Significance
Human impacts and climatic changes are widely considered to be responsible for rapid species extinction. However, determining their effects is challenging owing to the lack of long-term spatial–temporal data. In this study, we quantified the distinctive associations of anthropogenic and climatic stressors with the local extinction of 11 medium- or large-sized mammals using historical records over the past 3 centuries. We found that the increased local extinction of mammals was associated with intensified human disturbance (particularly for large-sized mammals) and with extreme temperature change (both cooling and warming). Our results provide insight into biodiversity conservation during the Anthropocene.
Keywords: local extinction, mammals, climate change, human disturbance, conservation
Abstract
Accelerated anthropogenic impacts and climatic changes are widely considered to be responsible for unprecedented species extinction. However, determining their effects on extinction is challenging owing to the lack of long-term data with high spatial and temporal resolution. In this study, using historical occurrence records of 11 medium- to large-sized mammal species or groups of species in China from 905 BC to AD 2006, we quantified the distinctive associations of anthropogenic stressors (represented by cropland coverage and human population density) and climatic stressors (represented by air temperature) with the local extinction of these mammals. We found that both intensified human disturbances and extreme climate change were associated with the increased local extinction of the study mammals. In the cold phase (the premodern period of China), climate cooling was positively associated with increased local extinction, while in the warm phase (the modern period) global warming was associated with increased local extinction. Interactive effects between human disturbance and temperature change with the local extinction of elephants, rhinos, pandas, and water deer were found. Large-sized mammals, such as elephants, rhinos, and pandas, showed earlier and larger population declines than small-sized ones. The local extinction sensitivities of these mammals to the human population density and standardized temperature were estimated during 1700 to 2000. The quantitative evidence for anthropogenic and climatic associations with mammalian extinction provided insights into the driving processes of species extinction, which has important implications for biodiversity conservation under accelerating global changes.
Global biodiversity has been declining rapidly in modern times (1–5), imposing great threats on natural ecosystems and our society (6, 7). Anthropogenic disturbance is considered to be a key factor causing population decline and species extinction (8). Climate is another major culprit causing range shifts and local extinctions of animals in their primary habitat (9). The threat of global warming is particularly serious to species living in regions of high latitudes (10). Although the applications of various methods, such as population viability analysis (11), analyses of historical population decline (8), range contraction (12, 13), species–area relationships (13, 14), and Red Data lists (15), have advanced the assessment of species extinction, the quantitative relationships between local extinction of endangered species and anthropogenic or climatic factors have been rarely evaluated. This lack of information prevents us from disentangling the relative roles of human impacts and climate change in causing extinctions of these endangered species. Understanding the relative effects of different stressors on extinction is critical for taking conservation actions.
China has a long history of recording significant political and natural events for over 3000 y. Medium- or large-sized mammals, including panda, elephant, rhino, tiger, and others, were important attractions owing to their economic value, declarative usage, or conflict with humans. Sightings of these large animals, reports of human–animal conflicts, or gifts to the emperor and captures of these animals were frequently recorded in Chinese historical literature. Wen (16) compiled a compendium of historical distributions of some endangered mammal species by extracting original records from official or formally documented histories (e.g., Twenty-Four Histories, Comprehensive Mirror, and Aid in Government) and local gazetteers (provincial, prefectural, and district gazetteers), which provide an opportunity for reconstructing the local extinction of these mammals over past millennia.
The objectives of this study were to estimate the local extinction probability of 11 mammal species or groups of species using historical records of their occurrences by referring to Wen (16) and other data compiled from various sources (Methods) and to quantify the distinctive associations of anthropogenic and climatic factors with their local extinctions.
Results
Overall Temporal Survival Patterns.
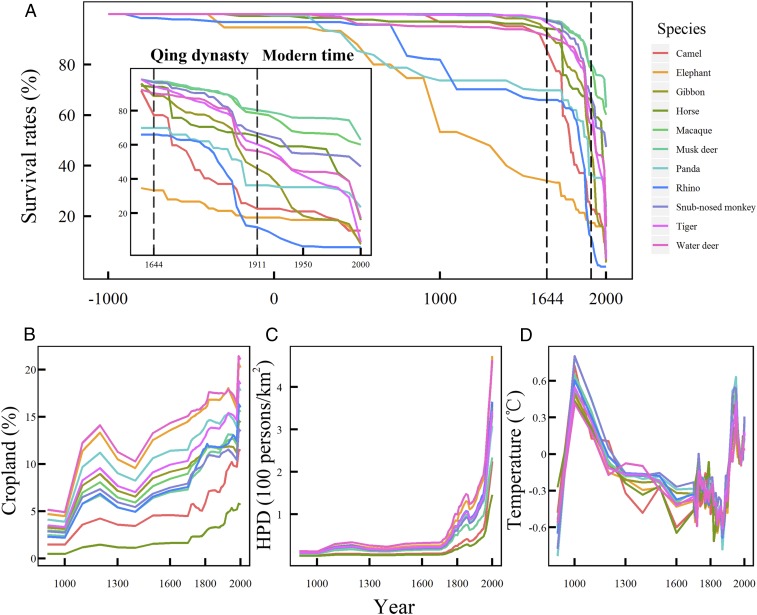
