Significance
An agnostic screen of our collection of stable sulfur fluoride exchange (SuFEx)able derivatives discovers a promising class of covalent and highly selective, elastase inhibitors. Their dormant behavior is a reflection of an exquisite sensitivity in choice of a protein reaction partner, like a “sleeping beauty finding the right prince.” Hence a special SuFEx catalysis environment is required for the perfect matchmaking event: a naturally folded protein with precise electrostatic and geometric setups, such that one of its own nucleophilic side chain ends up replacing the probe’s S–F link to become permanently covalent. In contrast, the same protein, when denatured, is incapable of being covalently modified by the same probe.
Keywords: click chemistry, SuFEx, agnostic, covalent inhibitor, elastase
Abstract
Sulfur fluoride exchange (SuFEx) has emerged as the new generation of click chemistry. We report here a SuFEx-enabled, agnostic approach for the discovery and optimization of covalent inhibitors of human neutrophil elastase (hNE). Evaluation of our ever-growing collection of SuFExable compounds toward various biological assays unexpectedly revealed a selective and covalent hNE inhibitor: benzene-1,2-disulfonyl fluoride. Synthetic derivatization of the initial hit led to a more potent agent, 2-(fluorosulfonyl)phenyl fluorosulfate with IC50 0.24 μM and greater than 833-fold selectivity over the homologous neutrophil serine protease, cathepsin G. The optimized, yet simple benzenoid probe only modified active hNE and not its denatured form.
Sulfur fluoride exchange (SuFEx)—the new-generation click chemistry, since first introduced in 2014 (1), has quickly found diverse applications across an array of fields (DOI: 10.1039/C8CS00960K), including chemical synthesis (2–12), material science (13–19), chemical biology (20–32), and drug discovery (33, 34). SuFEx creates robust intermolecular links between modules. The fidelity of SuFEx stems from the ability of otherwise very stable high oxidation state sulfur fluorides (35–39) to exchange the S–F bonds with incoming nucleophiles on separate modules under SuFEx catalysis conditions. The resulting new sulfur hub sites constitute permanent connections. These unique SuFEx reactions are thought to be made possible by the strict requirements needed to stabilize the departing fluoride ion in its transit away from the strong original covalent bond to sulfur. The facilitating agents are “H+” or “R3Si+.” The process especially favors a 1,8-diazabicyclo(5.4.0)undec-7-ene (DBU)-type amine catalyst (13, 40, 41), and is also thought to involve bifluoride counterion species (16, 17, 42).
In the context of selective in vitro or in vivo covalent capture of proteins by compounds containing a S-F bond that can undergo SuFEx reaction (i.e., SuFExable compounds), examples are rapidly accumulating (20–31). However, we are still far from making strong a priori inferences about the various factors that might relate to a given capture’s occurrence. That said, there is one remarkable fact shared by all these SuFEx-based protein captures, namely that the exchangeable S–F bonds in these probes are among the most demure electrophiles known to chemistry (1, 36–39). For example, no reaction occurred in the neat mixture of refluxing benzenesulfonyl fluoride (4 mL, ∼33 mmol) and aniline (45 mL, ∼500 mmol) at 184 °C for 3 h (35). Under the same conditions, electrophiles commonly studied as covalent “warheads” in medicinal chemistry including epoxide, acrylamide, vinyl sulfone, chloroacetamide, chloromethyl ketone, β-lactam, maleimide, and fluorophosphate do not survive (SI Appendix, Table S3).
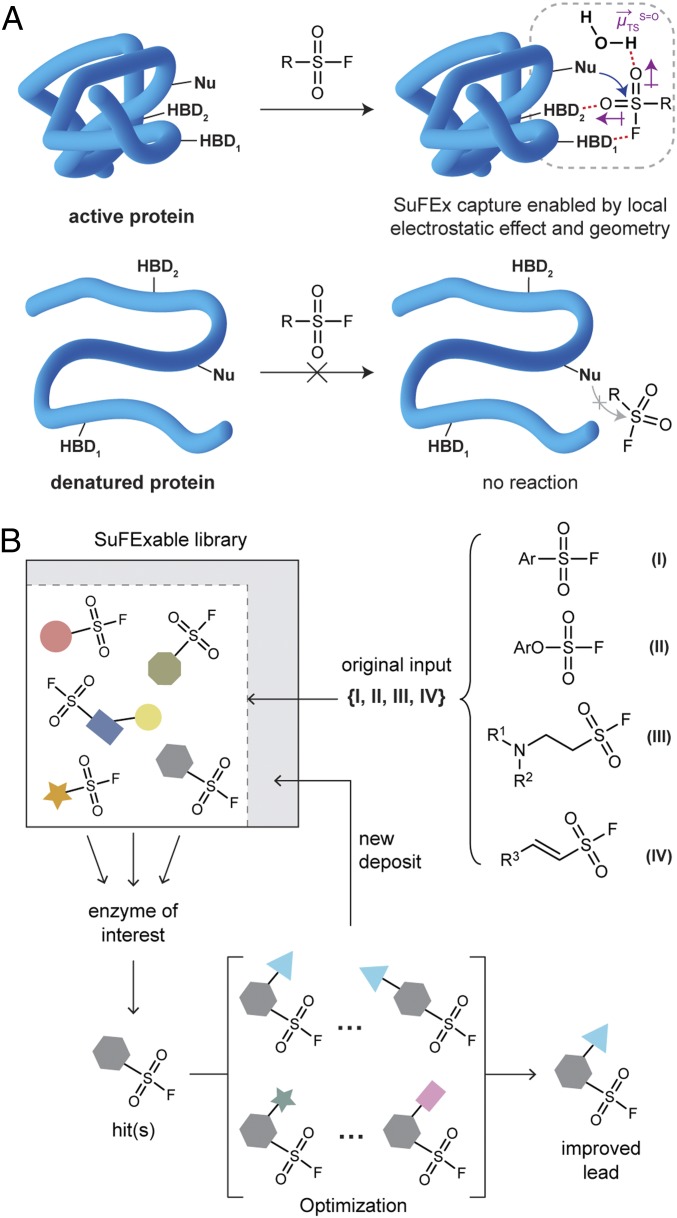
Only a correctly folded and functionally active protein can serve as a “catalyst” (43) for a SuFEx capture event upon one of its own nucleophilic amino acid side chains (Fig. 1A). These enzymic SuFEx capture reactions are, we posit, the result of a unique ensemble of factors between the naturally folded protein and its correct partner probe’s latent S–F electrophilic site. When the perfect S–F probe for a given protein encounters the denatured form of the latter, there is no detectable reaction (24, 33). In fact, in our experience with many S–F capture probes and various denatured proteins, even including entire denatured proteomes, is that misfolded proteins simply do not react with S–F electrophiles at all (24). Among the main factors present in the natural proteins to facilitate extraction and transport of fluoride will be special groups, e.g., arginine side chains juxtaposed to provide just so, hydrogen bonding networks (33). The latter are closely related to local electric fields, electrostatic effects which are unique for all natural proteins (44–47). Thus, it is not hard to imagine that the transit of “F−” away from a SuFExable probe in a capture event could be a very subtle outcome of electric field pulses for the specific reactant pair at hand. And yet, the enormous range of reactivity of, e.g., PhSO2–F (stable in “refluxing aniline test,” vide supra), does argue for something quite special going on with these nearly inert S–F-based covalent probes.
Fig. 1.
