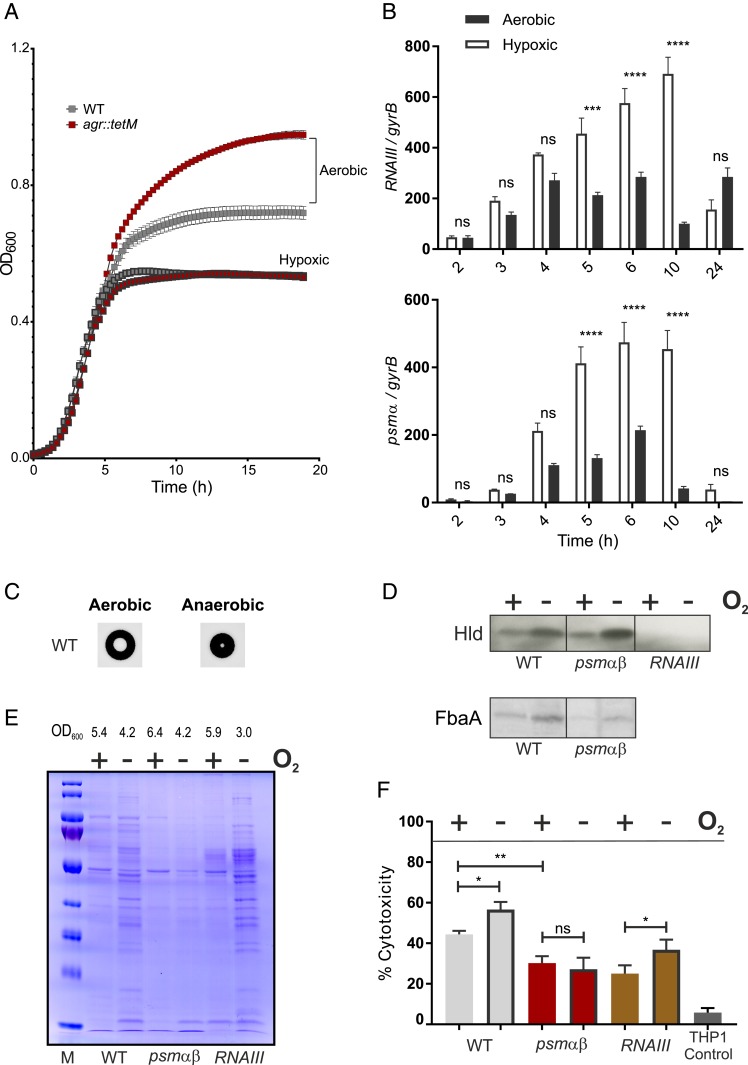
Growth under hypoxic conditions resulted in significantly lower final bacterial densities. However, the agr mutant no longer has a growth advantage under hypoxia (Fig. 5A). We reasoned that, perhaps, due to the lower cell density, the QS system might just not be active under low oxygen conditions. In contrast to this assumption, we found that Agr activity is even higher under hypoxic conditions. This was shown on the transcriptional level wherein both the Agr target genes RNAIII and psmα were significantly increased throughout growth under hypoxia compared to aerobic conditions (Fig. 5B). Interestingly, the transcription of pmt was not significantly influenced by hypoxia (SI Appendix, Fig. S4).
Fig. 5.
Increased Agr activity in low oxygen environments. (A) Growth of HG001 and agr::tetM under aerobic and hypoxic environments. Error bars represent SD (n = 3). (B) Bacteria were harvested at different time points (2–6, 10, 24 h) during growth. Total RNA from strain HG001 was isolated. RNAIII or psmα mRNA was quantified by qRT-PCR with reference to gyrB. Error bars indicate SEM (n = 3). Statistical significance determined by repeated measures two-way ANOVA with Bonferroni’s posttest. (C) Colony morphology and hemolysis zone of HG001 grown on blood agar under aerobic and anaerobic environments. (D–F) Bacterial supernatants from cultures (HG001, psmα psmβ, and RNAIII) grown either aerobically or under hypoxic conditions were analyzed by (D) Western blot for Hld and aldolase (FbaA) and (E) SDS-PAGE for extracellular proteome (loading control for Western blots). The final bacterial yield in each culture (OD600) is indicated. (F) Bacterial supernatants were analyzed for a cytotoxic potential against THP1 macrophages. Percentage of cytotoxicity shown was normalized to the Triton control. Error bars indicate SD (n = 6). Statistical significance determined by one-way ANOVA with Tukey’s posttest. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001.
