Abstract
PURPOSE:
To analyze optic disc hemorrhages (DH) associated with primary open-angle glaucoma by quantifying their geometric profile and comparing their densitometry with hemorrhages from retinal vein occlusions (RVO) and retinal macroaneurysms (MA), which have venous and arterial sources of bleeding, respectively.
DESIGN:
Retrospective cross-sectional study.
METHODS:
SETTING:
Massachusetts Eye & Ear.
POPULATION:
Fundus images of DH (n = 40), MA (n = 14), and RVO (n = 25) were identified. Patient clinical backgrounds and demographics were obtained.
MAIN OUTCOME MEASURES:
Grayscale pixel intensity units of hemorrhages and adjacent arteriole and venule over the same background tissue were measured. Densitometry differentials (arteriole or venule minus hemorrhage [ΔA and ΔV, respectively]) were calculated. The ratios of length (radial) to midpoint width for DH were calculated. Mean ΔA and ΔV between groups were compared with t tests. Multiple linear regression assessed the relation of retinal hemorrhage diagnosis to ΔA and ΔV and of DH shape to ΔA and ΔV.
RESULTS:
Mean (± standard deviation) ΔA and ΔV for DH (6.9 ±7.1 and −4.7 ± 8.0 pixel intensity units, respectively) and MA (5.3 ±5.9 and −6.0 ± 4.6, respectively) were comparable (P ≥.43). Mean ΔA (14.6 ± 7.7) and ΔV (6.4 ± 6.3) for RVO were significantly higher compared to DH and MA (P < .0001) and remained significant in multivariable analyses. A unit increase in DH length-to-width ratio was associated with 1.2 (0.5) and 1.3 (0.5) pixel intensity unit (standard error) decrease in ΔA and ΔV, respectively (P ≤ .014).
CONCLUSIONS:
DH have densitometry profiles comparable to MA and different from RVO, suggesting that DH in glaucoma have an arterial origin.
OPTIC DISC HEMORRHAGES (DH) ARE OBSERVED more frequently in patients with primary open-angle glaucoma (POAG) than in patients with primary angle-closure glaucoma patients or in healthy controls.1 DH occur in POAG over a wide range of intraocular pressures (IOP), but are more commonly seen in those who have untreated maximum IOP ≤ 21 mm Hg.2–4 Analyses performed in the Ocular Hypertension Treatment Study (OHTS), Collaborative Normal Tension Glaucoma Study (CNTGS), and Early Manifest Glaucoma Trial (EMGT) indicate that DH were associated with the transition to glaucoma or open-angle glaucoma progression even after controlling for the level of IOP.5–8
It is not known whether glaucomatous optic disc damage causes the hemorrhage or whether the hemorrhage is an upstream event, making the optic disc more susceptible to damage. Currently, there are different hypotheses on how DH develop. One hypothesis is that DH are attributable to an underlying vascular event, such as ischemia to the optic nerve head or damage to the blood-retinal barrier.9,10 Another theory is that DH develop from mechanical shearing of blood vessels at the lamina cribrosa.11 These hypotheses are not necessarily exclusive and the actual mechanism may involve both. Knowledge of the etiology of DH and whether they originate from an arterial or venous source may be helpful in elucidating their significance and identifying methods for prevention. An arterial source might suggest that DH may be attributable, in part, to vascular dysregulation, which has been shown to be present in POAG.12,13 The nitric oxide signaling pathway mediates autoregulation through smooth muscle cells, which are far more critical in controlling blood flow in arteries than in veins.14 In contrast, a venous source would suggest that DH may be predominantly independent of vascular dysregulation, perhaps owing to lamina cribrosa distortion secondary to IOP fluctuations or other factors, as blood typically experiences lower sheer stress levels on the venous than the arterial side.15–18
In this work, we describe a novel technique to analyze the densitometry and shape of DH. Using this approach, we attempt to determine the blood source of DH. To the best of our knowledge, no prior group has directly assessed the blood source in POAG patients with DH. By identifying the source of DH, we can learn more about the underlying pathophysiology of POAG, which can ultimately lead to new therapeutic approaches.
