Significance
A large family of membrane proteins, voltage-gated ion channels, regulate a vast array of physiological functions in essentially all life forms. How these molecules sense membrane potential and respond by creating ionic conduction is incompletely understood. The voltage sensors of these channels contain a “hydrophobic gasket,” a ring of hydrophobic amino acids near the center of the membrane, separating internal and external aqueous solutions. Although voltage-gated proton channels, HV1, resemble voltage-sensing domains of other channels, they differ fundamentally. On depolarization, HV1 conducts protons, whereas other voltage sensors open a physically distinct pore. We identify Val109, Phe150, Val177, and Val178 as the hHV1 hydrophobic gasket. Replacement with less hydrophobic amino acids accelerated channel opening and caused proton-selective leak through closed channels.
Keywords: HVCN1, voltage-sensing domain, voltage gating, ion channels, protons
Abstract
The hydrophobic gasket (HG), a ring of hydrophobic amino acids in the voltage-sensing domain of most voltage-gated ion channels, forms a constriction between internal and external aqueous vestibules. Cationic Arg or Lys side chains lining the S4 helix move through this “gating pore” when the channel opens. S4 movement may occur during gating of the human voltage-gated proton channel, hHV1, but proton current flows through the same pore in open channels. Here, we replaced putative HG residues with less hydrophobic residues or acidic Asp. Substitution of individuals, pairs, or all 3 HG positions did not impair proton selectivity. Evidently, the HG does not act as a secondary selectivity filter. However, 2 unexpected functions of the HG in HV1 were discovered. Mutating HG residues independently accelerated channel opening and compromised the closed state. Mutants exhibited open–closed gating, but strikingly, at negative voltages where “normal” gating produces a nonconducting closed state, the channel leaked protons. Closed-channel proton current was smaller than open-channel current and was inhibited by 10 μM Zn2+. Extreme hyperpolarization produced a deeper closed state through a weakly voltage-dependent transition. We functionally identify the HG as Val109, Phe150, Val177, and Val178, which play a critical and exclusive role in preventing H+ influx through closed channels. Molecular dynamics simulations revealed enhanced mobility of Arg208 in mutants exhibiting H+ leak. Mutation of HG residues produces gating pore currents reminiscent of several channelopathies.
Voltage-gated proton channels (HV1) exist in phylogenetically disparate species where they perform even more disparate functions, from calcification in coccolithophores (1) and mediating action potentials in bioluminescent dinoflagellates (2, 3), to numerous functions in various human tissues (4) such as compensating for electron flux in phagocytes (5–10) and enabling sperm capacitation (11). Identification of the gene (12, 13) revealed that HV1 has 4 transmembrane helices, S1 to S4, and is homologous to the voltage-sensing domains (VSDs) present in most voltage-gated ion channels, voltage-sensing phosphatases, and TMEM266 (14). Unlike VSDs of other channels that sense voltage and cause a separate pore to open or close, HV1 itself conducts protons (15), producing a direct readout of its gating state and making it a unique system for studying gating mechanisms.
Cysteine scanning studies of the aqueous accessibility of residues on the S4 helix of K+ channels revealed that VSDs contain 2 aqueous vestibules that are separated by a relatively short isthmus, termed the hydrophobic gasket (HG), within which S4 residues are inaccessible from either side of the membrane (16–18). In Shaker K+ channels, replacing each of the first 4 Arg in S4 individually with His further revealed that in each case anomalous proton transfer, by carrier or channel mechanisms, occurred within a specific voltage range (19–21). These results suggested that a proton, presumably as a hydronium ion, can access the HG at the center of the “gating pore” where the imidazole group of His accepts the proton, perhaps rotates, and then protonates a water molecule on the distal side.
Several amino acids contributing to the HG in Shaker K+ channels were identified, including I237 on S1 and F290 on S2 (22, 23); these positions correspond to Val109 and Phe150 in hHV1. The same highly conserved Phe in S2 together with 2 conserved acidic groups (Asp174 and Glu153 in hHV1) was proposed to act as a “charge transfer center” that temporarily stores each cationic group (Arg or Lys) in the Shaker S4 segment as it moves from the internal vestibule to the external vestibule (24). That hHV1 shares the architectural feature of a short hydrophobic barrier is suggested by the R205H mutant in which the His shuttles protons into the cell at negative voltages (25), and by efficient proton permeation through open channels.
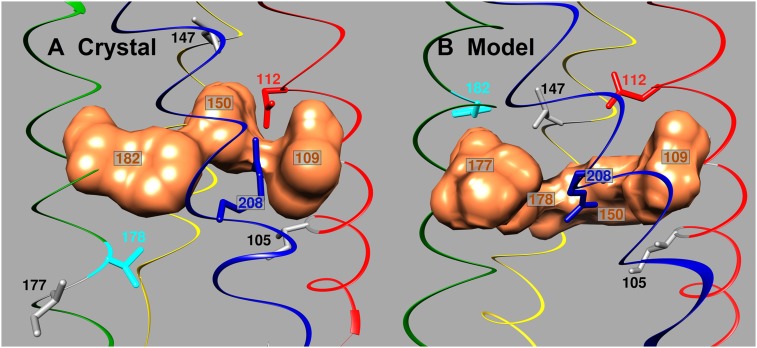
The Ciona intestinalis voltage-sensing phosphatase (CiVSP) is more closely related to HV1 phylogenetically than are K+ channels (3). Crystal structures of CiVSP were determined in both “down” and “up” positions, corresponding with closed and open states (26). Li et al. (27) also studied hHV1 using electron paramagnetic resonance (EPR) and produced closed and open models of hHV1 based on the CiVSP structures. These studies identified 3 conserved hydrophobic amino acids (Val109, Phe150, and Val178) (28) as plausible members of the HG in hHV1. The positions of S1 and S4, but not S2 and S3, in the hHV1 models corresponded well with the single existing crystal structure of HV1 in a presumed closed state. In the chimeric molecule successfully crystallized (29), the cytoplasmic ends of S2 and S3 segments of the mouse channel were replaced with the corresponding section of CiVSP. Li et al. (27) concluded that if S3 in the mHV1 crystal were shifted upward (toward the extracellular surface) and S2 downward by 1 helical turn, the structure and model would match. A significant consequence is that, in the mHV1 crystal structure, the third HG residue is predicted to be Phe182, not Val178 (both with hHV1 numbering). Fig. 1 illustrates the location of the putative HG residues in the homology model and crystal structure of hHV1 in relation to other important amino acids, including Asp112 that is required for proton selectivity (30) and Arg208, the central of 3 Arg in the S4 helix that contribute to gating charge (31).
Fig. 1.
Close-up side view of the central region of HV1 in a closed state (A) in the crystal structure, and (B) in the model of Li et al. (27). Structures were superimposed and are shown at the same viewing angle. The putative HG residues (surface representation of side chains in bronze) are V109, F150, and F182 in the crystal structure (human numbering), but V109, F150, V177, and V178 in the model; the disputed element in each is aqua. Asp112 is red, Arg208 in S4 is blue, and other residues mutated here are gray. Helices are color-coded: S1, red; S2, yellow; S3, green; S4, blue. Extracellular is above. Superimposed and drawn with the University of California, San Francisco (UCSF) Chimera package (Resource for Biocomputing, Visualization, and Informatics, UCSF, supported by National Institute of General Medical Sciences Grant P41-GM103311) (32).
Here, we address both the identity of amino acids contributing to, and the functional importance of, the HG in hHV1 by replacing several amino acids in this region with neutral but smaller and less hydrophobic ones, or with Asp, which may be charged. The results reaffirm that hHV1 contains a narrow barrier separating internal and external solutions like other VSDs, but in addition the HG ensures the occlusion of protons in the closed channel. We mutated both Val178 and Phe182 to determine which is more likely to be a member of the HG by exploiting our discovery of distinctive effects of mutating Val109 and Phe150. This approach identified Val178 and another HG residue, Val177.
Results
Experimental Strategy.
Molecular dynamics (MD) simulations of a homology model of the open state of hHV1 (33) were performed, and mutations designed to compromise the integrity of the HG were proposed for experimental testing. Increased pore hydration and a lowered barrier in the potential of mean force for the movement of Na+ along the channel suggested that some of these mutants might be permeable to Na+ in the open state (SI Appendix, Fig. S1 and Table S1). When these mutants were tested experimentally, the channel did not conduct Na+ (Table 1) but was discovered to be leaky to H+ in the closed state.
Table 1.
