Significance
Reactive oxygen species (ROS) are key signaling molecules that play an important role in the regulation of stomatal movements in response to stress conditions. However, how basal cellular ROS levels are regulated in stomatal guard cells is not yet known. Our results revealed that autophagy maintains ROS homeostasis by eliminating oxidized peroxisomes, which allows the optimization of stomatal opening for photosynthetic CO2 fixation and plant growth. This study provides insights on regulatory mechanisms of ROS homeostasis in guard cells and the physiological significance of plant peroxisome-specific autophagy, that is, pexophagy.
Keywords: Arabidopsis, autophagy, peroxisome, ROS, stomata
Abstract
Reactive oxygen species (ROS) function as key signaling molecules to inhibit stomatal opening and promote stomatal closure in response to diverse environmental stresses. However, how guard cells maintain basal intracellular ROS levels is not yet known. This study aimed to determine the role of autophagy in the maintenance of basal ROS levels in guard cells. We isolated the Arabidopsis autophagy-related 2 (atg2) mutant, which is impaired in stomatal opening in response to light and low CO2 concentrations. Disruption of other autophagy genes, including ATG5, ATG7, ATG10, and ATG12, also caused similar stomatal defects. The atg mutants constitutively accumulated high levels of ROS in guard cells, and antioxidants such as ascorbate and glutathione rescued ROS accumulation and stomatal opening. Furthermore, the atg mutations increased the number and aggregation of peroxisomes in guard cells, and these peroxisomes exhibited reduced activity of the ROS scavenger catalase and elevated hydrogen peroxide (H2O2) as visualized using the peroxisome-targeted H2O2 sensor HyPer. Moreover, such ROS accumulation decreased by the application of 2-hydroxy-3-butynoate, an inhibitor of peroxisomal H2O2-producing glycolate oxidase. Our results showed that autophagy controls guard cell ROS homeostasis by eliminating oxidized peroxisomes, thereby allowing stomatal opening.
Guard cells surround adjustable stomatal pores on the leaf epidermis and control gas exchange between plants and the atmosphere, allowing CO2 influx for photosynthetic carbon fixation and nutrient uptake by roots via the transpirational stream. Because plants are sessile, guard cells sense and integrate multiple endogenous and environmental signals such as light, water status, hormones, and CO2 concentrations, thereby optimizing stomatal aperture to promote plant growth and survival under environmental conditions (1–5). Reactive oxygen species (ROS), including hydrogen peroxide (H2O2), act as important second messengers in abscisic acid (ABA) signaling and cause a reduction in stomatal aperture (6, 7). In order to be effective as a second messenger and avoid toxicity, the basal intracellular ROS concentrations, which might increase rapidly in response to stress, need to be maintained at low levels.
Numerous studies have shown that various environmental conditions and phytohormones also trigger ROS production in guard cells (6). Elevated ROS cause an increase in guard cell intracellular Ca2+ concentration by activating voltage-dependent Ca2+-permeable channels, which leads to the activation of slow-sustained (S-type) anion channels in the plasma membrane (8, 9). Anion efflux from guard cells results in membrane depolarization that leads to K+ efflux through outward-rectifying K+ channels, which in turn causes water efflux and stomatal closure. A genetic study in Arabidopsis identified a plasma membrane leucine-rich repeat receptor-like pseudokinase GUARD CELL HYDROGEN PEROXIDE-RESISTANT1 that is essential for H2O2-mediated activation of Ca2+ channels and S-type anion channels (9, 10).
ROS have also been implicated in the inhibition of blue light-dependent stomatal opening, an effect essential for promoting stomatal closure under stress conditions during the day (11). Blue light perception by phototropins, light-activated receptor kinases (12), initiates signaling cascades by phosphorylating a Ser/Thr protein kinase BLUE LIGHT SIGNALING 1 (BLUS1) (13). The activated BLUS1 ultimately activates the plasma membrane H+-ATPase via phosphorylation (14). Consequently, H+ extrusion from guard cells hyperpolarizes the plasma membrane that facilitates K+ uptake through inward-rectifying K+ channels, eventually leading to water uptake and stomatal opening. H2O2 has been shown to inhibit blue light-dependent H+ pumping by suppressing the phosphorylation of H+-ATPase, which prevents membrane hyperpolarization and sustains the depolarized state (11). The H2O2-induced Ca2+ also inhibits H+-ATPase (15). Moreover, H2O2 inhibits inward-rectifying K+ channels (16). Therefore, ROS appear to play multifunctional roles in stomatal regulations.
Cellular ROS levels are regulated by the balance between the rate of ROS production and degradation. Two plasma membrane NADPH oxidases, AtrbohD and AtrbohF, have been implicated as a major source of ROS production in Arabidopsis guard cells (6, 7). A recent study on H2O2 influx into guard cells suggested the involvement of plasma membrane aquaporin PIP2; 1 in H2O2 transport (17). Furthermore, ROS are generated via normal energy and metabolic processes in chloroplasts and peroxisomes (18, 19). The increased ROS in guard cells are removed by antioxidant enzymes such as ascorbate peroxidase (APX1) and catalase (CAT3) (20, 21). Although some mechanistic details of ROS production and signaling by various physiological signals have been elucidated, little is known about how basal ROS homeostasis is maintained in guard cells.
In this study, we isolated and characterized the Arabidopsis autophagy-related (atg) mutants that are impaired in light and low CO2-induced stomatal opening. We showed that autophagy is essential for maintaining basal ROS levels by eliminating oxidized peroxisomes in guard cells, which allows stomatal opening.
Results
Isolation of an Arabidopsis atg2-5 Mutant Defective in Stomatal Opening Responses.
