Significance
Precise alloying in nanoparticles with the structure retained has long been a major challenge. In addition, little has been achieved for alloy manipulation at the atomic level. Although several structures of bimetallic nanoclusters have been reported, the monodispersity of dopants remains challenging, not to mention the tri- or multimetallic nanoclusters. Here we present a maneuverable nanosystem based on an M29 (M = Au/Ag/Pt/Pd/Cu) nanocluster with the tetra-stratified configuration M1(center)@M12(first shell)@M12(SR)18(second shell)@(M-PPh3)4(vertex). A rich library of 21 nanoclusters ranging from monometallic to tetrametallic compositions has been rationally constructed. The synergetic effects on optical properties and stability have been explicitly mapped out. Our methodology provides fundamental principles toward controllable alloying at the nanoscale with multimetallic compositions and atomically monodispersed dopants.
Keywords: nanocluster, alloy, intermetallic synergy, photoluminescence
Abstract
Exploring intermetallic synergy has allowed a series of alloy nanoparticles with prominent chemical–physical properties to be produced. However, precise alloying based on a maintained template has long been a challenging pursuit, and little has been achieved for manipulation at the atomic level. Here, a nanosystem based on M29(S-Adm)18(PPh3)4 (where S-Adm is the adamantane mercaptan and M is Ag/Cu/Au/Pt/Pd) has been established, which leads to the atomically precise operation on each site in this M29 template. Specifically, a library of 21 species of nanoclusters ranging from monometallic to tetrametallic constitutions has been successfully prepared step by step with in situ synthesis, target metal-exchange, and forced metal-exchange methods. More importantly, owing to the monodispersity of each nanocluster in this M29 library, the synergetic effects on the optical properties and stability have been mapped out. This nanocluster methodology not only provides fundamental principles to produce alloy nanoclusters with multimetallic compositions and monodispersed dopants but also provides an intriguing nanomodel that enables us to grasp the intermetallic synergy at the atomic level.
It has been ca. 5,000 y since the Bronze Age in which “alloying” was first practiced. Alloying has indeed become the most important strategy in tailoring the physical/chemical performances of metal materials. In recent decades, colloidal nanoparticles have been of tremendous academic and industrial interest due to their extraordinary catalytic, optical, magnetic, and electrochemical properties, because alloying of metal nanoparticles with heterometals has proven to be a versatile strategy for improving the nanoparticle performances (1–5). In general, owing to the synergistic effect, alloys often display enhanced properties relative to the monometallic homologs, which largely broadens the applications of such nanomaterials (1–5). However, a detailed understanding of how the synergistic effect arises remains elusive for 2 main reasons: 1) nanoparticles that are uniform at the atomic level are difficult to prepare (usually, a distribution of size was obtained) and 2) their surface chemistry (e.g., metal–ligand interactions) is difficult to study (6). Nanochemists are often frustrated by the well-known fact that no 2 nanoparticles are the same, which precludes the fundamental study on many properties of colloidal nanoparticles in which the total structures must be known. Besides, the atomic-level tailoring of specific sites of nanoparticles with specific numbers of heterometals remains so far the least feasible. Atomic-level understanding of the synergism and atomically precise control over alloy compositions require precise molecular entities to serve as model nanosystems and precise molecular tools.
Atomically precise metal nanoclusters with ultrasmall sizes (e.g., <2-nm diameter of the metallic core) provide an exciting opportunity for investigating structure–property correlations at the atomic level, owing to their uniform size and precise structure (6–23). The quantum size effects of nanoclusters also lead to unprecedented intermetallic quantum synergisms (24–34), and such knowledge will serve as the key to fine tailoring of the physical/chemical performances of alloy nanoparticles. As to the composition/structure of alloy nanoclusters, due to the differences in size and electronegativity each type of heteroatom may prefer different site(s) in the parent nanocluster (35, 36). Taking the most researched Au25(SR)18 as an example, the Ag dopants are preferentially doped at the icosahedral shell, whereas the Cu dopants go to the staple motifs, albeit Cu and Ag are in the same group of gold (37, 38). By comparison, a single Pt or Pd heteroatom can be doped in the Au25 nanocluster template, namely M1Au24(SR)18, wherein the Pt or Pd dopant occupies the central position (39, 40). However, monodispersity of the dopants such as Ag and Cu remains extremely challenging in the MxAu25-x(SR)18 (where x is often a range of Ag or Cu heteroatoms), and this phenomenon extends to other cluster templates. The attainment of monodispersity (i.e., x being a definite number, rather than a range) from bimetallic to multimetallic nanoclusters with the preserved structure is highly desirable for revealing the precise composition–property correlations, and such correlations will allow the controllable syntheses of new alloy nanomaterials with novel structure/composition and tailored performance.
In this work, we exploit the nanocluster system M29(S-Adm)18(PPh3)4 (where M = Ag/Au/Cu/Pt/Pd and S-Adm is adamantanethiolate) to prepare a rich library of multimetallic nanoclusters, with which the atomic-level tailoring has been accomplished in terms of the doping sites, the heterometal types, and the alloying contents. The M29 template possesses a tetra-stratified configuration: M(center)@M12(first shell)@M12(SR)18(second shell)@(Ag-PPh3)4(vertex). In particular, owing to the accessibility of each site with multichoices of metals, a rich library of 21 species of nanoclusters ranging from monometallic to tetrametallic compositions has been successfully prepared step by step via the combination of the in situ synthesis, the targeted metal exchange, and the forced metal exchange. The atomic monodispersity of each nanocluster in this M29 library has been characterized by electrospray ionization mass spectrometry (ESI-MS) analysis. Among them, Pt1Ag12Cu12Au4(SR)18(PPh3)4 and Pd1Ag12Cu12Au4(SR)18(PPh3)4 are tetrametallic nanoclusters with atomic monodispersity. More importantly, the optical properties and thermal stability of these M29 nanoclusters have been compared, and the synergetic effects on these properties have been mapped out at the atomic level. Overall, this M29 (M = Ag/Au/Cu/Pt/Pd) nanosystem offers an ideal platform for unprecedented systematic evaluation of the synergistic effects, which provides impetus for future experimental and theoretical developments on controllable alloy nanomaterials with enhanced performance.
Results and Discussion
The M29 Alloy Library.