Overview. (A) The SuFExable probes (e.g., sulfonyl fluoride) only capture a specific, naturally folded protein where a nucleophilic side chain (Nu) and hydrogen bonding donors (HBDs, including amino acid side chains and bound or free water) constitute such precise networks that meet the local geometric and electrostatic requirements (dipole moment changes in transition state) for the protein’s own SuFEx reaction. (B) Schematic of the SuFExable library-enabled covalent lead compound discovery process.
In this light, the awakening of a SuFExable probe by the captured protein (24, 33) mirrors very closely our earlier femtomolar, freeze-frame, yet reversible, inhibitors made inside acetylcholinesterase (AChE) as a “reaction vessel” (48–51), regarding the little understood, yet enormous rate acceleration achieved by the AChE protein. We named these enzyme templating processes in situ click chemistry, more recently also termed kinetic target-guided synthesis (52–54).
Taking inspiration from the original click chemistry manifesto (55) that molecular diversity can be achieved with ease through the connection of small modules, using just a few good reactions, in sequences of usually no more than 3 steps, we demonstrate here 2 distinct but logically connected, sequential SuFEx-enabled entities: 1) efficient construction of a pool of SuFExable compounds via click chemistry principles, and 2) bioprospecting this library for covalent capture agents for important protein targets. The expectation, based on previous experience, is that evaluation of a SuFEx library often turns up a few hits. The latter can then be followed up by short and simple syntheses of analogs for structure–activity relationship evaluation. This “compound-bank” mode of the SuFEx-enabled platform, itself contributes to the ever-growing library of SuFExable modules and candidates, and like “compounding interest,” rewards the principle library with expanding opportunities for discovering new kinds of phenotypic modulators, including useful new functions for medicines in the long term (Fig. 1B).
Over the past 5 y, we (1, 5, 8, 56) and others (12, 57–60) have found a collection of efficient methods to synthesize SuFExable compounds from either simple or complex organic molecules using highly connective SuFEx core electrophiles (e.g., SO2F2, O = SF4, CH2 = CHSO2F). Collective work within the Sharpless laboratory continues to build a library of SuFExable small modules. There are >1,000 compounds in this collection. The individual compounds are dissolved as 10 mM dimethyl sulfoxide (DMSO) stock solutions and stored in −20 °C freezer, with most being stable at −20 °C as evidenced by periodic inspection by liquid chromatography-mass spectrometry (LC-MS) of randomly sampled compounds over 3 y. Thanks to the great stability of the SuFExable S–F links toward water and oxygen, the oldest library deposits (as DMSO solutions) are still pure. To date, the library and/or its sublibraries have been screened with collaborators at Scripps Research against multiple targets. Here we report a case study using the SuFEx-enabled approach to the discovery of selective, covalent inhibitors of human neutrophil elastase (hNE).
hNE, a member of the serine protease superfamily, is aberrantly active in cystic fibrosis, chronic obstructive pulmonary disease, and inflammatory bowel diseases (61–77). This protease is therefore a key target for the development of antiinflammatory agents to combat these diseases. Alkyl and aryl sulfonyl fluorides have a long history as rather promiscuous covalent inhibitors of serine proteases (78–87). In the late 1970s, the Powers group demonstrated the intrinsic reactivity differences across several serine proteases, including elastase, with 2-amido(peptido) benzenesulfonyl fluoride inhibitors (88, 89). Their results encouraged us to investigate our library of SuFExable compounds as potential covalent inhibitors of hNE.
Results and Discussion
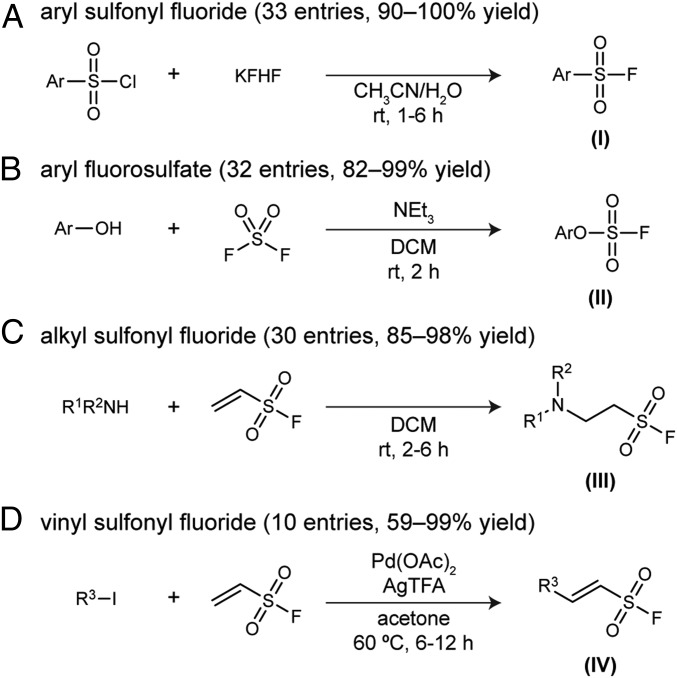
A set of 105 compounds (SI Appendix, Table S1), many of which appear to be new compounds, were selected without bias, to form a primary library for the screen against hNE. The selected compounds can be categorized into 4 subsets by the nature of S–F functional groups (Fig. 2). Each group tends to have its own intrinsic zone of reactivity in acid–base environments. To give a general impression, the relative rate measured on a representative molecule of each subset under SuFEx catalysis was found to be 28.5 (I), 1.0 (II, reference), 14.1 (III), and 4.1 (IV), respectively (SI Appendix, Fig. S6). Aryl sulfonyl fluorides (I), despite their own extreme resistance toward nucleophiles, top the SuFEx reactivity hierarchy among the 4 subsets, while aryl fluorosulfates (II) lie at the bottom.
Fig. 2.
Efficient SuFEx derivatization of abundant building blocks yields a SuFExable library with 4 subset groups, categorized according to their S–F links’ inherent reactivity. (A) Aryl sulfonyl fluorides (Subset I). (B) Aryl fluorosulfates (Subset II). (C) Alkyl sulfonyl fluorides (Subset III). (D) (E)-Vinyl sulfonyl fluorides (Subset IV). Ar, aryl, or heteroaryl; NEt3, triethylamine; R1,2, H, alkyl, aryl, or heteroaryl; R3, alkenyl, aryl, or heteroaryl; Pd(OAc)2, palladium (II) acetate; AgTFA, silver (I) trifluoroacetate.
