Abstract
Aims
Edoxaban is a direct factor Xa inhibitor approved for stroke prevention in atrial fibrillation (AF). Uninterrupted edoxaban therapy in patients undergoing AF ablation has not been tested.
Methods and results
The ELIMINATE-AF trial, a multinational, multicentre, randomized, open-label, parallel-group study, was conducted to assess the safety and efficacy of once-daily edoxaban 60 mg (30 mg in patients indicated for dose reduction) vs. vitamin K antagonists (VKAs) in AF patients undergoing catheter ablation. Patients were randomized 2:1 to edoxaban vs. VKA. The primary endpoint (per-protocol population) was time to first occurrence of all-cause death, stroke, or International Society of Thrombosis and Haemostasis-defined major bleeding during the period from the end of the ablation procedure to end of treatment (90 days). Overall, 632 patients were enrolled, 614 randomized, and 553 received study drug and underwent ablation; 177 subjects underwent brain magnetic resonance imaging to assess silent cerebral infarcts. The primary endpoint (only major bleeds occurred) was observed in 0.3% (1 patient) on edoxaban and 2.0% (2 patients) on VKA [hazard ratio (95% confidence interval): 0.16 (0.02–1.73)]. In the ablation population (modified intent-to-treat population including patients with ablation), the primary endpoint was observed in 2.7% of edoxaban (N = 10) and 1.7% of VKA patients (N = 3) between start of ablation and end of treatment. There were one ischaemic and one haemorrhagic stroke, both in patients on edoxaban. Cerebral microemboli were detected in 13.8% (16) patients who received edoxaban and 9.6% (5) patients in the VKA group (nominal P = 0.62).
Conclusion
Uninterrupted edoxaban therapy represents an alternative to uninterrupted VKA treatment in patients undergoing AF ablation.
Keywords: Atrial fibrillation, Ablation, Anticoagulation, Bleeding events, Stroke
Introduction
Catheter ablation is a safe and effective strategy for rhythm control in patients with symptomatic atrial fibrillation (AF). The most important peri-procedural complications comprise stroke, transient ischaemic attack (TIA), and cardiac tamponade. To reduce the risk of thromboembolic events, systemic anticoagulation before, during, and after ablation is mandatory.1,2 In addition to the intraoperative administration of heparin to maintain the activated clotting time (ACT) >300 s, uninterrupted therapy with warfarin is recommended. This strategy is associated with a lower risk of bleeding compared to temporary vitamin K antagonist (VKA) discontinuation covered by a bridging strategy with low-molecular-weight heparin.3 Although non-vitamin K antagonist oral anticoagulants (NOACs) have been demonstrated to have superior efficacy and similar (dabigatran, rivaroxaban) or lower (apixaban, edoxaban) risk of bleeding in the long-term treatment of patients with AF compared with VKAs,4 there has been a lack of data to support their uninterrupted peri-procedural use during AF ablation. Recently, three studies comparing uninterrupted rivaroxaban, dabigatran, and apixaban to VKA showed similarly low rates of bleeding associated with uninterrupted NOAC vs. uninterrupted VKA therapy.5–7 These results led to the guideline recommendation to continue uninterrupted treatment with NOACs during and after catheter ablation for patients who were previously receiving these NOACs.1
In the ENGAGE AF-TIMI 48 trial,8 once-daily treatment with edoxaban 60/30 mg regimens was non-inferior compared with well-managed warfarin for the prevention of stroke or systemic embolism but with superior safety, however, experience in ablation was very limited.9 Since there has been no randomized controlled trial on the use of edoxaban for catheter ablation of AF, the ELIMINATE-AF trial [a Prospective, Randomized, Open-Label, Blinded Endpoint Evaluation (PROBE) Parallel Group Study Comparing Edoxaban vs. VKA in Subjects Undergoing Catheter Ablation of Non-valvular Atrial Fibrillation], was conducted to investigate the efficacy and safety of uninterrupted edoxaban vs. VKA in patients undergoing catheter ablation for AF.
Patients and methods
Trial design
ELIMINATE-AF (ClinicalTrials.gov: NCT02942576) was a multinational, multicentre, randomized, open-label, parallel-group, blinded-endpoint evaluation (PROBE) study, the design and rationale of which has been previously published in detail.10 The study was conducted between March 2017 and September 2018 in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, and local regulations on clinical research. The protocol was approved by the institutional review board or independent ethics committee at each participating study centre. All patients provided written informed consent prior to enrolment. An independent Data and Safety Monitoring Board reviewed the safety data during the conduct of the trial to protect patient rights, safety, and well-being.
Trial population
Adult patients (≥18 years of age) with documented non-valvular AF (paroxysmal, ≤7 days; persistent, >7 days but ≤12 months; long-standing persistent, >12 months) scheduled for their first or repeated catheter ablation for AF were eligible. Full inclusion and exclusion criteria can be found in the design paper.10 Patients scheduled for catheter ablation with energy sources other than radiofrequency or cryoballoon were excluded from participating, as were patients with mechanical heart valves, moderate to severe mitral stenosis, transient AF or AF of a reversible nature, and patients who had a bioprosthetic heart valve implanted within 3 months prior to randomization.