In this study, 9,365 occurrence records of 11 mammal species or groups of species in 1,364 grids were identified in China, including camel (Camelus ferus), elephant (Elephas maximus), gibbon (genus Hylobates, Nomascus, and Hoolock), horse (genus Equus), macaque (genus Macaca), musk deer (genus Moschus), panda (Ailuropoda melanoleuca), rhinos (genus Dicerorhinus and Rhinoceros), snub-nosed monkey (genus Rhinopithecus), tiger (Panthera tigris), and water deer (Hydropotes inermis) (SI Appendix, Table S1 and Fig. S1). We treated gibbon, horse, macaque, musk deer, rhinos, and snub-nosed monkey each as a mammal taxon group because individual species were not distinguished in the literature. In the following, we refer to the 11 species or groups of species simply as “species” unless there is a necessity to distinguish them. As shown in Fig. 1A, before the Qing Dynasty (before 1644), the survival rates (proportion of survived grids) of rhinos, elephant, and panda dramatically decreased due to massive local extinctions, while the other mammal species showed a minor decrease in survival rate. During the Qing Dynasty (1644 to 1911, the premodern period in China) and the modern period (after 1911), all mammal species (except for snub-nosed monkey, macaque, and musk deer) showed large decreases in survival rates. Cropland coverage slowly increased before the Qing Dynasty but rapidly increased during and after the Qing Dynasty (Fig. 1B). Human population density was low and stable before the Qing Dynasty but increased rapidly during and after the Qing Dynasty, particularly in the modern period after 1911 (Fig. 1C). Temperature (as measured by average summer air temperature) steadily decreased until the end of the Qing Dynasty then rapidly increased after the Qing Dynasty (Fig. 1D). The decreasing trends in survival rate of these mammals were significantly positively correlated with cropland coverage (n = 48) and human population density (n = 48) during AD 0 to 2000 but not with temperature (n = 38) during AD 900 to 1999 (SI Appendix, Table S2).
Fig. 1.
Temporal variation of the survival rates, cropland coverage, human population density, and temperature for the 11 mammal species or groups of species at the county or prefecture level. (A) Survival rates (percent, i.e., the proportion of survived grids compared to all distributed grids in history) of 11 large mammal species or groups of species caused by local extinctions in China (the same below). (B) Average cropland coverage (percent, from AD 900 to 2000). (C) Average human population density (HPD, persons per km2, from AD 900 to 2000). (D) Average summer air temperature (degrees Celsius, from AD 900 to 1999). Time resolution was 10 y after 1700 and 100 y before 1700. Different colored lines represent the survival rates (A), or average cropland coverage (B), average human population density (C), and average summer air temperature (D) of the 11 mammal species or groups of species in their distributed grids.
There were significant negative correlations between the body size (as measured by body mass in kilograms) of the study mammals and their average survival rates over 4 time periods (905 BC to AD 2000, AD 1000 to 2000, AD 1500 to 2000, and AD 1911 to 2000) (Fig. 2), indicating that large-sized mammals (e.g., elephant, rhino, and panda) had larger population declines as measured by the proportion of survived grids than small-sized ones.
Fig. 2.
Relationship between body mass (kilograms) and the average proportion of survived grids of the 11 mammal species or groups of species during 4 study periods from 905 BC to AD 2000 (A), from AD 1000 to 2000 (B), from AD 1500 to 2000 (C), and from AD 1911 to 2000. (D) Body mass was log-transformed. Blue shadows are the confidence intervals of the fitted linear regression models (indicated by the blue lines).
Associations of Stressors with the Local Extinction Probability.
Using spatial–temporal generalized additive model (stGAM) analysis, we found that human population density (representing the human disturbance) had a significantly positive association with the local extinction probability of elephant, gibbon, macaque, musk deer, panda, rhino, tiger, and water deer during 1700 to 2000 (Table 1). For the premodern period, human population density had significantly positive associations with the local extinction probability of macaque, musk deer, rhinos, tiger, and water deer (Table 1 and Fig. 3A); for the modern period, human population density had positive associations with the local extinction probability of elephant, gibbon, musk deer, tiger, and water deer (Table 1 and Fig. 3B). The responses of local extinction probability (0 to 100%) of these mammals became saturated with increased human population density (Fig. 3B). The local extinction sensitivity of these mammals to human population density ranged from 42.11 to 63.53% for the premodern period, with the order of water deer > musk deer > rhinos > macaque > tiger and from 34.54 to 99.37% for modern period with the order of elephant > musk deer > gibbon > water deer > tiger (SI Appendix, Table S3). The local extinction sensitivity of panda to human population density was 50.62% over the whole study period (SI Appendix, Fig. S2). No significant association between these sensitivities and body size was found.
Table 1.