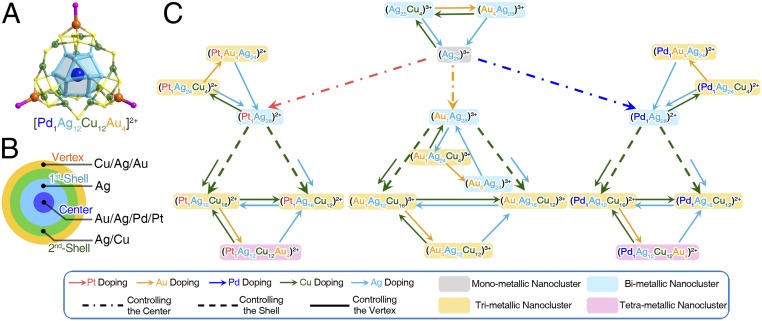
A nanocluster methodology involving a stepwise approach (in situ synthesis–targeted metal exchange–forced metal exchange) has been exploited to alloy the monometallic Ag29 nanocluster with different metals and to form a rich library of multimetallic nanoclusters with a unified configuration as M1(kernel)@M12(first shell)@M12(SR)18(second shell)@(M-PPh3)4(vertex) (M = Ag/Au/Pd/Pt/Cu) (Fig. 1). Specifically, bimetallic M1Ag28(SR)18(PPh3)4 (M = Au/Pt/Pd) nanoclusters are all composed of 28 silver atoms and a single heteroatom. From each bimetallic M1Ag28 nanocluster, 4 trimetallic nanoclusters with different second-shell@vertex compositions have been prepared, for example from Pt1Ag28 to Pt1Ag24Cu4, Pt1Ag24Au4, Pt1Ag12Cu16, and Pt1Ag16Cu12. Note that the central-Pt/Pd/Au atom as well as the Ag12 first shell in such bimetallic nanoclusters is retained in the subsequent alloying processes. Furthermore, the Au-doping operation (i.e., the forced metal-exchange method, discussed below) on the (Pt/Pd)1Ag12Cu16 trimetallic nanoclusters creates the corresponding tetrametallic nanoclusters that follow an arrangement of tetra-stratified Pt/Pd(center)@Ag12(first shell)@Cu12(SR)18(second shell)@(AuPPh3)4(vertex). Each nanocluster (except Pt/Pd doping) adopts a “+3” charge state, while the M29 nanoclusters containing a single Pt or Pd heteroatom are in a “+2” charge state. Since the central Pt/Pd atom contributes no free valence electron, the nominal electron count of each nanocluster in this M29 library is 8e.
Fig. 1.
The library of M29 nanoclusters. (A) Structure of the tetrametallic Pd1Ag12Cu12Au4(S-Adm)18(PPh3)4 nanocluster. (B) Structural anatomy of the center, first shell, second shell, and vertex. (C) Schematic illustration of the stepwise alloying process from monometallic to bi-, tri-, and tetrametallic nanoclusters on the basis of the M29 template. The pink, orange, blue, green, and cerulean arrows indicate the alloying pathways of doping Pt, Au, Pd, Cu, and Ag, respectively. The gray, blue, orange, and purple backgrounds indicate the mono-, bi-, tri-, and tetrametallic compositions of each nanocluster, respectively. For clarity, the ligand parts (SR and PPh3) are omitted in labels (the same below).
Controlling the Central Atom in the M29 Template.
The parent Ag29 and single-heteroatom doped M1Ag28 (M = Au/Pt/Pd) nanoclusters have been successfully prepared via an in situ synthetic procedure. The optical absorption, emission, and ESI-MS results of the obtained Pt1Ag28 are the same as those reported previously (41), where the single-Pt atom occupies the innermost center of the face-centered (fcc) Pt1Ag12 core (Fig. 2; also see SI Appendix, Fig. S1 for the structural anatomy of the M1Ag28 nanoclusters). Similarly, the central occupation of the single heteroatom has been observed in the crystal structure of Au1Ag28(SR)18(PPh3)4. Considering 1) the homolog of these M1Ag28(SR)18(PPh3)4 nanoclusters, 2) the central occupying tendency of the Pd heteroatom in the M13 kernel (25, 40), and 3) the same Pd X-ray photoelectron spectroscopy (XPS) signal between Pd1Ag28(SR)18(PPh3)4 and Pd1Ag24(S-PhMe2)18 (SI Appendix, Fig. S2), we thus propose the similar construction of the Pd1Ag28 with the determined structures of Pt1Ag28 and Au1Ag28 nanoclusters. In the fcc M1Ag12 core, the coordination number of the innermost metal is 12, the same as that in the fcc crystal lattice of the bulk Au/Ag/Pt/Pt or their alloys.
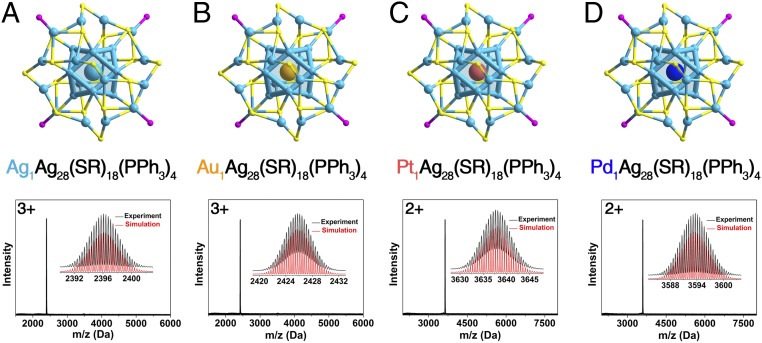
Fig. 2.
M29 nanoclusters from monometallic to bimetallic. Structures and ESI-MS spectra of (A) Ag29 and its central doped (B) Au1Ag28, (C) Pt1Ag28, and (D) Pd1Ag28 nanoclusters. (Insets) Experimental and simulated isotope patterns of each nanocluster. Color codes: cerulean sphere, Ag; orange sphere, Au; pink sphere, Pt; blue sphere, Pd; yellow sphere, S; purple sphere, P. For clarity, the carbon and hydrogen atoms are not shown.
ESI-MS was performed to validate the monodispersity of each nanocluster (Fig. 2, Bottom). First of all, the ESI-MS of each purified product shows a single intense peak, demonstrating the monodispersity of each nanocluster. In addition, the spacing of the mass peaks indicates a “3+” charge state of Ag29 and Au1Ag28 nanoclusters but a “2+” charge state for the single Pt or Pd-doped M1Ag28, indicating no free valence electron contribution from the Pt or Pd dopant. Accordingly, these 4 nanoclusters all represent an 8-electron closed-shell electronic configuration (i.e., 29-18-3 = 8e for Ag29 and Au1Ag28 and 28-18-2 = 8e for Pt1Ag28 and Pd1Ag28).
Controlling the Shell Atoms in the M29 Template.