Next we analyzed whether the increased transcription of RNAIII and psmα is accompanied by Agr-dependent changes in hemolysis. Increased expression of hemolysis could be verified after anaerobic growth on blood agar plates (Fig. 5C). The hemolytic zone relative to the size of single colonies was significantly higher (P < 0.005) in anaerobic (20.3 ± 5.002) compared to aerobic (4.204 ± 0.2803) grown bacteria. Microtitration of supernatants from bacterial cultures using red blood cells confirmed the higher hemolytic activity under low oxygen (SI Appendix, Fig. S5). Moreover, under hypoxia, the RNAIII encoded peptide Hld was found to be more abundant in the supernatants (Fig. 5D) consistent with the increased transcription of RNAIII. The release of cytoplasmic proteins and lipoproteins into the extracellular milieu was shown previously to be dependent on PSMs (49, 50). Accordingly, fewer proteins are detectable in the supernatants of the psm mutant (Fig. 5E). Of note, this effect was mainly seen under hypoxia. There was also an increased level of the cytoplasmic protein aldolase in the secretome under hypoxia (Fig. 5D). Aldolase was previously used as a marker protein for excretion of cytoplasmic proteins (49).
These data demonstrate that Agr activity is clearly higher under hypoxia without imposing a fitness cost. Furthermore, the data indicate that the metabolic burden imposed by the synthesis of RNAIII or PSMs does not explain the fitness differences. If this was the case, we would expect the fitness differences between WT and agr mutants under hypoxia to be higher and not lower. To solve this conundrum, we speculated that PSMs may be inactivated under hypoxic conditions, e.g., through proteolytic cleavage and, thus, cannot impose a selection pressure. We, therefore, tested the cytotoxicity of bacterial culture supernatants on THP1 cells (Fig. 5F). There was a significant increase in cytotoxicity of supernatants from WT and RNAIII mutants grown under hypoxia. This was abrogated in a psmαβ mutant, indicating that PSMs produced under hypoxia are not inactive with regard to their cytotoxic effects.
PSMs Induce ROS and Possess Antimicrobial Activity under Aerobic Conditions.
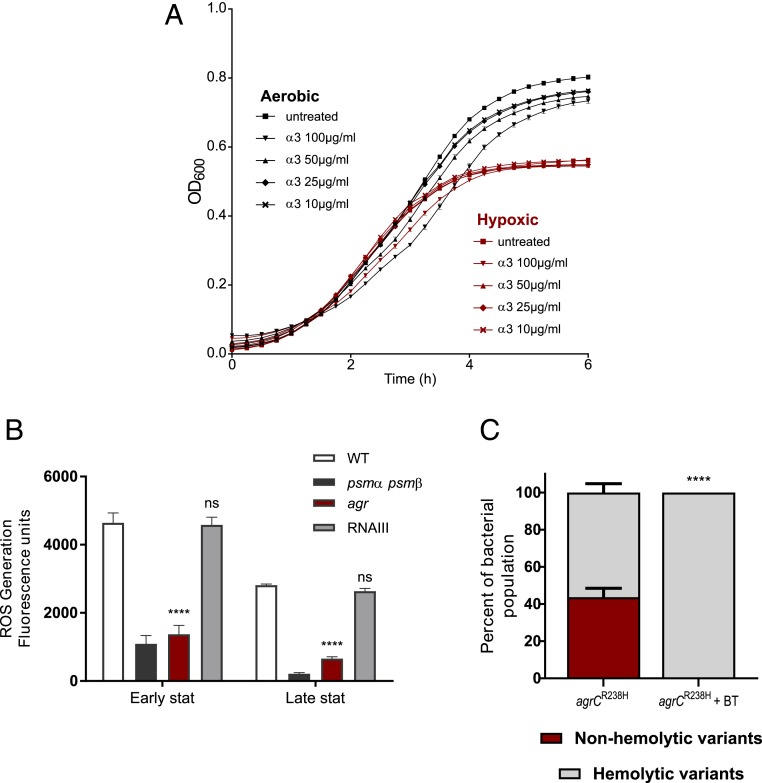
We next hypothesized that the bacterial cells are themselves not susceptible to PSMs under low O2. In an aerobic environment, PSMα3 resulted in a concentration-dependent growth defect wherein as low as 10 µg/mL affected bacterial yield; while up to 50 µg/mL had no effect under hypoxia (Fig. 6A). Therefore, under hypoxia, the Agr system is highly active with increased secretion of toxic PSMs. However, bacteria seem to be protected from their antimicrobial effects under these conditions.