Associations of human population density and temperature with the local extinction probability of mammal species or groups of species
| Mammal species or groups of species | Anthropogenic coefficients | Temperature coefficients | ||||
| Premodern period | Modern period | The whole study period | Premodern period | Modern period | The whole study period | |
| Camel | 2.13 | 0.17 | 0.96 | 1.61 | 4.51 | 1.44 |
| Elephant | 6.85 | 17.63** | 9.29** | 0.23 | 0.76 | −0.33 |
| Gibbon | 0.36 | 0.56* | 0.35* | −1.46** | 2.16*** | 0.66*** |
| Horse | 318.46 | 1.18 | 1.45 | 2.12 | 0.22 | 0.13 |
| Macaque | 0.62* | 0.19 | 0.22* | −2.76*** | 2.26*** | 0.54** |
| Musk deer | 0.67* | 0.45*** | 0.48*** | −0.4 | −0.38 | −0.34* |
| Panda | 0.49 | 0.75 | 0.67* | −3.07*** | −1.94** | −1.34*** |
| Rhinoceros | 0.71* | −95.86 | 0.51* | −2.42*** | 115.65 | −0.02 |
| Snub-nosed monkey | 0.51 | 0.26 | 0.3 | −0.85 | 1.1* | 0.29 |
| Tiger | 0.49*** | 0.42*** | 0.4*** | −1.52*** | 1.01*** | 0.4*** |
| Water deer | 0.91** | 0.53*** | 0.47*** | −3.43*** | 1.35*** | 0.07 |
Associations are represented by the positive or negative regression coefficients ( and ) from Eq. 1. The anthropogenic stressor was human population density (100 persons per km2). The analysis was conducted using stGAM methods for 3 periods: the premodern period (1700 to 1911), the modern period (1911 to 2000), and the whole study period (1700 to 2000). Boldfaced values indicate statistically significant regression coefficients (*P < 0.05, **P < 0.01, ***P < 0.001).
Fig. 3.
Relationship of the local extinction probability with the average human population density (A, premodern period; B, modern period) and the average standardized summer air temperature (C, premodern period; D, modern period). The analysis was conducted using stGAM methods. The average local extinction probability for human population density (or standardized temperature) was estimated using the standardized temperature (or human population density) and coordinates of each grid in Eq. 1. Different colored lines represent the responses of local extinction probability of the 11 mammal species or groups of species to different stressors.
We found that temperature had significantly positive associations with local extinction probability of gibbon, macaque, and tiger but negative associations with local extinction probability of musk deer and panda during 1700 to 2000 (Table 1). For the premodern period, temperature had significantly negative associations with the local extinction probability of gibbon, macaque, panda, rhino, tiger, and water deer (Table 1 and Fig. 3C). For the modern period, temperature showed significantly positive associations with local extinction probability of gibbon, macaque, snub-nosed monkey, tiger, and water deer but negative associations with panda (Table 1 and Fig. 3D). The local extinction probability of some mammals (panda, water deer, macaque, and rhinos during the premodern period) had a sigmoid shape with temperature with a noticeable critical value of extinction at approximately −0.5 SD for standardized temperature change. The local extinction sensitivity of mammals to the standardized temperature (equal to the temperature deviation from the mean relative to SD) during 1700 to 2000 ranged from 34.48 to 67.47% for mammals in the premodern period. The order of local extinction sensitivity to climate cooling is water deer > panda > macaque > rhinos > tiger > gibbon (SI Appendix, Table S3). The local extinction sensitivity of mammals to the standardized temperature ranged from 23.85 to 45.75% in the modern period (excluding panda, which had a negative association with temperature). The order of local extinction sensitivity to climate warming is macaque > gibbon > water deer > snub-nosed monkey > tiger. The local extinction sensitivity of musk deer to climate cooling was 8.08% over the whole study period (SI Appendix, Fig. S2). No significant association between extinction sensitivity and body size was detected.
We found significantly positive interactions between human population density and temperature on local extinction probability of rhinos during the premodern period, on that of panda and water deer during the modern period, and on that of elephant during the whole study period (SI Appendix, Table S4 and Fig. S3). Model diagnostics using the partial autocorrelation function (see SI Appendix, Fig. S4 for the whole study period, SI Appendix, Fig. S5 for the premodern, and SI Appendix, Fig. S6 for the modern period) and residual semivariograms (see SI Appendix, Fig. S7 for the model using Eq. 1 and SI Appendix, Fig. S8 for the model using Eq. 2) revealed no (or minor) residual temporal autocorrelation and no (or minor) residual spatial autocorrelation.
Discussion
By using long-term historical records in China, we found large-sized mammals showed larger and earlier population decline than small-sized mammals over the past 2 millennia, and all mammals showed precipitous population declines since the Qing Dynasty (approximately 1644). We found that both intensified anthropogenic and extreme climate change were associated with increased local extinction of the 11 studied mammal species during 1700 to 2000. The local extinction sensitivity of these mammals to change in human population density was similar, except for elephant, which had a very high sensitivity, while the local extinction sensitivity to temperature change was much larger. Our study provides insights into the mechanisms of historical extinctions of mammals and should have important conservation implications, as discussed below. However, due to the limitation of our historical data (e.g., uncertainty of historical records, biased recording efforts in space and time, and various spatial or temporal resolutions) conclusions should be cautiously interpreted, particularly for species with a small sample size.
Impacts of Human Disturbances.