Closed channel conductance and selectivity of HG mutants
| Mutant | gH,closed | gH(C/O) pH 7 | ΔVrev pH 7→6 | ΔVrev pH 7→8 | ΔVrev pH 6→5 | ΔVrev TMA+→Na+ |
| I105G | No | 0 | 56.7 ± 1.6 (6) | 53.7 ± 3.5 (5) | — | |
| I105D | No | 0 | 49.4 ± 2.3 (5) | 49 (1) | 60 (1) | 1.8 ± 0.8 (3) |
| L108T | No | 0 | 53 (1) | 55.5 ± 3.5 (2) | −2.4 (1) | |
| V109T | No | 0 | 52.8 ± 1.1 (4) | 55.7 ± 1.9 (3) | 1.6 (1) | |
| V109A | No | 0 | 56.5 ± 1.0 (4) | 54.0 ± 6.0 (2) | 1.1 ± 1.3 (3) | |
| L147T | No | 0 | 51.3 ± 3.0 (3) | — | ||
| L147D | No | 0 | 50.7 ± 1.7 (3) | 53 (1) | 0.2 ± 0.4 (5) | |
| F150A | No | 0 | 47.5 ± 1.5 (2) | 52 (1) | −0.5 ± 0.8 (4) | |
| V178T | No | 0 | 49 (1) | |||
| F182A | No | 0 | 55.8 ± 1.1 (4) | 50.1 ± 3.0 (2) | 45 | −0.9 ± 1.1 (3) |
| V177T | No | 0 | 47.0 ± 4.0 (3) | 53.8 ± 2.5 (5) | −0.3 ± 1.0 (3) | |
| V177D | Yes | 0.103 ± 0.014 (8) | 55.3 ± 3.7 (3) | 0.5 ± 0.2 (3) | ||
| V178A | Yes | 0.017 ± 0.010 (5) | 50.2 ± 1.4 (4) | 47 (1) | 0.6 ± 0.7 (3) | |
| V109A/F150A | Yes | 0.164 ± 0.044 (6) | 49.7 ± 3.8 (3) | 34 (1) | 0.8 ± 1.1 (4) | |
| V109A/V178A | Yes | 0.028 ± 0.012 (7) | 49.9 ± 1.3 (4) | 0.8 ± 0.8 (3) | ||
| F150A/V178A | Yes | 0.144 ± 0.015 (3) | 44.5 ± 0.5 (2) | 70 (1) | −0.6 ± 0.6 (3) | |
| V178D | Yes | 0.036 ± 0.019 (4) | 57.3 ± 1.4 (4) | 50.7 ± 2.7 (3) | 55 (1) | 0.6 ± 1.2 (6) |
| V109A/F150A/V178A | Yes | 0.35 ± 0.05 (9) | 44.0 ± 2.8 (5) | 36.3 ± 4.9 (3) | 29.7 ± 11.9 (3) | −0.3 ± 0.4 (4) |
| V109D | Yes | 0.40 ± 0.06 (5) | 59 (1) | 3.0 ± 0.9 (3) | ||
| F150D | Yes | 0.38 ± 0.09 (4) | 39.3 ± 3.5 (3) | 45 (1) | 39 ± 6 (2) | 1.2 ± 1.4 (4) |
| F150D/R211G | Yes | 0.67 ± 0.04 (6) | 39.6 ± 8.2 (5) | 33.3 ± 8.4 (4) | 41.0 ± 6.4 (4) | 1.8 ± 0.4 (5) |
The existence of gH,closed was defined as consistent reversible changes in the holding current of >1 pA when pH was changed or with addition of Zn2+ or both. Absolute values of Vrev changes with pH are given for both whole-cell and inside-out patch measurements. Mutants with large gH,closed tend to have smaller and more variable Vrev changes with pH because proton leak at Vhold continually dissipates the pH gradient even if the channel is perfectly proton selective, to an extent dependent on geometry and other factors. Parameter gH(C/O) is the ratio of closed to open gH (Materials and Methods). Monovalent cation substitutions were done at pH 7//7, and measured liquid junction potentials have been corrected.
In order to evaluate the role and importance of the HG in hHV1, we mutated the putative HG residues themselves (V109, F150, and V178) as well as several nearby hydrophobic amino acids (Fig. 1). Specifically, L108 and V177 neighbor putative HG positions; I105, L147, and F182 are 1 tier below or above the HG in our model. We mutated one or more HG residues to less hydrophobic and smaller amino acids, or to the negatively charged Asp. The background was the full-length wild-type (WT) hHV1, which assembles as a dimer. Distinctive features common to the last 10 constructs in Table 1 are discussed using F150D and V109D as examples. The similar phenomenology of these mutants reinforces the idea that their behavior is a consequence of decreased hydrophobicity.
Lower Hydrophobicity at the HG Did Not Impair H+ Selectivity.
For each construct, voltage-clamp current families were collected at several pH to evaluate the general effects of the mutation. Essentially all hHV1 mutants studied were strongly proton selective (Table 1). Selectivity was assessed by determining the reversal potential, Vrev, at several pH. For most mutants in the upper half of the table, changes in pHo (in whole-cell studies) or pHi (in inside-out patch studies) shifted Vrev by ≥50 mV/unit, which indicates H+ selectivity. Imperfect pH control accounts for deviations from the Nernst potential for H+ (34, 35). We specifically examined possible Na+ permeability. Changing the predominant cation from TMA+ to Na+ shifted Vrev by only a few millivolts, within the range of liquid junction potentials, which we consider negligible. Replacing TMA+ with Na+ should shift Vrev by 17.5 mV if PH/PNa were 106 and by 2.4 mV if PH/PNa were 107, calculated with the Goldman–Hodgkin–Katz voltage equation. We cannot rule out Na+ permeation altogether; we simply cannot measure it.
Increasing the Hydrophilicity of the HG Compromises the Impermeability of the Closed Channel.
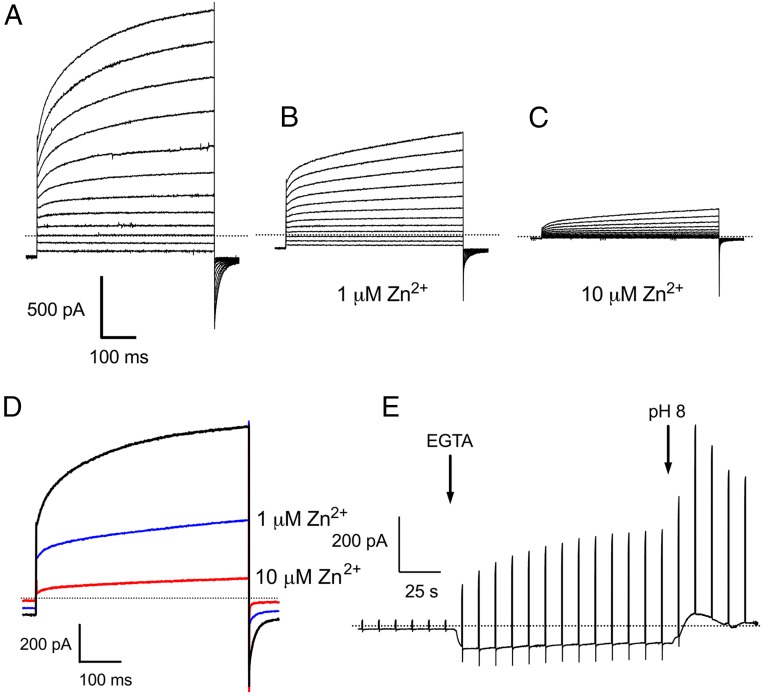
Increasing the hydrophilicity of the HG impaired the ability of hHV1 channel closure to occlude proton leak through the channel. Fig. 2 illustrates this dramatic consequence in the F150D mutant. Three families of currents in the same cell are shown, nominally at pHo 7, pHi 7. The family in Fig. 2A generally resembles WT hHV1 currents, but with some differences. Time-dependent turn-on of outward current appears at 0 mV and above (∼20 mV earlier than WT). Most remarkably, the cell appears leaky, with a substantial time-independent current at Vhold and with large instantaneous jumps at the start of each depolarizing pulse. Four kinds of evidence support the idea that this “leak” conductance (gH,closed) is carried largely or entirely by protons through a closed state of the mutant proton channel. First, it was inhibited by Zn2+. Zn2+ has no effect on leak current in cells transfected with WT hHV1 (36). Fig. 2 B and C illustrates the inhibition of both open and closed F150D channels by 1 μM Zn2+ and 10 μM Zn2+. The currents during pulses to +80 mV superimposed in Fig. 2D illustrate that the holding current at −40 mV, and the outward currents appear similarly sensitive to inhibition by Zn2+. We interpret the inward current at −40 mV as closed-channel current, because time-dependent activation of proton current was evident only at 0 mV or more positive (Fig. 2A). We interpret the instantaneous jump in outward current during depolarizing pulses as current flowing through conducting closed channels, which is followed by a slow rise in current that we attribute to channels opening in a relatively normal time-dependent manner.
Fig. 2.
Zn2+-sensitive closed-channel proton conductance in a cell expressing the F150D mutant. (A) A family of currents elicited by pulses applied from Vhold = −40 mV to −30 through +80 mV in increments of 10 mV. The dotted line indicates zero current. The nominal pH was 7.0//7.0, but Vrev was −10 mV, indicating that pHi was in fact 6.83. Families of currents at the same voltages in the same cell in the presence of 1 μM Zn2+ (B) or 10 μM Zn2+ (C) show substantial reduction of both inward and outward currents. (D) Superimposed currents at 80 mV in this cell reveal similar Zn2+ sensitivity of open and closed channels. Application of 1 μM Zn2+ or 10 μM Zn2+, respectively, reduced the inward current at −40 mV to 57% and 8%, the initial current at the start of the pulse to 80 mV to 56% and 8%, and the current at the end of the pulse to 38% and 15%. (E) The time course of changes in closed-channel current at −40 mV and open-channel current during test pulses to 40 mV applied every 10 s in the same cell as A–D. Initially, the bath contained 10 μM Zn2+. At the arrow, the bath was washed with pH 7 solution containing EGTA, producing both rapid and gradual changes. Then pH 8 solution was applied, resulting in outward current at −40 mV.