Fig. 6.
PSMs induce ROS and possess antimicrobial activity under aerobic conditions. (A) Different concentrations of PSMα3 (100, 50, 25, and 10 µg/mL) were added to bacterial cultures of HG001 agr. Growth under aerobic and hypoxic environments was monitored. Error bars indicate SEM (n = 3). Bacterial yield compared to the untreated control was significant only under aerobic growth (****P ≤ 0.0001) and not under hypoxia. (B) Early and late stationary phase cultures of HG001, psmα psmβ, agr, and RNAIII were compared for ROS levels. Error bars indicate SEM (n = 3). Asterisks indicate statistical significance in comparison to HG001. (C) HG001 agrCR238H evolved for 3 d in the presence of antioxidants (1 mM 2,2'-bipyridyl and 100 mM thiourea). Percentage of nonhemolytic and hemolytic subpopulations was determined based on colony phenotype on blood agar. Error bars indicate SEM (n = 3). Asterisks indicate statistical significance in comparison to the HG001 agrCR238H. Statistical significance determined by (A) one-way ANOVA with Tukey’s posttest and (B and C) two-way ANOVA with Bonferroni’s posttest. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001.
We speculated that PSMs may induce ROS under aerobic conditions contributing to the toxicity. Indeed, we found significantly higher levels of ROS in the WT compared to the psmαβ or agr mutant (Fig. 6B). Thus, under hypoxia cytotoxic PSM production is enhanced without harming the producer suggesting that the selection of agr mutants under aerobic conditions is to avoid PSM-mediated ROS production. Indeed, addition of 2,2'-bipyridyl and thiourea to block hydroxyl radical accumulation could completely prevent selection of agr mutants in the constitutive agrCR238H background (Fig. 6C).
However, we also found that RNAIII deletion was sufficient to prevent selection of agr mutants (Fig. 3C), although ROS production was comparable to the WT (Fig. 6B). The glutathione peroxidase BsaA was reported to be derepressed in agr mutants, thus, enabling bacteria to survive oxidative stress (51). We, therefore, tested whether bsaA deletion would increase the selection pressure in a RNAIII mutant (Fig. 3D). However, this was not the case. Moreover, we could not confirm that agrA or RNAIII deletion significantly increases bsaA expression under our growth conditions (SI Appendix, Fig. S6). Taken together, these results suggest that other RNAIII-regulated factors are involved in the protective effect against ROS in a RNAIII mutant.
Discussion
The social trait of QS sensing in proteobacteria has been widely studied and, depending on the model organism analyzed, different mechanisms to balance the relationship between QS-positive bacteria and QS-negative cheaters have been discovered (2, 3, 8). To gain insight into such processes in peptide-based QS systems, we employed evolution and competition experiments. We investigated conditions and factors driving the evolution and selection of QS-negative S. aureus mutants and found the antimicrobial activity of the QS-dependent PSMs to be a major driver for the evolution of QS mutants. Thus, a QS-controlled gene product PSMα can drive the selection of QS mutants. This demarcates the Agr system from other QS systems that are described to have evolved mechanisms to avoid cheating (8, 52).
Fitness Advantage of agr Mutants.