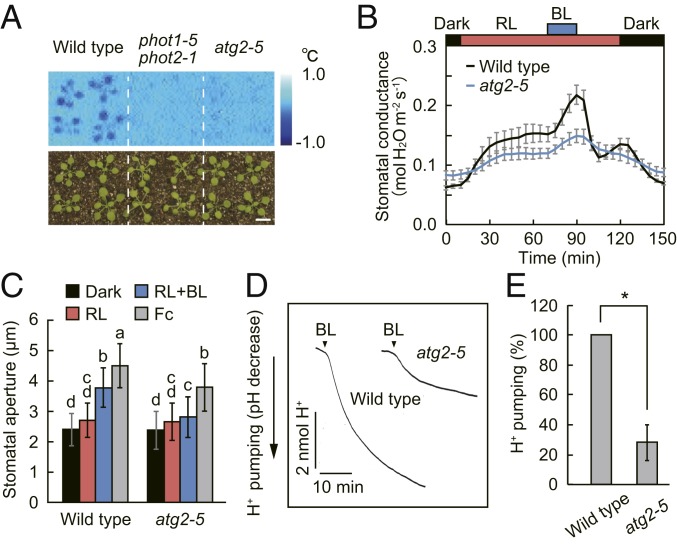
Leaf temperature provides a sensitive assay to monitor stomatal opening via transpirational water loss. We developed a system that detects blue light-dependent stomatal opening by thermal imaging (13). By screening Arabidopsis T-DNA insertion and ethyl methanesulfonate-mutagenized lines, we isolated a recessive mutant showing defects in leaf temperature decrease in response to blue light (Fig. 1A). We named this mutant atg2-5, since it is allelic to the atg2 mutants described later. The stomatal conductance in intact leaves of wild type increased in response to red light, and superimposition of weak blue light elicited additional stomatal opening (Fig. 1B). In contrast, both red light- and blue light-dependent stomatal opening were reduced in the atg2-5 mutant (Fig. 1B). Measurements of stomatal aperture in epidermis revealed that atg2-5 showed reduced stomatal opening in response to blue light and fusicoccin (Fc), an activator of the H+-ATPase (Fig. 1C). Furthermore, the atg2-5 mutation impaired low CO2-induced stomatal opening (SI Appendix, Fig. S1).
Fig. 1.
Impairment of light-dependent stomatal responses in the atg2-5 mutant. (A) Thermal image of a blue light-dependent leaf temperature decrease. Dark-adapted plants were illuminated with red light (RL: 80 µmol m−2 s−1) for 50 min, and then blue light (BL: 5 µmol m−2 s−1) was superimposed on RL. Subtractive images were obtained by subtracting an initial thermal image (before BL illumination) from an image taken 15 min after BL illumination. (Scale bar: 1 cm.) (B) Light-dependent changes in stomatal conductance in intact leaves. Leaves of dark-adapted plants were illuminated with RL (300 µmol m−2 s−1) for 1 h, and then BL (10 µmol m−2 s−1) was superimposed as indicated. Data represent means ± SEM (n = 5). (C) Light- and fusicoccin-dependent stomatal opening in the epidermis. Epidermal strips were incubated in 5 mM MES-bistrispropane (pH 6.5), 50 mM KCl, and 0.1 mM CaCl2 in the dark or under RL (50 µmol m−2 s−1) with or without BL (10 µmol m−2 s−1) for 2 h. Fusicoccin (Fc) at 1 µM was added to the epidermis and incubated in the dark for 2 h. Data represent means ± SD (n = 75, pooled from triplicate experiments). Different letters indicate significant difference (ANOVA with Tukey’s test, P < 0.01). (D) Blue light-dependent H+ pumping. Guard cell protoplasts were illuminated with RL (50 µmol m−2 s−1) for 2 h, and then BL (10 µmol m−2 s−1) was superimposed on RL as indicated. (E) Quantification of the magnitude of H+ pumping. Data represent means ± SEM (n = 4). Asterisk indicates significant difference from the wild type (P < 0.01; Student’s t test).
To determine whether atg2-5 impairs blue light signaling that leads to the activation of H+-ATPase, we measured H+ pumping from guard cell protoplasts. In addition to stomatal opening, H+ pumping by blue light was attenuated in the atg2-5 mutant compared with that in wild-type control (Fig. 1 D and E). Similarly, blue light-dependent phosphorylation of H+-ATPase was reduced in the atg2-5 mutant (SI Appendix, Fig. S2 A and B). We found that the amount of H+-ATPase was slightly decreased in atg2-5 (SI Appendix, Fig. S2 A and C). However, such a decrease could not completely account for the reduced stomatal opening since the disruption of Arabidopsis AHA1, a major H+-ATPase isoform expressed in guard cells, remarkably reduces the total amount of H+-ATPase, but exhibits a lesser degree of inhibition in stomatal opening and H+ pumping (22). Taken together, these results suggest that atg2-5 mutation blocks multiple stomatal opening systems.
Identification of the ATG2 Gene.
