Significance
We report the identification and characterization of the small molecule aspects of the Odontosyllis undecimdonta bioluminescence system. The chemical structures of the 4 best-known marine luciferins are as diverse as their phylogenetic distribution. The unique structure of Odontosyllis luciferin provides a key insight into a completely novel chemical basis of bioluminescence. Odontosyllis oxyluciferin is the only green primary emitter described for any known bioluminescent marine organism. Together with Odontosyllis luciferase, our recent findings provide insight into the biochemistry and photochemistry of a new light-emitting system. Our studies represent a crucial step in the development of orthogonal luminescence-based analytical methods for a variety of applications, including live animal imaging and pharmaceutical development.
Keywords: bioluminescence, Odontosyllis luciferin, oxyluciferin, heterocycles
Abstract
Marine polychaetes Odontosyllis undecimdonta, commonly known as fireworms, emit bright blue-green bioluminescence. Until the recent identification of the Odontosyllis luciferase enzyme, little progress had been made toward characterizing the key components of this bioluminescence system. Here we present the biomolecular mechanisms of enzymatic (leading to light emission) and nonenzymatic (dark) oxidation pathways of newly described O. undecimdonta luciferin. Spectral studies, including 1D and 2D NMR spectroscopy, mass spectrometry, and X-ray diffraction, of isolated substances allowed us to characterize the luciferin as an unusual tricyclic sulfur-containing heterocycle. Odontosyllis luciferin does not share structural similarity with any other known luciferins. The structures of the Odontosyllis bioluminescent system’s low molecular weight components have enabled us to propose chemical transformation pathways for the enzymatic and nonspecific oxidation of luciferin.
Having appeared dozens of times during evolution, bioluminescence serves as one of the prime means of intraspecies and interspecies communication among marine organisms. The chemical nature and mechanisms of action of the few known types of bioluminescence substrates (luciferins) are as diverse as their phylogenetic distribution. Advances in the development of a wide diversity of applied procedures have been primarily the result of increased understanding of the basic biochemistry of bioluminescence from a very few well-characterized systems, including beetles and coelenterates. Important medical applications, especially those related to gene expression and calcium ion detection, have been spawned by characterizing bioluminescence systems at the molecular level.
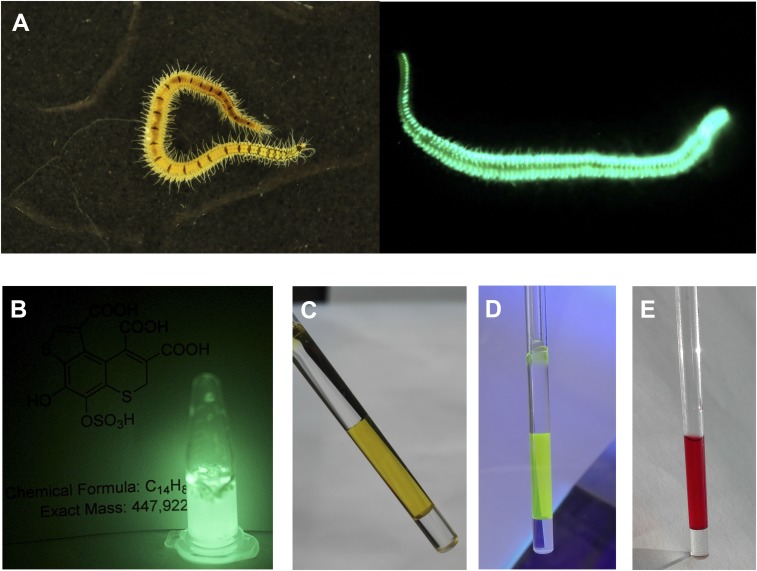
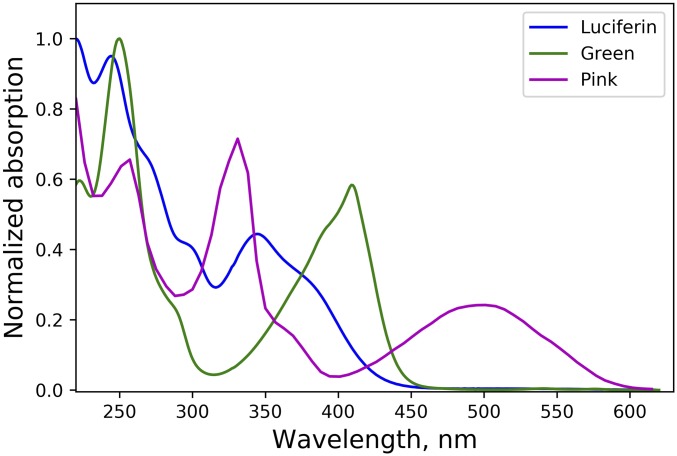
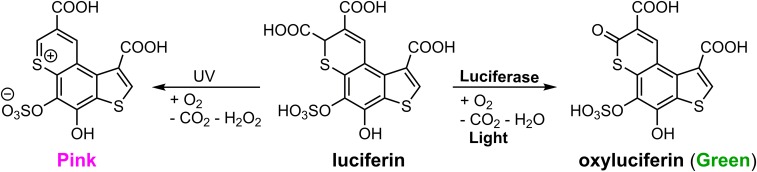
Odontosyllis undecimdonta fireworms are small (12 to 20 mm long) but display a spectacular bioluminescence (Fig. 1A), correlated with the lunar cycle. Luciferin-luciferase reactions of Odontosyllis phosphorea and Odontosyllis enopla were first demonstrated by Harvey in 1952 (1). Further in-depth investigations conducted by Shimomura et al. (2, 3) and Trainor (4) established that the Odontosyllis bioluminescence reaction requires only oxygen, a luciferin, and a luciferase enzyme to emit greenish-blue light. During the enzymatic luminescent reaction, luciferin is converted into an oxyluciferin (light emitter), which we refer to here as “Green,” a compound with fluorescence (λabs = 445 nm, λem = 507 nm) highly similar to Odontosyllis bioluminescence. Nonenzymatic autooxidation on air produces a pink-colored substance (λabs = 520 nm), which we refer to here as “Pink.” The susceptibility of the luciferin to decomposition presented a serious challenge to investigations of the Odontosyllis bioluminescence system due to the difficulty obtaining the pure substrate.