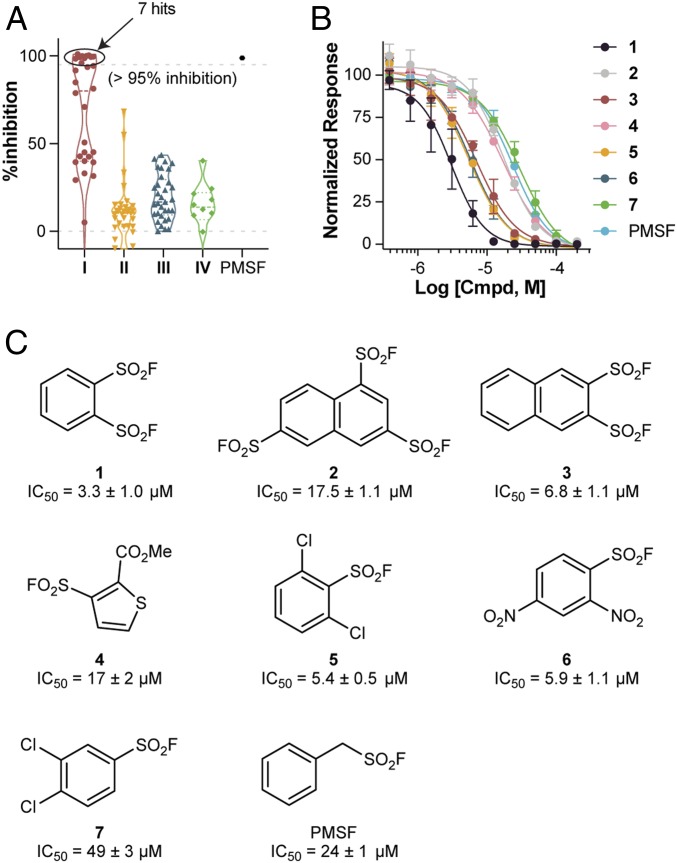
Elastase (5 nM) was incubated with each entry of the primary SuFEx library (final compound concentration 200 μM) for 10 min at room temperature prior to the addition of peptide substrate MeOSuc-AAPV-AMC (50 μM). Increase in fluorescence was measured for 30 min at 30-s intervals. The assay was well behaved, as evidenced by a Z′ of 0.86, and signal-to-background of ∼3,000:1. Reasoning that a covalent inhibitor, which targets a catalytic residue, should completely inactivate the enzyme at high compound concentration (200 μM), a high threshold (95%) was chosen for hit identification. Under this criterion, the screen yielded 7 hits as probable covalent inhibitors (Fig. 3A, 6.7% overall hit rate and 23% hit rate within subset I). All 7 compounds belong to subset I, which suggests a rough cutoff based on the S-link’s inherent reactivity. After validation by NMR, LC-MS, and dose-dependent response, benzene-1,2-disulfonyl fluoride (1) proved to be the leading candidate, inhibiting hNE with IC50 = 3.3 ± 1.0 μM (Fig. 3 B and C).
Fig. 3.
Screen of the primary SuFEx library toward elastase inhibitory activity. (A) Initial screen with 105 SuFExable compounds yielded 7 hits with >95% inhibition at 200 µM. (B) Dose–response curves of hit compounds (1–7, and phenylmethanesulfonyl fluoride [PMSF] as a reference inhibitor) against hNE (AAPV-AMC fluorescence assay). Each compound was assessed over a 2-fold logarithmic dilution series. (C) Structures and IC50 values of compounds 1–7 and PMSF. IC50 values were measured based on 10-min incubation and are shown in mean ± SD (n ≥ 3).
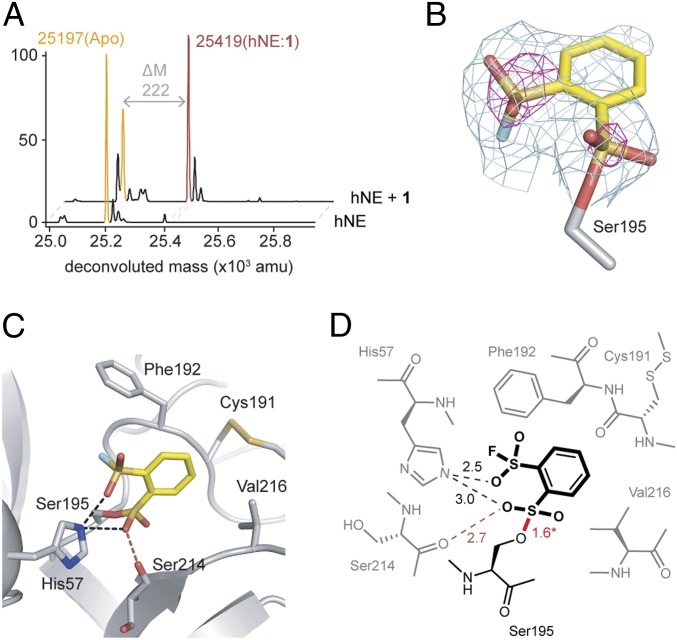
The covalent inhibition of hNE by 1 was examined by high-resolution matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (Fig. 4A). Incubation of 1 (exact mass 242 Da) with hNE yielded a peak shift from the protein mass by 222 Da. Increased mass corresponds to: 1) a single molecule of 1 covalently captured by hNE; 2) the loss of 1 hydrogen from the protease (possibly from the catalytic serine); and (3) the loss of 1 fluorine from 1.
Fig. 4.
Compound 1 (exact mass 241.95) is a covalent inhibitor of hNE. (A) MALDI-TOF mass spectrometry evidence of covalent complex hNE:1 formation. (B) Naive Fo-Fc map contoured at 1.5σ (green) and 4σ (magenta) clearly delineates the binding orientation of 1 to Ser195 and the specific location of the sulfur groups, respectively. Residue of 1 is shown as a stick model with yellow carbon, red oxygen, light-blue fluorine, mustard sulfur. (C) Active site residues that provide potential hydrogen bonds (black dashes), repulsive interactions (brown dash), and hydrophobic residues that bind 1 (gray elastase carbon, nitrogen blue). (D) Schematic of bond distances between 1 and elastase with potential hydrogen bonds (black dashes) and negative repulsive interactions (brown dash) with bond distances in Å. The covalent bond (red) between Ser195 and the sulfur of 1 were set at 1.57 Å during structure refinement.
We also subjected hNE to cocrystallization with 1 in order to corroborate the covalent binding. The structure was determined using molecular replacement with Protein Data Bank (PDB) ID code 5adw and the cocomplex was refined to 2.33-Å resolution (SI Appendix, Table S2). Importantly the naive Fo-Fc electron density maps contoured to 4σ clearly position 1, as a result of the strong diffraction of sulfurs (Fig. 4B). The aryl group of 1 is nestled into a hydrophobic pocket consisting of residues Phe192 and Val216 and the compound is covalently bound to the catalytic Ser195, as highlighted by continuous electron density and a bond distance of ∼1.6 Å (Fig. 4 C and D). The covalent inhibition of hNE via sulfonylation by 1 appeared to be permanent—dialyzing away small molecules after incubation did not recover enzyme function (SI Appendix, Fig. S2).
Considering the monocovalent attachment mode of 1, with the second –SO2F intact, it was envisaged that 1 of the 2 sulfonyl fluoride groups could be substituted so as to perhaps improve the capture rate, and/or selective binding (Table 1). A set of benzenesulfonyl fluoride cores carrying ortho-substituents (8–24) was therefore examined. Compounds 8–18 (90) were synthesized by the efficient aqueous potassium bifluoride exchange procedure from commercially available sulfonyl chlorides but showed poorer reactivity/binding with hNE. The monovinylogous derivative 19 was a more active inhibitor with IC50 = 2.2 ± 0.7 μM. The ortho-sulfamoyl benzenesulfonyl fluorides (20, 21) led to considerably lower activity. A recently developed potassium bifluoride catalyzed SuFEx perfluoroalkylation of aryl sulfonyl fluorides (91) enabled us to quickly convert 1 to the monoperfluoroalkyl sulfones (22, 23). Compound 22 with the ortho-triflyl group was identified as a better lead molecule with IC50 = 1.1 ± 0.1 μM. To our surprise, a simple benzenoid compound 24, where the ortho-substituent is another SuFEx functionality, aryl fluorosulfate, emerged as the best agent to date with much improved potency, IC50 = 0.24 ± 0.02 µM.
Table 1.