Second, the inward current at the holding potential Vhold lowered pHi, consistent with proton-selective leak current. When inward current at Vhold was increased by removing Zn2+ (Fig. 2E) or lowering pHo (Fig. 3), the test pulse current amplitude increased over several minutes as the greater proton influx lowered pHi. This phenomenon made quantitative study of the properties of mutants displaying gH,closed quite difficult, because essentially all HV1 properties depend strongly on pH. In general, constitutive gH will act to dissipate the applied pH gradient. Because we typically held the membrane at negative voltages (to close the channels), the presence of a closed-channel gH will tend to drive the Nernst potential for H+, EH, toward Vhold. At symmetrical pHo 7, pHi 7, EH is nominally 0 mV, but continuous H+ influx at Vhold will lower pHi. Indeed, in 4 cells expressing F150D, the holding current at −40 mV averaged −49 ± 10 pA (mean ± SEM) and Vrev was −10.8 ± 1.6 mV. Measured by tail currents, Vrev became more negative when Vhold was more negative, because there was a larger steady H+ influx. Fig. 2E illustrates the mutable nature of pHi in these experiments. The record begins at symmetrical pH 7 (nominal) in the presence of 10 μM Zn2+, which had greatly reduced both inward and outward currents. Application of pHo 7 solution with EGTA to this cell relieved inhibition, rapidly increasing the inward holding current at −40 mV as well as the outward current during the +40-mV test pulses. The inward current then gradually decreased, reflecting a decreasing driving force as H+ influx progressively lowered pHi, moving EH closer to Vhold. The progressive increase in outward current during the test pulses to +40 mV also results from the lower pHi, which increases both gH and the driving force (40 mV − Vrev). Changing the bath to pHo 8 not only decreased the inward current at −40 mV but produced an overshoot of outward current, demonstrating that EH was now well negative to −40 mV. The overshoot dissipated as H+ efflux increased pHi. This example shows clearly that H+ flux through closed channels changes pHi profoundly and that all numerical results presented here must be taken as approximate.
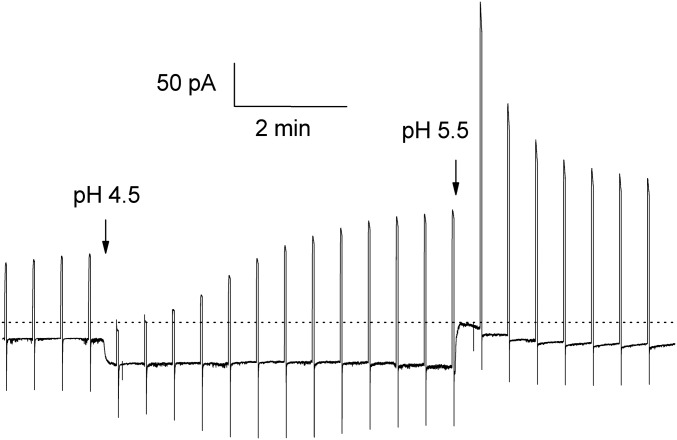
Fig. 3.
Proton influx through closed V109D channels lowers pHi. Whole-cell currents were recorded initially in symmetrical pH 5.5 solutions, with the membrane held at −60 mV and 2-s test pulses to 20 mV applied every 30 s. The arrows indicate when pHo was changed. When pHo was lowered from 5.5 to 4.5, the holding current increased immediately, reflecting proton influx through closed (C1) channels. The test pulse current at 20 mV decreased at first, reflecting the positive shift of EH (which decreases or even inverts the driving force) as well as the expected positive shift of the gH–V relationship due to the ΔpH dependence of gating (37). However, during subsequent pulses the test current increased progressively as the H+ influx that occurs continuously at Vhold lowered pHi and reversed these 2 changes, resulting in both EH and the gH–V relationship shifting negatively over the course of several minutes. Eventually, the test current at 20 mV is even larger at pHo 4.5 than it was at nominally symmetrical pH, presumably indicating that the true pHi has dropped lower than it was initially at pHo 5.5. Lowering pHi increases gH of WT HV1 channels by roughly 2-fold/unit in most whole-cell studies of voltage-gated proton channels (38). Upon return to pHo 5.5, the test current during the first pulse is much larger than it was previously in the same solution, reflecting the lower pHi. The holding current at −60 mV rapidly approaches 0 pA, showing that the true pH gradient shortly after the bath change was roughly pHo 5.5, pHi 4.5, and thus EH was near Vhold at −60 mV. Gradually, H+ directly extruded by the large outward test pulse currents and decreased H+ influx at Vhold restores pHi toward its previous value.
A third type of evidence of H+-selective closed-channel conduction is that the magnitude of the leak at Vhold varied according to pHo, as expected for changes in the permeant ion concentration. The current at Vhold increased at lower pHo and decreased at higher pHo in whole-cell studies, consistent with protons being a major charge carrier. Figs. 2E and 3 illustrate this phenomenon in F150D and V109D mutants, respectively. The manifestations in inside-out patches were inverted, and the inward current at Vhold became smaller when pHi was lowered. In cells lacking hHV1 or with WT hHV1, the leak current at Vhold did not change with pHo. When pHo was decreased in cells with V109D, closed-channel proton currents increased. Lowering pHo from 5.5 to 4.5 (with nominally pHi 5.5) increased the holding current at −40 or −60 mV by a factor 3.0 ± 0.2 in 5 cells (mean ± SEM), as illustrated in Fig. 3. However, correcting for the change in driving force decreases this difference. Evidently gH,closed increases as pHo is lowered considerably less than in proportion to the proton concentration.
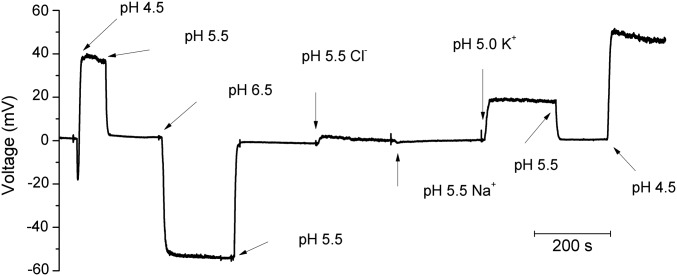
Finally, if HG mutant channels conduct protons selectively in both open and closed states, and any other conductances present are negligibly small, then the membrane should act as a pH electrode. This prediction is borne out in Fig. 4, which shows the effects of pH changes on membrane potential in a cell with V109D channels studied under current-clamp conditions. Beginning with symmetrical pH 5.5 solutions, the membrane potential was near 0 mV. Changing pHo to 4.5 resulted in a rapid 40-mV depolarization that was reversed upon return to pH 5.5. Increasing pHo to 6.5 hyperpolarized the membrane by a nearly Nernstian amount (∼58 mV/unit pH), showing that the membrane conductance is overwhelmingly proton selective. Returning to pHo 5.5 resulted in rapid depolarization to ∼0 mV. Replacing CH3SO3− with Cl− produced only a transient blip, indicating negligible anion permeability. Replacing TMA+ with Na+ or K+ had similarly minor effects. That the membrane potential of this cell approached the Nernst potential for protons (EH) and was minimally affected by other ions indicates that the conductance was proton selective. If a small fraction of channels remain open at Vrev (0 mV at symmetrical pH), the membrane potential changes most likely reflect proton conductance through a mixture of open and closed channels.
Fig. 4.
Membrane potential response to pH changes in a cell with the V109D mutant confirms proton selectivity. The initial condition was symmetrical pH 5.5 TMA+ CH3SO3− solutions, with membrane potential recorded under current clamp. At the arrows, pHo was changed, Cl− was substituted for CH3SO3−, or Na+ or K+ (at pH 5.0) replaced TMA+, as indicated. The membrane behaves as a pH electrode.
We tested the proton selectivity of closed channels by comparing the holding current in TMA+ or Na+ solutions at pH 7. Na+ sometimes produced small increases in the holding current in some mutants, but these were insensitive to Zn2+, which blocked gH,closed (Fig. 2). Furthermore, we sometimes saw similar effects of Na+ in nontransfected HEK-293 cells. We conclude that closed hHV1 channels are not detectably permeable to Na+.
Estimating the Conductance of Closed Channels.
Given that the existence of gH,closed changes pHi and consequently Vrev, accurate conductance estimates are improbable. However, in cells in which 10 to 100 μM Zn2+ was applied (e.g., Fig. 2), typically up to 90% of the holding current was eliminated. The error is thus not inordinate. Rough estimates were obtained at symmetrical pH 7.0 in 2 ways (Materials and Methods). The holding current, usually at −40 or −60 mV, was considered to reflect mainly gH,closed. The time-independent (or weakly time-dependent) currents at negative voltages reversed at very nearly the same voltage as the time-dependent “normal” gH based on traditional tail current measurements, consistent with both being largely or entirely proton selective. In mutants with large gH,closed, there was an instantaneous jump upon depolarization, which we ascribe to closed-channel conductance, followed by a slower time-dependent increase in current, which we attribute to the normal opening process. We assume that the time course reflects the increasing proportion of open channels, and the final current reflects the fully open-channel conductance.
The amplitude of gH,closed relative to the gH of open channels is given in Table 1 as gH(C/O). Replacing individual HG residues with Ala produced detectable gH,closed only for V178A. However, all 3 double Ala mutants (V109A/F150A, V109A/V178A, and F150A/V178A) leaked protons when closed, and the triple Ala mutant had a large leak with gH,closed one-third of the open conductance. Each HG residue replaced by Asp produced distinct gH,closed.