Treatments
Patients were centrally randomized in a 2:1 ratio to receive edoxaban or VKA using a block randomization method. Patients randomized to edoxaban received once-daily edoxaban 60 mg (30 mg if they met one or more of the criteria for dose reduction).10 The preferred VKA varied across countries (phenprocoumon in Germany and Belgium, acenocoumarol in Spain, and warfarin in Belgium and in all other countries). Patients randomized to a VKA were required to maintain an international normalized ratio (INR) of 2.0–3.0 at least for the last 10 days prior to ablation. International normalized ratio values were documented at least once a week during the pre-ablation period and once a month during the post-ablation period. Patients with INR between 1.5 and 3.5 at the last measurement before the ablation procedure could still undergo ablation at the discretion of the investigator. During the study period, including on the day before the procedure, patients randomized to once-daily edoxaban took their scheduled dose in the evening.
Pre-ablation anticoagulant treatment was mandated for 21–28 days according to current guidelines.1,11 Transoesophageal echocardiography or intracardiac echocardiography was performed prior to ablation to identify cardiac clots. If clots were identified, ablation was not performed, and patients were switched to standard of care and entered the 30-day follow-up period. The maximum interval between the last pre-ablation edoxaban dose and the ablation procedure was 18 h in order to maintain sufficient inhibition of endogenous factor Xa.
During ablation, unfractionated heparin (UFH) was used according to European Heart Rhythm Association guidelines11 targeting an ACT of 300–400 s. Study medication was restarted at least 6 h post-sheath removal after achieving adequate haemostasis. Treatment continued for 90 days post-ablation. Clinical visits occurred at 30, 60, and 90 days post-ablation. Patients discontinued study medication at end of treatment and were transitioned to an oral anticoagulant at the investigator’s discretion.
Magnetic resonance imaging sub-study
A magnetic resonance imaging (MRI) sub-study was performed to detect silent cerebral lesions. An MRI examination of the head was conducted either at 1.5 Tesla (T) or at 3 T field strength within 4 ± 2 days after ablation. The following MRI sequences were used: a T1-weighted spin echo sequence to rule out acute cerebral haemorrhage, diffusion-weighted imaging (DWI), and (post-processed) apparent diffusion coefficient maps to assess acute cerebral infarction, as well as a fluid-attenuated inversion recovery (FLAIR) sequence to differentiate acute from chronic ischaemic brain lesions (sequence parameters given in Supplementary material online, Table S1). FLAIR images were also assessed for the burden of white matter hyperintensities usually attributed to chronic small vessel disease according to the Fazekas scale.12 The Fazekas scale is the most widely used scale for quantifying periventricular and deep white matter disease severity on T2-weighted images.12
Images failing the immediate quality check were repeated whenever feasible. All images were independently evaluated by two experienced neuroradiologists who were blinded to treatment allocation.
Trial endpoints
The primary study endpoint was time to first occurrence of all-cause death, stroke (ischaemic, haemorrhagic, or undetermined), or International Society of Thrombosis and Haemostasis-defined major bleeding during the period from the end of the ablation procedure to end of treatment (90 days). The primary safety endpoint was the time to the first occurrence of International Society on Thrombosis and Haemostasis (ISTH)-defined major bleeding from the date of the first intake of study medication to end of treatment.
Secondary efficacy endpoints included a variety of other composite and individual thromboembolic events, and secondary safety endpoints included other bleeding events.10 All clinical events were centrally adjudicated by a committee of experts blinded as to patient treatment allocation.
Trial size and statistical analysis
ELIMINATE-AF was an exploratory study aiming at enrolment of approximately 560 patients to document about 450 patients who underwent ablation and had no major protocol violation [per-protocol (PP) analysis set]. This sample size was considered appropriate by the steering committee to generate clinically meaningful data. The sample size necessary to establish formal non-inferiority between edoxaban and VKA would have made the trial unfeasible.
The pre-specified main analysis for the primary outcome parameter and key secondary efficacy parameters was defined by the PP analysis set and the ‘post-ablation period’ (end of the ablation procedure to day 90/end of treatment visit), and for the primary and key secondary safety parameters based on the modified intent-to-treat (mITT) analysis set and the ‘overall period’ (date of first intake of study medication to the day 90/end of treatment). The mITT analysis set consisted of all randomized patients who received at least one dose of study medication (safety analysis set). To allow for a comparison of our study results with other NOAC ablation trials,5–7 an analysis period from the start of ablation to the day 90/end-of-treatment visit was also evaluated (ablation population).
Based on adjudicated events, the number and percentage of patients experiencing specific events were summarized by treatment group. Results of a time-to-first event analysis using a Cox proportional hazard model are presented as hazard ratios (HRs) including 2-sided 95% confidence intervals (CIs) and nominal P-values. Due to the exploratory nature of the study, all P-values should be interpreted in an exploratory, non-confirmatory way.
Descriptive statistics for continuous and categorical variables were summarized as means (standard deviation), median (lower–upper quartile), and numbers (percentages), respectively.
The safety analysis set was used to summarize treatment emergent adverse events (TEAE) defined as adverse events which started on or after first dose of the study medication or started prior to but then worsened after the first dose of the assigned study medication.