We show that under aerobic conditions agr mutants possess a fitness advantage and grow to higher cell densities compared to the WT. Agr mutants were shown to outcompete the WT consistent with previous in vitro (11) and in vivo reports (9). Interestingly, the WT is not lost from the population but remains in the culture at low numbers (Fig. 1). This is likely because the AIP concentration falls below the threshold for Agr activation. Accordingly, a constitutive AIP producer in which AIP synthesis is uncoupled from QS is lost from the population after prolonged coculture (Fig. 2B). When producers are scarce, the autoregulatory properties of the system restrict AgrA activity and thereby maintain the producer in the population (SI Appendix, Fig. S1). Other mechanisms to inactivate Agr during infection, e.g., through hemoglobin (53), lipoproteins (54), oxidants (55), or microbial interference (28, 56, 57) may similarly function to maintain producer–cheater homeostasis.
QS Fitness Cost Is Not due to Metabolic Burden.
It was proposed that the fitness advantage of QS mutants is due to the costs associated with producing a QS signal and/or the regulated public goods (1, 8, 45). The metabolic cost to just produce AIP in S. aureus was estimated to be high with 184 ATP for a single AgrD preprotein AIP (1). However, our data show that that the fitness advantage of agr mutants is independent of the assumed metabolic burden. First, an AIP nonproducer showed a fitness advantage in monoculture but interestingly not in competition with an AIP producer. Thus, AIP synthesis itself does not impose a fitness burden. Second, the syntheses of downstream targets (public goods) are also unlikely to drive evolution of agr mutants based on the metabolic burden to produce them. This is based on our observation that, under anaerobic conditions, significantly more public goods, such as PSMs or RNAIII regulated factors, are synthesized (Fig. 5). However, under this specific condition, the increased Agr activity has no impact on the fitness monitored either by growth (Fig. 5A) or in competition experiments (Fig. 4D). Of note, also for other QS systems, experimental evidence for the assumption that QS cheaters arise because of the metabolic cost is limited (45) and warrants revisiting. This observation conforms to the idea that the function and not production of a factor downstream of QS signaling imposes the fitness burden under aerobic conditions.
Increased Agr Activity under Hypoxia.
Here, we demonstrated that Agr activity is increased during hypoxic growth, despite lower bacterial cell densities. Hypoxia results in significantly enhanced transcription of the main AgrA targets (RNAIII and psm), Hld synthesis, PSM-mediated protein release, and cell toxicity (Fig. 5). The results confirm recent data from Wilde et al. (47). It has been shown that oxidizing conditions induce disulphide bond formation of AgrA involving the conserved cysteine Cys-199 (51). Of note, the same residue also reacts with nitric oxide (58). Both oxidation and S-nitrosylation of Cys-199 were shown to inhibit DNA binding of AgrA to its cognate promoters. Therefore, under aerobic conditions, AgrA function might be partially inhibited through oxidation.
Agr/PSM Does Not Impose a Fitness Cost under Hypoxia Due to Diminished ROS Formation.
Under aerobic conditions, the fitness cost could be linked to PSM expression. Under hypoxia, the higher level of PSMs does not affect fitness since bacteria are protected from the antimicrobial activity of PSMs. We could further show that PSM synthesis results in increased ROS formation (Fig. 6B). This explains the fitness burden of psm-positive strains under aerobic conditions only. However, it does not explain the fitness advantage of the RNAIII mutant. AgrA mutants were previously found to be more resistant toward certain kinds of oxidative stress which was linked to higher expression of the glutathione peroxidase BsaA (51, 59). The role of BsaA could not be confirmed here. However, other ROS protective factors might be up-regulated in the RNAIII mutant to compensate for PSM toxicity.
Conclusion and Outlook: Role of Agr QS In Vivo.
Several epidemiological and virulence studies indicate that Agr activity is required only under certain infection settings (5, 42). Nevertheless, Agr activity is maintained in most colonizing isolates despite the obvious fitness disadvantage. This indicates that, on the population level, several mechanisms may have evolved to maintain the balance. The Agr system was found to be inactive during nose colonization (60), possibly due to Agr interference imposed by other staphylococci (57) in the skin microbiome. Many of the globally spreading haMRSA strains are characterized by low Agr activity (30) which could also be seen as a strategy to avoid agr mutant selection.