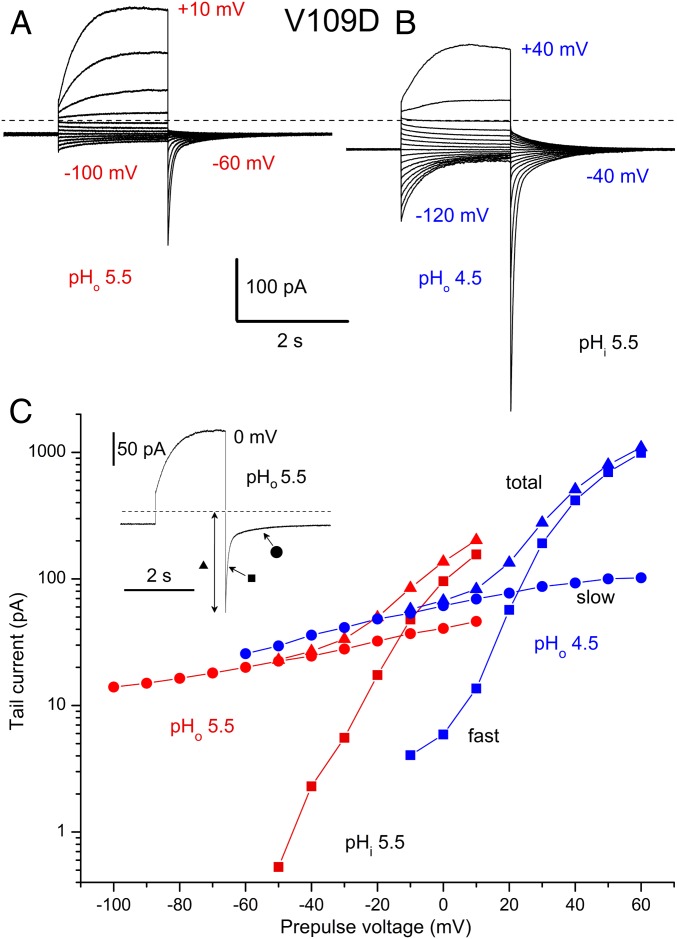
A Deeper Closed State Revealed in V109D.
Fig. 5A shows a typical family of V109D currents at symmetrical pH 5.5//5.5. From Vhold = −60 mV pulses to more negative voltages elicited slowly decaying inward currents. A superficial interpretation might be that these are “tail currents” due to a population of channels open at −60 mV that close at more negative voltages. Consistent with this idea, repolarization to −60 mV results in slowly activating inward currents, which are more obvious in Fig. 5B. Above about –30 mV, a proton conductance with more normal appearance begins to turn on. Its activation is faster than WT hHV1, and the gH appears to activate at somewhat more negative voltages. For reasons discussed above, we suspect that the apparently closed channels still conducted proton current, even at −60 mV. One consequence is that Vrev determined by tail currents in this cell was −28 mV. The continuous proton influx at Vhold lowered pHi well below the nominal pH 5.5 of the pipette solution to ∼5.02.
Fig. 5.
Tail current kinetics in a cell with the V109D mutant reveals 2 steps in gating. (A) A family of currents elicited by pulses applied from Vhold = −60 mV to −100 through 10 mV in increments of 10 mV. The dashed line indicates zero current. Nominal pH was 5.5//5.5, but Vrev was −28 mV, indicating pHi was 5.0. (B) A family of currents in the same cell at pHo 4.5 during pulses from Vhold = −40 mV to −120 through 40 mV in 10-mV increments. Because Vrev was +21 mV, pHi was 4.86. Note slow tail currents after pulses negative to Vhold (τslow ∼ 700 ms) and that a fast tail current component appears after repolarization from voltages that activate the normal time-dependent gH (τfast ∼ 20 ms at pHo 5.5 and 70 ms at pHo 4.5). (C) Tail current amplitudes from these measurements. Inset identifies fast (■), slow (●), and total (▲) tail amplitudes. Slow and total are absolute “instantaneous” currents, and fast component is relative. Tails were monoexponential for negative prepulse voltages but required a double exponential for more positive prepulses.
Lowering pHo shifts the gH–V relationship of all known proton channels by ∼40 mV to more positive voltages (4, 37, 38). We therefore lowered pHo to 4.5 (Fig. 5B), to ensure that all of the channels at Vhold were closed. The well-spaced currents at positive voltages now appear to activate about 40 mV more positively, with increased spacing above +10 mV, but the inward (proton-selective) holding current at −40 mV was increased, and the decaying inward currents during pulses to more negative voltages were more pronounced. Both from time-dependent relaxation of tail currents and the reversal of total current, Vrev was +21 mV, indicating that pHi was now 4.86. Thus, Vrev shifted 49 mV when pHo was lowered from 5.5 to 4.5, and ΔpH shifted by 0.84 units. The Vrev shift was thus 58.3 mV/unit (49 mV/0.84 units) indicating perfect proton selectivity. Examination of the tail currents seen upon repolarization at both pHo reveal that, following pulses in the negative voltage range, the currents exhibit a monoexponential time course. Following depolarizing pulses that activate normal time-dependent outward currents, the tail currents are distinctly double exponential. The amplitudes of each kinetic component as well as their sum are plotted in Fig. 5C. The fast tail currents are associated with activation of the normal gH and are steeply voltage dependent. The slow tail currents evidently reflect a slow gating process between closed but conducting channels and a deeper closed state that appears not to conduct detectably.
The behavior of V109D in Fig. 5 suggests the following gating transitions:
| [1] |
The Closed2 (C2) state does not conduct or conducts negligibly. Closed1 (C1) and Open (O) both conduct protons but O has a higher conductance. The transition between C2 and C1 has very weak voltage dependence. The C1→O transition has strong voltage dependence that presumably reflects movement of most of the gating charge.
The experiment in SI Appendix, Fig. S2 provides additional evidence that C2↔C1 transitions are kinetically distinct from the normal opening transition. Activation kinetics and amplitude depend on prepulse voltage. Similar behavior was observed in many cells expressing V109D, both at pH 7 and at pH 5.5. According to [1], the most negative prepulses produce C2→C1→O transitions, but more positive prepulses result in mostly C1→O transitions. SI Appendix, Fig. S2 confirms that the slow decay of current at large negative voltages reflects transitions unrelated to the C1↔O transition, presumably C1→C2.
Decreasing Hydrophobicity Outside the HG Does Not Impair Selectivity or Induce gH,closed.
Table 1 indicates whether each mutant exhibited closed-channel gH, and its magnitude relative to the open-channel gH, gH(C/O). Mutations expected to decrease the hydrophobicity at positions I105, L108, L147, F150, V177, V178, or F182 did not detectably impair H+ selectivity. Of the positions tested, the only mutations that exhibited closed-state conductance were of the 3 predicted HG residues, V109, F150, and V178, as well as V177. Because Val178 is the central of 5 consecutive valines, and Val177 obliquely faces the pore, we suspect that V177 and V178 act interchangeably. Convincing gH,closed was not observed for another HG neighbor (L108T) or for positions 1 helical turn above (F182A) or below the HG (I105G, I105D, L147D).
Is Phe182 Rather than Val178 Part of the HG?
Homology models identify Val178 as part of the HG (Fig. 1), but in the crystal structure of mHV1, this position appears to be occupied by Phe178 (Phe182 in hHV1) (29). If the crystal structure is closer to reality than the homology models, Phe182 mutants might be expected to share the properties of mutations at the other 2 elements of the HG. Unfortunately, F182D mutants did not produce detectable currents, despite the transfected cells being green, indicating likely membrane expression. The lack of current in F182D may indicate that it does not face the pore in hHV1, consistent with the homology model but not the crystal structure (Fig. 1). We then tried F182A, because a neutral substituent might be less disruptive than introducing a charge. Currents were detected and were proton selective, but convincing gH,closed was not detected (Table 1). In contrast, V178A, V178D, and all double or triple Ala mutants that included Val178 exhibited gH,closed. That V177D also exhibits gH,closed further supports the role of its neighbor Val178 in the HG, because both are at a similar “height” in the membrane. Combined, these results strongly support the idea that Val178 (in concert with Val177) and not Phe182 completes the HG in hHV1.
The Double Mutant F150D/R211G.
Our open-channel model of hHV1 exhibited frequent interaction between F150 and R211 in the HG region, at the peak energy barrier for cation permeation (33). We therefore tested a double mutant, F150D/R211G, but it retained high proton selectivity (Table 1). Like F150D alone, this double mutant exhibited gH,closed.
Increasing the Hydrophilicity of the HG Region Facilitates Channel Opening.