Results
Patient population
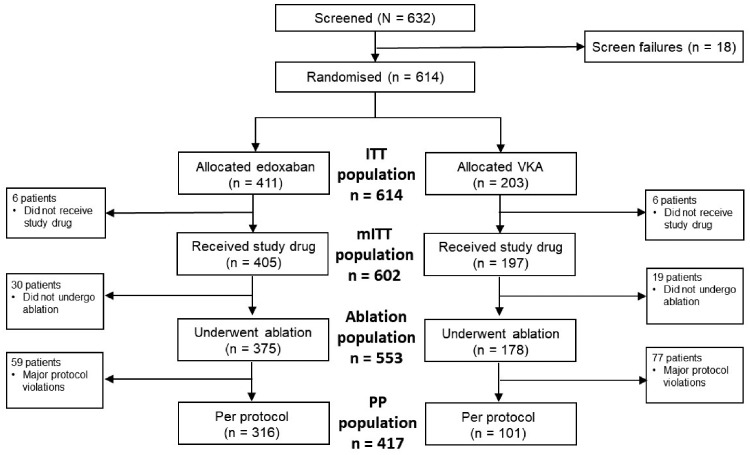
A total of 632 patients were enrolled at 58 sites across 11 countries in Europe, Asia, and Canada. Of these, 614 patients were randomized and 602 received at least one dose of study medication (mITT population). A total of 553 subjects received study drug and underwent catheter ablation (ablation population). The PP population consisted of 417 patients (Figure 1). Reasons for exclusion from the PP population are given in Supplementary material online, Table S2.
Figure 1.
CONSORT diagram. ITT, intent-to-treat; mITT, modified intent-to-treat; PP, per-protocol; VKA, vitamin K antagonist.
Baseline characteristics were well balanced between treatment groups (intent-to-treat population, Table 1, and PP population, Supplementary material online, Table S3). Edoxaban adherence was calculated on the basis of number of tablets taken (mean > 97%) and VKA adherence was measured by monitoring the INR and calculating the time in therapeutic range (TTR).13 The median TTR in the control group was 65% (mITT) and 73% (PP). Time in therapeutic range was 84% (mITT) and 91% (PP) when an extended INR range of 1.8–3.2 was applied. The majority of patients with ablation (96.4%) received trial medication until the end of treatment/day 90.
Table 1.
Patient demographics (intent-to-treat analysis set, N = 614)
| Total (N = 614) | Edoxaban (N = 411) | VKAa (N = 203) | |
|---|---|---|---|
| Age (years) | 60.5 (53–67) | 60.0 (53–67) | 61.0 (52–67) |
| Male gender, n (%) | 439 (71.5) | 290 (70.6) | 149 (73.4) |
| BMI (kg/m2) | 28.1 (25.4–31.2) | 28.1 (25.1–31.1) | 27.8 (25.7–31.2) |
| CHA2DS2-VASc score, n (%) | |||
| 0 | 140 (22.8) | 96 (23.4) | 44 (21.7) |
| 1 | 166 (27.0) | 109 (26.5) | 57 (28.1) |
| ≥2 | 308 (50.2) | 206 (50.1) | 102 (50.2) |
| Medical history, n (%) | |||
| Congestive heart failure | 110 (17.9) | 71 (17.3) | 39 (19.2) |
| Previous CAD (prior MI, prior PCI, or prior CABG) | 117 (19.2) | 76 (18.6) | 41 (20.3) |
| Previous MI | 24 (3.9) | 19 (4.6) | 5 (2.5) |
| Previous stroke/TIAb | 30 (4.9) | 22 (5.4) | 8 (3.9) |
| PAD | 10 (1.6) | 7 (1.7) | 3 (1.5) |
| Diabetes mellitus | 87 (14.2) | 55 (13.4) | 32 (15.8) |
| Hypertension | 371 (60.4) | 250 (60.8) | 121 (59.6) |
| Mild valvular heart disease | 52 (8.5) | 32 (7.8) | 20 (9.9) |
| Creatinine clearance (mL/min) | 96.5 (79.1–118.3) | 95.8 (77.6–117.6) | 97.2 (79.9–118.7) |
| AF type, n (%) | |||
| Paroxysmal | 415 (67.6) | 284 (69.1) | 131 (64.5) |
| Persistent | 166 (27.0) | 105 (25.5) | 61 (30.0) |
| Long-standing persistent | 33 (5.4) | 22 (5.4) | 11 (5.4) |
| Previous cardioversion, n (%) | 310 (50.5) | 209 (50.9) | 101 (49.8) |
| ECG at randomization, n (%) | |||
| AF | 148 (24.5) | 98 (24.3) | 50 (25.0) |
| Atrial flutter | 28 (4.6) | 18 (4.5) | 10 (5.9) |
| TOE/ICE (ablation population = mITT with ablation analysis set) c | (N = 553) | (N = 375) | (N = 178) |
| TOE | 393 (74.6) | 266 (74.3) | 127 (75.1) |
| Intracardiac echocardiography | 134 (25.4) | 92 (25.7) | 42 (24.9) |
| Last dose of study anticoagulant to start of ablation procedure (h) | 15.4 (13.5–17.1) | 14.8 (13.3–16.5) | 16.5 (14.8–19.5) |
| Sheath removal to next dose of study anticoagulant (h) | 6.7 (6.1–8.0) | 6.7 (6.1–8.0) | 6.6 (6.1–7.9) |
| Medication use at baseline, n (%) | |||
| VKA | 242 (39.4) | 146 (35.5) | 96 (47.3) |
| Amiodarone | 156 (25.9) | 97 (24) | 59 (29.9) |
| Other antiarrhythmic drugs | 419 (69.6) | 278 (68.6) | 141 (71.6) |
| Digitalis glycosides | 30 (5.0) | 22 (5.4) | 8 (4.0) |
| Betablocker | 455 (75.6) | 312 (77) | 142 (72.6) |
| Calcium channel antagonists | 136 (22.6) | 90 (22.2) | 46 (23.4) |
| ACE or angiotensin receptor inhibitors | 303 (50.3) | 201 (49.6) | 102 (51.8) |
| Diuretics | 153 (25.4) | 100 (24.7) | 53 (26.9) |
| Aspirin | 13 (3.2) | 7 (1.7) | 6 (3.0) |
| Clopidogrel | 10 (1.7) | 7 (1.7) | 3 (1.5) |
| Proton-pump inhibitors | 267 (44.4) | 184 (45.4) | 83 (42.1) |
| NSAIDs | 30 (5.0) | 24 (5.9) | 6 (3.0) |
Data are presented as median (Q1–Q3) unless otherwise indicated.