Aerobic growth conditions and the clinically relevant quinolone antibiotic ciprofloxacin accelerates the selection of agr mutants. Ciprofloxacin leads to induction of the SOS response and, thus, an increased mutation rate. However, ciprofloxacin might also favor agr mutations because they are better adapted to survive oxidative stress imposed by the antibiotic (59). Thus, ROS formation by the bacterium’s own products (PSMs), ciprofloxacin, or other infection-related stresses, such as phagocytosis may favor the selection of QS mutants. PSMs have been shown to disrupt membrane potential via depolarization (49) which might be a trigger for ROS accumulation. Bacteria have evolved mechanisms to dampen Agr activity under such conditions by, e.g., AgrA oxidation/nitrosylation (51, 58) or inactivation of the AIP (55). Hypoxic conditions in contrast favor the maintenance of the WT population and even allow the hyperactivation of the system without imposing a fitness burden.
S. aureus is a major skin pathogen and the oxygen-driven tuning of the Agr system might especially be of importance to provide the pathogen with different features beneficial for disease progression. Under high O2 conditions, such as at the skin surface, agr mutants are selected (33), and one may also speculate that this might be the source for agr mutants often found in bacteremic patients (34–36). On the other hand, Agr activity is crucial for pathogenicity in skin abscesses (42). Here, S. aureus is entrapped in a hypoxic environment in which the bacteria can now afford to increase Agr activity without the accompanied fitness disadvantage. The increase in Agr-regulated toxins, such as PSMs may, thus, function to facilitate escape from the abscess. In this manner, the environmental oxygen might act to alter the bacterial perception of a self-inflicted insult, such as the PSMs in order to change the course of microbial evolution.
Materials and Methods
More information on the methods used in this study is available in SI Appendix.
Bacterial Strains and Growth Conditions.
Strains and plasmids are listed in SI Appendix, Table S1. All S. aureus strains were grown in tryptic soy broth (TSB), and antibiotics at 3 µg/mL tetracycline, 10 µg/mL erythromycin, 150 µg/mL streptomycin, or 10 µg/mL chloramphenicol, were added to the respective resistant strains in the overnight cultures. For evolution experiments under aerobic or anaerobic environments, bacteria were transferred daily to an optical density OD600 of 0.05 with or without subinhibitory concentration of ciprofloxacin (0.125 µg/mL). All strains tested had the same minimum inhibitory concentration (MIC) of 0.25 µg/mL as determined by Etest (bioMérieux). For evolution experiments with antioxidants, the combination of 2,2'-bipyridyl and thiourea was used as described (59) but at lower concentrations of 1 and 100 mM, respectively. Cultures were evaluated on columbia agar with sheep blood (Oxoid) at indicated intervals (2, 3, 5, 10, 15, and 20 d) to determine the percentage of nonhemolytic variants that had accumulated within the population. For growth under anaerobic conditions, the cultures were incubated in an anaerobic jar with an anaerobic gas pack (Oxoid AnaeroGen, Thermo Scientific). This pack reduces ambient oxygen to below 0.1% within 2.5 h and increases carbon dioxide to 13% within 24 h. For hypoxic growth, the corks were replaced with rubber stoppers similar to that described previously (47). For growth under a microaerophilic environment, the Campy Pouch system (BD Diagnostics) was used to create an oxygen concentration of 5–15%.
Bacterial Competition.
Bacterial strains were set up in competition in a 1:1 or 1:9 or 9:1 ratio at an initial OD600 of 0.05. The culture was transferred (1% v/v) daily to fresh media. At the end of the competition experiment, the 2 subpopulations were measured by their respective markers by resistance to streptomycin (150 µg/mL) or fluorescence (PsarA-Cerulean or PsarA-Venus).
Fluorescence of competing bacterial strains were measured in an automated reader (Tecan Infinite 200 PRO). For Venus, an excitation wavelength of 505 nm with a bandwidth of 9 nm and emission wavelength of 535 nm with a bandwidth of 20 nm were used. For Cerulean, an excitation wavelength of 434 nm with a bandwidth of 9 nm and emission wavelength of 485 nm with a bandwidth of 20 nm were used.
Growth Curves.