Fig. 1.
O. undecimdonta worms and components of its bioluminescence system. (A) The fireworm in daylight and in the dark. (B) Light emission of lyophilized worms in water. (C) NMR tube with the purified oxyluciferin (Green), visible light. (D) Fluorescence of Green, UV light. (E) NMR tube with the purified product of nonenzymatic oxidation of luciferin (Pink), visible light.
The Odontosyllis luciferase has recently been sequenced and cloned (5, 6). This enzyme has proven to be unique among sequenced polychaetes and all other sequenced organisms, suggesting that chemical basis of Odontosyllis bioluminescence is not similar to that of any other species.
To elucidate the structures of the components of O. undecimdonta luminescence system, we designed the purification procedure from the polychaete biomass. We used 2 sources of target compounds: lyophilized O. undecimdonta worms and lyophilized substrate fractions of the O. undecimdonta biomass, collected and partially purified by Inoue et al. (7) via acetone extraction and thin-layer chromatography separation. The lyophilized worms produced bright luminescence on addition of water (Fig. 1B), indicating that all components of the bioluminescent system remained active. Lyophilized luciferin samples also demonstrated luminescence activity on addition of “cold extract” prepared from lyophilized worms as described previously (4). These observations allowed us to conclude that we had functionally active luciferin for further structural and mechanistic studies.
We observed that aqueous extracts from lyophilized worms emit bright light at 25 °C, with the emission significantly decreasing at 4 °C but then increasing back to the initial intensity on thawing. This means that low temperatures reversibly inhibit the luminescent reaction, thereby enabling the extraction and separation of bioluminescence system components from Odontosyllis biomass. The highly labile nature and extremely low content of Odontosyllis luciferin dictated that the protocol for purification should include low temperatures, fast lyophilization, and shielding from light at all times. Failure to comply with the protocol led to the rapid visible conversion of colorless luciferin to Pink and to the irreversible loss of bioluminescence activity.
Ion-exchange chromatography of a chilled water extract of O. undecimdonta worms allowed the separation of substrate and enzyme-containing fractions in the first chromatographic step (SI Appendix, Fig. S1). None of the fractions alone possessed bioluminescence activity. Pairwise combination of all the fractions allowed the identification of those containing luciferin and luciferase. Luciferin-containing fractions showed visible yellow-green coloration (Fig. 1C) and were fluorescent under UV light (Fig. 1D). Taking into account the earlier reports by Trainor (4), we considered that the observable yellow-green color was likely caused by the presence of oxyluciferin, which necessitated further purification steps (SI Appendix, Fig. S2). Fractions containing luciferin or Green (SI Appendix, Fig. S2) were identified by bioluminescence assay or absorbance at 410 nm and were collected separately, lyophilized, and used for further analysis. The UV spectrum of Green (Fig. 2), while strongly pH-dependent, was similar to that of oxyluciferin reported by Shimomura et al. (3).