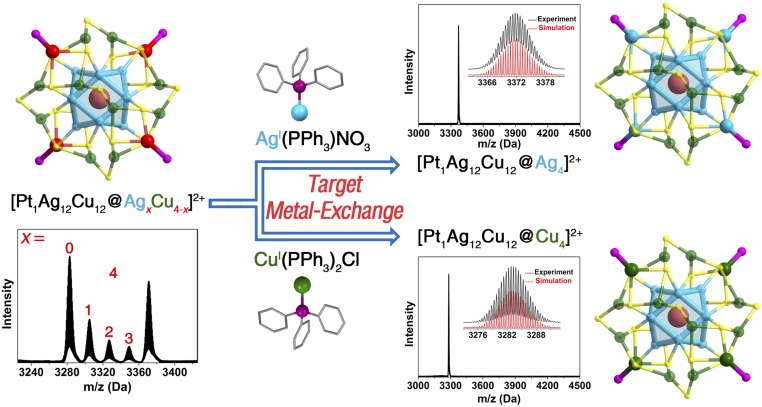
Transforming the M-Ag (M = Au/Pt/Pd) “precursor” into the M-Ag-Cu results in the generation of trimetallic M1Ag16-xCu12+x nanoclusters with polydispersed x = 0–4. Considering that the number of the alternative occupying sites (i.e., 4) is the same as that of the vertex Ag-PPh3 units in the Ag29 template, these vertex sites are proposed to be the Ag-to-Cu exchange locations; in other words, the Ag12(first shell)@Cu12(second shell) constructions in such nanoclusters are nonswappable. Density functional theory (DFT) calculations also demonstrated that the metal-exchange location is the vertex, instead of sites on the second shell (SI Appendix, Figs. S3 and S4). Besides, by summing up the previous alloy nanoclusters that containing the Au/Ag and Cu metals (42–44), it is suggested that the first shell in this M29 template is totally occupied by the 12 Ag atoms, and the second-shell metal is Cu (such arrangements have also been confirmed by the crystal structure of Pt1Ag12Cu16(SR)18(PPh3)4). Taking the Pt-centered nanosystem as an example (Fig. 3, Left), the ESI-MS spectrum of the in situ-prepared product displays 5 peaks centered at 3283.3, 3305.3, 3327.3, 3349.8, and 3371.8 Da, corresponding to the Pt1Ag12Cu16(SR)18(PPh3)4, Pt1Ag13Cu15(SR)18(PPh3)4, Pt1Ag14Cu14(SR)18(PPh3)4, Pt1Ag15Cu13(SR)18(PPh3)4, and Pt1Ag16Cu12(SR)18(PPh3)4, respectively (SI Appendix, Figs. S5 and S6). The mass spacing of adjacent peaks is 22 Da, which matches the expected gap between Ag and Cu atoms, that is, [M(Ag)-M(Cu)]/2(charge) = 22 Da. Consequently, the trimetallic Pt@Ag@Cu nanoclusters have been successfully synthesized, albeit with polydispersed dopant numbers (Fig. 3, Left).
Fig. 3.
M29 nanoclusters with trimetallic compositions. Structures and ESI-MS spectra of Pt1Ag12Cu12@AgxCu4-x(SR)18(PPh3)4 (x = 0–4) and its metal-exchanged products: trimetallic Pt1Ag16Cu12(SR)18(PPh3)4 and Pt1Ag12Cu16(SR)18(PPh3)4 nanoclusters with monodispersity. (Insets) Experimental and simulated isotope patterns of each nanocluster. Color codes: cerulean sphere, Ag; pink sphere, Pt; green sphere, Cu; red sphere, mixed Ag/Cu; yellow sphere, S; purple sphere, P. Carbon and hydrogen atoms are not shown. For clarity, the chemical formula of each nanocluster is only marked with the corresponding metal composition (the same below), because all nanoclusters in this M29 system follow the same configuration as M1@M12@M12(SR)18@(M-PPh3)4 (M = Ag/Au/Pd/Pt/Cu).
The metal-exchange method has been extensively exploited to alloy the parent nanocluster with a retained framework (28, 35, 36). For example, the icosahedral-shell Au atoms are substituted by Ag atoms when alloying Au25(SR)18 with the AgI-SR complex (35). Besides, the reversible process has been observed by reacting the as-alloyed AgxAu25-x(SR)18 with the AuI–SR complex (35). In this context, we are motivated to “focus” the distribution of Pt1Ag16-xCu12+x(SR)18(PPh3)4 into monodispersed nanoclusters (i.e., a single x value) by way of a targeted metal-exchange method. As depicted in Fig. 3, we indeed found that reaction of the Pt1Ag16-xCu12+x(SR)18(PPh3)4 with a large amount of AgI(PPh3)NO3 complex generates the monodispersed Pt1Ag16Cu12(SR)18(PPh3)4 nanocluster, which exhibits a single mass peak centered at 3,371.8 Da in the ESI-MS spectrum. Accordingly, the substitution of vertex Cu atoms by Ag atoms occurs in the parent nanoclusters. Furthermore, the detected [CuI(PPh3)2]+ (centered at 587.11 Da; SI Appendix, Fig. S7; ESI-MS spectra of the starting Pt1Ag16-xCu12+x(SR)18(PPh3)4 are shown in SI Appendix, Figs. S5 and S6) also validates the targeted metal-exchange process, which can be summarized as
On the other hand, reacting the Pt1Ag16-xCu12+x(SR)18(PPh3)4 with CuI(PPh3)2Cl results in the monodispersed Pt1Ag12Cu16(SR)18(PPh3)4 nanocluster, whose composition and construction have been verified by the ESI-MS analysis and X-ray crystallography (Fig. 3, Lower Right and SI Appendix, Fig. S8). Besides, Pd-centered and Au-centered trimetallic nanoclusters have also been obtained with a procedure similar to that of the Pt-centered nanosystem (see SI Appendix, Figs. S9–S13 for the Pd-centered nanosystem and SI Appendix, Figs. S14–S18 for the Au-centered nanosystem).
Controlling the Vertex Atoms in the M29 Template.