Growth curves were generated using an automated reader (Tecan Infinite 200 PRO). Overnight cultures were diluted to an OD600 of 0.05. Measurements were made using either a 24-well flat-bottom or a 96-well U-bottom plate format (Greiner) with 1 mL and 100 µL culture volumes, respectively. In order to create a hypoxic environment, wells in the 96-well plates were sealed with an adhesive optical film (BZO Seal film; Biozym). Plates were incubated at 37 °C with a 3 mm orbital shaking amplitude. The OD600 was measured every 15 or 30 min.
Toxins and Cytoplasmic Proteins in Bacterial Supernatants.
Overnight cultures were subcultured to OD600 0.05 and then grown for ∼22 h under aerobic or hypoxic conditions. Bacterial supernatants were harvested and filtered using a 0.45 µm filter (Millipore) and analyzed for cytotoxic potential, hemolytic activity, extracellular toxins, and proteome analysis.
ROS Measurement.
ROS measurements were performed as described (61) with the following modifications: 10×PBS pH7.4 (without calcium and magnesium; Gibco) was used to stop the reaction to yield the final dichlorofluorescin (DCF) reagent (2′,7′-DCF diacetate). Bacteria were harvested from overnight cultures (late stationary) or after 6 h of growth (early stationary). A 1 OD600 equivalent of bacteria was resuspended in 100 µL of DCF reagent and incubated for 40 min. Fluorescence was measured using an automated reader (Tecan Infinite 200 PRO) where an excitation wavelength of 488 nm with a bandwidth of 9 nm and emission wavelength of 515 nm with a bandwidth of 20 nm were used.
Statistical Analyses.
All statistical analyses are based on biological replicates as detailed in the figure legends. Data were analyzed with one-way or two-way ANOVA. Tukey’s or Bonferroni’s posttests were used as appropriate. P values ≤0.05 were considered significant. All data show the mean and SEM except the cytotoxicity assay where mean and SD are depicted.
Supplementary Material
Acknowledgments
We thank Vittoria Bisanzio, Isabell Samp, Janina Bayer, Alexander Mathias Ott, and Petra Horvatek for technical assistance. We thank Jan Liese for the gift of plasmids. The work was supported by Grants from the Deutsche Forschungsgemeinschaft TR766/A7, TR156/A01, and GRK1708 to C. W. and by infrastructural funding from the Deutsche Forschungsgemeinschaft (DFG), Cluster of Excellence EXC 2124 “Controlling Microbes to Fight Infections.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1902752116/-/DCSupplemental.
References
- 1.Keller L., Surette M. G., Communication in bacteria: An ecological and evolutionary perspective. Nat. Rev. Microbiol. 4, 249–258 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Whiteley M., Diggle S. P., Greenberg E. P., Progress in and promise of bacterial quorum sensing research. Nature 551, 313–320 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abisado R. G., Benomar S., Klaus J. R., Dandekar A. A., Chandler J. R., Bacterial quorum sensing and microbial community interactions. MBio 9, e01749-18 (2018). Erratum in: MBio9, e02331-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Özkaya Ö., Xavier K. B., Dionisio F., Balbontín R., Maintenance of microbial cooperation mediated by public goods in single and multiple traits scenarios. J. Bacteriol. 199, JB.00297-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shopsin B., Copin R., “Staphylococcus aureus adaptation during infection” in Antimicrobial Resistance in the 21st Century, Fong I. W., Shlaes D., Drlica K., Eds. (Springer International Publishing, Cham, 2018), pp. 431–459. [Google Scholar]
- 6.Le K. Y., Otto M., Quorum-sensing regulation in staphylococci-an overview. Front. Microbiol. 6, 1174 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Painter K. L., Krishna A., Wigneshweraraj S., Edwards A. M., What role does the quorum-sensing accessory gene regulator system play during Staphylococcus aureus bacteremia? Trends Microbiol. 22, 676–685 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Asfahl K. L., Schuster M., Social interactions in bacterial cell-cell signaling. FEMS Microbiol. Rev. 41, 92–107 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Pollitt E. J. G., West S. A., Crusz S. A., Burton-Chellew M. N., Diggle S. P., Cooperation, quorum sensing, and evolution of virulence in Staphylococcus aureus. Infect. Immun. 82, 1045–1051 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulander W., et al. , The agr quorum sensing system in Staphylococcus aureus cells mediates death of sub-population. BMC Res. Notes 11, 503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulander W., et al. , Antibiotic-mediated selection of quorum-sensing-negative Staphylococcus aureus. MBio 3, e00459-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novick R. P., Geisinger E., Quorum sensing in staphylococci. Annu. Rev. Genet. 