METHODS
IMAGE ACQUISITION:
In order to establish whether our technique could identify arterial or venous etiology in hemorrhages, we compared internet stock photographs of DH to hemorrhages from retinal vein occlusions (RVO) and retinal macroaneurysms (MA). RVO and MA represent venous and arterial sources of bleeding, respectively.19,20 These images were identified using a combination of keywords in Google: “glaucoma,” “disc hemorrhage,” “retinal vein occlusion,” and “retinal macroaneurysm.” These results, which support the feasibility of our approach, can be found in the Supplementary Material (available at AJO.com).
STUDY POPULATION:
We then applied our technique to cases where clinical information was available in a retrospective cross-sectional study. We identified patients at Massachusetts Eye & Ear with DH, RVO, and MA (Figure 1). Our research was approved by the Institutional Review Board at Massachusetts Eye & Ear and adhered to the tenets set forth by the Declaration of Helsinki. Patients with a history of POAG and photodocumentation of DH were identified by glaucoma specialists (B.J.S., L.Q.S., and L.R.P.) during clinic visits between June 2016 and June 2017. Their medical records were reviewed and we confirmed the presence of open angles and absence of secondary forms of elevated IOP such as presence of exfoliation material. Humphrey visual field 24–2 test data were collected if obtained within 6 months of the confirmatory fundus photograph with a Topcon DX50 camera (Topcon, Oakland, New Jersey, USA).
FIGURE 1.
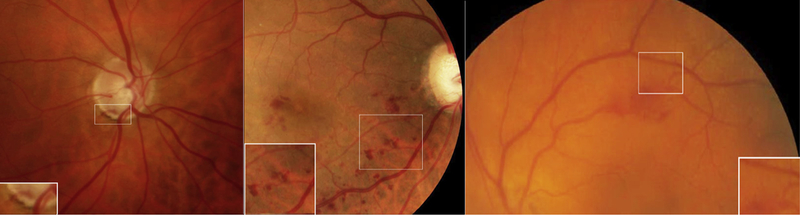
Images from patients with an optic disc hemorrhage (Left), retinal vein occlusion (Middle), and retinal macroaneurysm (Right) with the adjacent arteriole and venule used for analysis (inset).
Patients with RVO and MA were identified by a retina specialist (J.B.M.) and by documented ICD-10 codes H34.83 (tributary branch retinal vein occlusion) and H35.09 (other intraretinal microvascular abnormalities) from October 2015 to December 2016. To augment the sample, a second search based on ICD-9 coding 362.17 (other intraretinal microvascular abnormalities) was performed from January 2010 to October 2015. All images were taken with either the Optos 200Tx (Optos, Marlborough, Massachusetts, USA) or Topcon DX50 camera.
Clinical demographics including sex, age, logMAR visual acuity, IOP at the time of the hemorrhage, hypertension, diabetes, and use of blood thinning medication (antiplatelets and anticoagulants) were collected on all patients. Patients with POAG were subcategorized as having normal tension glaucoma (NTG; IOP ≤ 21 mm Hg) or high tension glaucoma (HTG; IOP > 21 mm Hg) based on untreated IOP maximum of either eye. Time from symptoms to photographic documentation of the hemorrhage was recorded for RVO and MA. This was unavailable for DH, as the patients were all asymptomatic at the time of image capture. Patients were excluded if there were no fundus photographs on initial examination at diagnosis or the images were of poor quality (eg, motion artifact and/or a poor view owing to media opacity).
DENSITOMETRY AND GEOMETRIC PROFILE:
ImageJ software (NIH, Bethesda, Maryland, USA) was used for all analyses. The densitometry, in pixel intensity units, of the hemorrhage was assessed using the “histogram” function. This provides mean grayscale pixel intensity over a selected area. Densitometry values range from 1 (black) to 255 (white) pixel intensity units. Sections of each hemorrhage used for the analysis were included if they were adjacent to an arteriole and venule of similar background intensity and excluded if they were overlying a vessel to ensure that the densitometry was only attributable to the hemorrhage itself. For RVO, where there were many hemorrhages, the measurements were made over the first randomly selected hemorrhage identified by the grader that fit the above criteria. The length (radial with respect to the disc) and width (measured at the midpoint of the hemorrhage) of the hemorrhage were measured in DH and the length-to-width ratio was calculated to define a geometric profile for the hemorrhage. It was not possible to calculate length-to-width ratio in RVO and MA, as the hemorrhage edges were irregular with coalescence of multiple areas of bleeding, leading to nondiscrete geometric profiles.