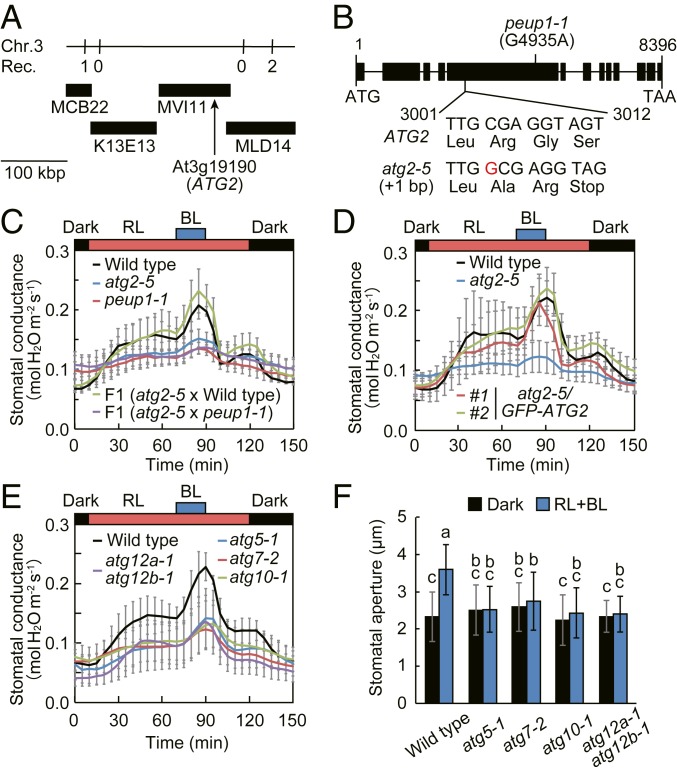
To identify the gene responsible for the atg2-5 mutant, we initially attempted to determine the T-DNA insertion site, since atg2-5 was isolated from the pool of T-DNA lines. However, such flanking sequence was not amplified by thermal asymmetric interlaced PCR (TAIL-PCR). Therefore, we used map-based cloning and whole-genome sequencing to identify the causal mutation in the atg2-5 mutant. The ATG2 locus was mapped to a 231 kbp region on the upper arm of chromosome 3 (Fig. 2A), and this region contains 70 annotated genes according to The Arabidopsis Information Resource. To narrow down candidate genes, we applied MutMap (23) to F2 progeny that showed the mutant phenotype. The highest SNP-index peak was observed on chromosome 3, consistent with the results of map-based cloning. Accordingly, sequence analysis of this region revealed a 1 bp insertion of G in the fifth exon of At3g19190, which encodes AUTOPHAGY-RELATED2 (ATG2), and this insertion resulted in a frameshift and premature stop codon (Fig. 2B). We examined stomatal opening in loss-of-function allele of peup1-1, which harbor a single point mutation within ATG2 (Fig. 2B) (24). The peup1-1 mutant was defective in light-dependent stomatal opening (Fig. 2C and SI Appendix, Fig. S3 A and B). The F1 plants from the cross between atg2-5 and wild type showed the same stomatal opening as in wild type, whereas those from the cross between atg2-5 and peup1-1 showed traits of both parents (Fig. 2C and SI Appendix, Fig. S3 A and B). The atg2-5 stomatal phenotypes were complemented by expressing GFP-ATG2 from its own promoter (Fig. 2D and SI Appendix, Fig. S3 C and D). We concluded that the mutation in ATG2 is the basis for the atg2-5 phenotype.
Fig. 2.
Identification of ATG2 gene and impairment of stomatal opening in other autophagy-related mutants. (A) Map-based cloning of the ATG2 gene. ATG2 was mapped to the 231 kbp region between BAC clones MCB22 and MLD14. Numbers indicate the recombination from a total of 154 chromosomes. (B) Genomic structure and mutation sites of ATG2 gene. Black rectangles indicate the protein-coding region. (C) Light-dependent stomatal opening in F1 progenies obtained from crosses between the atg2-5 and peup1-1 mutants. (D) Rescue of light-dependent stomatal opening by transformation of atg2-5 mutant with GFP-ATG2. (E and F) Impairment of light-dependent stomatal opening in intact leaves (E) and the epidermis (F) in other autophagy-related mutants. For C−E, stomatal conductance was measured as described in Fig. 1B. Data represent means ± SEM (n = 3). For F, epidermal strips were incubated as described in Fig. 1C. Data represent means ± SD (n = 75, pooled from triplicate experiments). Different letters indicate significant difference (ANOVA with Tukey’s test, P < 0.01).
The Autophagy-Defective Mutants Exhibit Impairments in Stomatal Opening.
Autophagy is a eukaryotic process that degrades cytoplasmic constituents and organelles and is strictly controlled by a set of highly conserved autophagy-related proteins, including ATG2. In yeast, ATG2 forms a complex with the phosphatidylinositol 3-phosphate (PI3P)-binding protein ATG18 at the preautophagosomal structure (PAS) to promote autophagosome formation (25, 26). In Arabidopsis, ATG2 has also been shown to be essential for autophagosome formation (27). To determine whether the defective stomatal phenotype of atg2 mutants is caused by the disruption of general autophagy or the ATG2 specific function, we examined light-dependent stomatal opening in Arabidopsis mutants lacking other autophagy-essential genes such as ATG5, ATG7, ATG10, and ATG12. ATG12 is a small ubiquitin-like protein that is specifically conjugated to ATG5 through the sequential actions of ubiquitin E1- and E2-like proteins ATG7 and ATG10, respectively. The ATG12–ATG5 conjugate further interacts with ATG16, and the resulting complex facilitates phagophore expansion and maturation (25, 26). Similar to that in atg2 mutants, the stomatal conductance of atg5-1, atg7-2, atg10-1, and atg12a-1 atg12b-1 was less responsive to both red light and blue light (Fig. 2E). Furthermore, the stomatal opening in epidermis was decreased in these mutants (Fig. 2F). Thus, the defect of autophagic process appeared to be linked to the inhibition of stomatal opening.
Autophagy-Defective Mutants Accumulate High Levels of ROS in Guard Cells, and Antioxidants Restore Stomatal Opening.