Fig. 2.
UV-visible spectra of Odontosyllis luciferin (blue line), Odontosyllis oxyluciferin (Green; green line), and the product of luciferin nonspecific oxidation (Pink; magenta line). Spectra were measured in the following conditions: luciferin in MeCN/H2O 35%/65% containing 0.1% trifluoroacetic acid; Green in MeCN/H2O 50%/50% containing 0.1% trifluoroacetic acid; Pink in MeCN/H2O 25%/75% containing 0.1% trifluoroacetic acid.
To confirm Green as the oxyluciferin, the purified Odontosyllis luciferin and luciferase preparations were mixed together in reaction buffer and monitored by high-performance liquid chromatography (HPLC). The reaction mixture produced visible light, and the Green concentration increased over the course of the reaction (SI Appendix, Fig. S3 A and C). HPLC and high-resolution mass spectroscopy (HRMS) analyses showed that the product of enzymatic luciferin bioluminescence oxidation and Green had similar absorption spectra (SI Appendix, Fig. S3B) and identical molecular ions and fragmentation patterns in mass spectra (SI Appendix, Fig. S4). These data indicate that Green is in fact the Odontosyllis oxyluciferin.
Meanwhile, the HPLC analysis of lyophilized luciferin samples revealed that the most abundant compound (SI Appendix, Fig. S5) corresponded to Pink—the product of nonenzymatic oxidation of luciferin (Fig. 1E), whose absorption spectrum (Fig. 2) was similar to that described by Shimomura et al. (3).
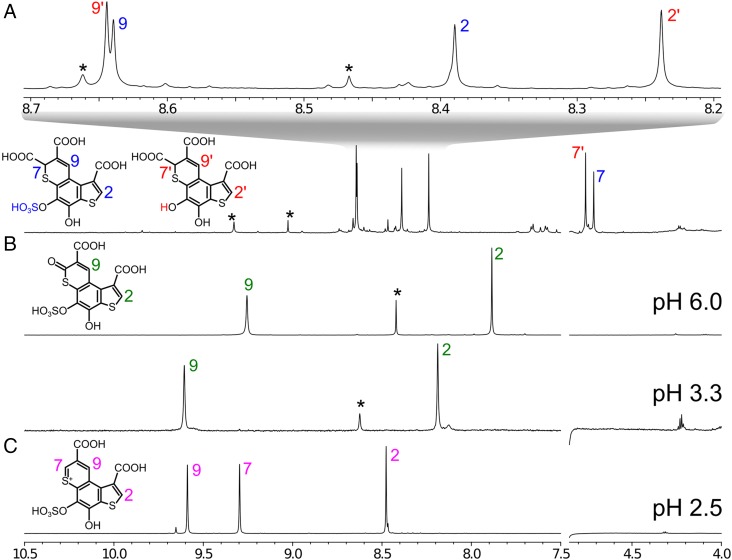
HPLC-purified fractions containing Pink were combined (yielding 7.2 absorbance units), lyophilized, and subjected to nuclear magnetic resonance (NMR) and HRMS analyses. Only 3 hydrogens (Fig. 3C) and 12 carbon atoms were observed in 1H, 13C, and 1H-13C heteronuclear multiple quantum correlation (HSQC) and heteronuclear multiple bond correlation (HMBC) NMR spectra of Pink (SI Appendix, Fig. S6 and Table S1). The analysis of NMR correlation data revealed an aromatic core of Pink containing 2 sulfur atoms. The HRMS spectra of Pink showed [M+H]+ with m/z = 402.9196, which corresponds to the formula C13H7O9S3+ (calcd m/z = 402.9245), and an [M−H]− ion peak at m/z = 400.9123 closely matching the formula C13H5O9S3− (calcd m/z = 400.9101). The MS fragmentation pattern of deprotonated Pink unambiguously showed the presence of a sulfate group and 2 carboxylic acid groups (SI Appendix, Fig. S7).
Fig. 3.