Optimization of the original hit 1 by examining 2-substituted benzenesulfonyl fluoride
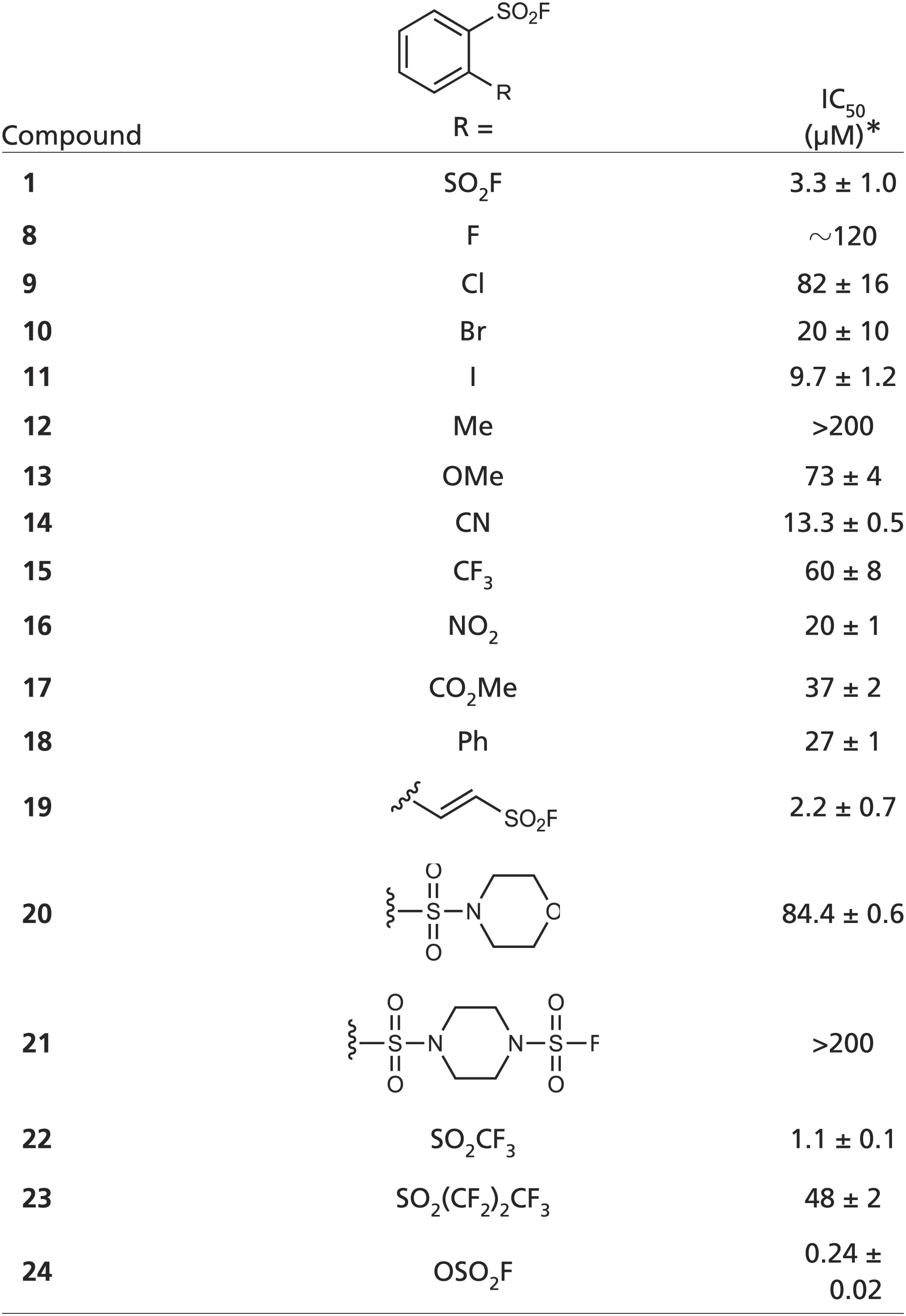 |
IC50 values were measured based on 10-min incubation and are shown in mean ± SD (n ≥ 3).
Next, we tested the lead compounds (1, 19, 22, and 24) against a panel of serine proteases; we found that three (1, 22, and 24) among the four effective hNE inhibitors did not inactivate the homologous serine protease, human cathepsin G (hCG), which has 37% sequence identity with hNE and a highly similar crystal structure [root-mean-square deviation (rmsd) = 0.82 Å, max rmsd = 5.89 Å for 180 out of 218 Cα residues of hNE]. Unlike PMSF (PhCH2SO2F) long known for ablating the hydrolytic activity of almost all serine proteases, the compounds 1, 22, and 24 identified in this study showed 58 and >182, and >833-fold specificity for hNE over hCG, respectively (Table 2). The selective inhibition of hNE could be partly attributed to a proximity factor as suggested by molecular modeling using a reactive docking protocol (SI Appendix, Fig. S5)
Table 2.
Selectivity of the lead compounds 1, 19, 22, and 24 against hNE and hCG
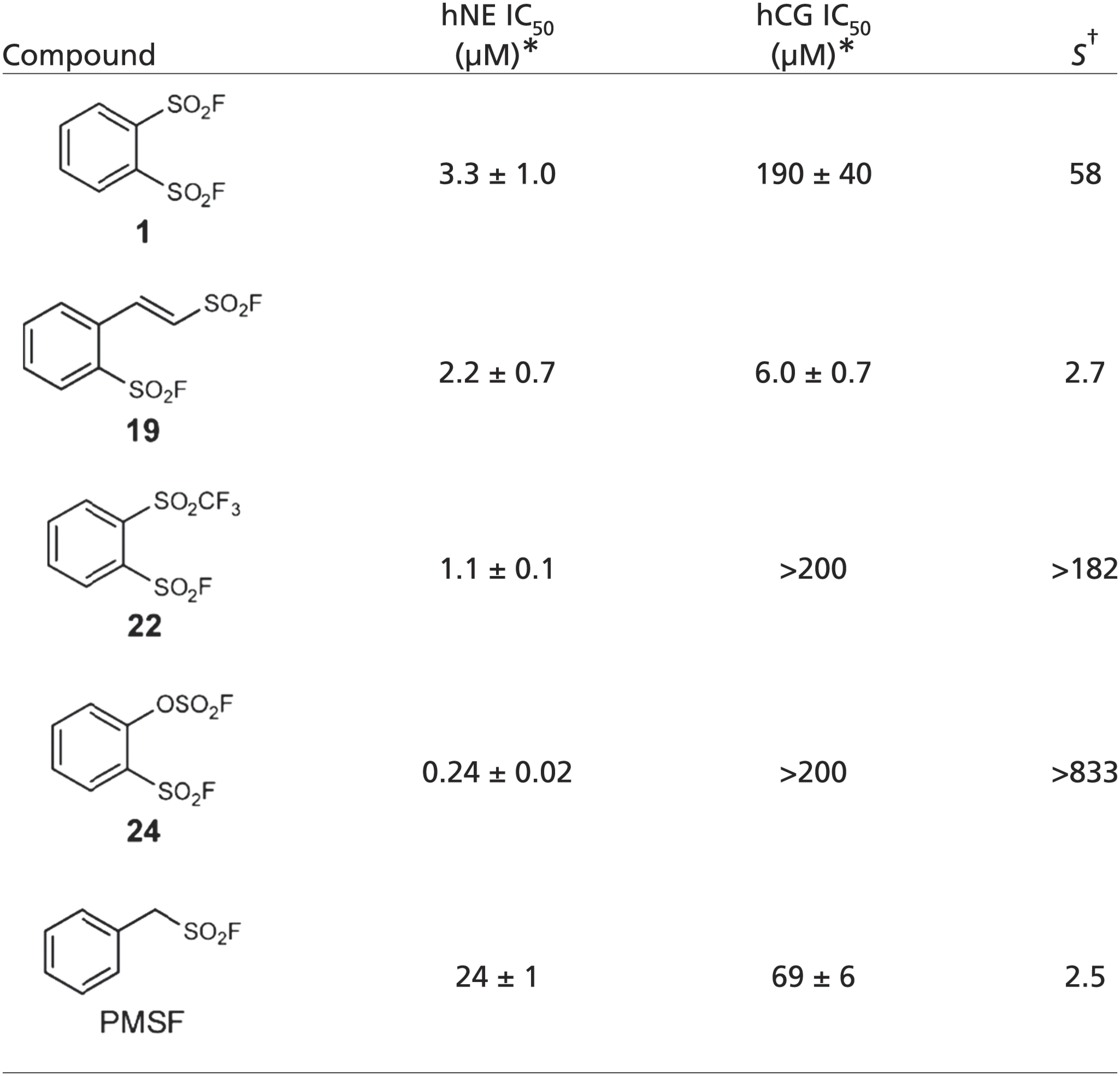 |
IC50 values were measured based on 10-min incubation and are shown in mean ± SD (n ≥ 3).