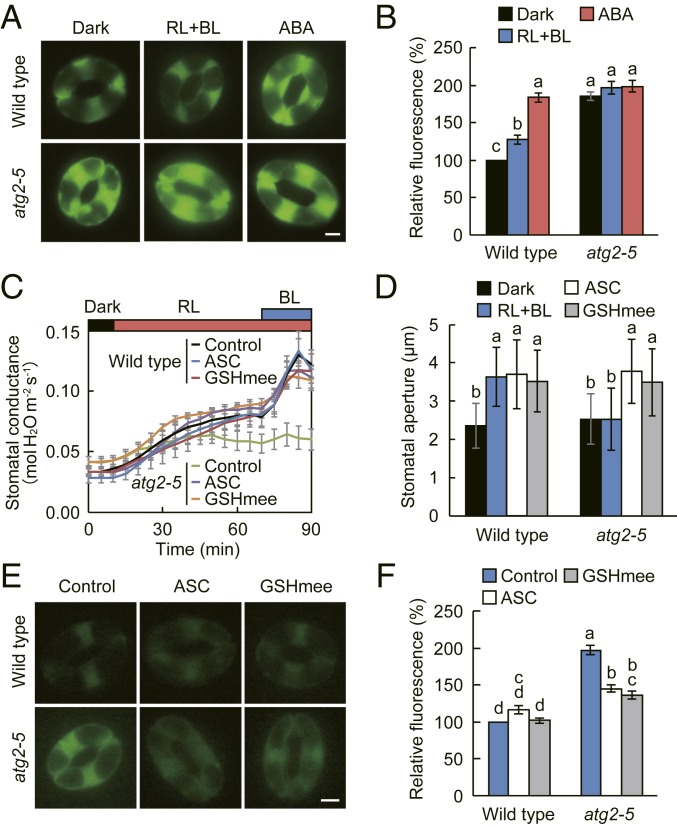
Arabidopsis autophagy-defective mutants show high levels of ROS in leaves (28). Considering that ROS play a central role in stomatal regulations (6, 7), we suspected that increased intracellular ROS might be responsible for the defective stomatal phenotype in atg mutants. To explore this possibility, we determined ROS levels in guard cells by using H2DCFDA, an oxidation-sensitive fluorescence probe. In wild type, intracellular ROS levels increased slightly after exposure to light and largely in response to 10 µM ABA (Fig. 3 A and B). In contrast, the atg2-5 mutant exhibited elevated ROS levels irrespective of ABA treatment (Fig. 3 A and B). Furthermore, similar elevated ROS levels were observed in other atg mutants (SI Appendix, Fig. S4 A and B).
Fig. 3.
ROS accumulation in atg2 guard cells and restoration of stomatal opening and ROS levels by antioxidants. (A and B) ROS accumulation in atg2 mutant guard cells. Images of ROS accumulation in guard cells indicated by the fluorescence dye H2DCFDA (A) and quantification of the relative ROS levels analyzed using ImageJ software (B). Epidermal strips were incubated in the dark or under red (RL: 50 µmol m−2 s−1) and blue light (BL: 10 µmol m−2 s−1) for 1.5 h, and then loaded with H2DCFDA for 30 min. After excess dye was removed by washing, abscisic acid (ABA) at 10 µM was added, and reactions were allowed to proceed under RL and BL for an additional 30 min. (Scale bar: 5 µm.) Data represent means ± SEM (n = 90, pooled from triplicate experiments). (C and D) Restoration of light-dependent stomatal opening in intact leaves (C) and epidermis (D) of atg2-5 mutant by ascorbic acid (ASC) and glutathione methyl ester (GSHmee). For C, leaves of dark-adapted plants were illuminated with RL and BL as described in Fig. 1B. During dark adaptation, 10 mM ASC or 10 µM GSHmee was applied to the soil, and plants were incubated for at least 9 h before the measurements. Data represent means ± SEM (n = 6). For D, epidermal strips were incubated as described in Fig. 1C in the presence of 10 mM ASC or 10 µM GSHmee. Data represent means ± SD (n = 75, pooled from triplicate experiments). (E and F) Scavenging of ROS by ASC and GSHmee in atg2-5 mutant guard cells. Epidermal strips were prepared from dark-adapted plants as described in Fig. 3C, and the ROS levels were determined using H2DCFDA. (Scale bar: 5 µm.) Data represent means ± SEM (n = 90, pooled from triplicate experiments). For (B, D, and F) lowercase letters a, b, c, and d indicate significant difference (ANOVA with Tukey’s test, P < 0.01).
To determine whether the observed constitutive ROS accumulation in atg mutants correlates with the inhibition of stomatal opening, we investigated the effect of antioxidants on light-dependent stomatal opening. Ascorbic acid (ASC) acts as an electron donor for the reduction of H2O2 into H2O, and reduced glutathione (GSH) participates in the regeneration of ASC (19). Application of ASC restored stomatal opening in the atg2-5 mutant, but had no effects on stomatal opening in the wild type (Fig. 3 C and D). A similar result was observed with the addition of glutathione monoethyl ester (GSHmee), a membrane-permeable form of GSH (Fig. 3 C and D) (29). Furthermore, these antioxidants recovered stomatal opening in other atg mutants (SI Appendix, Fig. S4C). Along with stomatal opening, we confirmed that the antioxidants scavenged intracellular ROS in atg2-5 guard cells (Fig. 3 E and F and SI Appendix, Fig. S4 D and E). Conversely, the double mutant of ASC- and GSH-deficient vtc1-1 and cad2-1, respectively, displayed ROS accumulation and reduced stomatal opening particularly under high light condition (SI Appendix, Fig. S5). Taken together, these data suggest that autophagy has a major function in regulating basal ROS levels and stomatal opening.
Autophagy-Defective Mutants Harbor Aggregated Peroxisomes with High Levels of H2O2 in Guard Cells.