Anthropogenic factors, for example overexploitation (17), habitat destruction (18), agricultural development (19), urbanization (20), deforestation (21), and human-introduced diseases (22), are widely considered to be the major factors driving excessively high local or global species extinctions, particularly for wildlife (8, 23). However, the effects of human disturbances on local extinction of animals have rarely been quantified. We found that intensified human disturbance was associated with increased local extinction probability for most mammals during 1700 to 2000 (Table 1), except for a few species, probably owing to their small sample sizes (camel, n = 22; horse, n = 72; and snub-nosed monkey, n = 74) (SI Appendix, Table S5). We demonstrated that human population density could drive massive local extinctions of rhinos, elephant, and panda before 1644 (Fig. 1). After 1644, especially in the modern period, human population density was positively associated with local extinction of most mammal species in China. Over the past 3 centuries, all of the mammals studied showed a sign of a catastrophic population crash (except snub-nosed monkey, macaque, and musk deer), likely associated with increased human populations (Table 1 and Fig. 3 A and B). According to the estimates of local extinction sensitivity in SI Appendix, Table S3, we found that if the human population density reached 400 persons per square kilometer within a grid, the mammals we studied suffered a local extinction sensitivity of 34.54 to 99.37% within a period of 50 y (Fig. 3B and SI Appendix, Table S3). Elephant, the largest mammal we studied, was very sensitive to human disturbances. This quantified local extinction sensitivity to human population density could have useful conservation implications for assessing the degree of human impacts on the long-term survival of these mammal species.
High human population density is also linked to extensive poaching and road kills, habitat loss due to agricultural cultivation (24), deforestation, and other land-use changes at the local scale (25). During the past 3 decades, China has experienced a rapid increase in population, as well as industrialization and urbanization, thus imposing great pressure on these mammals. High population density not only destroyed habitats of animals via increasing cropland coverage and deforestation but also poached large mammals for food and medicine or deterred the mammals by grazing livestock. Previous studies suggest that human disturbance may cause local extinctions of elephant, rhinos, and panda based on indirect evidence on the relationship between boundary contraction and population density (24, 26). Rhinoceros have been extensively hunted by people in the late Pleistocene (26, 27). The remains of rhinoceros have been found in 78% of anthropogenic sites (28). Hunting and poaching for the horn and habitat loss by logging or land conversion to agriculture have accelerated the range contraction of the rhinos (29) and tiger (30). Currently, 76 to 80% of nonhuman primate species in South and Southeast Asia are threatened with extinction (31). Previous studies indicated that gibbon is highly vulnerable to forest loss and hunting caused by human population expansion (32). Our study provides quantitative evidence of human disturbance driving local extinction of these mammals.
Impacts of Climate Change.
Current climate change influences species survival in a given area (13, 33). The potential association of climate change with local extinction is estimated to vary from 0 to 54% with an average of 7.9% (34). Previous studies indicated that both climate warming (10) and cooling (26) could cause range shifts and local extinction of animals, but quantitative evidence is rare.
In this study, we found temperature showed opposite associations with local extinction of gibbon, macaque, tiger, and water deer; negative associations between local extinction and temperature were found in the cold phase of the premodern period, while positive associations were found in the warm phase of the modern period (Table 1 and Fig. 3 C and D). These observations suggest that both climate warming and cooling were associated with increased local extinction probability of these mammals. This finding is likely because both global warming (27) and cooling (26) could cause boundary contractions of mammals, thus causing local extinctions of these mammals.
We found that local extinction of gibbon, macaque, panda, rhinos, tiger, and water deer was negatively associated with temperature in the cold phase of the premodern period (Table 1 and Fig. 3C). All these mammal species lived in tropical or subtropical regions, except for tiger, which was widely distributed in both northern and southern China (SI Appendix, Fig. S1). Our previous studies suggested that animals living in tropical or subtropical regions of the Northern Hemisphere would suffer northern boundary contraction toward the south under climate cooling (26), which explained the negative associations between temperature and the local extinction of these mammals. Other studies suggest that primates are likely to experience more temperature change (35), and climate could affect primate birth rates, timing of births, and infant and adult female survival and physical condition (36). Gibbons are more sensitive to climatic variations than other mammals (37). Climate often has great impacts on activity patterns, behavioral adaptations, and the foraging time budget of gibbons (38). Our results indicate that local extinction of gibbon was less sensitive to climate cooling (but more sensitive to warming) than that of the other mammals, while panda was more sensitive to climate cooling than the other mammals (SI Appendix, Table S3).
Chen et al. (39) reported that rapid range shifts of species were significantly associated with elevated global warming. Such a warming-induced range shift would increase local extinction of animals. We found that the local extinction of gibbon, macaque, snub-nosed monkey, tiger, and water deer was positively associated with temperature in the warm phase (and gibbon, macaque, and tiger also showed a general positive association with temperature for the whole period; Table 1). It is expected that global warming during the modern period would expand the northern boundary of these mammals. However, owing to massive habitat destruction and human encroachment in the modern period, animals were not able to expand their range under global warming. Instead, they were forced to leave their favorable but fragmented habitats and faced a high risk of extinction. Li et al. found that temperature and human disturbance had interactive effects on range shifts of the Asian elephant and the rhinos (26), which is consistent with our findings (SI Appendix, Table S4 and Fig. S3). This interactive effect likely resulted from habitat fragmentation preventing the movement of animals, either in the warming or cooling period. Global warming could force the Asian elephant and the rhinoceros to move north or east, but this trend has been impeded by intensified human activities. Although tiger and musk deer were widely distributed, tiger was found to occur in 8 provinces of southern China until the 1990s (40). There are 5 tiger species or subspecies living in different climate zones, and they could suffer extinction pressures under climate change as a whole. For example, climate cooling may have contributed to large extinctions of tiger subspecies in the west and north of China during the cold premodern time, while recent global warming might contribute to the complete extinction of tigers in southern China.