42, 541–564 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Wang B., Muir T. W., Regulation of virulence in Staphylococcus aureus: Molecular mechanisms and remaining puzzles. Cell Chem. Biol. 23, 214–224 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kavanaugh J. S., Horswill A. R., Impact of environmental cues on staphylococcal quorum sensing and biofilm development. J. Biol. Chem. 291, 12556–12564 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bronesky D., et al. , Staphylococcus aureus RNAIII and its regulon link quorum sensing, stress responses, metabolic adaptation, and regulation of virulence gene expression. Annu. Rev. Microbiol. 70, 299–316 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Janzon L., Löfdahl S., Arvidson S., Identification and nucleotide sequence of the delta-lysin gene, hld, adjacent to the accessory gene regulator (agr) of Staphylococcus aureus. Mol. Gen. Genet. 219, 480–485 (1989). [DOI] [PubMed] [Google Scholar]
- 17.Janzon L., Arvidson S., The role of the delta-lysin gene (hld) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. EMBO J. 9, 1391–1399 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Queck S. Y., et al. , RNAIII-independent target gene control by the agr quorum-sensing system: Insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 32, 150–158 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung G. Y., Joo H. S., Chatterjee S. S., Otto M., Phenol-soluble modulins–critical determinants of staphylococcal virulence. FEMS Microbiol. Rev. 38, 698–719 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peschel A., Otto M., Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 11, 667–673 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cogen A. L., et al. , Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J. Invest. Dermatol. 130, 192–200 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joo H. S., Cheung G. Y., Otto M., Antimicrobial activity of community-associated methicillin-resistant Staphylococcus aureus is caused by phenol-soluble modulin derivatives. J. Biol. Chem. 286, 8933–8940 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlatterer K., et al. , The mechanism behind bacterial lipoprotein release: Phenol-soluble modulins mediate toll-like receptor 2 activation via extracellular vesicle release from Staphylococcus aureus. MBio 9, e01851-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Thompson C. D., Weidenmaier C., Lee J. C., Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat. Commun. 9, 1379 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bojer M. S., Lindemose S., Vestergaard M., Ingmer H., Quorum sensing-regulated phenol-soluble modulins limit persister cell populations in Staphylococcus aureus. Front. Microbiol. 9, 255 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu T., et al. , The agr quorum sensing system represses persister formation through regulation of phenol soluble modulins in Staphylococcus aureus. Front. Microbiol. 8, 2189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee S. S., et al. , Essential Staphylococcus aureus toxin export system. Nat. Med. 19, 364–367 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piewngam P., et al. , Pathogen elimination by probiotic Bacillus via signalling interference. Nature 562, 532–537 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berube B. J., Bubeck Wardenburg J., Staphylococcus aureus α-toxin: Nearly a century of intrigue. Toxins (Basel) 5, 1140–1166 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R., et al. , Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13, 1510–1514 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Goerke C., et al. , Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect. Immun. 68, 1304–1311 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suligoy C. M., et al. , Mutation of agr is associated with the adaptation of Staphylococcus aureus to the host during chronic osteomyelitis. Front. Cell. Infect. Microbiol. 8, 18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soong G., et al. , Methicillin-resistant Staphylococcus aureus adaptation to human keratinocytes. MBio 6, e00289-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowler V. G., Jr, et al. , Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J. Infect. Dis. 190, 1140–1149 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Giulieri S. G., et al. , Genomic exploration of sequential clinical isolates reveals a distinctive molecular signature of persistent Staphylococcus aureus bacteraemia. Genome Med. 10, 65 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young B. C., et al. , Severe infections emerge from commensal bacteria by adaptive evolution. eLife 6, e30637 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schweizer M. L., et al. , Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob. Agents Chemother. 55, 1082–1087 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schröder W., Goerke C., Wolz C., Opposing effects of aminocoumarins and fluoroquinolones on the SOS response and adaptability in Staphylococcus aureus. J. Antimicrob. Chemother. 