86% of the patients on VKA received warfarin.
Includes ischaemic, embolic, and undetermined; haemorrhagic stroke prohibited.
In total, 5 patients with detected thrombus (edoxaban 3; VKA 2); none of these underwent ablation procedure.
ACE, angiotensin converting enzyme; AF, atrial fibrillation; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75, Diabetes mellitus, prior Stroke or transient ischaemic attack or thromboembolism, Vascular disease, Age 60–70, female Sex category; ECG, electrocardiography; ICE, intracardiac echocardiography; ITT, intent-to-treat; MI, myocardial infarction; mITT, modified intent-to-treat; NSAID, non-steroidal anti-inflammatory drugs; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack; TOE, transoesophageal echocardiography; VKA, vitamin K antagonist.
Peri-procedural heparin use
The study protocol called for peri-procedural administration of UFH to maintain the ACT >300 s which was achieved in 66.7% of cases. During the ablation procedure, patients assigned to edoxaban received on average 14.261 IU of UFH compared to 11.473 IU in the VKA arm (nominal P < 0.0001) (Table 2). Administration of UFH resulted in an average ACT of 303 s in the edoxaban and 338 s in the VKA arm (nominal P < 0.0001).
Table 2.
Use of unfractionated heparin and activated clotting time during catheter ablation (modified intent-to-treat with ablation population)
| Total (N = 553) | Edoxaban (N = 375) | VKA (N = 178) | P-value | |
|---|---|---|---|---|
| Total dose of UFH (IU) | ||||
| Mean (SD) | 13 362 (5945) | 14 261 (6397) | 11 473 (4300) | <0.0001 |
| Median | 12 301 | 13 000 | 10 225 | |
| Q1–Q3 | 10 000–16 000 | 10 000–17 500 | 8541–14 000 | |
| Mean ACT (s) | ||||
| Mean (SD) | 314.2 (51.45) | 302.8 (41.64) | 338.0 (61.15) | <0.0001 |
| Median | 307.7 | 301.4 | 332.6 | |
| Q1–Q3 | 281.6–341.5 | 277.0–330.4 | 300.5–371.0 | |
P-values are exploratory.
ACT, activated clotting time; SD, standard deviation; UFH, unfractionated heparin; VKA, vitamin K antagonist.
Primary endpoint and its components
The incidence of the primary endpoint (death, stroke, or ISTH-defined major bleeding during the post-ablation period) was 0.3% (1 patient) in the edoxaban group and 2.0% (2 patients) in the VKA group [HR (95% CI): 0.16 (0.02–1.73), Table 3]. All three events were major bleedings. Including all events that occurred from the start of the ablation procedure to the end of treatment in the PP population, the primary composite endpoint was observed in 1.3% (4 patients) in the edoxaban and in 3.0% (3 patients) in the VKA group [HR (95% CI): 0.42 (0.10–1.89)]. In the ablation population, the primary endpoint was observed in 2.7% (10 edoxaban patients) and 1.7% (3 VKA patients) from the start of the ablation procedure to end of treatment (Table 3).
Table 3.
Primary study endpoint [composite of all-cause death, stroke, and major bleeding (International Society on Thrombosis and Haemostasis)] in the per-protocol and the modified intent-to-treat population
| Edoxaban (ITT N = 411) | VKA (ITT N = 203) | HR (95% CI) | |
|---|---|---|---|
| PP population post-ablation a | |||
| N | 316 | 101 | |
| Primary endpoint events, n (%) | 1 (0.3) | 2 (2.0) | 0.16 (0.02–1.73) |
| PP population peri- and post-ablationb | |||
| N | 316 | 101 | |
| Primary endpoint events, n (%) | 4 (1.3) | 3 (3.0) | 0.42 (0.10–1.89) |
| mITT population peri- and post-ablation b | |||
| N | 375 | 178 | |
| Primary endpoint events, n (%) | 10 (2.7) | 3 (1.7) | 1.60 (0.44–5.78) |
From the end of catheter ablation to day 90/end of treatment.
From the start of catheter ablation to day 90/end of treatment.
CI, confidence interval; HR, hazard ratio; mITT, modified intent-to-treat; PP, per-protocol; VKA, vitamin K antagonist.
The primary safety endpoint (ISTH-defined major bleeding) in the mITT population and overall study period was observed in 2.5% (10 patients) in the edoxaban group and 1.5% (3 patients) in the VKA group (Table 4).
Table 4.