S value denotes the selectivity, defined by the ratio of IC50 (hCG) over IC50 (hNE).
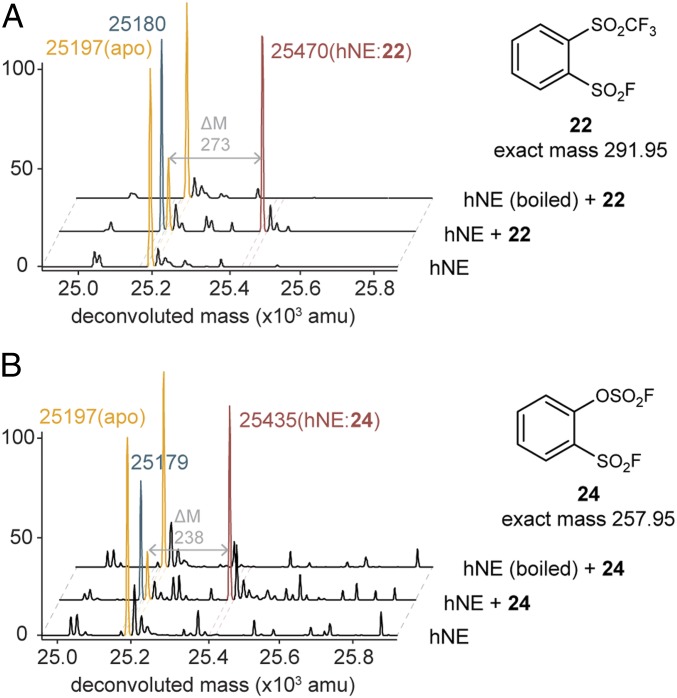
High-resolution MALDI-TOF mass spectrometry study supports the covalent inhibition mechanism of the more potent and selective agents 22 and 24 to be sulfonylation of hNE (maroon peaks, +273 Da for 22, and +238 Da for 24). In both cases, we observed the hNE dehydration product peak (M – 18, turquoise) suggesting both agents to effect the covalent modification at the same catalytic serine as 1 did (29, 32, 92–94). To further demonstrate the stringent dependence of SuFEx reactions on protein’s tertiary structure, compounds 22 and 24 were incubated, respectively, with inactive denatured hNE and no covalent modification of the enzyme was found (Fig. 5).
Fig. 5.
High-resolution MALDI-TOF mass spectrometry demonstrated 2 sulfonyl fluoride capture agents for hNE as selective, covalent inhibitors. (A) Compound 22. (B) Compound 24.
To conclude, we have demonstrated a SuFEx library-enabled approach to discover covalent deactivators of an enzyme’s function, the protein at hand being human neutrophil elastase. Its structure is known, including complexed with (ir)reversible inhibitors in the active site, but the library of sulfonyl fluorides used in the screen was chosen without regard to any enzyme:potential ligand relationships. In other words, agnostic of structural considerations, our approach rapidly identified 2 SuFExable probes (22, 24) that found and captured their own protein, hNE in this instance. This useful sulfur fluoride library is being used and augmented regularly at Scripps Research, and it will hopefully contribute to future SuFEx-driven covalent drug discovery endeavors.
Materials and Methods
General Procedure I for the Preparation of Aryl Sulfonyl Fluorides (Fig. 2A).
Aryl sulfonyl chloride (commercially available from Sigma-Aldrich or synthesized according to known procedures) dissolved in acetonitrile (Fisher HPLC grade, 0.5–1 M) was treated with saturated potassium bifluoride aqueous solution (Sigma-Aldrich, ∼5 M, 1.5–2.5 equiv). The emulsion was stirred vigorously for 1–4 h before being partitioned between ethyl acetate and water. The organic solution was collected, dried over anhydrous sodium sulfate (Na2SO4), concentrated, and purified by column chromatography, if necessary, to yield the desired aryl sulfonyl fluoride (33 examples, 90–100% isolated yield).
General Procedure II for the Preparation of Aryl Fluorosulfates (Fig. 2B).
Phenols (Sigma-Aldrich), and triethylamine (Alfa Aesar, 1.5 equiv) were dissolved in dichloromethane (DCM) (Fisher). The flask sealed with a rubber septum was evacuated, and sulfuryl fluoride gas (SynQuest Laboratories, Inc.) in a balloon was introduced to the flask via a needle. The reaction was stirred vigorously for 2 h. Upon completion, solvent was removed in vacuo. The residue was partitioned between ethyl acetate and water. The organic phase was washed with brine, dried over anhydrous Na2SO4, then concentrated and purified by flash column chromatography to give the desired aryl fluorosulfate (32 examples, 82–99% isolated yield).
General Procedure III for the Preparation of Alkyl Sulfonyl Fluorides (Fig. 2C).
To a solution of primary or secondary alkyl amine (Sigma-Aldrich, or Combi-Blocks) in DCM or glacial acetic acid (Sigma-Aldrich, 0.5–1 M), ethenesulfonyl fluoride (ESF) (homemade, 2.2 equiv) (56) was added dropwise. The mixture was stirred at room temperature for 6–12 h. Upon completion, volatiles were removed in vacuo. The residue was purified by flash column chromatography to give the desired sulfonyl fluoride adducts of primary or secondary amines (30 examples, 85–98% isolated yield).
General Procedure IV for the Preparation of Vinyl Sulfonyl Fluorides (Fig. 2D).
An oven-dried Schlenk tube was charged with (hetero)aryl iodide (Sigma-Aldrich, or Combi-Blocks), AgTFA (Acros, 1.2 equiv), Pd(OAc)2 (Alfa Aesar, 2 mol %), acetone (anhydrous over 4-Å molecular sieves, Acros), and ESF (homemade, 2 equiv) were added. The resulting mixture was refluxed at 60 °C. Upon full conversion of (hetero)aryl iodide (6–12 h), solvent was removed in vacuo. The crude was purified by flash column chromatography to give the desired product (10 examples, 59–99% yield).