IMAGE ANALYSIS:
Densitometry of each hemorrhage was compared to the most adjacent retinal arteriole and venule over similar background tissue (Figure 1). Measurements of arterioles and venules were not made over copper wiring if present in the vessel. Figure 2 shows the corresponding histogram of the hemorrhages and adjacent vessels from Figure 1. The mean pixel intensity in the arteriole minus the mean pixel intensity in the hemorrhage (ΔA) and the mean pixel intensity in the venule minus the mean pixel intensity in the hemorrhage (ΔV) were calculated (Figure 3).
FIGURE 2.
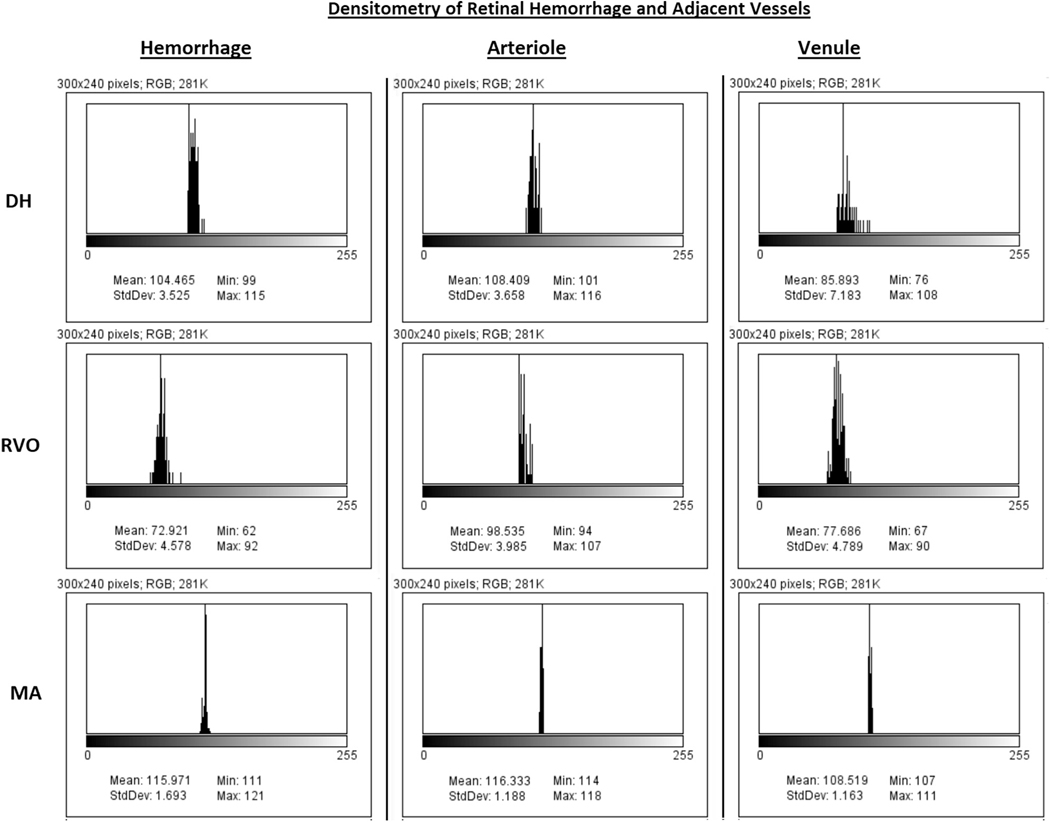
Densitometry of retinal hemorrhage and adjacent vessels. Histogram of the hemorrhages (Left column), adjacent arte-riole (Center column), and venule (Right column) from Figure 1. X-axis corresponds to densitometry value ranging from 1 (black) to 256 (white) pixel intensity. Y-axis corresponds to relative number of pixels at each densitometry value. Values listed correspond to the minimum, maximum, and mean densitometry.