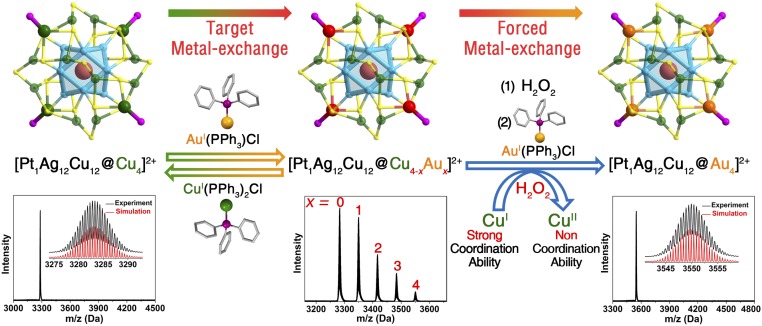
Based on the above understandings of the vertex metal atoms (bonded with the PPh3 ligands) with exchangeable characteristic, we perceive a good opportunity to obtain the alloyed M29 nanoclusters containing more than 3 types of metals. Recently, Negishi and coworkers (36) reported the synthesis of tetrametallic Pd1AgxCuyAu24-x-y(SR)18 nanoclusters by exploiting the metal-exchange rules that different metal dopants preferentially occupy different sites in the parent Au25(SR)18 nanocluster. However, alloying in the Au25 template always results in a mixture product with distributed dopant numbers, for example AgxAu25-x and CuxAu25-x nanoclusters (35, 37, 38). In comparison, the M29 nanosystem can act as a perfect model to obtain the multimetallic nanocluster in atomic monodispersity. As shown in Fig. 4 and SI Appendix, Figs. S19 and S20, the further metal-exchange operation on the trimetallic Pt1Ag12Cu16(SR)18(PPh3)4 has been performed by reacting the nanocluster with AuI(PPh3)Cl. On the one hand, the alloying outcomes follow an incompletely metal-exchanged pattern [even with the overdose of AuI(PPh3)Cl] and 5 peaks including Pt1Ag12Cu12@Cu4(SR)18(PPh3)4, Pt1Ag12Cu12@Cu3Au1(SR)18(PPh3)4, Pt1Ag12Cu12@Cu2Au2(SR)18(PPh3)4, Pt1Ag12Cu12@Cu1Au3(SR)18(PPh3)4, and Pt1Ag12Cu12@Au4(SR)18(PPh3)4 are detected in the ESI-MS (note that the maximum Au alloying number is 4, validating the vertex alloying mode in these M29 nanosystems). On the other hand, reacting the mixture of Pt1Ag12Cu16-xAux(SR)18(PPh3)4 (x = 0–4) nanoclusters with only tiny amounts of CuI(PPh3)2Cl results in the monodispersed Pt1Ag12Cu16(SR)18(PPh3)4 nanocluster (Fig. 4). Combining the above 2 aspects, it is suggested that the bonding ability of Cu atoms in these 4 vertex sites is far stronger than that of the Au atoms. Considering that 1) the bonding mode of these vertex metals is μ3-M (M = Cu/Au) and 2) μ3-Cu has been reported several times (however, μ3-Au has not been observed yet) (42–44), we speculate that the stability of such μ3-Au is not as high as that of the μ3-Cu in the M29 nanosystem (SI Appendix, Fig. S21). The instability of vertex-Au nanoclusters has also been proved by the stability tests (discussed below) as well as DFT calculations (SI Appendix, Fig. S3).
Fig. 4.
M29 nanoclusters from trimetallic to tetrametallic. Illustration of the synthetic procedures from Pt1Ag12Cu16 to poly-dispersed Pt1Ag12Cu16-xAux (x = 0–4), and then to monodispersed Pt1Ag12Cu12Au4 nanocluster. The blue curve represents the oxidation from Cu(I) to Cu(II) effected by H2O2, thus the noncoordinate ability of Cu(II) leads to the monodispersity of the final tetrametallic Pt1Ag12Cu12Au4 nanocluster. Color codes: cerulean sphere, Ag; pink sphere, Pt; orange sphere, Au; green sphere, Cu; red sphere, mixed Au/Cu; yellow sphere, S; purple sphere, P. For clarity, the carbon and hydrogen atoms are not shown.
For obtaining the tetrametallic Pt1Ag12Cu12Au4 nanocluster with monodispersity, the 4 vertex Cu atoms should be substituted by the Au dopants completely. By analyzing the ESI-MS results of metal-exchange processes from Pt1Ag16-xCu12+x to Pt1Ag12Cu16 (or Pt1Ag16Cu12), no oxidation-reduction but just metal-exchange processes Ag(I)→Cu(I) or Cu(I)→Ag(I) occur, indicating that the vertex Cu atoms are almost in the Cu(I) valence state. By noting that adding the CuII(PPh3)2Cl2 to the Pt1Ag16-xCu12+x nanoclusters cannot convert them into monodispersed Pt1Ag12Cu16 (SI Appendix, Fig. S22), we hypothesize that the Cu(II) atoms have no coordination ability to act as the μ3-Cu at the vertex sites. Therefore, considering the competitive relationship of Au and Cu in these sites, the oxidation capability of H2O2 has been exploited to weaken the coordination ability of Cu atoms and this indeed allows us to prepare the monodispersed Pt1Ag12Cu12Au4 nanocluster (Fig. 4, Right). In this “forced metal-exchange” process, the addition of H2O2 turns the Cu(I) into the oxidized state [Cu(II), shown in SI Appendix, Fig. S23], and further addition of the AuI(PPh3)Cl guarantees the existence of the Au(I) that is able to occupy the vertex sites. The H2O2/AuI(PPh3)Cl addition is repeated 3 times to eliminate the CuI coordination completely, and then ESI-MS demonstrates the generation of the tetrametallic Pt1Ag12Cu12Au4(SR)18(PPh3)4 with monodispersity (the Pd-centered procedure and results are in SI Appendix, Figs. S24–S26). This result is exciting since it not only produces a tetrametallic nanocluster with precise metallic occupancy but also evokes the possibility for revealing the intermetallic synergy in detail in a coherent monodispersed nanosystem from monometallic to bi-, tri-, and tetrametallic compositions. However, the obtained Au-vertex nanoclusters (Pt1Ag12Cu12Au4 or Pd1Ag12Cu12Au4) are less stable and decompose in solution (SI Appendix, Fig. S23), in agreement with the DFT results that the energy of Au-vertex nanoclusters is much higher than that of Cu-vertex nanoclusters (SI Appendix, Fig. S3).
Overview of the Unique M29 Nanosystem.
As summarized in SI Appendix, Fig. S27, a unique nanosystem has been established based on the Pt-centered M29 template (see SI Appendix, Fig. S28 for the Pd-centered nanosystem). With the controllable synthetic procedure (combining the in situ synthesis and metal-exchange method), the monometallic Ag29 has been precisely alloyed to bimetallic Pt1Ag28, trimetallic Pt1Ag12Cu16, and tetrametallic Pt1Ag12Cu12Au4 nanoclusters (SI Appendix, Fig. S27A). SI Appendix, Fig. S27B shows the structural anatomy of the Pt1Ag12Cu12Au4 nanocluster. Specifically, the single Pt atom occupies the innermost position and is further enclosed by 12 Ag atoms, constituting the Pt1Ag12 kernel. Then, four 3-fold symmetric Cu3(S-Adm)6 staple motifs wrap up the Pt1Ag12 kernel. Because these Cu3(S-Adm)6 motifs connect each other by sharing the end thiolates, a cage-like Cu12(SR)18 is formed. Finally, 4 vertex Au-PPh3 units block the bare corners and the whole structure is constructed, formulated Pt1Ag12Cu12Au4(S-Adm)18(PPh3)4 with a tetra-stratified configuration—Pt(center)@Ag12(first shell)@Cu12(S-Adm)18(second shell)@(Ag-PPh3)4(vertex).