Protease Activity Assays and SuFExable Library Screen.
hNE activity was measured in a total volume of 100 µL in a reaction buffer of phosphate-buffered saline (PBS) (pH 7.4) and 0.05% (vol/vol) Nonidet P 40 Substitute (Sigma). Final composition of each reaction was 5 nM hNE (Elastin Products Corp.), 50 µM MeOSuc-AAPV-AMC substrate (Millipore), ∼2.5% DMSO (Fisher), and various concentrations of fragments as inhibitors. Elastase was incubated with various concentrations of inhibitors for 10 min at room temperature before addition of MeOSuc-AAPV-AMC. Residual proteolytic activity was measured at 25 °C using an Envision microplate reader for a total of 30 min at 30-s intervals. Only data points reflecting linear substrate conversion were used to determine relative protease activity. IC50 values were obtained by fitting the data to a dose–response inhibition, log (inhibitor) vs. response–variable slope (4 parameters) using GraphPad Prism 8. The physicochemical parameters were obtained from a reference, and the correlation analysis was carried out using GraphPad Prism 8.
MALDI-TOF Mass Spectrometry of SuFExable Probes Bound to Neutrophil Elastase.
Elastase was resuspended in 50 mM sodium acetate (pH 4.5), 100 mM NaCl to 0.2 mg/mL final concentration. DMSO solution of each compound (10 mM) was diluted 1:10 in the above buffer, then added in a compound:hNE ratio = 3:1 and incubated at rt for 1 h prior to analysis by MALDI-TOF mass spectrometry.
Crystallization and X-Ray Data Collection.
Inhibitor 1 was added in a 1.2 molar excess to hNE [10 mg/mL in 10 mM Hepes (pH 6.5)], incubated for 1 h at 25 °C, and immediately used for crystallization. Crystals were grown by sitting drop-vapor diffusion by mixing equal volumes (1.5 µL) of hNE:1 complex and reservoir solution consisting of 0.3 M ammonium citrate (pH 5.0), 14% (wt/vol) PEG 3350 at 25 °C. Data were collected on single, flash-cooled crystals at 100 K in cryoprotectant consisting of 0.2 M ammonium citrate (pH 5.0), 20% (wt/vol) PEG 3350, and 20% (vol/vol) glycerol, and were processed with HKL2000 in orthorhombic space group P212121. The calculated Matthews’ coefficient (VM = 2.77 Å3Da−1) suggested 4 monomers per asymmetric unit with a solvent content of 56%. X-ray data were collected to 2.33-Å resolution on beamline 12.2 at the Stanford Synchrotron Radiation Lightsource.
Supplementary Material
Acknowledgments
We are very grateful to the following coworkers for their contribution to the SuFExable compound library: Hua Wang (library manager); Larisa Krasnova; Suhua Li; Bing Gao; Grant A. L. Bare; Gerui Ren; Feng Zhou; Feng Liu; Hamid R. Safaei (Department of Chemistry); Peng Wu and his group (Department of Molecular Medicine, Scripps Research); Jiajia Dong and his group (Shanghai Institute of Organic Chemistry); the J.E.M. group (La Trobe University); Hua-Li Qin and his group (Wuhan University of Technology); En-Xuan Zhang and his group (AsymChem Inc.); Bo Qin; and Mike Petrassi (Calibr, Scripps Research). The current work was financially supported by the National Institutes of Health R01GM117145 (K.B.S.), R01GM069832 (S.F.), T32AI7354-27 (J.L.W.), The Scripps Research Institute (D.W.W.), and the ARC FT170100156 (J.E.M.). We thank Prof. Hugh Rosen and Prof. Jin-Quan Yu (Scripps Research) for access to instrumentation, Dr. Yongxuan Su of the Molecular Mass Spectrometry Facility (University of California San Diego) for atmospheric-pressure chemical ionization MS, Dr. John Cappiello (Scripps Research) for proofreading, and the staff of the Stanford Synchrotron Radiation Lightsource.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID code 6e69).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909972116/-/DCSupplemental.
References
- 1.Dong J., Krasnova L., Finn M. G., Sharpless K. B., Sulfur(VI) fluoride exchange (SuFEx): Another good reaction for click chemistry. Angew. Chem. Int. Ed. Engl. 53, 9430–9448 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Hanley P. S., Ober M. S., Krasovskiy A. L., Whiteker G. T., Kruper W. J., Nickel- and Palladium-catalyzed coupling of aryl fluorosulfonates with aryl boronic acids enabled by sulfuryl fluoride. ACS Catal. 5, 5041–5046 (2015). [Google Scholar]
- 3.Qin H. L., Zheng Q., Bare G. A. L., Wu P., Sharpless K. B., A Heck-Matsuda process for the synthesis of β-arylethenesulfonyl fluorides: Selectively addressable bis-electrophiles for SuFEx click chemistry. Angew. Chem. Int. Ed. Engl. 55, 14155–14158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrow A. S., Moses J. E., Synthesis of sulfonyl azides via Lewis base activation of sulfonyl fluorides and trimethylsilyl azide. Synlett 27, 1840–1843 (2016). [Google Scholar]
- 5.Li S., Wu P., Moses J. E., Sharpless K. B., Multidimensional SuFEx click chemistry: Sequential sulfur(VI) fluoride exchange connections of diverse modules launched from an SOF4 hub. Angew. Chem. Int. Ed. Engl. 56, 2903–2908 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schimler S. D., et al. , Nucleophilic deoxyfluorination of phenols via aryl fluorosulfonate intermediates. J. Am. Chem. Soc. 139, 1452–1455 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Zelli R., Tommasone S., Dumy P., Marra A., Dondoni A., A click ligation based on SuFEx for the metal-free synthesis of sugar and iminosugar clusters. Eur. J. Org. Chem. 2016, 5102–5116 (2016). [Google Scholar]
- 8.Zha G. F., et al. , Palladium-catalyzed fluorosulfonylvinylation of organic iodides. Angew. Chem. Int. Ed. Engl. 56, 4849–4852 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren G., Zheng Q., Wang H., Aryl fluorosulfate trapped Staudinger reduction. Org. Lett. 19, 1582–1585 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smedley C. J., et al. , Sulfur-fluoride exchange (SuFEx)-mediated synthesis of sterically hindered and electron-deficient secondary and tertiary amides via acyl fluoride intermediates. Chemistry 23, 9990–9995 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Gao B., Li S., Wu P., Moses J. E., Sharpless K. B., SuFEx chemistry of thionyl tetrafluoride (SOF4) with organolithium nucleophiles: Synthesis of sulfonimidoyl fluorides, sulfoximines, sulfonimidamides, and sulfonimidates. Angew. Chem. Int. Ed. Engl. 57, 1939–1943 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo T., et al. , A new portal to SuFEx click chemistry: A stable fluorosulfuryl imidazolium salt emerging as an “F-SO2+” donor of unprecedented reactivity, selectivity, and scope. Angew. Chem. Int. Ed. Engl. 57, 2605–2610 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Dong J., Sharpless K. B., Kwisnek L., Oakdale J. S., Fokin V. V., SuFEx-based synthesis of polysulfates. Angew. Chem. Int. Ed. Engl. 53, 9466–9470 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yatvin J., Brooks K., Locklin J., SuFEx on the surface: A flexible platform for postpolymerization modification of polymer brushes. Angew. Chem. Int. Ed. Engl. 54, 13370–13373 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Oakdale J. S., Kwisnek L., Fokin V. V., Selective and orthogonal post-polymerization modification using sulfur(VI) fluoride exchange (SuFEx) and copper-catalyzed azide-alkyne cycloaddition (CuAAC) reactions. Macromolecules 49, 4473–4479 (2016). [Google Scholar]
- 16.Gao B., et al. , Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates. Nat. Chem. 9, 1083–1088 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H., et al. , SuFEx-based polysulfonate formation from ethenesulfonyl fluoride-amine adducts. Angew. Chem. Int. Ed. Engl. 56, 11203–11208 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gahtory D., et al. , Quantitative and orthogonal formation and reactivity of SuFEx platforms. Chemistry 24, 10550–10556 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks K., et al. , SuFEx postpolymerization modification kinetics and reactivity in polymer brushes. Macromolecules 51, 297–305 (2018). [Google Scholar]