FIGURE 3.
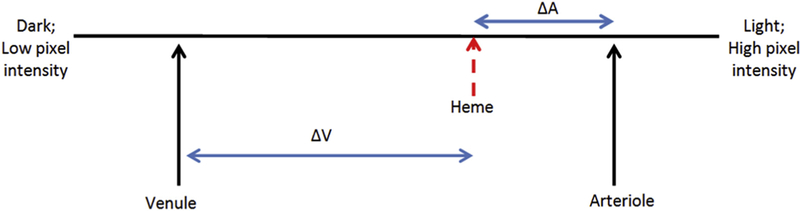
Illustrative model of the densitometry spectrum for a hemorrhage, adjacent arteriole, and adjacent venule. The differences in arterial and hemorrhaged blood (ΔA) and venous and hemorrhaged blood (ΔV) are shown. This example suggests the hemorrhage is arterial in origin. Venous hemorrhages do not produce hemorrhages that are brighter than venous blood. As the hemorrhage ages, the densitometry becomes darker, presumably owing to deoxygenation, and ΔA and ΔV increase.
Two observers unaware of the patient’s clinical information other than hemorrhage group performed all image analyses described. It was not possible to mask the diagnostic group because such information was apparent upon examination of the fundus photograph.
STATISTICAL ANALYSIS:
All statistical analyses were performed in R version 0.98.1091 (R Foundation for Statistical Computing, Vienna, Austria). All tests were 2-sided and the threshold for significance was set at P < .05. Where we performed 3 pairwise comparisons (DH vs RVO, DH vs MA, and MA vs RVO), P < .0167 was considered significant based on Bonferroni correction. Mann-Whitney U test, χ2 test, and Fisher exact test were used, as appropriate, to determine significant differences in clinical demographics among the groups. Intraclass correlation coefficient (ICC) was used to analyze inter-rater agreement. We compared the ΔA values, ΔV values, and length-to-width ratios obtained by reader 1 with those obtained by reader 2 for all hemorrhage images analyzed. Because the purpose of our inter-rater reliability evaluation was to assess the reliability of the 2 particular raters in our study and because their values were averaged together for our final analyses, we used the 2-way mixed-effect model for the mean of 2 raters for our ICC. The unpaired t test was used to compare mean densitometries between groups in univariate analyses. Multiple linear regression analyses adjusting for age, sex, hypertension, diabetes, and use of blood thinning medication were performed to determine whether subject type was a predictor of densitometry in 3 pairwise comparisons (DH vs RVO, DH vs MA, and MA vs RVO). We selected the above covariates for the multivariable models because they may be associated with vascular dysfunction or tendency for bleeding. We used linear regression adjusting for age to assess the relation of densitometry to length-to-width ratio in DH cases. Finally, we stratified DH cases by maximum IOP to determine if DH in NTG or HTG differed in densitometry or geometric profile.
RESULTS
BASELINE DEMOGRAPHICS:
A total of 94 DH, MA, and RVO patients were identified with photographic documentation of the hemorrhage. Thirteen patients were excluded owing to poor image quality (DH = 6, RVO = 5, MA = 2) and 2 patients were excluded owing to extensive vitreous hemorrhage obscuring visualization of the posterior pole (MA = 2). As a result, 79 patients with DH (n = 40), MA (n = 14), or RVO (n = 25) were included in the final analysis.
There was no significant difference in age, history of diabetes, or IOP at the time of hemorrhage among the 3 groups (Table 1). Patients with DH and MA were more likely to be female (80.0% and 85.7%, respectively) compared to RVO (48.0%; P = .0073 and P = .020, respectively). Patients with RVO and MA were more likely to have hypertension (76.0% and 71.4%, respectively) compared with DH patients (45.0%; P = .014 and P = .029, respectively). Patients with MA were also more likely to be on a blood thinner compared to DH patients (71.4% vs 37.5%, P = .028). At the time of hemorrhage documentation, patients with DH had better logMAR visual acuity (0.07) compared to RVO (0.40, P < .0001) and MA (0.98, P < .0001). No patients with DH had evidence of diabetic retinopathy on clinical examination.