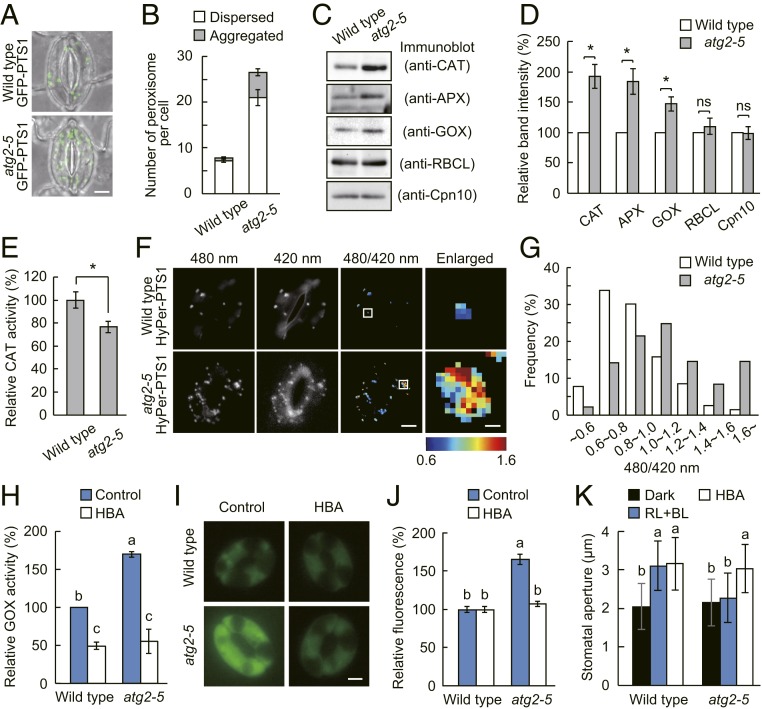
If autophagy plays a role in maintaining intracellular ROS in guard cells, it might control the degradation of oxidized or damaged organelles. In plant cells, peroxisomes and chloroplasts are the major sites of ROS production (18, 19, 30). Therefore, we rationalized that autophagy could impact ROS homeostasis by eliminating dysfunctional peroxisomes or chloroplasts, known as pexophagy and chlorophagy, respectively (31). To address this, we crossed atg mutants with transgenic plants expressing GFP fused with the peroxisome targeting signal 1 (GFP-PTS1), a peroxisomal marker (32), and analyzed peroxisome number in guard cells. A significant increase in peroxisome number was observed in atg2-5 as well as other atg mutants compared with that in wild type (Fig. 4 A and B and SI Appendix, Fig. S6 A and B). In contrast, such an increase was not noted in chloroplasts, as visualized by chlorophyll fluorescence images (SI Appendix, Fig. S6 C and D). In accordance with these results, the atg2-5 guard cells exhibited excess accumulation of peroxisomal proteins such as CAT, APX, and glycolate oxidase (GOX) but not chloroplastic ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (RBCL) and mitochondrial chaperonin 10 (Cpn10) (Fig. 4 C and D).
Fig. 4.
Autophagy deficiency causes an increase of peroxisome number and H2O2 accumulation in guard cells. (A) Images of GFP-PTS1 fluorescence and differential interference contrast (DIC) in guard cells. (Scale bar: 5 µm.) (B) Quantification of peroxisome number per guard cell. Data represent means ± SEM (n = 16). (C) Levels of peroxisomal (CAT, APX, and GOX), chloroplastic (RBCL), and mitochondrial proteins (Cpn10) in the atg2-5 mutant guard cells. (D) Quantification of the protein levels using ImageJ software. Data represent means ± SEM (n = 4). (E) Relative CAT activity in the crude extracts from guard cell protoplasts. The values of the total CAT activity were divided by the band intensity values of corresponding CAT. Data represent means ± SEM (n = 4). (F) Images of HyPer-PTS1 fluorescence excited with 420 nm and 480 nm light, and the corresponding fluorescence ratio (480/420) in guard cells. (Scale bar: 5 µm.) The boxed areas in the 480/420 nm images are enlarged in the Right. (Scale bar: 0.5 µm.) (G) Distribution of the fluorescence ratio (480/420 nm) in peroxisomes of wild type (n = 272) and atg2-5 (n = 275). (H) Relative GOX activity in the crude extracts from epidermal strips. Epidermal strips were preincubated in the dark for at least 9 h in the presence or absence of 10 µM 2-hydroxy-3-butynoate (HBA), and then illuminated with red light (RL: 50 µmol m−2 s−1) and blue light (BL: 10 µmol m−2 s−1) for 2 h. Data represent means ± SEM (n = 3). (I and J) Restorations of ROS accumulation in atg2-5 guard cells by HBA. Epidermal strips were preincubated as described in H, and the ROS levels were determined using H2DCFDA. (Scale bar: 5 µm.) Data represent means ± SEM (n = 90, pooled from triplicate experiments). (K) Restoration of stomatal opening in atg2-5 mutant by HBA. Data represent means ± SD (n = 75, pooled from triplicate experiments). For D and E, asterisks indicate significant difference from the wild type (P < 0.01; Student’s t test). For H, J, and K, different letters indicate significant difference (ANOVA with Tukey’s test, P < 0.01).
Notably, the atg mutants exhibited aggregation of peroxisomes in guard cells (Fig. 4 A and B and SI Appendix, Fig. S6 A and B). The aggregated peroxisomes accumulate enzymatically inactive forms of CAT, a main antioxidative enzyme in peroxisomes (24, 33). Disruptions of CAT3, the predominant isoform expressed in Arabidopsis guard cells (21), and NO CATALASE ACTIVITY1 (NCA1), a molecular chaperone required for CAT activity (34, 35), resulted in ROS accumulation and inhibition of stomatal opening (SI Appendix, Fig. S7). Thus, such peroxisome aggregates could involve ROS production. Our attempts to isolate insoluble fraction from guard cell protoplasts and measure its CAT activity were unsuccessful. Alternatively, we calculated the relative CAT activity by dividing the total CAT activity measured in the crude extracts from guard cell protoplasts by the band intensity of CAT protein in immunoblot analysis (Fig. 4 C and D). The relative CAT activity decreased in atg2-5 mutant guard cells in comparison to wild type (Fig. 4E). Consequently, we determined the basal H2O2 levels in peroxisomes by using genetically encoded H2O2 sensor HyPer fused with PTS1 (HyPer-PTS1) (36). After oxidation with H2O2, the excitation peak of HyPer shifts from 420 to 500 nm. Therefore, the ratiometric fluorescent signal provides a sensitive measurement of cellular H2O2. The HyPer-PTS1 signal was apparent in guard cells of both wild-type and atg2-5 mutant (Fig. 4F). Unlike in wild type, the 480/420 nm ratio was higher in atg2-5 mutant, indicating that H2O2 is accumulated in the peroxisomes of atg2-5 mutant guard cells (Fig. 4 F and G). These findings indicate that autophagy might control ROS levels by selectively eliminating oxidized peroxisomes in guard cells.