Primary safety endpoint: major bleeding events and study periods (safety set, modified intent-to-treat population)
| Study period | Major bleeding events |
||
|---|---|---|---|
| Edoxaban (N = 405), n (%) |
VKA (N = 197), n (%) |
Edoxaban vs. VKA HR (95% CI) |
|
| Overall events | 10 (2.5) | 3 (1.5) | 1.68 (0.46–6.07) |
| n (%) | n (%) | Diagnosisa Edoxaban/VKA (n/n) | |
| Pre-ablation period (day of randomization to start of ablation procedure) | 1 (0.3) | 0 (0.0) | Haemorrhagic stroke |
| Peri-ablation period (from sheath insertion to sheath removal) | 4 (1.0) | 1 (0.5) | Cardiac tamponade 1/1 Bleeding at puncture site 2/0 Lower GI bleeding 1/0 |
| Post-ablation period ≤48 h after ablation >48 h after ablation to end of treatment | 3 (0.7) 2 (0.5) | 2 (1.0) 0 (0.0) | Cardiac tamponade 2/1 b Bleeding at puncture site 1/1 b Menstrual bleeding 1b/0 Lower GI bleeding 1/0 |
Ablation procedure-related events given in italics.
Events also included in the per-protocol analysis set.
CI, confidence interval; GI, gastrointestinal; HR, hazard ratio; VKA, vitamin K antagonist.
Specifically, the type and number of bleeding events in the edoxaban vs. the VKA group were the following: pericardial tamponade requiring drainage 3 vs. 2; bleeding at puncture site 3 vs. 1; intracranial bleeding 1 vs. 0; gastrointestinal bleeding 2 vs. 0; and other bleedings 1 vs. 0 (Table 4).
During the overall study period (mITT), there were one ischaemic and one haemorrhagic stroke, both in patients assigned to edoxaban. The ischaemic stroke occurred in a 50-year-old male patient on day 4 after ablation. This patient with a history of heart failure and reduced left ventricular ejection fraction was overweight (144 kg), had supra-normal creatinine clearance and was inappropriately dose-reduced to edoxaban 30 mg. The haemorrhagic stroke occurred in a 71-year-old male with a body weight of 71 kg (creatinine clearance of 86 ml/min, and apart from hypertension, no additional risk factors) prior to ablation on study day 17. This patient received edoxaban 60 mg, as no dose reduction criterion were present.
Other types of bleeding
Clinically relevant non-major (CRNM) bleeding events occurred in 7.9% (32 patients) in the edoxaban and in 3.6% (7 patients) in the VKA group. There were no CRNM bleeding events during ablation in either treatment group. The majority of CRNM bleeding events occurred during the first days after ablation.
Magnetic resonance imaging sub-study
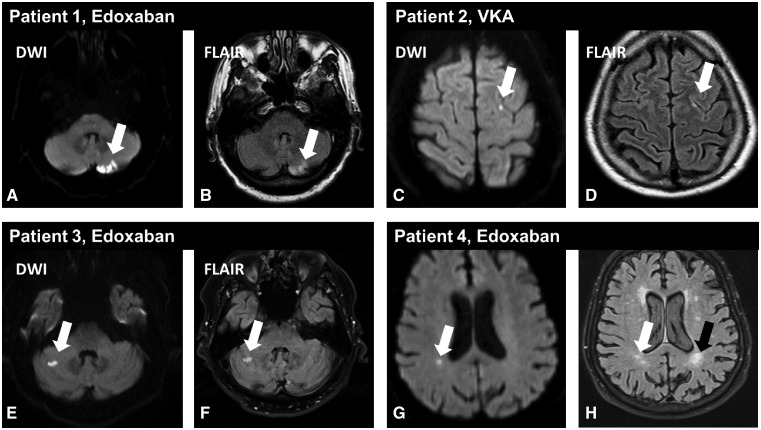
An MRI of the head was acquired in 177 of 207 (85.5%) eligible patients across 14 centres participating in the MRI sub-study. Non-diagnostic image quality led to the exclusion of 2 of 177 patients. Patients who participated in the sub-study showed similar baseline characteristics as those who did not. There were 168 (116 edoxaban, 52 VKA) analysable MRIs of good diagnostic image quality for T1 and DWI and 173 (118 edoxaban, 55 VKA) for FLAIR sequencing. Acute cerebral microembolism ≤10 mm size (Figure 2) was detected in 13.8% (16) of patients (95% CI 7.52–20.07%) who received edoxaban, and 9.6% (5) of patients (95% CI 1.60–17.63%) in the VKA group (nominal P = 0.62). The distribution of lesions between treatment groups was very similar. An acute left cerebellar embolism of 20 × 15 mm in diameter was observed in a clinically asymptomatic patient receiving edoxaban. The burden of subcortical and deep white matter hyperintensities according to the Fazekas scale was well balanced between MRI sub-study treatment groups (Supplementary material online, Table S4).
Figure 2.
Examples of acute cerebral microembolism after atrial fibrillation ablation in the magnetic resonance imaging sub-study. Acute cerebral microembolism are depicted by diffusion-weighted imaging (white arrows in A, C, E, G) with corresponding demarcation on fluid-attenuated inversion recovery images (white arrows in B, D, F, H) suggesting an appearance over 24 h ago. (A and B) A larger clinically asymptomatic left cerebellar embolism in an asymptomatic patient randomized to edoxaban. The fluid-attenuated inversion recovery images also revealed chronic white matter hyperintensities (black arrow in H). DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery.
Treatment emerging adverse events
The number of patients with TEAEs was balanced between study arms with 51.9% (N = 210) and 50.3% (N = 99) in the edoxaban and the VKA groups, respectively.