According to the aforementioned nanocluster methodology (including the in situ synthesis, targeted metal-exchange, and forced metal-exchange methods), a library of 21 species of M29 (M = Ag/Cu/Pt/Pd/Au) nanoclusters has been successfully constructed. The “in situ synthesis” guarantees the single-metal occupation of the kernel, the first shell, and the second shell. Besides, a combination of “targeted metal-exchange” and “forced metal-exchange” methods guarantees the single-metal occupation of the vertex. SI Appendix, Fig. S29 generalizes the ESI-MS spectra and metal arrangements of these nanoclusters. ESI-MS results demonstrated the monodispersity of each nanocluster, which is of significance for the subsequent investigation on the metal synergistic effect at the atomic level. The monodispersity of each nanocluster was also verified by the elemental analysis and the inductively coupled plasma-atomic emission spectrometry results of these M29 nanoclusters (SI Appendix, Tables S1 and S2). The NMR result of the M29 nanoclusters (here we tested Pt1Ag28, Au1Ag28, and Pt1Ag12Cu16 nanoclusters that had been crystallized) presented only one 31P peak, demonstrating the same chemical environment of the 4 PPh3 ligands as well as the symmetry of the nanocluster’s configuration (SI Appendix, Fig. S30).
Seven subsystems have been established on the basis of the different metal arrangement, including Ag25@M4, Au1Ag24@M4, Pt1Ag24@M4, Pd1Ag24@M4, Au1Ag12Cu12@M4, Pt1Ag12Cu12@M4, and Pd1Ag12Cu12@M4 subsystems. Each subsystem has the same M1(center)@M12(first shell)@M12(SR)18(second shell) metal configuration. For each subsystem, 3 different types of metals could be arranged (i.e., Ag, Cu, or Au) on the vertex sites, giving rise to 3 different nanocluster members in each subsystem (SI Appendix, Figs. S31–S37). Accordingly, altogether 21 nanoclusters (7 × 3 = 21) with monodispersity have been obtained for the M29 library. Evidenced by the mass spectra, the nanocluster’s overall charge is “+3” when the kernel position is occupied by Ag or Au, whereas the charge is “+2” when Pt or Pd is at the innermost of the nanocluster (SI Appendix, Figs. S30–S37).
Synergy Effect on the Nanocluster Properties.
The intermetallic synergism has been experimentally and theoretically investigated based on alloy nanoclusters, and has been proved to be the decisive effect that influences the chemical and physical properties of nanoclusters (e.g., optical, catalytic, electrochemical properties, and stability) (24–26, 30, 32–34, 45–49). The available M29 nanosystem now allows us to evaluate the synergistic effects in detail. Here we focus on the synergistic effects on the electronic structure of nanoclusters, and optical absorption, photoluminescence (PL), and stability.
First of all, we find that each nanocluster in the M29 system possesses the 8e free-electronic structure. In this context, a comparison of the oxidation states of metals in different M29 nanoclusters can help us infer the synergistic effects on electronic structures of these nanoclusters. SI Appendix, Figs. S38–S40 present the XPS results of different M29 nanoclusters. From the XPS results, the substitution of Ag atoms in the second shell or vertex positions by Cu leads to the concentration of free valence electrons to the cluster’s kernel, and thus the XPS peaks of inner metals shift to the M(0) peak. Collectively, owing to the differences of the metal electronegativity, substituting the parent shell metal (Ag) with more active metal (Cu) would make the free electrons less bound to the outermost shell and concentrating to the kernel. Thus, the kernel metals would tend to present a metallic state (i.e., M0), and the shell metals will be in a more oxidized state (i.e., Mδ+).
SI Appendix, Figs. S41–S44 present the optical absorption and PL of the 21 nanoclusters, sorted by the metal occupation of the kernel. Some important structure-optical absorption correlations have been mapped out: 1) For the kernel, substituting the central Ag atom by a Pd heteroatom hardly changes the optical absorption, whereas doping a Pt/Au heteroatom into the kernel significantly red-shifts the absorption; 2) for the second shell, exchanging the Ag12(SR)18 into Cu12(SR)18 blue-shifts the optical absorption while maintaining their initial profiles; 3) for the vertex, the characteristic absorption profiles are almost retained when the vertex Ag atoms are substituted by Cu; by contrast, metal exchanging of these vertex atoms into Au not only red-shifts the initial absorption but also generates a new peak at the range of higher wavelength.
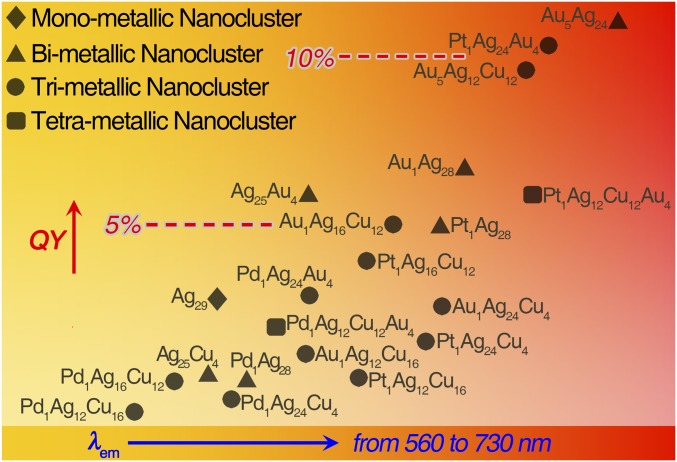
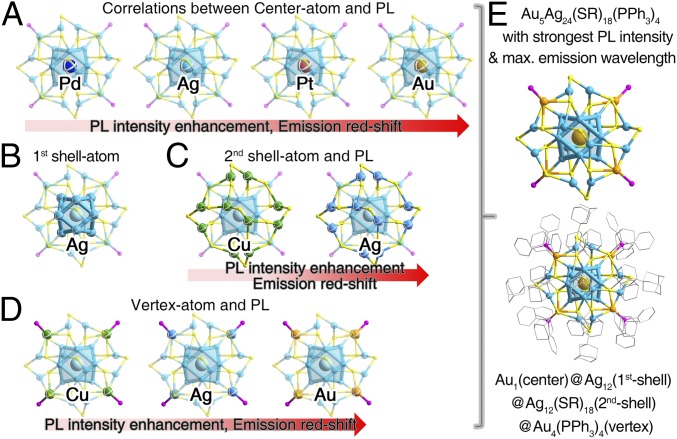
Interestingly, all nanoclusters in the M29 nanosystem fluoresce when illuminated at 445 nm (SI Appendix, Figs. S41–S44). By comparing these PL spectra, synergy effects on PL characteristics in terms of PL intensity and emission wavelength are analyzed (Fig. 5 and SI Appendix, Figs. S45–S48). For the kernel, doping a Pd heteroatom into the center of M29 weakens the PL intensity and blue-shifts the emission; on the contrary, enhanced PL intensity as well as red-shifted emission has been observed by substituting the center Ag with a Pt or Au atom (SI Appendix, Fig. S45). For the second shell, alloying the Ag12(SR)18 shell into Cu12(SR)18 blue-shifts the emission wavelength and slightly reduces the PL intensity (SI Appendix, Fig. S46). For the vertex, substituting the vertex Ag atoms by Au cannot only red-shift the emission but also significantly enhance the PL intensity; opposite phenomena have been observed in exchanging the vertex Ag atoms with Cu (SI Appendix, Fig. S47). According to the above observations, the Au1@Ag12@Ag12(SR)18@(Au-PPh3)4 displays the highest PL intensity and maximum emission wavelength (with quantum yield of 11.6% and emission of 715 nm) among the 21 nanoclusters (Figs. 5 and 6). Collectively, based on the abovementioned correlations between the metal arrangement in the M29 template and PL properties, we find that controlling the nanocluster kernel or vertex metals with large electron affinity [note: metal electron affinity subsequence Au(2.309) > Pt(2.128) > Ag(1.302) > Cu(1.228) > Pd(0.562)] is in favor of preparing emissive nanoclusters with higher PL intensity. The obtained general trends of PL with different doping modes will provide guidelines for future work on fluorescent alloy nanoclusters.