- 20.Grimster N. P., et al. , Aromatic sulfonyl fluorides covalently kinetically stabilize transthyretin to prevent amyloidogenesis while affording a fluorescent conjugate. J. Am. Chem. Soc. 135, 5656–5668 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baranczak A., et al. , A fluorogenic aryl fluorosulfate for intraorganellar transthyretin imaging in living cells and in Caenorhabditis elegans. J. Am. Chem. Soc. 137, 7404–7414 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narayanan A., Jones L. H., Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. (Camb.) 6, 2650–2659 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hett E. C., et al. , Rational targeting of active-site tyrosine residues using sulfonyl fluoride probes. ACS Chem. Biol. 10, 1094–1098 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Chen W., et al. , Arylfluorosulfates inactivate intracellular lipid binding protein(s) through chemoselective SuFEx reaction with a binding site Tyr residue. J. Am. Chem. Soc. 138, 7353–7364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W., et al. , Synthesis of sulfotyrosine-containing peptides by incorporating fluorosulfated tyrosine using an Fmoc-based solid-phase strategy. Angew. Chem. Int. Ed. Engl. 55, 1835–1838 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoppmann C., Wang L., Proximity-enabled bioreactivity to generate covalent peptide inhibitors of p53-Mdm4. Chem. Commun. (Camb.) 52, 5140–5143 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Fadeyi O., et al. , Chemoselective preparation of clickable aryl sulfonyl fluoride monomers: A toolbox of highly functionalized intermediates for chemical biology probe synthesis. ChemBioChem 17, 1925–1930 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Li S. H., et al. , Direct introduction of R-SO2F moieties into proteins and protein-polymer conjugation using SuFEx chemistry. Polymer 99, 7–12 (2016). [Google Scholar]
- 29.Fadeyi O. O., et al. , Covalent enzyme inhibition through fluorosulfate modification of a noncatalytic serine residue. ACS Chem. Biol. 12, 2015–2020 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Wang N., et al. , Genetically encoding fluorosulfate-L-tyrosine to react with lysine, histidine, and tyrosine via SuFEx in proteins in vivo. J. Am. Chem. Soc. 140, 4995–4999 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang B., et al. , Proximity-enhanced SuFEx chemical cross-linker for specific and multitargeting cross-linking mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 115, 11162–11167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang B., et al. , Genetically introducing biochemically reactive amino acids dehydroalanine and dehydrobutyrine in proteins. J. Am. Chem. Soc. 141, 7698–7703 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortenson D. E., et al. , “Inverse drug discovery” strategy to identify proteins that are targeted by latent electrophiles as exemplified by aryl fluorosulfates. J. Am. Chem. Soc. 140, 200–210 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z., et al. , SuFEx click chemistry enabled late-stage drug functionalization. J. Am. Chem. Soc. 140, 2919–2925 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suter C., “Derivatives of aromatic sulfonic acids. 1. Sulfonyl halides, esters, and anhydrides” in The Organic Chemistry of Sulfur: Tetracovalent Sulfur Compounds (Wiley, New York, 1944), pp. 452–458. [Google Scholar]
- 36.Steinkopf W., Aromatic sulphuric fluoride. J. Prakt. Chem. 117, 1–82 (1927). [Google Scholar]
- 37.Steinkopf W., On aromatic sulpho-fluoride. J. Prakt. Chem. 128, 63–88 (1930). [Google Scholar]
- 38.Davies W., Dick J. H., Aliphatic sulphonyl flurorides. J. Chem. Soc. 1932, 483–486 (1932). [Google Scholar]
- 39.Davies W., Dick J. H., Benzenesulphonyl fluoride derivatives. J. Chem. Soc. 1932, 2042–2046 (1932). [Google Scholar]
- 40.Gembus V., Marsais F., Levacher V., An efficient organocatalyzed interconversion of silyl ethers to tosylates using DBU and p-toluenesulfonyl fluoride. Synlett 2008, 1463–1466 (2008). [Google Scholar]
- 41.Choi E. J., Jung D., Kim J. S., Lee Y., Kim B. M., Chemoselective tyrosine bioconjugation through sulfate click reaction. Chemistry 24, 10948–10952 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Hmissa T., et al. , Autocatalytic synthesis of bifluoride ionic liquids by SuFEx click chemistry. Angew. Chem. Int. Ed. Engl. 57, 16005–16009 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Bourassa J. L., Ives E. P., Marqueling A. L., Shimanovich R., Groves J. T., Myoglobin catalyzes its own nitration. J. Am. Chem. Soc. 123, 5142–5143 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Warshel A., Bora R. P., Perspective: Defining and quantifying the role of dynamics in enzyme catalysis. J. Chem. Phys. 144, 180901 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suydam I. T., Snow C. D., Pande V. S., Boxer S. G., Electric fields at the active site of an enzyme: Direct comparison of experiment with theory. Science 313, 200–204 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Fried S. D., Boxer S. G., Electric fields and enzyme catalysis. Annu. Rev. Biochem. 86, 387–415 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fried S. D., Bagchi S., Boxer S. G., Extreme electric fields power catalysis in the active site of ketosteroid isomerase. Science 346, 1510–1514 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis W. G., et al. , Click chemistry in situ: Acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks. Angew. Chem. Int. Ed. Engl. 41, 1053–1057 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Bourne Y., et al. , Freeze-frame inhibitor captures acetylcholinesterase in a unique conformation. Proc. Natl. Acad. Sci. U.S.A. 101, 1449–1454 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agnew H. D., et al. , Iterative in situ click chemistry creates antibody-like protein-capture agents. Angew. Chem. Int. Ed. Engl. 48, 4944–4948 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agnew H. D., et al. , Protein-catalyzed capture agents. Chem. Rev. 10.1021/acs.chemrev.8b00660 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oueis E., Sabot C., Renard P.-Y., New insights into the kinetic target-guided synthesis of protein ligands. Chem. Commun. (Camb.) 51, 12158–12169 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Bosc D., Jakhlal J., Deprez B., Deprez-Poulain R., Kinetic target-guided synthesis in drug discovery and chemical biology: A comprehensive facts and figures survey. Future Med. Chem. 8, 381–404 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Jaegle M., et al. , Protein-templated fragment ligations—From molecular recognition to drug discovery. Angew. Chem. Int. Ed. Engl. 56, 7358–7378 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kolb H. C., Finn M. G., Sharpless K. B., Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. Engl. 40, 2004–2021 (2001). [DOI] [PubMed] [Google Scholar]