68, 529–538 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Butterfield J. M., et al. , Predictors of agr dysfunction in methicillin-resistant Staphylococcus aureus (MRSA) isolates among patients with MRSA bloodstream infections. Antimicrob. Agents Chemother. 55, 5433–5437 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goerke C., et al. , High phenotypic diversity in infecting but not in colonizing Staphylococcus aureus populations. Environ. Microbiol. 9, 3134–3142 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Traber K. E., et al. , Agr function in clinical Staphylococcus aureus isolates. Microbiology 154, 2265–2274 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He L., Le K. Y., Khan B. A., Resistance to leukocytes ties benefits of quorum sensing dysfunctionality to biofilm infection. Nat. Microbiol. 4, 1114–1119 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.George S. E., et al. , Phenotypic heterogeneity and temporal expression of the capsular polysaccharide in Staphylococcus aureus. Mol. Microbiol. 98, 1073–1088 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Geisinger E., Muir T. W., Novick R. P., Agr receptor mutants reveal distinct modes of inhibition by staphylococcal autoinducing peptides. Proc. Natl. Acad. Sci. U.S.A. 106, 1216–1221 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruparell A., et al. , The fitness burden imposed by synthesising quorum sensing signals. Sci. Rep. 6, 33101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carreau A., El Hafny-Rahbi B., Matejuk A., Grillon C., Kieda C., Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 15, 1239–1253 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilde A. D., et al. , Bacterial hypoxic responses revealed as critical determinants of the host-pathogen outcome by TnSeq analysis of Staphylococcus aureus invasive infection. PLoS Pathog. 11, e1005341 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevens E., Laabei M., Gardner S., Somerville G. A., Massey R. C., Cytolytic toxin production by Staphylococcus aureus is dependent upon the activity of the protoheme IX farnesyltransferase. Sci. Rep. 7, 13744 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ebner P., et al. , Non-classical protein excretion is boosted by PSMα-induced cell leakage. Cell Rep. 20, 1278–1286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanzelmann D., et al. , Toll-like receptor 2 activation depends on lipopeptide shedding by bacterial surfactants. Nat. Commun. 7, 12304 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun F., et al. , Quorum-sensing agr mediates bacterial oxidation response via an intramolecular disulfide redox switch in the response regulator AgrA. Proc. Natl. Acad. Sci. U.S.A. 109, 9095–9100 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M., Schaefer A. L., Dandekar A. A., Greenberg E. P., Quorum sensing and policing of Pseudomonas aeruginosa social cheaters. Proc. Natl. Acad. Sci. U.S.A. 112, 2187–2191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pynnonen M., Stephenson R. E., Schwartz K., Hernandez M., Boles B. R., Hemoglobin promotes Staphylococcus aureus nasal colonization. PLoS Pathog. 7, e1002104 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall P. R., et al. , Nox2 modification of LDL is essential for optimal apolipoprotein B-mediated control of agr type III Staphylococcus aureus quorum-sensing. PLoS Pathog. 9, e1003166 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothfork J. M., et al. , Inactivation of a bacterial virulence pheromone by phagocyte-derived oxidants: New role for the NADPH oxidase in host defense. Proc. Natl. Acad. Sci. U.S.A. 101, 13867–13872 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paharik A. E., et al. , Coagulase-negative staphylococcal strain prevents Staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell Host Microbe 22, 746–756.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams M. R., et al. , Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci. Transl. Med. 11, eaat8329 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urbano R., et al. , Host nitric oxide disrupts microbial cell-to-cell communication to inhibit staphylococcal virulence. Cell Host Microbe 23, 594–606.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar K., Chen J., Drlica K., Shopsin B., Tuning of the lethal response to multiple stressors with a single-site mutation during clinical infection by Staphylococcus aureus. MBio 8, e01476-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burian M., Wolz C., Goerke C., Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLoS One 5, e10040 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim H. Y., Go J., Lee K. M., Oh Y. T., Yoon S. S., Guanosine tetra- and pentaphosphate increase antibiotic tolerance by reducing reactive oxygen species production in Vibrio cholerae. J. Biol. Chem. 293, 5679–5694 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.