Fig. 5.
Comparison of PL property of the M29 nanoclusters. The rhomboid, triangular, circular, and square symbols represent the mono-, bi-, tri-, and tetrametallic nanoclusters, respectively.
Fig. 6.
(A–D) Illustration of the strategies to enhance the PL intensity of the M29 nanocluster. (E) Au5Ag24(SR)18(PPh3)4 nanocluster with the strongest PL intensity and the maximum emission wavelength, among these 21 nanoclusters of M29.
Synergy effects on the thermal stability of these nanoclusters have also been investigated (SI Appendix, Figs. S48–S53). Time-dependent optical absorption of these nanoclusters (dissolved in CH2Cl2) was monitored at room temperature (SI Appendix, Figs. S48–S51). By comparing the variation trends of the absorptions, we conclude that 1) for the kernel, the stability sequence is Pd1M28 < Ag1M28 < Au1M28 ∼ Pt1M28; 2) for the second shell, alloying the Ag12(SR)18 shell into Cu12(SR)18 enhances the stability of nanoclusters; and 3) for the vertex, the stability sequence is M25Cu4 > M25Ag4 >> M25Au4 (SI Appendix, Figs. S52 and S53). The correlations between the metal occupation in vertex and the stability of nanoclusters agree with the energy changes of nanoclusters derived from the DFT calculations (SI Appendix, Fig. S3).
Conclusion
We have developed a methodology to prepare atomically precise alloy nanoclusters based on the M29(S-Adm)18(PPh3)4 template (M = Au/Ag/Pd/Pt/Cu) with a tetra-stratified configuration—M1(center)@M12(first shell)@M12(SR)18(second shell)@(M-PPh3)4(vertex). Owing to the easy maneuverability of each site in this M29 template, a library of 21 nanoclusters with atomic monodispersity has been synthesized by exploiting the in situ synthesis, targeted metal-exchange, and forced metal-exchange methods, and these nanoclusters range from monometallic to bi-, tri-, and tetrametallic constitutions. The monodispersity of each nanocluster has been verified by the ESI-MS measurement. In addition, the precise structures of these M29 nanoclusters enable us to uncover the intermetallic synergy at the atomic level, which provides guidelines for future work on alloy nanoclusters. Overall, the M29 nanosystem presented in this work is of significance not only because it provides a platform to generate alloyed nanoclusters with multimetallic compositions and monodispersed dopants but also it offers atomic-level insight into the intermetallic synergy.
Methods
Synthesis of the Monometallic [Ag29(S-Adm)18(PPh3)4]3+.
For the nanocluster synthesis, AgNO3 (30 mg, 0.18 mmol) was dissolved in CH3OH (5 mL) and CH3COOC2H5 (35 mL) with sonication. The solution was vigorously stirred (∼1,200 rpm) with magnetic stirring for 15 min. Then, Adm-SH (0.1 g) and PPh3 (0.1 g) were added and the reaction was vigorously stirred (∼1,200 rpm) for another 90 min. After that, NaBH4 (1 mL) aqueous solution (20 mg⋅mL−1) was added quickly to the above mixture. The reaction was allowed to proceed for 36 h under a N2 atmosphere. After that, the aqueous layer was removed, and the mixture in the organic phase was rotavaporated under vacuum. Then ∼15 × 3 mL of CH3OH was used to wash the synthesized nanoclusters. The precipitate was dissolved in CH2Cl2, which produced the [Ag29(S-Adm)18(PPh3)4]3+ nanocluster. The yield is 16% based on the Ag element (calculated from the AgNO3).
Synthesis of the Bimetallic [Pt1Ag28(S-Adm)18(PPh3)4]2+.
Specifically, the metal source for synthesizing [Ag29(S-Adm)18(PPh3)4]3+ nanocluster (i.e., AgNO3, 0.18 mmol) was altered to Ag/Pt mixture (AgNO3/H2PtCl6·6H2O = 0.17/0.01 mmol in the [Pt1Ag28(S-Adm)18(PPh3)4]2+ synthesis) and the other conditions were not changed, then the [Pt1Ag28(S-Adm)18(PPh3)4]2+ nanoclusters were obtained. The yield is 45% based on the Ag element (calculated from the AgNO3).
Synthesis of Trimetallic [Pt1Ag12+xCu16-x(S-Adm)18(PPh3)4]2+ (x = 0–4).
Specifically, the metal source for synthesizing [Ag29(S-Adm)18(PPh3)4]3+ nanocluster (i.e., AgNO3, 0.18 mmol) was altered to Ag/Cu/Pt mixture (AgNO3/CuI(PPh3)2Cl/H2PtCl6·6H2O = 0.1/0.07/0.01 mmol in the [Pt1Ag12+xCu16-x(S-Adm)18(PPh3)4]2+ synthesis) and the other conditions were not changed, then the [Pt1Ag12+xCu16-x(S-Adm)18(PPh3)4]2+ nanoclusters were obtained. The yield is 15% based on the Ag element (calculated from the AgNO3).
Synthesis of Trimetallic [Pt1Ag12Cu16(S-Adm)18(PPh3)4]2+ and [Pt1Ag16Cu12(S-Adm)18(PPh3)4]2+.