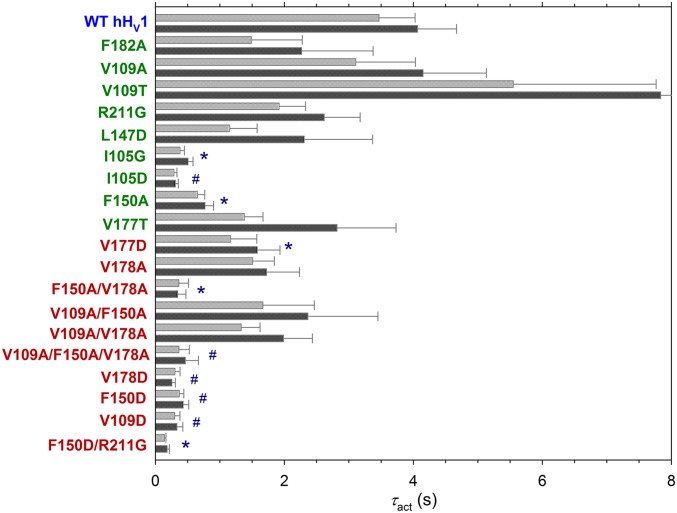
Fig. 6 illustrates that most of the mutations intended to make the HG less hydrophobic accelerated channel opening. Mutation to Ala significantly accelerated activation in F150A, but mutations to Asp had greater impact. Each single Asp (V109D, F150D, V178D) mutant opened substantially faster than WT. The ubiquity of faster activation suggests that, in WT channels, the HG acts to retard channel opening. The speeding of activation and induction of gH,closed appear to be independent and mechanistically unrelated consequences of HG mutation. There was no correlation between the magnitude of gH,closed in each mutant and the speeding of τact (R2 is 0.19 and 0.17 for linear regression on +40- or +60-mV data, respectively). Notably, some mutations (e.g., I105G, I105D, and F150A) profoundly accelerated activation, but did not produce gH,closed. Conversely, V178A, V109A/F150A, and V109A/V178A exhibited gH,closed without a significant decrease in τact.
Fig. 6.
Many HG mutants have faster activation kinetics. Mutants without detectable gH,closed are labeled in green, and those with gH,closed are red. Time constants of activation (τact) were determined by single- or occasionally by double-exponential fits to increasing currents. Plotted are mean ± SEM τact at 60 mV (light gray) and 40 mV (dark gray) in each mutant. When 2 exponentials were required to fit the currents reasonably, the slower one is plotted here. Significant differences from τact in WT hHV1 indicated by *P < 0.05, #P < 0.01 by Student’s t test. SI Appendix, Table S2 gives statistical details of these studies. An error bar for V109T is truncated.
Further evidence that reducing hydrophobicity of the HG region promotes channel opening is a substantial negative shift of the gH–V relationship in I105A and I105D (SI Appendix, Fig. S3). On average, the voltage at which the gH was 10% maximal (39) shifted from 14.1 ± 3.4 mV (mean ± SEM) in 7 cells transfected with WT hHV1 to −13.3 ± 3.6 mV (n = 7) in I105G mutants and −39.0 ± 8.3 mV (n = 5) in I105D (P < 0.0001 for both). We did not attempt to quantify gH–V data for other mutants, because the additional time- and voltage-dependent gH,closed process [1] made unambiguous interpretation difficult. Other constructs did not appear as obviously shifted as Ile105 mutants, however. That Ile105 mutation produced a large negative shift without producing gH,closed further indicates that the 2 key functions of the HG, namely preventing proton influx through closed channels and retarding channel opening, are mechanistically independent.
The HG Prevents the Guanidinium of Arg208 from Moving Above Residue 150 in the Closed State.
To investigate the molecular basis for closed-state proton leakiness in these HG mutants, extensive simulations totaling 6.5 µs were performed using the spectroscopically and biochemically constructed model of the resting state of hHV1 by Li et al. (27). The effect of these mutations on the size and nature of the HG was analyzed by computing the average hydration along the channel (SI Appendix, Fig. S4). Increased pore hydration relative to WT was observed in the intracellular vestibule (−0.9 < z < −0.5 nm) in single aspartate mutants; however, little to no change was seen in leaky alanine mutants. Therefore, we conclude that H+ leakiness in the closed state is not due to increased hydration of the HG.
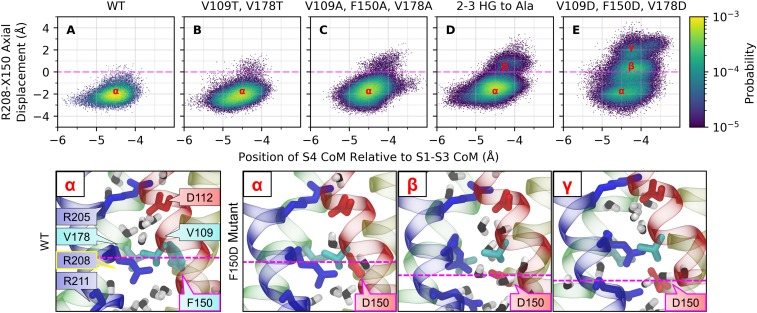
We then monitored the position of the guanidinium group of Arg208 relative to the side chain of residue 150 versus the position of the S4 helix center of mass relative to the rest of the channel (S1 to S3; Fig. 7 and SI Appendix, Fig. S5). Consistent with the gating charge distribution of the closed state, the guanidinium group remained below Phe150 most of the time in the systems considered. However, transient excursions (∼2 to 3 Å) of the guanidinium group of Arg208 above residue 150 were observed in single threonine mutants (Fig. 7B). These excursions became more likely in single alanine mutants (Fig. 7C). In double- and triple-alanine HG mutants, 2 distinct metastable conformational states were sampled in which the guanidinium group of Arg208 is positioned either ∼1 Å above or ∼1 to 2 Å below residue 150 (Fig. 7D). Finally, a third conformation was populated in single aspartate mutants with the side chain of Arg208 positioned 2 Å above Phe150 (Fig. 7E). At most, the upward shift of the Arg208 side chain is correlated to an 0.5- to 1-Å upward shift of the S4 helix, falling well short of the >4-Å S4 translation to the activated state in other homologous voltage sensing domains (26, 40). In our open-state model, Arg208 faces Asp112 (33). Importantly, dynamic fluctuations of the guanidinium group of Arg208 past residue 150 were significant and metastable in all leaky mutants but were transient and occurred very rarely in the WT channel (Fig. 7A) or in nonleaky mutants (Fig. 7 B and C).
Fig. 7.
Conformational fluctuations of WT and HG mutants of the resting state of hHV1 from MD simulations. The axial position of the guanidinium group of Arg208 relative to the side chain of residue 150 is shown versus the axial position of the center of mass of the S4 helix relative to the center of mass (CoM) of the rest of the channel (helices S1 to S3). (A) WT hHV1 samples a single basin (α), with the position of the guanidinium group remaining below residue 150 (dashed line). (B) Rare and transient excursions of the guanidinium group above residue 150 are observed in the (nonleaky) threonine HG mutants. (C) These excursions become more likely in single alanine mutants, including leaky V178A, and (D) populate a distinct conformational state (β) in double- and triple-alanine mutants. (E) Finally, aspartate mutants sample 3 distinct basins, 2 of which are located at or above residue 150 (β and γ). Representative snapshots of the single basin in WT (α) and the 3 basins (α, β, γ) in F150D are shown below the distributions. S1 (red), S2 (yellow), S3 (green), and S4 (blue) helices are shown as ribbons with side chains labeled and colored based on their residue type: hydrophobic (cyan), acidic (red), and basic (blue), with pore-associated water molecules (black). Individual distributions are included in SI Appendix, Fig. S5.
Taken together, our simulation results suggest that the HG acts as a hydrophobic and steric barrier that prevents the Arg208 guanidinium from slipping above the gating charge transfer center in the resting state.
Discussion
The HG Retards Channel Opening in WT hHV1.
Many of the HG mutants studied activated about an order of magnitude faster than WT hHV1 (Fig. 6). This result is consistent with the report that, in V109A, F150A, and V178A, the gH–V relationship is shifted negatively by 10 to 27 mV (41); and the present observation that activation of I105A and especially I105D are shifted dramatically toward negative values. Together, these findings indicate that the HG in WT channels impedes channel opening. Mutations in the HG region did not affect activation kinetics equally. Although most Asp mutations (I105D, V109D, F150D, V177D, and V178D) strongly accelerated opening, among the single neutral mutants only F150A (42) and I105G did. There was no correlation between the speeding of activation and the magnitude of gH,closed, which shows that these consequences have different mechanisms. The proximity of Ile105 to the HG is evidently sufficient that its mutation speeds activation, but without producing gH,closed. We conclude that activation kinetics is influenced by the overall hydrophobicity of the region, whereas inducing gH,closed requires decreased hydrophobicity specifically in the locations occupied by Val109, Phe150, Val177, and Val178. Surprisingly, the HG in the Shaker K+ channel VSD appears to have the opposite effect on gating. Replacing Ile237 (which corresponds with Val109 in hHV1) with less hydrophobic amino acids promoted the closed state, shifting g–V relationships positively (23).
Teleologically, that the HG resists channel opening may serve to ensure that hHV1 opens at appropriate voltages. The ΔpH dependence of its gating means that, under all conditions, hHV1 is poised to open just above EH, when there is an outward electrochemical gradient for protons. Opening at more negative voltages would allow proton influx, which in most situations in most cells is deleterious.
Reducing the Hydrophobicity of the HG Produces Closed-State Proton Conduction.
The most dramatic and consistent effect of reducing the hydrophobicity of HG residues was to produce a closed-channel proton-selective leak conductance. The conductance of closed channels, gH,closed, was smaller than that of the open channel, gH, but substantial, and dependent on many factors, especially pHo. The magnitude of gH,closed increased with the number of substitutions to the HG. The single-mutant V178A had distinct but tiny gH,closed averaging only 1.7% of the open gH. All 3 double Ala mutants produced moderate gH,closed 3 to 16% of gH (Table 1). The triple-mutant V109A/F150A/V178A produced robust gH,closed (35% of gH) comparable to that seen in V109D or F150D. We conclude that the HG in HV1 ensures that the closed channel is fully occluded—that no ions, not even protons, are allowed into the cell.