1H NMR spectra of compounds from Odontosyllis. (A) Luciferin in methanol-d4 at 10 °C, 800 MHz. (B) Oxyluciferin in H2O + 10% D2O at 15 °C, pH 3.3 (800 MHz) and pH 6.0 (600 MHz). (C) Pink in H2O + 10% D2O at 15 °C, pH 2.5 (800 MHz). Impurities are labeled by asterisks; signals of the compounds are labeled with numbers (SI Appendix, Table S1 and Figs. S6, S8, and S14).
A consecutive series of chromatographic experiments allowed the isolation of 10 and 2.6 absorbance units of pure oxyluciferin (Green) and luciferin, respectively, from 6 g of lyophilized worm biomass. The following NMR spectra were acquired for Green: 1H, 13C, 2D 13C-HSQC, and 2D 13C-HMBC (SI Appendix, Fig. S8 and Table S1). The observed NMR chemical shifts (Fig. 3B) and correlations coincided with the Pink carbon core, except for the loss of 1 aromatic singlet at position 7 (Fig. 3B) and the appearance of deshielded carbon atom in the same position (SI Appendix, Fig. S8). Electrospray ionization (ESI)-HRMS spectra of Green showed an [M+H]+ ion at m/z = 418.9207 for which the closest molecular formula is C13H7O10S3+ (calcd m/z = 418.9196). HRMS spectra of oxyluciferin recorded in negative-ion mode showed an [M−H]− ion with m/z = 416.9067, closely matching the formula C13H5O10S3− (calcd m/z = 416.9050) (SI Appendix, Fig. S9).
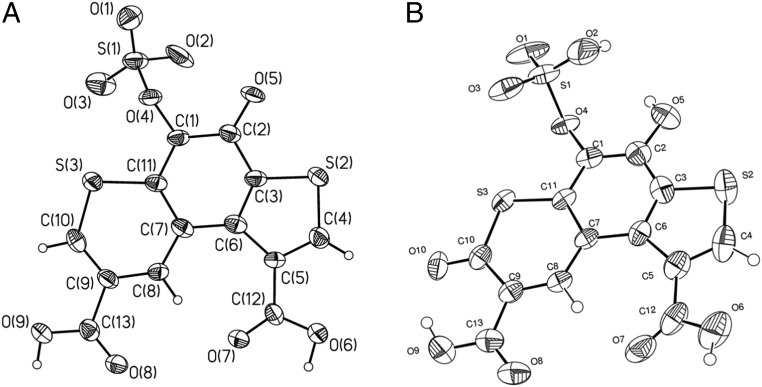
As the NMR data were insufficient for unequivocal structure determination, single crystals of purified Pink and oxyluciferin were grown and subjected to X-ray diffraction studies (Fig. 4) to unequivocally establish the chemical structures of nonenzymatic luciferin oxidation product and oxyluciferin. Both Pink and oxyluciferin have identical tricyclic thieno[3,2-f]thiochromene skeletons, carboxylated at the C1 and C8 positions. The sole difference between the 2 molecules is the absence of a carbonyl group in the α-position with respect to the sulfur atom in thiopyran moiety of Pink compared with oxyluciferin. The NMR data for Pink and oxyluciferin were fully consistent with the X-ray structures (SI Appendix, Figs. S6 and S8).
Fig. 4.
Structures of Odontosyllis oxyluciferin (Green) and product of nonspecific luciferin oxidation (Pink). (A) General view of Odontosyllis luciferin nonenzymatic oxidation product (Pink) as revealed by X-ray diffraction; nonhydrogen atoms are shown as thermal ellipsoids (P = 50%). (B) General view of oxyluciferin (Green) as revealed by X-ray diffraction; nonhydrogen atoms are shown as thermal ellipsoids (P = 50%).
The X-ray structures of Pink and Green played a key role in the subsequent elucidation of the parental Odontosyllis luciferin structure. ESI-HRMS spectra of luciferin showed an [M−H]−ion with m/z = 446.9167, while in positive mode an [M+H]+ molecular ion was observed with m/z = 448.9246. These data are consistent with the molecular formula C14H8O11S3 (calcd [M−H]− m/z = 446.9156 and [M+H]+ m/z = 448.9301). HPLC-MS analysis also revealed a second compound in the luciferin sample with an [M−H]−ion at 366.9594, for which the closest molecular formula is C14H7O8S2−. Both compounds showed similar retention times and almost identical fragmentation patterns (SI Appendix, Fig. S10). The tandem mass spectrometry (MS/MS) fragmentation pattern of deprotonated luciferin (m/z = 446.9156) showed a characteristic loss of SO3 followed by elimination of water and sequential triple decarboxylation, leading to a heterocyclic core (SI Appendix, Fig. S11). In the mass spectrum of the second compound (m/z = 366.9594), an identical fragmentation pattern was observed, differing only in the absence of the elimination of SO3. It seemed reasonable to conclude that the second compound was O-desulfated luciferin (SI Appendix, Figs. S10–S13).