We found climate cooling was associated with increases local extinction of pandas living in subtropical regions, which is consistent with our previous observations (26). Climate cooling and intensified human impacts during the past millennia caused boundary contractions of the panda toward the southwest of China (41). Pandas have very restrictive bamboo diets; warm and wet weather conditions are ideal for the growth of bamboo forests (41). In China, climate cooling often causes more droughts due to weakening summer monsoons (42), reducing bamboo forests, as well as habitats or food for other mammals.
To further illustrate how climate cooling or warming may change the distributions of the study mammals, we projected the potential distribution ranges as represented by the suitable habitats of these mammals in the Last Glacial Maximum (LGM, cold period) and Holocene (warm period) (SI Appendix, Fig. S9). We found that in the LGM all species showed clear contraction of their potential distribution as reflected by the projected suitable habitats; elephant, gibbon, macaque, musk deer, panda, snub-nosed monkey, tiger, and water deer showed obvious contraction of their northern boundary (SI Appendix, Fig. S9). The projected potential distribution range of elephant, panda, and water deer showed obvious expansion of their northward boundaries in the Holocene; horse showed contraction of its southern boundary (SI Appendix, Fig. S9). These results indicate that both climate warming and cooling could result in local extinction of mammals wherever range contraction occurred.
Impacts of Body Mass.
Body mass of the study mammals had significantly negative correlations with the average proportion of survived grids over 4 periods (905 BC to AD 2000, AD 1000 to 2000, AD 1500 to 2000, and AD 1911 to 2000) (Fig. 2). Large-sized mammals, such as rhino, elephant, and panda, showed a population decline before the Qing Dynasty (Fig. 1). This result is consistent with previous findings that larger-bodied species had greater extinction rates (8). Larger mammals are more desirably hunted as they are more nutritious and economically valuable. Extensive overhunting for meat is likely to be the most important factor responsible for large herbivore population decline (43). Large mammals need large habitats and are more susceptible to habitat destruction and fragmentation caused by cultivation, urbanization, and road construction (24). Large mammals also often have a larger range shift (26, 39). Thus, they would suffer a higher risk of local extinction under extreme climate change. However, we did not find any significant association of the local extinction sensitivity of mammals to either human population density or temperature with body mass (as well as their distribution area or the average latitude range of their distribution) during 1700 to 2000 (all P > 0.05). This result is probably due to too few species studied (i.e., small sample size). Further studies are necessary to understand the taxon-specific differences of local extinction sensitivity to stressors.
Implications for Conservation.
In this study, we assessed the associations of human and climatic factors with the local extinction of 11 mammal species or groups of species in China and estimated their local extinction sensitivity to human disturbance and climate change. The results derived from the study should have important implications for the conservation of mammal species in China and elsewhere. Because current global warming was associated with increased local extinction of gibbon, macaque, snub-nosed monkey, tiger, and water deer and would probably force them to move out of their favorable habitats, imposing high risk of local extinction, it is necessary to expand their nature reserves into high altitudes or latitudes. Because human disturbances have consistently been a primary factor causing local extinctions of mammals, it is essential to reduce the impacts of human disturbances, such as cultivation, hunting, deforestation, and grazing, on endangered species. The constructed response curves of local extinction of the study mammals to human population density can be useful in evaluating the local extinction risk of these mammals in a protected region or national park; human access should be kept below that level to ensure long-term survival of these mammals. Habitat fragmentation caused by humans would have an interactive effect with climate change, which could accelerate extinctions (particularly for elephant, panda, rhinos, and water deer) driven by climate change. A 1 °C increase or decrease could drive large mammals to move approximately 150 to 200 km toward the north or south (27). Most nature reserves in China (44) are not large enough (75% < 471 km2) to allow large-scale animal movement to adapt to climate change. Future priority should be given to building large reserves or wildlife corridors connecting isolated habitats to facilitate animal movement along altitudes or latitudes under accelerated climate warming.
Methods
Species Occurrence Data.
We obtained the historical distribution data of the 11 mammal species or groups of species from a compendium complied by Wen (16), in which the occurrence time and sites of large mammals are presented based on standard histories and local gazetteers as well as physical remains discovered over the last 3 millennia, uncovering distribution data (spatial resolution: 0.5 × 0.5 arc degrees, approximately 50 × 50 km2) before the modern period (i.e., before 1911). In the compendium, the use of historical literature records was conservative; records lacking other supporting observations were excluded, and only confirmed records were included (16). We extracted the information of mammal occurrence (i.e., year and location) from the compendium and then reconstructed the longitude and latitude of locations by referring to the book by Liang (45), which contains detailed information on location names. After data verification (for details see SI Appendix), a total of 7,040 records, with a resolution of a specific year or decade at the county or prefecture level in the compendium edited by Wen (16), was used in this study, which included 63.19% of all records of the compendium.