The number of serious TEAEs was low and also comparable among groups with 9.9% (N = 40) in the edoxaban and 7.6% (N = 15) in the VKA group. Severe TEAEs occurred in 2.2% (N = 9) and 2.5% (N = 5) in the edoxaban and the VKA groups, respectively (Supplementary material online, Table S5).
Discussion
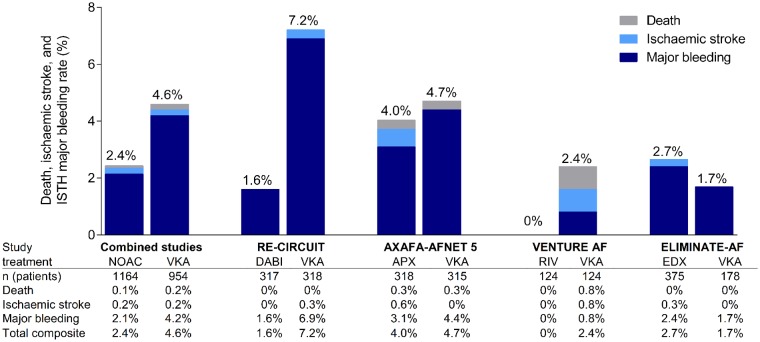
The observations made in ELIMINATE-AF show that uninterrupted administration of edoxaban represents an alternative to continuous anticoagulation with VKA in patients undergoing catheter ablation of AF. The management of anticoagulation around ablation is much easier with edoxaban as there is no need for regular INR controls. The incidence of the composite primary endpoint (death, stroke, and ISTH major bleeding) in the main analysis was low [1 vs. 2 events; HR (95% CI): 0.16 (0.02–1.73)]. International Society on Thrombosis and Haemostasis-defined major bleeding events (primary safety endpoint) in the overall study period were also low with 2.5% in the edoxaban group compared to 1.5% in patients assigned to VKA. There was no statistically significant difference in the incidence of major bleedings between edoxaban and VKA. This event rate for edoxaban 60 mg QD is similar to those observed with dabigatran 150 mg BID in the RE-CIRCUIT trial (1.6%) and with apixaban 5 mg BID in the AXAFA trial (3.1%) (all bleeding rates according to ISTH definition; Figure 3).5,7 In contrast, bleeding rates observed in the VKA arms of the three studies varied considerably, ranging between 1.7% in our study, 4.4% in AXAFA, and 6.9% in RE-CIRCUIT.5,7 Of note, the incidence of the most feared bleeding complication, cardiac tamponade, was similar in the edoxaban group (0.7%) and in the VKA group (1.0%). Importantly, patients in the VKA arm of our study had excellent INR control with a TTR of 65% or even 84% in the extended TTR range of 1.8–3.2. It is possible that the major bleeding rate differences in the VKA groups among these four studies reporting TTR between 65% and 84% represent a by-chance finding or were due to differences in patient populations and in different VKAs used (short- vs. long-acting VKA). Whereas there were no statistically significant differences in the incidence of major bleedings between edoxaban and VKA in our study, there was a trend towards higher CRNM bleeding events in the edoxaban arm. Not unexpectedly, most of the bleeding events occurred during the ablation procedure or in the first 48 h after completion. Hence, it is tempting to speculate that this may be in part related to the cumulative dose of intra-procedural heparin. Comparable to apixaban in the AXAFA trial,7 the mean ACT achieved during ablation was significantly lower in the edoxaban than in the VKA arm. As a consequence, investigators may have been inclined to administer a higher dose of UFH in patients assigned to edoxaban (on average, 24% more heparin than in the VKA arm). Other studies comparing factor Xa inhibitors with VKA have also reported use of higher heparin dosages in NOAC compared with VKA patients.6,14 It can be speculated that the higher dose of heparin may have contributed to an increased likelihood of bleeding events (e.g. from the vascular access sites) in the edoxaban group. This observation carries important clinical implications regarding surveillance of patients during the post-ablation period.
Figure 3.
Incidence rates of combined primary endpoint in ELIMINATE-AF (death, stroke, and International Society on Thrombosis and Haemostasis-defined major bleeding) in the non-vitamin K oral anticoagulant and vitamin K antagonist arms in the modified intent-to-treat peri- and post-ablation study period. Comparison with combined event rates in the three other randomized trials comparing uninterrupted non-vitamin K oral anticoagulant therapy with uninterrupted vitamin K antagonist therapy are shown. For all trials, rates of International Society on Thrombosis and Haemostasis-defined major bleeding events were included. AF, atrial fibrillation; APX, apixaban; DABI, dabigatran; EDX, edoxaban; ISTH, International Society on Thrombosis and Haemostasis; NOAC, non-vitamin K oral anticoagulant; RIV, rivaroxaban; VKA, vitamin K antagonist.