To gain further insight into the metabolic source of ROS in atg mutants, we investigated the involvement of GOX, which catalyzes the oxidation of glycolate to glyoxylate and concomitantly produces H2O2. The Arabidopsis genome contains 3 genes encoding GOX, and a public database indicated that GOX1 and GOX2 are preferentially expressed in the guard cells (Arabidopsis eFP Browser; ref. 37). However, simultaneous knock-down of these 2 genes causes severe growth defects (38). We, thus, utilized 2-hydroxy-3-butynoate (HBA), an irreversible inhibitor of GOX (39). We confirmed that HBA impaired GOX activity for recombinant GOX1 and GOX2 proteins (SI Appendix, Fig. S8). In concordance with GOX protein levels (Fig. 4 C and D), the atg2-5 mutant showed higher GOX activity than that of the wild type, and application of HBA reduced GOX activity in both wild-type and atg2-5 mutant (Fig. 4H). Consistently, HBA decreased ROS levels and recovered stomatal opening in the atg2-5 mutant (Fig. 4 I–K). Together, these results suggest that peroxisomal GOX activity is responsible for the primary source of ROS accumulation in atg mutant guard cells.
Discussion
In the present study, we showed that autophagy controls ROS homeostasis in guard cells, which is crucial for stomatal opening. Using thermal imaging, we isolated the Arabidopsis autophagy-defective mutant and found that deletion of autophagy-essential genes results in the impairment of stomatal opening in response to various signals, including light, Fc, and low CO2 (Fig. 1 A–C and SI Appendix, Fig. S1). We also found that intracellular ROS were constitutively increased in guard cells of these autophagy-defective mutants (Fig. 3 A and B and SI Appendix, Fig. S4). These findings are consistent with the enhanced ROS accumulation in the leaves of Arabidopsis atg mutants (28). Furthermore, exogenous application of antioxidants such as ascorbic acid and glutathione decreased the levels of ROS and recovered stomatal opening in the atg mutants (Fig. 3 C–F and SI Appendix, Fig. S4). From these results, we concluded that higher ROS accumulation is responsible for stomatal phenotypes in the atg mutants. Such ROS accumulation might cause the activation and inactivation of the stomatal closing and opening systems, respectively (8–11, 16). Supporting this, blue light-mediated activation of H+-ATPase was inhibited in the atg2-5 mutant (Fig. 1 D and E).
Peroxisomes are eukaryotic organelles that have diverse metabolic functions. In plant peroxisomes, ROS, particularly H2O2, are produced during metabolic processes, including photorespiration, fatty acid β-oxidation, and other oxidative reactions (30). We hypothesized that autophagy maintains the quality of peroxisomes by eliminating dysfunctional and oxidized peroxisomes, thereby preventing excess ROS accumulation in guard cells. In support of this hypothesis, the atg mutants showed an increased number and aggregation of peroxisomes in guard cells (Fig. 4 A and B and SI Appendix, Fig. S6 A and B). This aggregate contains enzymatically inactive CAT, implying a decrease in ROS detoxification in these peroxisomes (24, 33). Indeed, the atg2-5 mutant peroxisomes exhibited high levels of H2O2 with reduced CAT activity (Fig. 4 D–F). The atg mutants also display accumulation of oxidative peroxisomes in leaves but not in roots (24, 33). These findings, combined with those of previous studies, indicate that pexophagy plays crucial roles in ROS homeostasis in aerial plant tissues, including guard cells.
Our data suggest that peroxisomal GOX activity is the predominant metabolic source of ROS accumulation in the atg mutant guard cells (Fig. 4 H–K). However, it is currently unknown why excessive ROS was accumulated in atg guard cells even under dark and low light condition, in which photorespiration is not active (Fig. 3 A and B). We note that a GOX substrate glycolate is provided by conversion not only from the photorespiratory 2-phosphoglycolate (2-PG) metabolism with 2-PG phosphatase (PGLP) but also from glyoxylate with cytosolic and plastidial glyoxylate reductase (GLYR) (40). Glycolate is also produced by the oxidation of the 1,2-dihydroxyethyl-thiamin-diphosphate intermediate of transketolase (41). Conversely, ROS accumulation of cat3-1 and nca1-1 mutants under low light condition was limited compared to that of atg mutants (SI Appendix, Fig. S7). One possibility is that the highly oxidized peroxisomes are removed immediately as long as autophagy is functional. Further genetic analysis of atg mutants will help to dissect the nature of the ROS homeostasis in guard cells.
A recent investigation indicated that phototropins mediate the breakdown of triacylglycerols in guard cells, which leads to the production of ATP through peroxisomal β-oxidation and thus impacts light-dependent stomatal opening (42). We cannot exclude the possibility that intracellular ATP levels are diminished in atg mutant guard cells. However, Arabidopsis atg mutants retain functional β-oxidation activity (24, 43). Furthermore, mutation of the Arabidopsis KAT2/PED1/PKT3, a major isoform of 3-ketoacyl-CoA thiolase that catalyzes the last step of the β-oxidation, exhibited light-dependent stomatal opening comparable to that in wild type (SI Appendix, Fig. S9; ref. 44). Thus, β-oxidation is unlikely to be a major cause of the stomatal defects in atg mutants.