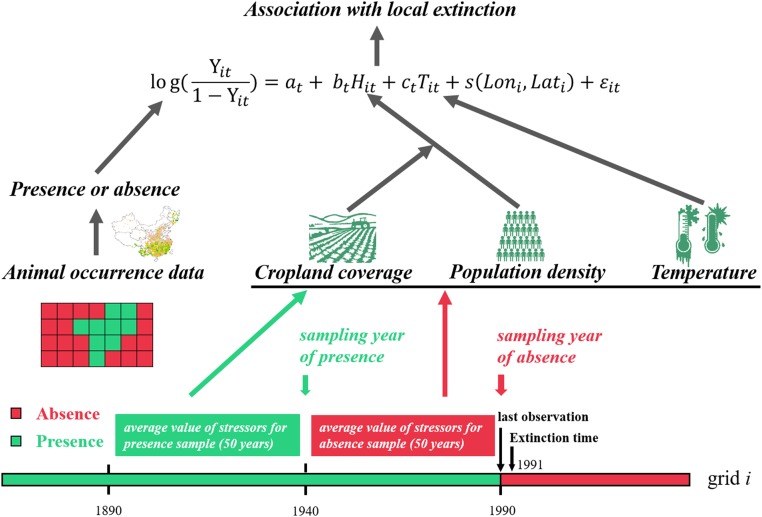
Since modern times (after 1911) in China, the distribution of animals was surveyed based on scientific field investigations. We obtained distribution data with accurate year and coordinate information (n = 2,325, covering the period of 1913 to 2006) for 10 mammal species (except rhinos) from the China Species Information Service (CSIS) by Xie et al. (46) as supplementary data to the compendium compiled by Wen (16). The occurrence time and sites of these mammals compiled in the CSIS database were collected from a large amount of scientific literature (46). To reconfirm the presence or absence of the 11 mammal species after 2006, we used the current International Union for Conservation of Nature (IUCN) distribution information (updated in 2014) about these mammal species from METADATA (Digital Distribution Maps on The IUCN Red List of Threatened Species, version 4) to determine the time of extinction in local grids of 0.5° × 0.5° (47). For example, if a species was last recorded in a grid in 1990 (last observation time) in our database but the occurrence of the species in that grid was not reported in the 2014 IUCN distribution map, then the year 1991 and the years after were defined as years of absence (i.e., extinction), and the years before 1991 were defined as years of presence (Fig. 4). Using the IUCN data, the definition of the local extinction time based on the last observation time is more conservative because it usually takes over 20 y to conclude that a species is extinct. The presence and absence data of mammals during 1700 to 2000 are supplied in SI Appendix.
Fig. 4.
Diagram of the stGAM method to estimate the associations of anthropogenic stressors (human population density) and climatic stressor (temperature) with the local extinction probability (presence or absence) of mammals. For each grid of each species’ distributional range, only data of 2 sampling years were used (i.e., the sampling year of presence and absence). The absence year (i.e., extinction year) was 1 y after the last observation. The presence year was the year 50 y before the absence year. The average value of stressors of the last 50 y before the sampling years of presence or absence was used to examine their associations with the local extinction probability (presence or absence) of mammal species of this grid.
Anthropogenic and Climate Proxy Data.
Cropland coverage, defined as the proportion of cropland area (percent) in each spatial grid, and human population density, defined as the number of persons per square kilometer in each spatial grid in a given time from the History Database of the Global Environment (48), were used to represent human disturbances over time (AD 0 to 1700, 100-y resolution; AD 1700 to 2000, 10-y resolution). Because human population density and cropland coverage are highly correlated, we only used human population density for modeling local extinction probability. The Asian summer (June through August) average temperature reconstructions (the only available high temporal resolution matching our species occurrence data) over the past 1,100 y (yearly, 900 to 1999), based on 357 publicly available proxy climate data (tree-ring-dominant) series from the World Data Center for Paleoclimatology archives, were used as a proxy of temperature change (downloaded from https://www.ncdc.noaa.gov/paleo-search/study/18635) (49). Details on the anthropogenic and climate proxy data can be found in SI Appendix. Because we are interested in the association of changes in the local temporal temperature with local extinction of these mammals, it is necessary to remove the effects of variation in regional temperature. To do this, the temperature series of each grid was standardized by (temperature − mean)/SD.
Statistical Analysis.
All historical occurrence data from 905 BC to AD 2006 were assigned to grids of 0.5 × 0.5 arc degrees (approximately 50 × 50 km2, for a total of 3,568 grids in China; the grid number of each species is shown in SI Appendix, Table S1). The year of last observation of a species or groups of species within each grid in our database and the lack of reporting of a grid in the 2014 IUCN distribution map were used to determine the time of local extinction (Fig. 4). For each grid, the years after the last observation were considered as the years of absence (local extinction); the years of last observation and before the last observation were considered as the years of presence. Years of presence or absence were adjusted to a decadal resolution to match the resolution of the environment variables.
stGAM analyses were used to model the associations of human population density and temperature with local extinction of these mammals in each grid from 1700 to 2000 (Fig. 4). (The sample size before 1700 is not large enough to support the analysis and the data were not considered.)