In ELIMINATE-AF, two patients suffered from stroke, one ischaemic and the other haemorrhagic (incidence 0.36%), both in patients receiving edoxaban. This stroke incidence is comparable to those observed in other studies. Specifically, in the AXAFA study, two strokes occurred in 633 patients (0.3%), one stroke in VENTURE AF among 248 patients (incidence 0.4%), and one TIA in RE-CIRCUIT among 635 patients (0.2%; Figure 3).5–7 As in RE-CIRCUIT,5 none of the patients enrolled in our study died. These findings from the four uninterrupted NOAC vs. VKA randomized studies indicate that the risk of thromboembolic events is low and is similar to those reported in retrospective registry studies.15
With the exception of AXAFA,7 the present study is the only one which systematically used MRI for detection of silent brain lesions. Asymptomatic cerebral infarcts have been described in patients undergoing AF ablation at incidences of 2% up to 41% of subjects.16–18 Our MRI sub-study revealed similar rates of acute cerebral microemboli (13.8% vs. 9.6%) after catheter ablation under edoxaban compared to VKA. The higher incidence of microemboli described in the AXAFA study7 is likely due to different timing of image acquisition. In contrast to the AXAFA study, in which a brain MRI was performed within 48 h after the ablation procedure,7 the MRIs in our study were acquired 4 ± 2 days after ablation, which is why the cerebral microemboli were already demarcated on the FLAIR images.16,19 Considering the very low incidence of ischaemic stroke and silent cerebral ischaemic lesions in our study population, it is likely that not only thromboembolism but also procedure-related air microembolizations may have contributed to the detected cerebral lesions on DWI.
As with previous trials examining rivaroxaban, dabigatran, and apixaban in the context of catheter ablation, ELIMINATE-AF has the limitation of not being a formal non-inferiority trial of edoxaban vs. VKA. However, such a trial was deemed not to be affordable given the need to enrol at least 3000 patients for a non-inferiority margin of 1.38 and a power of 80%. Like the other studies examining uninterrupted NOAC therapy for AF ablation, the present study is therefore an exploratory evaluation of the safety and efficacy of edoxaban in patients undergoing catheter ablation of AF. Another limitation consists of the exclusion of a considerable proportion of patients from the PP analysis due to predefined protocol violation criteria, including time out of INR range. This resulted in a relatively uneven exclusion of patients in the VKA vs. the edoxaban treatment groups. Finally, ELIMINATE-AF (like the other three NOAC ablation studies) is an unblinded study, which may allow for some residual bias.
In conclusion, results from ELIMINATE-AF demonstrate that edoxaban represents an alternative to VKA in this clinical scenario.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
Editorial assistance with formatting and figure preparation was provided by Kathleen Pieper, PhD, of AlphaBioCom (King of Prussia, PA, USA), and funded by Daiichi Sankyo, Inc. Participating sites are listed in the Supplementary material online, Appendix.
Funding
Funding for ELIMINATE-AF was provided by Daiichi Sankyo Europe GmbH.
Assurances
The first author wrote the manuscript draft and all co-authors provided input and comments.
Conflict of interest: S.H.H. reports personal fees from Bayer Healthcare, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer, personal fees, SJM, and from Zoll, outside the submitted work. J.C. has received personal fees for attending advisory committees and lecturing and his institution has received research grants from Daiichi Sankyo. R.C. reports research grants from and was a speaker consultant for Boston Scientific, Bayer, Medtronic, Abbott, St. Jude Medical, Pfizer, Daiichi Sankyo, and Biosense Webster during the conduct of this study. H.-C.D. reports grants and personal fees from Boehringer Ingelheim, during the conduct of the study; grants and personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Janssen-Cilag, Novartis, Sanofi Aventis; personal fees from Abbott, Allergan, Bayer Vital, Bristol-Myers Squibb Brainsgate, CoAxia, Corimmun, Covidien, Daiichi Sankyo, D-Pharm, EV3, Fresenius, Knoll, Merck Sharp & Dohme, Lilly, Medtronic, Mind-Frame, Neurobiological Technologies, Novo Nordisk, Paion, Parke-Davis, Pfizer, Schering-Plough, Servier, Solvay, St. Jude, Thrombogenics, Wyeth; and grants from Lundbeck, Syngis, Talecris, German Research Council (DFG), German Ministry of Education and Research BMBF), European Union, Bertelsmann Foundation, Heinz-Nixdorf Foundation, and the National Institutes of Health, outside the submitted work. H.H. reports personal fees from Pfizer/BMS, Daiichi Sankyo, Boehringer-Ingelheim, Cardiome, and Medtronic; grants and personal fees from Bayer and Abbott; and grants from Bracco Imaging Europe outside the submitted work. L.M. reports no conflicts. C.A.M. reports grants and personal fees from Bayer; personal fees from Pfizer and Servier; and was on a steering committee for Daiichi Sankyo during the conduct of this work. K.A. reports speaker fees from Daiichi Sankyo. M.G. reports an agreement with Johnson and Johnson—Biosense Webster outside the submitted work. H.R., R.S., and P.-E.R. are an employee of Daiichi Sankyo, GmbH. C.M. reports grants from Daiichi Sankyo during the conduct of the study and personal fees from Bayer Medical outside the submitted work. J.K. reports personal fees and other from Daiichi Sankyo, during the conduct of the study; advisory boards, proctoring, and education events and received personal fees from Biosense Webster, advisory board and personal fees from Boston Scientific; lectures, educational events and personal fees from Biotronik, advisory board, educational events, and personal fees Medtronic, and advisory board and educational fees from Abbott (St. Jude Medical), personal fees from Epix, and MicroPace outside the submitted work.