The HRMS analysis was further supported by NMR data (SI Appendix, Fig. S14 and Table S1). 1H NMR of luciferin revealed 2 distinct molecules with a 1:1.25 mol/mol ratio (Fig. 3A). Both compounds had 3 singlets in 1H NMR. Their 13C correlations in HSQC and HMBC NMR spectra were coincident (SI Appendix, Fig. S14), and the fragmentation patterns in HRMS identified the presence of SO3 group as the only difference between these substances. The signals at 4.713/4.759 ppm in the 1H NMR spectrum that were absent in Pink and Green support the loss of aromaticity in the core of luciferin otherwise shared with Pink and Green. The pattern of 2D NMR correlations assigned these signals to position 7 of the luciferin core (SI Appendix, Fig. S14). The integrals in 1H NMR and the cross-peak sign in multiplicity-edited 13C-HSQC unambiguously demonstrated that the nonaromatic carbon at position 7 has only one directly attached proton, and thus the last C7 valence should be occupied with the third carboxylic group.
The resulting chemical structure of Odontosyllis luciferin (Fig. 3A) is in full agreement with the C14H8O11S3 molecular formula, the neutral loss of 3 CO2 molecules observed in MS/MS fragmentation pattern (SI Appendix, Fig. S11), and the structures of Pink and oxyluciferin (Fig. 4 A and B). The proposed structure of luciferin is supported by literature data on the bioluminescence mechanisms of fireflies (8) and earthworms Fridericia heliota (9), where enzymatic decarboxylation of luciferins leads to the formation of a keto group in the corresponding oxyluciferins, as is seen in the chemical structure of Green.
Based on the chemical structure of Green, we hypothesize that Odontosyllis luciferin undergoes an Odontosyllis luciferase-catalyzed oxidative decarboxylation at the α-position of thiopyran moiety, thereby generating oxyluciferin (Fig. 5). The structure of the nonenzymatic oxidation product suggests a visible-light–induced oxidative decarboxylation in the presence of O2 as the oxidant (Fig. 5). Presumably, photoinduced oxidation of luciferin accompanied by the generation of H2O2 leads to formation of an Odontosyllis luciferin thiopyrylium cation (SI Appendix, Fig. S15), the α-decarboxylation of which affords Pink, as was previously reported for pyrylium salts (10, 11).
Fig. 5.
Chemical structures of Odontosyllis luciferin, oxyluciferin (Green), and a product of nonspecific luciferin oxidation (Pink). Biochemical and chemical transformations lead to the oxyluciferin bioluminescence emitter and the nonspecific oxidation product.
The structures of the newly identified compounds imply 2 possible pathways of Odontosyllis bioluminescence. In the first pathway, the reaction is initiated by the deprotonation at the C7 position of luciferin under luciferase catalysis, followed by addition of O2 via single electron transfer (SET) oxidation (8), leading to the formation of a dioxetanone intermediate and concomitant elimination of water (SI Appendix, Fig. S16). In an alternative possible mechanism, luciferase catalyzes the formation of cyclic anhydride intermediate, thereby increasing the acidity of neighboring α-hydrogen and facilitating its deprotonation. The resulting carbanion is readily oxygenated by O2 to form a peroxide anion, the cyclization of which leads to dioxetanone (SI Appendix, Fig. S16). In the final stage of both pathways, the release of CO2 generates oxyluciferin in an electronically excited state, which produces blue-green light on relaxation to the ground state.