The HG mutants still opened and closed in a voltage- and time-dependent manner, but in a voltage range negative to that producing normal time-dependent gating, closed channels allowed continuous proton influx. It might be argued that these mutations disrupt the integrity of the protein or disturb gating, and that consequently the proton leak is not very meaningful, simply indicating a broken channel. Three observations argue against this interpretation. First, these mutants still exhibited voltage- and time-dependent gating, suggesting that the gating mechanism was not grossly altered. Second, the strong proton selectivity of the closed-channel leak conductance suggests that the selectivity filter remained intact. Proton-selective conduction occurs only under the specific conditions of an appropriately juxtaposed Asp in S1 and Arg in S4 (43–45), and thus requires integrity of the selectivity filter of mutant proteins. Finally, potent Zn2+ inhibition of depolarization-activated H+ current suggests that the Zn2+ binding site remained largely intact. In WT hHV1 Zn2+ is coordinated tetrahedrally by His140, His193, Asp123, and Glu119 (residues residing on S1, S2, and the S3–S4 linker) based on mutation studies (12, 36), IR spectroscopy (46), and on the X-ray structure of the mHV1 crystal, which included a Zn2+ atom at its external binding site (29). That Zn2+ still potently inhibits both closed- and open-channel currents supports the idea that the HG mutants were globally functionally intact.
Which Amino Acids Comprise the HG?
Homology models identify Val178 as part of the HG (Fig. 1), analogous to Ile190 in CiVSP or Ile320 in Shaker (28, 47). However, in the crystal structure of mHV1, this position appears to be occupied by Phe178 (Phe182 in hHV1), in a region called the inner hydrophobic layer (29). A subsequent EPR study of hHV1 concluded that in the crystal structure of mHV1, replacing a 25-amino acid region of S2 through S3 with amino acids spliced from CiVSP resulted in a register shift of 1 turn of the helix up for S2 helix and down for S3 (27). When the crystal structure is “corrected” for these shifts, Val178 aligns with Phe150 and Val109 to complete the HG (27), as shown in Fig. 1B. Given this ambiguity, we mutated Phe182 in hHV1. If the crystal structure is closer to reality than the homology models, Phe182 mutants might be expected to share the manifestations of mutations to the other 2 HG elements. The F182D mutant did not produce detectable currents, despite the transfected cells being green, indicating likely membrane expression. In a previous study, we introduced Asp at each of 11 contiguous locations along the S1 transmembrane helix and observed current only with Asp at the 3 positions judged to face the pore in our homology model (43). Although other explanations are possible, the lack of current in F182D may indicate that it does not face the pore in hHV1, consistent with homology model predictions (27). The less obtrusive F182A mutant generated proton-selective currents but exhibited no gH,closed. We conclude that Val178 and not Phe182 is part of the HG.
To further define the location of the HG, we mutated hydrophobic residues that face the pore in the model and reside 1 helical turn above (Leu147, Phe182) or below (Ile105) the putative HG. All remained proton selective and none exhibited detectable gH,closed. It is surprising that L147T or L147D did not, because at the corresponding Shaker position, I287H does leak protons (22), but position 147 in S2 of hHV1 is at the level of Asp112, the selectivity filter (30), which has no parallel in the Shaker VSD. We mutated Val177, which neighbors Val178, the central member of a string of 5 valines. In a closed hHV1 model (27), Val178 faces the pore directly, but with a small rotation Val177 would face the pore. V177D produced a distinct gH,closed with faster activation. Evidently decreasing the hydrophobicity of either Val178 or Val177 compromises the closed channel. Taken together, these results support the identification of the HG in hHV1 as Val109, Phe150, Val177, and Val178.
What Is the Proton-Selective Pathway through Closed HG Mutant Channels?
One might imagine that decreased hydrophobicity in the HG region allows a more continuous water wire or, in the case of Asp mutants (V109D, F150D, V177D, and V178D), may directly create a proton transfer site. MD simulations in the closed-channel model show little change in hydration (SI Appendix, Fig. S4) and also indicate that the most hydrophobic region of the pore and the peak of the energetic barrier to cation permeation in the open state are at the level of the HG (33, 43, 45) (SI Appendix, Fig. S1). This led to the hypothesis that the HG might contribute to the exquisite proton selectivity of HV1, but this prediction was not borne out, as the HG mutants retained H+ selectivity.
Proton translocation in protein interiors is thought to occur by means of a Grotthuss relay mechanism along hydrogen-bonded chains of water molecules and titratable groups (35, 38, 48, 49). The involvement of water in HV1 permeation is ambiguous (50–52). The closed mHV1 crystal structure (29) and a closed CiHV1 channel model (53) contain 2 hydrophobic regions. However, open-channel models also exhibit hydrophobic regions (33, 45) that do not obviously differ in presumed closed states (50) or in Asp112 mutants that conduct protons, anions, or nothing (43). The present results demonstrate that the HG region alone is sufficient to preclude H+ conduction in closed hHV1 channels, and furthermore, that other bottlenecks are not.
The fact that closed hHV1 channels conduct protons if the HG is made less hydrophobic, even by single point mutations, is consistent with the view that the physical barrier separating protons from one side of the closed channel to the other is relatively thin. Also consistent with this view is the observation that the R205H mutant of hHV1 can conduct protons at negative voltages where the channel is presumably closed (25). A crucial property of the HG in other channels is that most of the membrane potential drops across this relatively short distance (∼4 to 10 Å) (17–21, 54–56). Consequently, charged groups that move through the HG essentially transfer their charge from intracellular to extracellular while moving across only a fraction of the membrane thickness. Steep voltage dependence can thus be achieved with limited physical movement, illustrating the parsimony of natural selection. Like other VSDs, HV1 has a focused electric field that enables gating with minimal motion of the protein.
It is clear that a conserved Asp in the middle of the S1 transmembrane helix is crucial for the H+ selectivity of HV1, because mutations that neutralize Asp result in anion permeability in 3 species (3, 30, 57). In most open-state homology models, Asp interacts almost continuously with one or more Arg in S4 (33, 43, 45, 53, 58), while quantum mechanical calculations on a reduced model of the HV1 selectivity filter showed that Asp–Arg interaction can explain both proton permeation and proton selectivity (44). That gH,closed is proton selective supports the idea that the sole selectivity filter, comprising Asp112 in S1 interacting with one or more Arg from S4, remains intact in the (C1) closed state of hHV1.
H+ Leakiness in HG Mutants Is Due to a More Open-Like Conformation in the Resting State.
Consistent with the above considerations on the nature and the thickness of the barrier opposing proton permeation in the resting state of the channel, the HG mutants in which the guanidinium group of Arg208 was most likely to slip past residue 150 correspond to the experimentally determined H+ leaky mutants (Fig. 7 and SI Appendix, Fig. S5). In this compromised conformational state, the channel topologically resembles the activated state in that Arg208 faces the extracellular vestibule of the pore, with a gating charge distribution intermediate between that of the closed state, in which Arg208 sits below Phe150 in the intracellular vestibule, and that of the open state, in which Arg208 is near Asp112 in the extracellular vestibule. These results suggest that H+ leakiness in HG mutants is a result of the inability of the gasket to prevent the guanidinium group of Arg208 from crossing the charge transfer center in a process where reduced steric interactions and new charge–charge interactions compromise the integrity of the HG.
Why Does Zn2+ Inhibit Closed-Channel H+ Current?
The bulk of evidence supports the idea that Zn2+ inhibits WT hHV1 currents by binding to the closed WT hHV1 channel and preventing opening (59–61). In a recent proposal, protons permeate hHV1 by binding consecutively to 3 sites, each with 2 acidic groups; the internal site includes E153 and D174, the central site D112 and D185, and the outer site E119 and D123 (62). Although the amino acids most critical for Zn2+ binding to mammalian HV1 are His140 and His193 (12, 29, 36), the crystal structure of the closed mHV1 also implicates E119 and D123 in Zn2+ coordination (29). Thus, both acids comprising the external proton binding site (62) would be busy binding Zn2+, and unavailable to shuttle protons out of the pore. Because E119 and D123 are both on S1, their relative positions would likely be uninfluenced by opening or closing of the channel. Although decreased hydrophobicity of HG mutants allows proton permeation through the HG constriction in closed (C1) channels, the proton might still need the outer pair of acids to complete its journey. Despite the attractiveness of this mechanism, we cannot rule out an alternative possibility that Zn2+ inhibits closed-channel H+ current by the simple fact of binding in the outer vestibule and occluding the proton pathway by electrostatic repulsion and steric obstruction.
Implications for Gating Mechanisms.