A target metal-exchange method was exploited to “focus” the polydispersed [Pt1Ag12+xCu16-x(S-Adm)18(PPh3)4]2+ (x = 0–4) nanoclusters into the [Pt1Ag12Cu16(S-Adm)18(PPh3)4]2+ or [Pt1Ag16Cu12(S-Adm)18(PPh3)4]2+. Specifically, 0.1 mmol [Pt1Ag12+xCu16-x(S-Adm)18(PPh3)4]2+ was dissolved in 20 mL CH2Cl2; 1 mmol CuI(PPh3)2Cl was added to the above solution and the solution was further vigorously stirred (∼1,200 rpm) for 30 min. The organic phase was rotavaporated under vacuum and washed several times with CH3OH. The precipitate was dissolved in CH2Cl2, which produced pure [Pt1Ag12Cu16(S-Adm)18(PPh3)4]2+. The yield is 80% based on the [Pt1Ag12+xCu16-x(S-Adm)18(PPh3)4]2+ nanoclusters. Changing the CuI(PPh3)Cl into the AgI(PPh3)NO3 generated pure [Pt1Ag16Cu12(S-Adm)18(PPh3)4]2+ with a 70% yield.
Synthesis of Tetrametallic [Pt1Ag12Cu16-xAux(S-Adm)18(PPh3)4]2+ (x = 0–4).
A “target metal-exchange” method was exploited to alloy the trimetallic [Pt1Ag12Cu16(S-Adm)18(PPh3)4]2+ nanocluster into the tetrametallic [Pt1Ag12Cu16-xAux(S-Adm)18(PPh3)4]2+ (x = 0–4) nanoclusters. Specifically, 0.1 mmol [Pt1Ag12Cu16(S-Adm)18(PPh3)4]2+ was dissolved in 20 mL CH2Cl2; 0.5 mmol AuI(PPh3)Cl was added to the above solution and the solution was further vigorously stirred (∼1,200 rpm) for 60 min. The organic phase was rotavaporated under vacuum. The precipitate was dissolved in CH2Cl2, which produced the tetrametallic [Pt1Ag12Cu16-xAux(S-Adm)18(PPh3)4]2+ nanocluster. The yield is 85% based on the [Pt1Ag12Cu16(S-Adm)18(PPh3)4]2+.
Synthesis of Tetrametallic [Pt1Ag12Cu12Au4(S-Adm)18(PPh3)4]2+.
The “forced metal-exchange” method was exploited to alloy the polydispersed [Pt1Ag12Cu16-xAux(S-Adm)18(PPh3)4]2+ (x = 0–4) nanoclusters into the monodispersed [Pt1Ag12Cu12Au4(S-Adm)18(PPh3)4]2+ nanocluster. Specifically, 0.1 mmol [Pt1Ag12Cu16-xAux(S-Adm)18(PPh3)4]2+ was dissolved in 20 mL CH2Cl2; 200 μL H2O2 was added and the solution was further vigorously stirred (∼1,200 rpm) for 3 min. Then, 0.5 mmol AuI(PPh3)Cl was added to the above solution and further vigorously stirred for 30 min. The H2O2@AuI(PPh3)Cl addition was repeated 3 times to eliminate the CuI coordination completely. The organic phase was then precipitated with a large amount of CH3OH. Finally, the precipitate was dissolved in CH2Cl2, which produced the monodispersed [Pt1Ag12Cu16Au4(S-Adm)18(PPh3)4]2+ nanocluster. The yield is 20% based on the [Pt1Ag12Cu16-xAux(S-Adm)18(PPh3)4]2+.
Crystallization of Au1Ag28(S-Adm)18(PPh3)4 and Pt1Ag12Cu16(S-Adm)18(PPh3)4.
Single crystals of Au1Ag28(S-Adm)18(PPh3)4 and Pt1Ag12Cu16(S-Adm)18(PPh3)4 nanoclusters were grown at room temperature for 7 d in CH2Cl2/CH3OH. Then, red crystals were collected and the structures of Au1Ag28(S-Adm)18(PPh3)4 and Pt1Ag12Cu16(S-Adm)18(PPh3)4 were determined by X-ray crystallography.
Other than the nanoclusters in the Pt-centered system, details of synthesis of other cases are provided in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China Grants U1532141, 21631001, 21871001, and 21803001; the Ministry of Education; the Education Department of Anhui Province (KJ2017A010); and the 211 Project of Anhui University. R.J. is financially supported by the Air Force Office of Scientific Research under Award FA9550-15-1-9999 (FA9550-15-1-0154).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, https://www.ccdc.cam.ac.uk (accession codes 1872534 and 1872544).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1912719116/-/DCSupplemental.
References
- 1.Chen P. C., et al. , Polyelemental nanoparticle libraries. Science 352, 1565–1569 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Gilroy K. D., Ruditskiy A., Peng H. C., Qin D., Xia Y., Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 116, 10414–10472 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Zeb Gul Sial M. A., Ud Din M. A., Wang X., Multimetallic nanosheets: Synthesis and applications in fuel cells. Chem. Soc. Rev. 47, 6175–6200 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Wang S., et al. , Shuttling single metal atom into and out of a metal nanoparticle. Nat. Commun. 8, 848 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenton J. L., Steimle B. C., Schaak R. E., Tunable intraparticle frameworks for creating complex heterostructured nanoparticle libraries. Science 360, 513–517 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Jin R., Zeng C., Zhou M., Chen Y., Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chem. Rev. 116, 10346–10413 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Chakraborty I., Pradeep T., Atomically precise clusters of noble metals: Emerging link between atoms and nanoparticles. Chem. Rev. 117, 8208–8271 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Liu P., Qin R., Fu G., Zheng N., Surface coordination chemistry of metal nanomaterials. J. Am. Chem. Soc. 139, 2122–2131 (2017). [DOI] [PubMed] [Google Scholar]
- 9.van der Linden M., et al. , Single Au atom doping of silver nanoclusters. ACS Nano 12, 12751–12760 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higaki T., et al. , Anomalous phonon relaxation in Au333(SR)79 nanoparticles with nascent plasmons. Proc. Natl. Acad. Sci. U.S.A. 116, 13215–13220 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou M., et al. , Three-orders-of-magnitude variation of carrier lifetimes with crystal phase of gold nanoclusters. Science 364, 279–282 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Agrachev M., Ruzzi M., Venzo A., Maran F., Nuclear and electron magnetic resonance spectroscopies of atomically precise gold nanoclusters. Acc. Chem. Res. 52, 44–52 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Cook A. W., Hayton T. W., Case studies in nanocluster synthesis and characterization: Challenges and opportunities. Acc. Chem. Res. 51, 2456–2464 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Yao Q., Yuan X., Chen T., Leong D. T., Xie J., Engineering functional metal materials at the atomic level. Adv. Mater. 