In addition to peroxisomes, photosynthetic electron transport chain in chloroplasts represents the major site of ROS generation in plant cells (18, 19). Recent studies have provided evidence for the selective elimination of chloroplasts via autophagy. In the leaves of wild-type Arabidopsis, the number of chloroplasts decreased following exposure to dark or UV-B, but such decrease was not found in atg mutants (45, 46). In contrast, under nonstress conditions, the number of chloroplasts and maximum quantum yield of photosystem II (Fv/Fm) were not significantly different between wild type and atg mutants (24, 46). In line with these findings, our data revealed no significant difference in chloroplast number in guard cells (SI Appendix, Fig. S6 C and D). We found 2 additional chloroplast degradation pathways that are not dependent on autophagy, which include the formation of senescence-associated vacuoles and CV-containing vesicles (47). Furthermore, recent studies have identified selective autophagy of the endoplasmic reticulum and mitochondria in plant cells (31). Further investigation will be required for elucidating the role of autophagy-mediated elimination of these organelles in ROS homeostasis in plant cells.
Plasma membrane NADPH oxidases mediate ROS production in guard cells in response to ABA (48). In addition, cell wall peroxidase is implicated in salicylic acid-induced ROS generation and stomatal closure (49). However, these pathways are unlikely to account for ROS accumulation in atg mutants because diphenyleneiodonium chloride and salicylhydroxamic acid, inhibitors of NADPH oxidase and peroxidase, respectively (16, 49), had little effect on ROS levels in the atg2-5 mutant (SI Appendix, Fig. S10).
In conclusion, this study identified autophagy-mediated ROS homeostasis mechanisms in guard cells. In general, autophagy has been recognized to play a role in nutrient recycling under starvation conditions. However, in sharp contrast, our findings showed that autophagy selectively degrades oxidized peroxisomes in guard cells under nutrient and nonstress conditions. Although selective elimination of organelles via autophagy is thought to maintain the quality control of organelles, the physiological significance of pexophagy in plants has remained unclear. Thus, we propose that plant pexophagy sustains stomatal opening and photosynthetic CO2 fixation by regulating basal ROS in guard cells. Maintenance of ROS homeostasis by pexophagy might also allow rapid ROS increase and stomatal closure in response to diverse environmental changes; this acts as an adaptive mechanism under natural conditions. Further studies are warranted to elucidate the selective mechanisms underlying plant pexophagy.
Materials and Methods
Plant materials and growth conditions, measurements of stomatal opening, isolation of guard cell protoplasts, measurement of H+ pumping, immunoblot analysis, identification of ATG2 gene, construction of transgenic plants, detection of ROS, detection of H2O2 in peroxisomes, confocal microscopy, measurement of CAT activity, preparation of recombinant proteins, and measurement of GOX activity are described in SI Appendix, SI Methods.
Supplementary Material
Acknowledgments
We thank Jyunichi Mano, Masaru Shibata, Tsuneaki Takami, Koichi Sugimoto, Shino Goto-Yamada, and Noriyuki Suetsugu for valuable discussion; Michitaro Shibata for technical information; Michito Tsuyama for equipment support; Ann Cuypers and Takahiro Ishikawa for providing vtc1-1, cad2-1, and vtc1-1 cad2-1 mutants; the Salk Institute Genomic Analysis Laboratory for providing the sequence-indexed Arabidopsis T-DNA insertion mutants; the Arabidopsis Biological Resource Center and NASC for providing seeds; the Model Plant Research Facility, National Institute for Basic Biology BioResource Center, and Spectrography and Bioimaging Facility, NIBB Core Research Facilities, for technical support; and the Iwate Biotechnology Research Center (IBRC) for providing MutMap pipeline. This work was supported by Japan Society for the Promotion of Science KAKENHI (Grant numbers: 18H02468, 26711019, 15K14552 [to A.T.]; 19K16171 [to S.Y.]; 17K07457 [to S.M.]); the Japan Foundation for Applied Enzymology (to A.T.); the Cooperative Research Grant of the Plant Transgenic Design Initiative (PTraD) at Gene Research Center, University of Tsukuba (to A.T.); the Cooperative Research Project Program of the Medical Institute of Bioregulation, Kyushu University (to A.T.); and NIBB Collaborative Research Program Number 17-518 (to K.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910886116/-/DCSupplemental.