We present strong evidence for at least 2 distinct closed states, consistent with many previous studies proposing multiple gating states (31, 37, 42, 63–67). That the shallow C1 closed state conducts protons in HG mutants uniquely enables distinguishing this state electrophysiologically. Because a variety of mutations produced similar phenomenology, it appears that the gating mechanism itself was not grossly altered; hence, the C1 state likely exists in WT channels. At voltages negative to the normal gating process, larger hyperpolarization produced a slow tail current indicating a second closing step (Fig. 5). The voltage dependence of this (C1↔C2) gating process is extremely weak. Similarly, Cherny et al. (37) observed a steeply voltage-dependent closing process in rat HV1 near threshold voltages, with a weakly voltage-dependent component at more negative voltages. Several previous studies have concluded that most of the gating charge moves between closed states, prior to the opening transition (25, 65–67). The weak voltage dependence of C2↔C1 transitions and the steep voltage dependence of the C1↔O step (Fig. 5 and SI Appendix, Fig. S2) observed here appear to contradict this conclusion, although the C1↔O step may encompass several substates of a more complete model. An intriguing possibility is that the deep closed-state C2 occurs when extreme hyperpolarization traps Arg208 below the HG where it resides in WT hHV1 (Fig. 7A).
Many if not most gated ion channels contain a hydrophobic gate that undergoes sharp wetting and dewetting transitions resulting from conformational changes upon opening and closing, respectively (53, 68–71). Whether or not the HG functions as a hydrophobic gate in HV1 is unclear. First, hydrophobic gates tend to extend over distances of 1 to 1.5 nm, longer than the length of the HG. Second, we did not find systematic differences in hydration between HG mutants exhibiting proton permeation or not (SI Appendix, Fig. S4). Previously, we found hydration to be indistinguishable in H+-selective, anion-selective, and nonconducting hHV1 mutants (43). It is possible that the HG widens upon opening (53) or that open and closed states differ in side-chain orientations (27). In light of the ΔpH dependence of HV1 activation (37), protonation of internal residues at low pHi or deprotonation of external residues at high pHo might alter the channel conformation in a way that permits proton permeation. Answering these questions will require detailed comparisons of open and closed states of the channel.
Implications for Genetic Disease.
Intriguingly, episodic ataxia in the human KV1.1 channel is associated with mutations at positions corresponding to Val109 (72) and Val178 (73) in hHV1. We predict that an individual with mutations to HG residues in hHV1 might exhibit closed-channel proton current. We and others (22, 25) have noticed that mutations that produce constitutive proton leak seem to decrease the vitality and longevity of cells that express these channels. Specific mutations to the VSD of other voltage-gated ion channels produce “omega currents” or “gating pore currents” in which the VSD becomes permeable to protons (74) or simply to cations in general. These gating pore currents are associated with a variety of hereditary diseases that mostly afflict excitable cells, nerve and muscle (75). The COSMIC database (76) has an entry for an F150C mutation in the HVCN1 gene from a 70-y-old male with bladder carcinoma, raising intriguing possibilities for future study.
Materials and Methods
MD Simulations.
MD methods are provided in SI Appendix.
Gene Expression.
Site-directed mutants were created using the Stratagene QuikChange (Agilent) procedure according to the manufacturer’s instructions. Transfection into HEK-293 cells was done as described (33). The following mutants were produced by GenScript: L147D, F150A, F150D, R211G, F150D/R211G, V109A/F150A/V178A, and V109A/V178A. No other voltage- or time-dependent conductances were observed under the conditions of this study. Most mutations were introduced into the WT background. In a few cases, mutations were introduced into a Zn2+-insensitive background (H140A/H193A), which we have used previously to distinguish the mutant channels from endogenous HV1 (30). In most cases, the level of expression of all mutants studied here was sufficiently high that contamination by native HV1 was negligible.
Electrophysiology.
In most experiments, cells expressing GFP-tagged proton channels were identified using Nikon inverted microscopes with fluorescence capability. For constructs that lacked the GFP tag, GFP was cotransfected. Conventional patch-clamp techniques were used (33) at room temperature (20 to 26 °C). Bath and pipette solutions contained 60 to 100 mM buffer, 1 to 2 mM CaCl2 or MgCl2 (intracellular solutions were Ca2+-free), 1 to 2 mM EGTA, and TMAMeSO3 to adjust the osmolality to ∼300 mOsm, titrated with TMAOH. Buffers used were Homopipes at pH 4.5 to 5.0, Mes at pH 5.5 to 6.0, BisTris at pH 6.5, Pipes at pH 7.0, Hepes at pH 7.5, and Tricine at pH 8.0. Currents are shown without leak correction. To minimize pHi changes due to large H+ fluxes, pulses for large depolarizations in pulse families were sometimes shortened. Reversal potentials (Vrev) were determined by 2 methods, as described previously (77).
Closed-Channel Conduction.
Proton current amplitude (IH) was usually determined by fitting the rising current with a single exponential and extrapolating to infinite time. Proton conductance (gH) was calculated from IH and Vrev measured in each solution, which was often well negative to the nominal EH: gH = IH/(V − Vrev). The open-state gH was calculated from the largest outward current measured. In some cases where current activation kinetics was difficult to evaluate, gH was calculated from tail current amplitudes instead of IH. Closed-channel gH (gH,closed) was calculated in 2 different ways, depending on its amplitude. In mutants in which gH,closed was small, it was calculated from the holding current, usually at −40 mV, and Vrev measured under the same conditions, ignoring any non-H+ leak. For this purpose, Vrev was simply the zero current potential, which was typically similar or identical to that determined using tail currents. If both open and closed conductances are proton selective, their Vrev should be the same. There were no instances in which these values were convincingly different, except in leaky cells. Cells with large leak currents were excluded from analysis. Without an objective way to estimate genuine leak, it is likely that gH,closed is systematically overestimated. In cells with large gH,closed, we estimated its relative amplitude (gH,closed/gH) from the difference between the initial and final current during a large depolarizing pulse (It = 0/It=∞), again ignoring leak. This value does not require knowledge of Vrev and also is not affected by intrinsic rectification of the gH,closed current–voltage relationship. Because this method does not correct for leak it will tend to overestimate the ratio. Because Zn2+ inhibited most of the holding current, we image the error is not inordinate. It should be emphasized that these estimates are rather arbitrary and will depend on Vhold and pH, as well as pipette geometry, cell volume, nonproton leak current, the completeness of equilibration, and the assumptions that all channels at Vhold are in Closed1 (not Closed2) and all proceed to Open during a large depolarization [1].
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant GM102336 (to T.E.D. and S.M.E.S.), National Institutes of Health Grants GM121462 and GM126902 (to T.E.D.), National Science Foundation Grant MCB-0943362 (to T.E.D. and S.M.E.S.), Bears Care (D.M.), Deutsche Forschungsgemeinschaft Grant MU 3574/4-1 (to B.M.), and Canadian Institutes of Health Research Grant MOP-130461 (to R.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors appreciate detailed criticism by Valerij Sokolov, and thank Qufei (Francis) Li and Eduardo Perozo for providing structural information.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1905462116/-/DCSupplemental.