A logistic GAM of the local extinction against human population density and temperature was fitted using the formula
| [1] |
where is the local extinction probability of a species in the ith grid (1 denotes absence of the species from the grid, i.e., extinction; 0 denotes presence of the species, i.e., survival) at time t, is the human population density, is the temperature; is a 2D smoothing function (with k value, dimension of the basis = 6) for modeling the spatial autocorrelation effects (50), and is uncorrelated random errors of zero mean and finite variance. , and are constants ( is an intercept and and represent the association of human population density and temperature with the local extinction, , of a species). To avoid temporal autocorrelation of local extinction, we only used data of 2 sampling years representing the year of presence or absence of the species in the grid in our modeling analysis (Fig. 4). The sampling year of absence was 1 y after the last observation, while the sampling year of presence was the year 50 y before the sampling year of absence. The anthropogenic and climatic variables of the sampling year of presence or absence were the average values of human population density and the standardized temperature variable of last 50 y of the sampling years (Fig. 4). See SI Appendix for details on the GAM analysis.
Based on the relationship between local extinction probability and human population density or standardized temperature (Fig. 3), the local extinction sensitivity of each species to human disturbance (or climate) was measured by the difference of local extinction probability when human population density changes from zero to 400 persons per km2 or the standardized temperature changes from zero to +1 SD (sensitivity to climate warming) or −1 SD (sensitivity to climate cooling) (SI Appendix, Table S3).
To illustrate the impacts of temperature change on potential distribution range (represented by suitable habitats) of the study mammals, the maximum entropy method (Maxent) for species distributions models (SDMs) (51) was used to project the potential distribution range of these mammal species in the late Pleistocene and Holocene. Details on the SDMs analysis can be found in SI Appendix.
All R code for modeling is presented in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by the National Key R&D Program of China (2017YFA0603304), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB31000000), and the International Society of Zoological Sciences/International Union of Biological Sciences Program of Biological Consequence of Global Change. F.H. acknowledges the support of the Natural Sciences and Engineering Research Council of Canada.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1818019116/-/DCSupplemental.
References
- 1.Foden W. B., et al. , Identifying the world’s most climate change vulnerable species: A systematic trait-based assessment of all birds, amphibians and corals. PLoS One 8, e65427 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira H. M., et al. , Scenarios for global biodiversity in the 21st century. Science 330, 1496–1501 (2010). [DOI] [PubMed] [Google Scholar]
- 3.May R. M., Ecological science and tomorrow’s world. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 41–47 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap T. A., Koo M. S., Ambrose R. F., Wake D. B., Vredenburg V. T., BIODIVERSITY. Averting a North American biodiversity crisis. Science 349, 481–482 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Rahbek C., Colwell R. K., Biodiversity: Species loss revisited. Nature 473, 288–289 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Ceballos G., et al. , Conservation challenges for the austral and neotropical America section. Conserv. Biol. 23, 811–817 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Hooper D. U., et al. , A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105–108 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Dirzo R., et al. , Defaunation in the Anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Koch P. L., Barnosky A. D., Late quaternary extinctions: State of the debate. Annu. Rev. Ecol. Evol. Syst. 37, pp. 215–250. [Google Scholar]
- 10.IPCC , Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Pachauri R. K., Meyer L. A.; Core Writing Team, Eds. (IPCC, Geneva, 2014). [Google Scholar]
- 11.Boyce M. S., Population viability analysis. Annu. Rev. Ecol. Syst. 23, 481–506 (1992). [Google Scholar]
- 12.Ceballos G., Ehrlich P. R., Mammal population losses and the extinction crisis. Science 296, 904–907 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Hei F., Area-based assessment of extinction risk. Ecology 93, 974–980 (2012). [DOI] [PubMed] [Google Scholar]
- 14.He F., Hubbell S., Estimating extinction from species–Area relationships: Why the numbers do not add up. Ecology 94, 1905–1912 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Stork N. E., Re-assessing current extinction rates. Biodivers. Conserv. 19, 357–371 (2010). [Google Scholar]
- 16.Wen R., The Distributions and Changes of Rare Wild Animals in China (Shandong Science and Technology Press, Jinan, 2009) [in Chinese]. [Google Scholar]
- 17.Rosser A. M., Mainka S. A., Overexploitation and species extinctions. Conserv. Biol. 16, 584–586 (2002). [Google Scholar]
- 18.Trombulak S. C., Frissell C. A., Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 14, 18–30 (2000). [Google Scholar]
- 19.Mckee J. K., Chambers E. N., “Behavioral mediators of the human population effect on global biodiversity losses” in Human Population, Cincotta R., Gorenflo L., Eds. (Ecological Studies (Analysis and Synthesis), Springer, 2011) , vol. 214, 47–59. [Google Scholar]