30, e1802751 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Bhattarai B., et al. , Chemistry and structure of silver molecular nanoparticles. Acc. Chem. Res. 51, 3104–3113 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Kwak K., Lee D., Electrochemistry of atomically precise metal nanoclusters. Acc. Chem. Res. 52, 12–22 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Nieto-Ortega B., Bürgi T., Vibrational properties of thiolate-protected gold nanoclusters. Acc. Chem. Res. 51, 2811–2819 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Weerawardene K. L. D. M., Häkkinen H., Aikens C. M., Connections between theory and experiment for gold and silver nanoclusters. Annu. Rev. Phys. Chem. 69, 205–229 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Zhao T., Herbert P. J., Zheng H., Knappenberger K. L. Jr, State-resolved metal nanoparticle dynamics viewed through the combined lenses of ultrafast and magneto-optical spectroscopies. Acc. Chem. Res. 51, 1433–1442 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Kang X., Zhu M., Tailoring the photoluminescence of atomically precise nanoclusters. Chem. Soc. Rev. 48, 2422–2457 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Kenzler S., Schrenk C., Schnepf A., Au108S24(PPh3)16: A highly symmetric nanoscale gold cluster confirms the general concept of metalloid clusters. Angew. Chem. Int. Ed. Engl. 56, 393–396 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Sugiuchi M., Shichibu Y., Konishi K., An inherently chiral Au24 framework with double-helical hexagold strands. Angew. Chem. Int. Ed. Engl. 57, 7855–7859 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Chen W., Chen S., Oxygen electroreduction catalyzed by gold nanoclusters: Strong core size effects. Angew. Chem. Int. Ed. Engl. 48, 4386–4389 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Kwak K., et al. , A molecule-like PtAu24(SC6H13)18 nanocluster as an electrocatalyst for hydrogen production. Nat. Commun. 8, 14723 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie S., Tsunoyama H., Kurashige W., Negishi Y., Tsukuda T., Enhancement in aerobic alcohol oxidation catalysis of Au25 clusters by single Pd atom doping. ACS Catal. 2, 1519–1523 (2012). [Google Scholar]
- 26.Bootharaju M. S., Joshi C. P., Parida M. R., Mohammed O. F., Bakr O. M., Templated atom-precise galvanic synthesis and structure elucidation of a [Ag24Au(SR)18]- nanocluster. Angew. Chem. Int. Ed. Engl. 55, 922–926 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Udayabhaskararao T., et al. , Ag7Au6: A 13-atom alloy quantum cluster. Angew. Chem. Int. Ed. Engl. 51, 2155–2159 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Chang W. T., et al. , Eight-electron silver and mixed gold/silver nanoclusters stabilized by selenium donor ligands. Angew. Chem. Int. Ed. Engl. 56, 10178–10182 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Lei Z., Pei X. L., Guan Z. J., Wang Q. M., Full protection of intensely luminescent gold(I)-silver(I) cluster by phosphine ligands and inorganic anions. Angew. Chem. Int. Ed. Engl. 56, 7117–7120 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Wang S., et al. , A 200-fold quantum yield boost in the photoluminescence of silver-doped Ag(x)Au(25-x) nanoclusters: The 13th silver atom matters. Angew. Chem. Int. Ed. Engl. 53, 2376–2380 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., et al. , Atomically precise alkynyl-protected metal nanoclusters as a model catalyst: Observation of promoting effect of surface ligands on catalysis by metal nanoparticles. J. Am. Chem. Soc. 138, 3278–3281 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Kim K., et al. , Elucidating the doping effect on the electronic structure of thiolate-protected silver superatoms by photoelectron spectroscopy. Angew. Chem. Int. Ed. Engl. 58, 11637–11641 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Hossain S., et al. , Alloy clusters: Precise synthesis and mixing effects. Acc. Chem. Res. 51, 3114–3124 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Gan Z., Xia N., Wu Z., Discovery, mechanism, and application of antigalvanic reaction. Acc. Chem. Res. 51, 2774–2783 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Wang S., et al. , Metal exchange method using Au25 nanoclusters as templates for alloy nanoclusters with atomic precision. J. Am. Chem. Soc. 137, 4018–4021 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Sharma S., et al. , Tuning the electronic structure of thiolate-protected 25-atom clusters by co-substitution with metals having different preferential sites. Dalton Trans. 45, 18064–18068 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Kumara C., Aikens C. M., Dass A., X-ray crystal structure and theoretical analysis of Au25-xAgx(SCH2CH2Ph)18- alloy. J. Phys. Chem. Lett. 5, 461–466 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Gottlieb E., Qian H., Jin R., Atomic-level alloying and de-alloying in doped gold nanoparticles. Chemistry 19, 4238–4243 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Qian H., et al. , Monoplatinum doping of gold nanoclusters and catalytic application. J. Am. Chem. Soc. 134, 16159–16162 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Tofanelli M. A., Ni T. W., Phillips B. D., Ackerson C. J., Crystal structure of the PdAu24(SR)180 superatom. Inorg. Chem. 55, 999–1001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang X., et al. , The tetrahedral structure and luminescence properties of Bi-metallic Pt1Ag28(SR)18(PPh3)4 nanocluster. Chem. Sci. 8, 2581–2587 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan J., et al. , Asymmetric synthesis of chiral vimetallic [Ag28Cu12(SR)24]4- nanoclusters via ion pairing. J. Am. Chem. Soc. 138, 12751–12754 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Yang H., et al. , Structural evolution of atomically precise thiolated bimetallic [Au(12+n)Cu32(SR)(30+n)]4− (n = 0, 2, 4, 6) nanoclusters. J. Am. Chem. Soc. 136, 7197–7200 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Wan X. K., et al. , Atomically precise bimetallic Au19Cu30 nanocluster with an icosidodecahedral Cu30 shell and an alkynyl-Cu interface. J. Am. Chem. Soc. 139, 9451–9454 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Kang X., Xiong L., Wang S., Pei Y., Zhu M., Combining the single-atom engineering and ligand-exchange strategies: Obtaining the single-heteroatom-doped Au16Ag1(S-Adm)13 nanocluster with atomically precise structure. Inorg. Chem. 57, 335–342 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Bootharaju M. S., Sinatra L., Bakr O. M., Distinct metal-exchange pathways of doped Ag25 nanoclusters. Nanoscale 8, 17333–17339 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Krishnadas K. R., Baksi A., Ghosh A., Natarajan G., Pradeep T., Structure-conserving spontaneous transformations between nanoparticles. Nat. Commun. 7, 13447 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan N., et al. , Bimetal doping in nanoclusters: Synergistic or counteractive? Chem. Mater. 28, 8240–8247 (2016). [Google Scholar]
- 49.Weerawardene K. L. D. M., Aikens C. M., Origin of photoluminescence of Ag25(SR)18- nanoparticles: Ligand and doping effect. J. Phys. Chem. C 122, 2440–2447 (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.