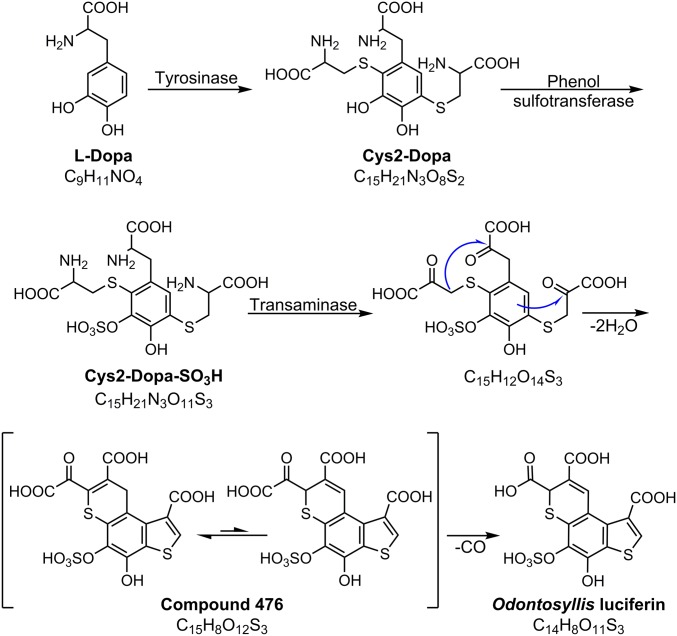
Based on the reasonable structural homology of Odontosyllis luciferin with benzothiazine moieties characteristic of pheomelanins (12), it is possible to propose a sequence of biosynthetic steps leading to the formation of luciferin (Fig. 6). It is likely that biosynthesis of luciferin starts with oxidation of a common tyrosine metabolite, l-DOPA, which undergoes oxidative coupling with 2 cysteine molecules, generating 2,5-dicysteinyldopa intermediate (Cys2-DOPA) (13). In turn, Cys2-DOPA may further undergo sulfation and pyridoxal-catalyzed transamination, followed by spontaneous dehydrative cyclization via aldol condensation and electrophilic thiophene ring closure to form a tricyclic luciferin precursor, compound 476 (Fig. 6). In the final step, oxidative decarboxylation of the latter yields Odontosyllis luciferin (14, 15).
Fig. 6.
Proposed pathway of Odontosyllis luciferin biosynthesis.
A strong argument in favor of this proposed mechanism is the presence of one of the predicted intermediates, compound 476, in the Odontosyllis biomass. The liquid chromatography–mass spectrometry profiles of the luciferin-containing fraction (SI Appendix, Fig. S17) show a major peak at m/z 476.9216 with a fragmentation pattern similar to that of the luciferin (SI Appendix, Fig. S18). The observed molecular ion corresponds to the molecular formula C15H9O12S3+ (calcd m/z = 476.9251), with the difference between this formula and that of the luciferin being CO. Further analysis of NMR spectra of the HPLC-purified substance (1H, 13C-HSQC and 13C HMBC) showed that compound 476 is a mixture of 2 tautomers (at a 3:1 ratio) with characteristic 1H and 13C chemical shifts and 2D cross-peak patterns coincident with the tricyclic thieno[3,2-f]thiochromene cores of Green, Pink, and luciferin (SI Appendix, Fig. S19). The significant change of chemical shift at the C7 position (155.3 ppm) of the major tautomer’s heterocyclic core results from conjugation of the double bond to the carbonyl group, indicating the presence of oxalyl moiety at this position. Thus, the proposed structure of compound 476 shown in Fig. 6 is supported by both MS/MS fragmentation pattern and NMR data.
In summary, we report the isolation and structural characterization of the novel luciferin, a key component of the fireworm O. undecimdonta bioluminescence system, Odontosyllis oxyluciferin (Green), and the product of luciferin nonspecific oxidation (Pink). The elucidation of Odontosyllis luciferin and oxyluciferin structures is a key step in the study of a new bioluminescent system, allowing us to propose the possible mechanisms of the luminescent and nonenzymatic oxidative decarboxylation pathways of this bioluminescent system, as well as shed light on the luciferin biosynthesis pathway. The unusual structures of these molecules present a difficult challenge for synthetic chemists. Once obtained, a new synthetic luciferin together with the recombinant luciferase (5) will further promote investigations of the biosynthetic pathway of luciferin and the chemical origin of the Odontosyllis bioluminescence, as was recently accomplished for fungal bioluminescence system (16). The structures and mechanisms presented here have the potential to stimulate the development of new bioluminescence-based applications in the future.