References
- 1.Schroeder J. I., Allen G. J., Hugouvieux V., Kwak J. M., Waner D., Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Hetherington A. M., Woodward F. I., The role of stomata in sensing and driving environmental change. Nature 424, 901–908 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Roelfsema M. R. G., Hedrich R., In the light of stomatal opening: New insights into ‘the Watergate’. New Phytol. 167, 665–691 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Shimazaki K., Doi M., Assmann S. M., Kinoshita T., Light regulation of stomatal movement. Annu. Rev. Plant Biol. 58, 219–247 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Assmann S. M., Jegla T., Guard cell sensory systems: Recent insights on stomatal responses to light, abscisic acid, and CO2. Curr. Opin. Plant Biol. 33, 157–167 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Song Y., Miao Y., Song C. P., Behind the scenes: The roles of reactive oxygen species in guard cells. New Phytol. 201, 1121–1140 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Sierla M., Waszczak C., Vahisalu T., Kangasjärvi J., Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 171, 1569–1580 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pei Z. M., et al. , Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Hua D., et al. , A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24, 2546–2561 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sierla M., et al. , The receptor-like pseudokinase GHR1 is required for stomatal closure. Plant Cell 30, 2813–2837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., et al. , Inhibition of blue light-dependent H+ pumping by abscisic acid through hydrogen peroxide-induced dephosphorylation of the plasma membrane H+-ATPase in guard cell protoplasts. Plant Physiol. 136, 4150–4158 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoshita T., et al. , Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414, 656–660 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Takemiya A., et al. , Phosphorylation of BLUS1 kinase by phototropins is a primary step in stomatal opening. Nat. Commun. 4, 2094 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita T., Shimazaki Ki., Blue light activates the plasma membrane H(+)-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 18, 5548–5558 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinoshita T., Nishimura M., Shimazaki K., Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard cells of fava bean. Plant Cell 7, 1333–1342 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X., et al. , Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 126, 1438–1448 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues O., et al. , Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. U.S.A. 114, 9200–9205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foyer C. H., Noctor G., Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 119, 355–364 (2003). [Google Scholar]
- 19.Asada K., Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 141, 391–396 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pnueli L., Liang H., Rozenberg M., Mittler R., Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J. 34, 187–203 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Jannat R., et al. , Roles of intracellular hydrogen peroxide accumulation in abscisic acid signaling in Arabidopsis guard cells. J. Plant Physiol. 168, 1919–1926 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamauchi S., et al. , The plasma membrane H+-ATPase AHA1 plays a major role in stomatal opening in response to blue light. Plant Physiol. 171, 2731–2743 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe A., et al. , Genome sequencing reveals agronomically important loci in rice using MutMap. Nat. Biotechnol. 30, 174–178 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Shibata M., et al. , Highly oxidized peroxisomes are selectively degraded via autophagy in Arabidopsis. Plant Cell 25, 4967–4983 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li F., Vierstra R. D., Autophagy: A multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci. 17, 526–537 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Liu Y., Bassham D. C., Autophagy: Pathways for self-eating in plant cells. Annu. Rev. Plant Biol. 63, 215–237 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Nishimura M. T., Zhao T., Tang D., ATG2, an autophagy-related protein, negatively affects powdery mildew resistance and mildew-induced cell death in Arabidopsis. Plant J. 68, 74–87 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Yoshimoto K., et al. , Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell 21, 2914–2927 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahan M. S., et al. , Deficient glutathione in guard cells facilitates abscisic acid-induced stomatal closure but does not affect light-induced stomatal opening. Biosci. Biotechnol. Biochem. 72, 2795–2798 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Del Río L. A., López-Huertas E., ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol. 57, 1364–1376 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Yoshimoto K., Ohsumi Y., Unveiling the molecular mechanisms of plant autophagy-from autophagosomes to vacuoles in plants. Plant Cell Physiol. 59, 1337–1344 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Mano S., et al. , Distribution and characterization of peroxisomes in Arabidopsis by visualization with GFP: Dynamic morphology and actin-dependent movement. Plant Cell Physiol. 43, 331–341 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Yoshimoto K., et al. , Organ-specific quality control of plant peroxisomes is mediated by autophagy. J. Cell Sci. 127, 1161–1168 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Hackenberg T., et al. , Catalase and NO CATALASE ACTIVITY1 promote autophagy-dependent cell death in Arabidopsis. Plant Cell 25, 4616–4626 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J., et al. , A chaperone function of NO CATALASE ACTIVITY1 is required to maintain catalase activity and for multiple stress responses in Arabidopsis. Plant Cell 27, 908–925 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa A., et al. , H2O2 in plant peroxisomes: An in vivo analysis uncovers a Ca(2+)-dependent scavenging system. Plant J. 62, 760–772 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winter D., et al. , An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS One 2, e718 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dellero Y., et al. , Decreased glycolate oxidase activity leads to altered carbon allocation and leaf senescence after a transfer from high CO2 to ambient air in Arabidopsis thaliana. J. Exp. Bot. 67, 3149–3163 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Jewess P. J., Kerr M. W., Whitaker D. P., Inhibition of glycollate oxidase from pea leaves. FEBS Lett. 53, 292–296 (1975). [DOI] [PubMed] [Google Scholar]
- 40.Dellero Y., Jossier M., Schmitz J., Maurino V. G., Hodges M., Photorespiratory glycolate-glyoxylate metabolism. J. Exp. Bot. 67, 3041–3052 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Christen P., Gasser A., Production of glycolate by oxidation of the 1,2-dihydroxyethyl-thamin-diphosphate intermediate of transketolase with hexacyanoferrate(III) or H2O2. Eur. J. Biochem. 107, 73–77 (1980). [DOI] [PubMed] [Google Scholar]
- 42.McLachlan D. H., et al. , The breakdown of stored triacylglycerols is required during light-induced stomatal opening. Curr. Biol. 26, 707–712 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J., et al. , Autophagy-related proteins are required for degradation of peroxisomes in Arabidopsis hypocotyls during seedling growth. Plant Cell 25, 4956–4966 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang T., Zhang X. F., Wang X. F., Zhang D. P., Arabidopsis 3-ketoacyl-CoA thiolase-2 (KAT2), an enzyme of fatty acid β-oxidation, is involved in ABA signal transduction. Plant Cell Physiol. 52, 528–538 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Wada S., et al. , Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves. Plant Physiol. 149, 885–893 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Izumi M., Ishida H., Nakamura S., Hidema J., Entire photodamaged chloroplasts are transported to the central vacuole by autophagy. Plant Cell 29, 377–394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie Q., Michaeli S., Peled-Zehavi H., Galili G., Chloroplast degradation: One organelle, multiple degradation pathways. Trends Plant Sci. 20, 264–265 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Kwak J. M., et al. , NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22, 2623–2633 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mori I. C., Pinontoan R., Kawano T., Muto S., Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol. 42, 1383–1388 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.