References
- 1.Taylor A. R., Chrachri A., Wheeler G., Goddard H., Brownlee C., A voltage-gated H+ channel underlying pH homeostasis in calcifying coccolithophores. PLoS Biol. 9, e1001085 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez J. D., et al. , Identification of a vacuolar proton channel that triggers the bioluminescent flash in dinoflagellates. PLoS One 12, e0171594 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith S. M. E., et al. , Voltage-gated proton channel in a dinoflagellate. Proc. Natl. Acad. Sci. U.S.A. 108, 18162–18167 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeCoursey T. E., Voltage-gated proton channels: Molecular biology, physiology, and pathophysiology of the HV family. Physiol. Rev. 93, 599–652 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeCoursey T. E., Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes. Physiology (Bethesda) 25, 27–40 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeCoursey T. E., Morgan D., Cherny V. V., The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature 422, 531–534 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Henderson L. M., Chappell J. B., Jones O. T. G., The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 246, 325–329 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan D., et al. , Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis. Proc. Natl. Acad. Sci. U.S.A. 106, 18022–18027 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsey I. S., Ruchti E., Kaczmarek J. S., Clapham D. E., Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc. Natl. Acad. Sci. U.S.A. 106, 7642–7647 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Chemaly A., et al. , VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification. J. Exp. Med. 207, 129–139 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lishko P. V., Botchkina I. L., Fedorenko A., Kirichok Y., Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140, 327–337 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Ramsey I. S., Moran M. M., Chong J. A., Clapham D. E., A voltage-gated proton-selective channel lacking the pore domain. Nature 440, 1213–1216 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki M., Takagi M., Okamura Y., A voltage sensor-domain protein is a voltage-gated proton channel. Science 312, 589–592 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Papp F., et al. , TMEM266 is a functional voltage sensor regulated by extracellular Zn2+. eLife 8, e42372 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S. Y., Letts J. A., MacKinnon R., Functional reconstitution of purified human Hv1 H+ channels. J. Mol. Biol. 387, 1055–1060 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang N., Horn R., Evidence for voltage-dependent S4 movement in sodium channels. Neuron 15, 213–218 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Yang N., George A. L. Jr, Horn R., Molecular basis of charge movement in voltage-gated sodium channels. Neuron 16, 113–122 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Larsson H. P., Baker O. S., Dhillon D. S., Isacoff E. Y., Transmembrane movement of the Shaker K+ channel S4. Neuron 16, 387–397 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Starace D. M., Stefani E., Bezanilla F., Voltage-dependent proton transport by the voltage sensor of the Shaker K+ channel. Neuron 19, 1319–1327 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Starace D. M., Bezanilla F., Histidine scanning mutagenesis of basic residues of the S4 segment of the Shaker K+ channel. J. Gen. Physiol. 117, 469–490 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starace D. M., Bezanilla F., A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature 427, 548–553 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Campos F. V., Chanda B., Roux B., Bezanilla F., Two atomic constraints unambiguously position the S4 segment relative to S1 and S2 segments in the closed state of Shaker K channel. Proc. Natl. Acad. Sci. U.S.A. 104, 7904–7909 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacroix J. J., Hyde H. C., Campos F. V., Bezanilla F., Moving gating charges through the gating pore in a Kv channel voltage sensor. Proc. Natl. Acad. Sci. U.S.A. 111, E1950–E1959 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao X., Lee A., Limapichat W., Dougherty D. A., MacKinnon R., A gating charge transfer center in voltage sensors. Science 328, 67–73 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randolph A. L., Mokrab Y., Bennett A. L., Sansom M. S., Ramsey I. S., Proton currents constrain structural models of voltage sensor activation. eLife 5, e18017 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q., et al. , Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain. Nat. Struct. Mol. Biol. 21, 244–252 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q., et al. , Resting state of the human proton channel dimer in a lipid bilayer. Proc. Natl. Acad. Sci. U.S.A. 112, E5926–E5935 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeCoursey T. E., Structural revelations of the human proton channel. Proc. Natl. Acad. Sci. U.S.A. 112, 13430–13431 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeshita K., et al. , X-ray crystal structure of voltage-gated proton channel. Nat. Struct. Mol. Biol. 21, 352–357 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Musset B., et al. , Aspartate 112 is the selectivity filter of the human voltage-gated proton channel. Nature 480, 273–277 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez C., Rebolledo S., Perez M. E., Larsson H. P., Molecular mechanism of voltage sensing in voltage-gated proton channels. J. Gen. Physiol. 141, 275–285 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettersen E. F., et al. , UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Kulleperuma K., et al. , Construction and validation of a homology model of the human voltage-gated proton channel hHV1. J. Gen. Physiol. 141, 445–465 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeCoursey T. E., Hydrogen ion currents in rat alveolar epithelial cells. Biophys. J. 60, 1243–1253 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeCoursey T. E., Hosler J., Philosophy of voltage-gated proton channels. J. R. Soc. Interface 11, 20130799 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musset B., et al. , Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. J. Physiol. 588, 1435–1449 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cherny V. V., Markin V. S., DeCoursey T. E., The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J. Gen. Physiol. 105, 861–896 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeCoursey T. E., Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83, 475–579 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Cherny V. V., et al. , Tryptophan 207 is crucial to the unique properties of the human voltage-gated proton channel, hHV1. J. Gen. Physiol. 146, 343–356 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nekouzadeh A., Rudy Y., Conformational changes of an ion-channel during gating and emerging electrophysiologic properties: Application of a computational approach to cardiac Kv7.1. Prog. Biophys. Mol. Biol. 120, 18–27 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong L., Kim I. H., Tombola F., Molecular determinants of Hv1 proton channel inhibition by guanidine derivatives. Proc. Natl. Acad. Sci. U.S.A. 111, 9971–9976 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong L., Pathak M. M., Kim I. H., Ta D., Tombola F., Voltage-sensing domain of voltage-gated proton channel Hv1 shares mechanism of block with pore domains. Neuron 77, 274–287 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan D., et al. , Peregrination of the selectivity filter delineates the pore of the human voltage-gated proton channel hHV1. J. Gen. Physiol. 142, 625–640 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudev T., et al. , Selectivity mechanism of the voltage-gated proton channel, HV1. Sci. Rep. 5, 10320 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chamberlin A., Qiu F., Wang Y., Noskov S. Y., Larsson H. P., Mapping the gating and permeation pathways in the voltage-gated proton channel Hv1. J. Mol. Biol. 427, 131–145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwaki M., et al. , Zn2+-binding to the voltage-gated proton channel Hv1/VSOP. J. Phys. Chem. B 122, 9076–9080 (2018). [DOI] [PubMed] [Google Scholar]
- 47.DeCoursey T. E., Morgan D., Musset B., Cherny V. V., Insights into the structure and function of HV1 from a meta-analysis of mutation studies. J. Gen. Physiol. 148, 97–118 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagle J. F., Morowitz H. J., Molecular mechanisms for proton transport in membranes. Proc. Natl. Acad. Sci. U.S.A. 75, 298–302 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pomès R., Roux B., Structure and dynamics of a proton wire: A theoretical study of H+ translocation along the single-file water chain in the gramicidin A channel. Biophys. J. 71, 19–39 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gianti E., Delemotte L., Klein M. L., Carnevale V., On the role of water density fluctuations in the inhibition of a proton channel. Proc. Natl. Acad. Sci. U.S.A. 113, E8359–E8368 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennett A. L., Ramsey I. S., CrossTalk opposing view: Proton transfer in Hv1 utilizes a water wire, and does not require transient protonation of a conserved aspartate in the S1 transmembrane helix. J. Physiol. 595, 6797–6799 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeCoursey T. E., CrossTalk proposal: Proton permeation through HV 1 requires transient protonation of a conserved aspartate in the S1 transmembrane helix. J. Physiol. 595, 6793–6795 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chamberlin A., et al. , Hydrophobic plug functions as a gate in voltage-gated proton channels. Proc. Natl. Acad. Sci. U.S.A. 111, E273–E282 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Islas L. D., Sigworth F. J., Electrostatics and the gating pore of Shaker potassium channels. J. Gen. Physiol. 117, 69–89 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tombola F., Pathak M. M., Isacoff E. Y., Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron 45, 379–388 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Ahern C. A., Horn R., Focused electric field across the voltage sensor of potassium channels. Neuron 48, 25–29 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Chaves G., et al. , Identification of an HV1 voltage-gated proton channel in insects. FEBS J. 283, 1453–1464 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Wood M. L., et al. , Water wires in atomistic models of the Hv1 proton channel. Biochim. Biophys. Acta 1818, 286–293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cherny V. V., DeCoursey T. E., pH-dependent inhibition of voltage-gated H+ currents in rat alveolar epithelial cells by Zn2+ and other divalent cations. J. Gen. Physiol. 114, 819–838 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu F., et al. , Molecular mechanism of Zn2+ inhibition of a voltage-gated proton channel. Proc. Natl. Acad. Sci. U.S.A. 113, E5962–E5971 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De La Rosa V., Bennett A. L., Ramsey I. S., Coupling between an electrostatic network and the Zn2+ binding site modulates Hv1 activation. J. Gen. Physiol. 150, 863–881 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Keulen S. C., et al. , Does proton conduction in the voltage-gated H+ channel hHv1 involve Grotthuss-like hopping via acidic residues? J. Phys. Chem. B 121, 3340–3351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeCoursey T. E., Cherny V. V., Voltage-activated hydrogen ion currents. J. Membr. Biol. 141, 203–223 (1994). [DOI] [PubMed] [Google Scholar]
- 64.Tombola F., Ulbrich M. H., Kohout S. C., Isacoff E. Y., The opening of the two pores of the Hv1 voltage-gated proton channel is tuned by cooperativity. Nat. Struct. Mol. Biol. 17, 44–50 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu F., Rebolledo S., Gonzalez C., Larsson H. P., Subunit interactions during cooperative opening of voltage-gated proton channels. Neuron 77, 288–298 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villalba-Galea C. A., Hv1 proton channel opening is preceded by a voltage-independent transition. Biophys. J. 107, 1564–1572 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carmona E. M., et al. , Gating charge displacement in a monomeric voltage-gated proton (Hv1) channel. Proc. Natl. Acad. Sci. U.S.A. 115, 9240–9245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zimmerberg J., Bezanilla F., Parsegian V. A., Solute inaccessible aqueous volume changes during opening of the potassium channel of the squid giant axon. Biophys. J. 57, 1049–1064 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Unwin N.; The Croonian Lecture , The Croonian Lecture 2000. Nicotinic acetylcholine receptor and the structural basis of fast synaptic transmission. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1813–1829 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamashita M., et al. , STIM1 activates CRAC channels through rotation of the pore helix to open a hydrophobic gate. Nat. Commun. 8, 14512 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rao S., et al. , Water and hydrophobic gates in ion channels and nanopores. Faraday Discuss. 209, 231–247 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imbrici P., D’Adamo M. C., Kullmann D. M., Pessia M., Episodic ataxia type 1 mutations in the KCNA1 gene impair the fast inactivation properties of the human potassium channels Kv1.4-1.1/Kvβ1.1 and Kv1.4-1.1/Kvβ1.2. Eur. J. Neurosci. 24, 3073–3083 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Zhu J., Alsaber R., Zhao J., Ribeiro-Hurley E., Thornhill W. B., Characterization of the Kv1.1 I262T and S342I mutations associated with episodic ataxia 1 with distinct phenotypes. Arch. Biochem. Biophys. 524, 99–105 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Struyk A. F., Cannon S. C., A Na+ channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore. J. Gen. Physiol. 130, 11–20 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moreau A., Gosselin-Badaroudine P., Chahine M., Biophysics, pathophysiology, and pharmacology of ion channel gating pores. Front. Pharmacol. 5, 53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Forbes S. A., et al. , COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res. 45, D777–D783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morgan D., DeCoursey T. E., Analysis of electrophysiological properties and responses of neutrophils. Methods Mol. Biol. 1124, 121–158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.