See page 3022 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz322)
References
- 1. Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen P-S, Chen S-A, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d’Avila A, de Groot NMS(N), Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao H-M, Verma A, Wilber DJ, Yamane T.. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Di Biase L, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R, Gallinghouse GJ, Themistoclakis S, Rossillo A, Lakkireddy D, Reddy M, Hao S, Hongo R, Beheiry S, Zagrodzky J, Rong B, Mohanty S, Elayi CS, Forleo G, Pelargonio G, Narducci ML, Dello Russo A, Casella M, Fassini G, Tondo C, Schweikert RA, Natale A.. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation 2014;129:2638–2644. [DOI] [PubMed] [Google Scholar]
- 4. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM.. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 5. Calkins H, Willems S, Gerstenfeld EP, Verma A, Schilling R, Hohnloser SH, Okumura K, Serota H, Nordaby M, Guiver K, Biss B, Brouwer MA, Grimaldi M, Circuit Investigators RE.. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med 2017;376:1627–1636. [DOI] [PubMed] [Google Scholar]
- 6. Cappato R, Marchlinski FE, Hohnloser SH, Naccarelli GV, Xiang J, Wilber DJ, Ma CS, Hess S, Wells DS, Juang G, Vijgen J, Hugl BJ, Balasubramaniam R, De Chillou C, Davies DW, Fields LE, Natale A; VENTURE-AF Investigators. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J 2015;36:1805–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirchhof P, Haeusler KG, Blank B, De Bono J, Callans D, Elvan A, Fetsch T, Van Gelder IC, Gentlesk P, Grimaldi M, Hansen J, Hindricks G, Al-Khalidi HR, Massaro T, Mont L, Nielsen JC, Nolker G, Piccini JP, De Potter T, Scherr D, Schotten U, Themistoclakis S, Todd D, Vijgen J, Di Biase L.. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J 2018;39:2942–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM, Engage AF, Timi I.. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 9. Steffel J, Ruff CT, Hamershock RA, Murphy SA, Senior R, Roy D, Lanz HJ, Mercuri MF, Antman EM, Giugliano RP.. First experience with edoxaban and atrial fibrillation ablation—insights from the ENGAGE AF-TIMI 48 trial. Int J Cardiol 2017;244:192–195. [DOI] [PubMed] [Google Scholar]
- 10. Hohnloser SH, Camm J, Cappato R, Diener HC, Heidbuchel H, Lanz HJ, Mont L, Morillo CA, Smolnik R, Yin OQP, Kautzner J.. Uninterrupted administration of edoxaban vs vitamin K antagonists in patients undergoing atrial fibrillation catheter ablation: rationale and design of the ELIMINATE-AF study. Clin Cardiol 2018;41:440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sticherling C, Marin F, Birnie D, Boriani G, Calkins H, Dan GA, Gulizia M, Halvorsen S, Hindricks G, Kuck KH, Moya A, Potpara T, Roldan V, Tilz R, Lip GY.. Antithrombotic management in patients undergoing electrophysiological procedures: a European Heart Rhythm Association (EHRA) position document endorsed by the ESC Working Group Thrombosis, Heart Rhythm Society (HRS), and Asia Pacific Heart Rhythm Society (APHRS). Europace 2015;17:1197–1214. [DOI] [PubMed] [Google Scholar]
- 12. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA.. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–356. [DOI] [PubMed] [Google Scholar]
- 13. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E.. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993;69:236–239. [PubMed] [Google Scholar]
- 14. Kottmaier M, Bourier F, Pausch H, Reents T, Semmler V, Telishevska M, Koch-Buttner K, Lennerz C, Lengauer S, Kornmayer M, Rousseva E, Brooks S, Brkic A, Ammar-Busch S, Kaess B, Dillier R, Grebmer C, Kolb C, Hessling G, Deisenhofer I.. Safety of uninterrupted periprocedural edoxaban versus phenprocoumon for patients who underwent left atrial catheter ablation procedures. Am J Cardiol 2018;121:445–449. [DOI] [PubMed] [Google Scholar]
- 15. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E.. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–38. [DOI] [PubMed] [Google Scholar]
- 16. Deneke T, Shin DI, Balta O, Bunz K, Fassbender F, Mugge A, Anders H, Horlitz M, Pasler M, Karthikapallil S, Arentz T, Beyer D, Bansmann M.. Postablation asymptomatic cerebral lesions: long-term follow-up using magnetic resonance imaging. Heart Rhythm 2011;8:1705–1711. [DOI] [PubMed] [Google Scholar]
- 17. Haeusler KG, Koch L, Herm J, Kopp UA, Heuschmann PU, Endres M, Schultheiss HP, Schirdewan A, Fiebach JB.. 3 Tesla MRI-detected brain lesions after pulmonary vein isolation for atrial fibrillation: results of the MACPAF study. J Cardiovasc Electrophysiol 2013;24:14–21. [DOI] [PubMed] [Google Scholar]
- 18. Kuwahara T, Abe M, Yamaki M, Fujieda H, Abe Y, Hashimoto K, Ishiba M, Sakai H, Hishikari K, Takigawa M, Okubo K, Takagi K, Tanaka Y, Nakajima J, Takahashi A.. Apixaban versus warfarin for the prevention of periprocedural cerebral thromboembolism in atrial fibrillation ablation: multicenter prospective randomized study. J Cardiovasc Electrophysiol 2016;27:549–554. [DOI] [PubMed] [Google Scholar]
- 19. Lickfett L, Hackenbroch M, Lewalter T, Selbach S, Schwab JO, Yang A, Balta O, Schrickel J, Bitzen A, Luderitz B, Sommer T.. Cerebral diffusion-weighted magnetic resonance imaging: a tool to monitor the thrombogenicity of left atrial catheter ablation. J Cardiovasc Electrophysiol 2006;17:1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.