- 56.Zheng Q., Dong J., Sharpless K. B., Ethenesulfonyl fluoride (ESF): An on-water procedure for the kilogram-scale preparation. J. Org. Chem. 81, 11360–11362 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Veryser C., Demaerel J., Bieliu Nas V., Gilles P., De Borggraeve W. M., Ex situ generation of sulfuryl fluoride for the synthesis of aryl fluorosulfates. Org. Lett. 19, 5244–5247 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Zhou H., et al. , Introduction of a crystalline, shelf-stable reagent for the synthesis of sulfur(VI) fluorides. Org. Lett. 20, 812–815 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Smedley C. J., et al. , 1-Bromoethene-1-sulfonyl fluoride (BESF) is another good connective hub for SuFEx click chemistry. Chem. Commun. (Camb.) 54, 6020–6023 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Leng J., Qin H. L., 1-Bromoethene-1-sulfonyl fluoride (1-Br-ESF), a new SuFEx clickable reagent, and its application for regioselective construction of 5-sulfonylfluoro isoxazoles. Chem. Commun. (Camb.) 54, 4477–4480 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Birrer P., et al. , Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 150, 207–213 (1994). [DOI] [PubMed] [Google Scholar]
- 62.Cantin A. M., Hartl D., Konstan M. W., Chmiel J. F., Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 14, 419–430 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Gehrig S., et al. , Lack of neutrophil elastase reduces inflammation, mucus hypersecretion, and emphysema, but not mucus obstruction, in mice with cystic fibrosis-like lung disease. Am. J. Respir. Crit. Care Med. 189, 1082–1092 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Gibson R. L., Burns J. L., Ramsey B. W., Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168, 918–951 (2003). [DOI] [PubMed] [Google Scholar]
- 65.Mayer-Hamblett N., et al. , Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am. J. Respir. Crit. Care Med. 175, 822–828 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakamura H., Yoshimura K., McElvaney N. G., Crystal R. G., Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J. Clin. Invest. 89, 1478–1484 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nichols D. P., Chmiel J. F., Inflammation and its genesis in cystic fibrosis. Pediatr. Pulmonol. 50 (suppl. 40), S39–S56 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Sagel S. D., Chmiel J. F., Konstan M. W., Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc. Am. Thorac. Soc. 4, 406–417 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sagel S. D., Wagner B. D., Anthony M. M., Emmett P., Zemanick E. T., Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 186, 857–865 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Twigg M. S., et al. , The role of serine proteases and antiproteases in the cystic fibrosis lung. Mediators Inflamm. 2015, 293053 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wagner C. J., Schultz C., Mall M. A., Neutrophil elastase and matrix metalloproteinase 12 in cystic fibrosis lung disease. Mol. Cell Pediatr. 3, 25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barnes P. J., Cytokines as mediators of chronic asthma. Am. J. Respir. Crit. Care Med. 150, S42–S49 (1994). [DOI] [PubMed] [Google Scholar]
- 73.Barnes P. J., Mediators of chronic obstructive pulmonary disease. Pharmacol. Rev. 56, 515–548 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Pandey K. C., De S., Mishra P. K., Role of proteases in chronic obstructive pulmonary disease. Front. Pharmacol. 8, 512 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu Y., et al. , Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 168, 968–975 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Belaaouaj A., et al. , Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat. Med. 4, 615–618 (1998). [DOI] [PubMed] [Google Scholar]
- 77.Motta J. P., et al. , Food-grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci. Transl. Med. 4, 158ra144 (2012). [DOI] [PubMed] [Google Scholar]
- 78.Myers D. K., Kemp A. Jr, Inhibition of esterases by the fluorides of organic acids. Nature 173, 33–34 (1954). [DOI] [PubMed] [Google Scholar]
- 79.Fahrney D. E., Gold A. M., Sulfonyl fluorides as inhibitors of esterases. 1. Rates of reaction with acetylcholinesterase, α-chymotrypsin, and trypsin. J. Am. Chem. Soc. 85, 997–1000 (1963). [Google Scholar]
- 80.Gold A. M., Fahrney D., Sulfonyl fluorides as inhibitors of esterases. 2. Formation and reactions of phenylmethanesulfonyl α-chymotrypsin. Biochemistry 3, 783–791 (1964). [DOI] [PubMed] [Google Scholar]
- 81.Gold A. M., Sulfonyl fluorides as inhibitors of esterases. 3. Identification of serine as site of sulfonylation in phenylmethanesulfonyl α-chymotrypsin. Biochemistry 4, 897–901 (1965). [DOI] [PubMed] [Google Scholar]
- 82.Baker B. R., Hurlbut J. A., Irreversible enzyme inhibitors. CXIV. Proteolytic enzymes. IV. Additional active-site-directed irreversible inhibitors of α-chymotrypsin derived from phenoxyacetamides bearing a terminal sulfonyl fluoride. J. Med. Chem. 11, 241–245 (1968). [DOI] [PubMed] [Google Scholar]
- 83.Baker B. R., Hurlbut J. A., Irreversible enzyme inhibitors. 113. Proteolytic enzymes. 3. Active-site-directed irreversible inhibitors of α-chymotrypsin derived from phenoxyacetamides with an N-fluorosulfonylphenyl substituent. J. Med. Chem. 11, 233–241 (1968). [DOI] [PubMed] [Google Scholar]
- 84.Baker B. R., Erickson E. H., Irreversible enzyme inhibitors. CXV. Proteolytic enzymes. V. Active-site-directed irreversible inhibitors of trypsin derived from p-(phenoxyalkoxy) benzamidines with a terminal sulfonyl fluoride. J. Med. Chem. 11, 245–249 (1968). [DOI] [PubMed] [Google Scholar]
- 85.Baker B. R., Specific irreversible enzyme inhibitors. Annu. Rev. Pharmacol. 10, 35–50 (1970). [DOI] [PubMed] [Google Scholar]
- 86.Laura R., Robison D. J., Bing D. H., (p-Amidinophenyl)methanesulfonyl fluoride, an irreversible inhibitor of serine proteases. Biochemistry 19, 4859–4864 (1980). [DOI] [PubMed] [Google Scholar]
- 87.Shannon D. A., et al. , Sulfonyl fluoride analogues as activity-based probes for serine proteases. ChemBioChem 13, 2327–2330 (2012). [DOI] [PubMed] [Google Scholar]
- 88.Lively M. O., Powers J. C., Specificity and reactivity of human granulocyte elastase and cathepsin G, porcine pancreatic elastase, bovine chymotrypsin and trypsin toward inhibition with sulfonyl fluorides. Biochim. Biophys. Acta 525, 171–179 (1978). [DOI] [PubMed] [Google Scholar]
- 89.Yoshimura T., Barker L. N., Powers J. C., Specificity and reactivity of human leukocyte elastase, porcine pancreatic elastase, human granulocyte cathepsin G, and bovine pancreatic chymotrypsin with arylsulfonyl fluorides. Discovery of a new series of potent and specific irreversible elastase inhibitors. J. Biol. Chem. 257, 5077–5084 (1982). [PubMed] [Google Scholar]
- 90.Sadlowski C., et al. , Nitro sulfonyl fluorides are a new pharmacophore for the development of antibiotics. Mol. Syst. Des. Eng. 3, 599–603 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smedley C. J., et al. , Bifluoride ion mediated SuFEx trifluoromethylation of sulfonyl fluorides and iminosulfur oxydifluorides. Angew. Chem. Int. Ed. Engl. 58, 4552–4556 (2019). [DOI] [PubMed] [Google Scholar]
- 92.Photaki I., Transformation of serine to cysteine–β-elimination reactions in serine derivatives. J. Am. Chem. Soc. 85, 1123–1126 (1963). [Google Scholar]
- 93.Strumeyer D. H., White W. N., Koshland D. E. Jr, Role of serine in chymotrypsin action–Conversion of active serine to dehydroalanine. Proc. Natl. Acad. Sci. U.S.A. 50, 931–935 (1963). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weiner H., White W. N., Hoare D. G., Koshland D. E. Jr, The formation of anhydrochymotrypsin by removing the elements of water from the serine at the active site. J. Am. Chem. Soc. 88, 3851–3859 (1966). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.