- 20.Menon M., Prashanthi Devi M., Nandagopalan V., Mohanraj R., “Species diversity and functional assemblages of bird fauna along the riverine habitats of Tiruchirappalli, India” in Environmental Management of River Basin Ecosystems, Ramkumar M., Kumaraswamy K., Mohanraj R., Eds. (Springer International Publishing, Cham, 2015), pp. 729–748. [Google Scholar]
- 21.Hill J. K., Hamer K. C., Determining impacts of habitat modification on diversity of tropical forest fauna: The importance of spatial scale. J. Appl. Ecol. 41, 744–754 (2004). [Google Scholar]
- 22.Smith K. F., Sax D. F., Lafferty K. D., Evidence for the role of infectious disease in species extinction and endangerment. Conserv. Biol. 20, 1349–1357 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Turvey S. T., Crees J. J., Li Z., Bielby J., Yuan J., Long-term archives reveal shifting extinction selectivity in China’s postglacial mammal fauna. Proc. Biol. Sci. 284, 20171979 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang G., et al. , Effects of environmental and anthropogenic drivers on Amur tiger distribution in northeastern China. Ecol. Res. 29, 801–813 (2014). [Google Scholar]
- 25.Kramer-Schadt S., Revilla E., Wiegand T., Breitenmoser U. R. S., Fragmented landscapes, road mortality and patch connectivity: Modelling influences on the dispersal of Eurasian lynx. J. Appl. Ecol. 41, 711–723 (2004). [Google Scholar]
- 26.Li X., et al. , Human impact and climate cooling caused range contraction of large mammals in China over the past two millennia. Ecography 38, 74–82 (2015). [Google Scholar]
- 27.Wan X., Zhang Z., Climate warming and humans played different roles in triggering late Quaternary extinctions in east and west Eurasia. Proc. Biol. Sci. 284, 20162438 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong H., Les rhinocéros des sites à fossiles humains de chine. Anthropologie 104, 523–529 (2000). [Google Scholar]
- 29.Wen H., Wen R., The Change of the Plant and Animal in China During Different Historical Period (Chongqing Publishing Group, Chongqing, 2006) [in Chinese]. [Google Scholar]
- 30.Jiang G., et al. , New hope for the survival of the Amur leopard in China. Sci. Rep. 5, 15475 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schipper J., et al. , The status of the world’s land and marine mammals: Diversity, threat, and knowledge. Science 322, 225–230 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Turvey S. T., Crees J. J., Di Fonzo M. M. I., Historical data as a baseline for conservation: Reconstructing long-term faunal extinction dynamics in late imperial-modern China. Proc. Biol. Sci. 282, 20151299 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson R. G., Dawson T. P., Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 12, 361–371 (2003). [Google Scholar]
- 34.Urban M. C., Climate change. Accelerating extinction risk from climate change. Science 348, 571–573 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Graham T. L., Matthews H. D., Turner S. E., A global-scale evaluation of primate exposure and vulnerability to climate change. Int. J. Primatol. 37, 158–174 (2016). [Google Scholar]
- 36.Wiederholt R., Post E., Birth seasonality and offspring production in threatened neotropical primates related to climate. Glob. Change Biol. 17, 3035–3045 (2011). [Google Scholar]
- 37.Alamgir M., Mukul S. A., Turton S. M., Modelling spatial distribution of critically endangered Asian elephant and Hoolock gibbon in Bangladesh forest ecosystems under a changing climate. Appl. Geogr. 60, 10–19 (2015). [Google Scholar]
- 38.Ni Q., et al. , Effects of food availability and climate on activity patterns of western black-crested gibbons in an isolated forest fragment in southern Yunnan, China. Primates 56, 351–363 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Chen I. C., Hill J. K., Ohlemüller R., Roy D. B., Thomas C. D., Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 (2011). [DOI] [PubMed] [Google Scholar]
- 40.State Forestry Administration , Investigation of Resources of Key Terrestrial Wildlife Animal in China (China Forestry Publishing, Beijing, 2009) [in Chinese]. [Google Scholar]
- 41.Zhao S., et al. , Whole-genome sequencing of giant pandas provides insights into demographic history and local adaptation. Nat. Genet. 45, 67–71 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Tian H., et al. , Scale-dependent climatic drivers of human epidemics in ancient China. Proc. Natl. Acad. Sci. U.S.A. 114, 12970–12975 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ripple W. J., et al. , Collapse of the world’s largest herbivores. Sci. Adv. 1, e1400103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan C., Xie Y., Li X., Holyoak M., Zhang Z., Species co-occurrence and phylogenetic structure of terrestrial vertebrates at regional scales. Glob. Ecol. Biogeogr. 25, 455–463 (2016). [Google Scholar]
- 45.Liang F., Dynastic Data of China’s Households, Cultivated Land and Land Taxation (Shanghai People’s Press, Shanghai, 1980) [In Chinese]. [Google Scholar]
- 46.Xie Y., Zhang S., Wang W., Biodiversity Atlas of China (Hunan Education Press, Changsha, 2009) [In Chinese]. [Google Scholar]
- 47.IUCN , The IUCN Red List of threatened species. Version 2018.1. (2018). https://www.iucnredlist.org. Accessed 1 January 2018.
- 48.Klein Goldewijk C. G. M., A historical land use data set for the Holocene; HYDE 3.2 (replaced). DANS (2016). 10.17026/dans-znk-cfy3. Accessed 10 May 2016. [DOI]
- 49.Feng S., et al. , A multi-proxy reconstruction of spatial and temporal variations in Asian summer temperatures over the last millennium. Clim. Change 131, 663–676 (2015). [Google Scholar]
- 50.Trevor H., Robert T., Generalized Additive Models (Chapman and Hall, 1990). [Google Scholar]
- 51.Phillips S. J., Dudík M., Schapire R. E., [Internet] Maxent software for modeling species niches and distributions (Version 3.4.1, 2019). http://biodiversityinformatics.amnh.org/open_source/maxent/. Accessed 1 January 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.