Materials and Methods
Details of the materials and methods used in this study, including experimental procedures, materials and equipment used for structural characterization of Odontosyllis luciferin, oxyluciferin (Green), and the product of luciferin nonspecific oxidation (Pink), and supplementary figures and tables, are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank the late Dr. Shoji Inoue and Dr. Hisae Kakoi (Meijo University) for providing Odontosyllis materials, Sergey Shakhov for photography, and Drs. Mikhail Baranov and Andrey Mikhaylov for discussions. Some experiments were carried out using equipment provided by the Institute of Bioorganic Chemistry of the Russian Academy of Sciences Сore Facility. Some experiments were supported by Planta LLC. Structural and mechanistic studies were supported by Russian Science Foundation Grant 18-74-10102. Isolation, purification, and biochemical studies were supported by Russian Science Foundation Grant 16-14-00052p. B.R.B. acknowledges support from the Air Force Office of Scientific Research (FA9550-18-1-0017).
Footnotes
Conflict of interest statement: I.V.Y. is shareholder of Planta LLC. Planta LLC filed patent applications related to the use of Odontosyllis luciferin.
This article is a PNAS Direct Submission.
Data deposition: Crystallographic data have been deposited in the Cambridge Crystallographic Data Centre database (CCDC nos. 1840215 and 1840216).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1902095116/-/DCSupplemental.
References
- 1.Harvey E. N., Bioluminescence (Academic Press, New York, 1952). [Google Scholar]
- 2.Shimomura O., Beers J. R., Johnson F. H., The cyanide activation of Odontosyllis luminescence. J. Cell. Comp. Physiol. 64, 15–21 (1964). [DOI] [PubMed] [Google Scholar]
- 3.Shimomura O., Johnson F. H., Saiga Y., Partial purification and properties of the Odontosyllis luminescence system. J. Cell. Comp. Physiol. 61, 275–292 (1963). [Google Scholar]
- 4.Trainor G. L., “Studies on the Odontosyllis bioluminescence system,” PhD dissertation, Harvard Univ, Cambridge, MA (1979).
- 5.Schultz D. T., et al. , Luciferase of the Japanese syllid polychaete Odontosyllis undecimdonta. Biochem. Biophys. Res. Commun. 502, 318–323 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Mitani Y., et al. , Novel gene encoding a unique luciferase from the fireworm Odontosyllis undecimdonta. Sci. Rep. 8, 12789 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue S., Okada K., Tanino H., Kakoi H., A new hexagonal cyclic enol phosphate of 6-beta-hydroxypropionyllumazines from the marine swimming polychaete, Odontosyllis undecimdonta. Heterocycles 35, 147–150 (1993). [Google Scholar]
- 8.Branchini B. R., et al. , Experimental support for a single electron-transfer oxidation mechanism in firefly bioluminescence. J. Am. Chem. Soc. 137, 7592–7595 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Dubinnyi M. A., et al. , Novel mechanism of bioluminescence: Oxidative decarboxylation of a moiety adjacent to the light emitter of Fridericia luciferin. Angew. Chem. Int. Ed. Engl. 54, 7065–7067 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Andreichikov Y. P., Kholodova N. V., Dorofeenko G. N., Generation of pyran-2-ylidene and its reaction with some carbonyl compounds. Dokl. Akad. Nauk SSSR 236, 1364–1366 (1977). [Google Scholar]
- 11.Kholodova N. V., Brovchenko V. G., Pyshchev A. I., Kuznetsov E. V., Dual reacting of monocyclic α-carboxypyrylium salts in reaction with secondary amines. Chem. Heterocycl. Compd. 25, 1312–1313 (1989). [Google Scholar]
- 12.Land E. J., Riley P. A., Spontaneous redox reactions of dopaquinone and the balance between the eumelanic and phaeomelanic pathways. Pigment Cell Res. 13, 273–277 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Agrup G., Hansson C., Rorsman H., Rosengren E., The effect of cysteine on oxidation of tyrosine, dopa, and cysteinyldopas. Arch. Dermatol. Res. 272, 103–115 (1982). [DOI] [PubMed] [Google Scholar]
- 14.Lopalco A., et al. , Mechanism of decarboxylation of pyruvic acid in the presence of hydrogen peroxide. J. Pharm. Sci. 105, 705–713 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopalco A., Stella V. J., Effect of molecular structure on the relative hydrogen peroxide scavenging ability of some α-keto carboxylic acids. J. Pharm. Sci. 105, 2879–2885 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Kotlobay A. A., et al. , Genetically encodable bioluminescent system from fungi. Proc. Natl. Acad. Sci. U.S.A. 115, 12728–12732 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.