TABLE 1.
Baseline Demographic and Clinical Features in Study Sample of Patients With Disc Hemorrhages, Retinal Vein Occlusions, and Retinal Macroaneurysms
| Demographic and Clinical Features | DH (N = 40) | RVO (N = 25) | MA (N = 14) |
P Value for DH vs RVO |
P Value for DH vs MA |
P Value for RVO vs MA |
|---|---|---|---|---|---|---|
| Age in years, mean (SD) | 66.0 (9.4) | 64.5 (12.8) | 73.5 (18.1) | .76a | .0026a | .0073a |
| Female sex, n (%) | 32 (80.0) | 12 (48.0) | 12 (85.7) | .0073b | .99c | .020b |
| Hypertension, n (%) | 18 (45.0) | 19 (76.0) | 10 (71.4) | .014b | .029b | .99c |
| Diabetes mellitus, n (%) | 5 (12.5) | 4 (16.0) | 3 (21.4) | .71c | .41c | .69c |
| Using blood thinning medication, n (%) | 15 (37.5) | 10 (40.0) | 10 (71.4) | .84b | .028b | .060b |
| LogMAR best-corrected visual acuity, mean (SD) | 0.07 (0.1) | 0.4 (0.4) | 0.98 (1.0) | <.0001a | <.0001a | .20a |
| Intraocular pressure in affected eye at time of hemorrhage discovery, in mm Hg, mean (SD) | 14.2 (2.8) | 15.8 (5.4) | 14.4 (2.7) | .25a | .88a | .42a |
| Maximum intraocular pressure in affected eye, in mm Hg, mean (SD) (n = 38) | 19.5 (5.0) | N/A | N/A | N/A | N/A | N/A |
| Cup-to-disc ratio in affected eye at time of hemorrhage discovery, mean (SD) | 0.7 (0.1) | N/A | N/A | N/A | N/A | N/A |
| Mean deviation on reliable visual field closest to time of hemorrhage discovery in affected eye in decibels, mean (SD) (n = 39) | −3.6 (3.0) | N/A | N/A | N/A | N/A | N/A |
| Time from symptoms to imaging in weeks, mean (SD) | N/A | 5.1 (5.0) | 3.2 (2.7) | N/A | N/A | .20a |
| Hemorrhage descriptors | ||||||
| ΔA (arterial – hemorrhage densitometry), mean (SD), pixel intensity units | 6.9 (7.1) | 14.6 (7.7) | 5.3 (5.9) | <.0001a | .49a | .00031a |
| ΔV (venous – hemorrhage densitometry), mean (SD), pixel intensity units | −4.7 (8.0) | 6.4 (6.3) | −6.0 (4.6) | <.0001a | .36a | <.0001a |
| Length-to-width ratio, mean (SD) | 4.9 (2.5) | N/A | N/A | N/A | N/A | N/A |
DH = disc hemorrhage; MA = microaneurysm; N/A = not available; RVO = retina vein occlusion; SD = standard deviation.
Mann-Whitney U test.
χ2 test.
Fisher exact test.
In the DH group, 28 of the 40 patients with DH (70%) were identified as having NTG. Average (± standard deviation [SD]) cup-to-disc ratio was 0.7 ± 0.1 and mean deviation on Humphrey visual field was −3.60 ± 2.96 decibels. Only 1 POAG patient did not have a Humphrey visual field within 6 months. For RVO and MA patients, average time from symptoms to imaging was 5.1 ± 5.0 weeks and 3.2 ± 2.7 weeks, respectively.
ARTERIOLE-HEMORRHAGE DIFFERENTIALS (ΔA) IN OPTIC DISC HEMORRHAGES AND MACROANEURYSMS WERE SMALLER THAN IN RETINAL VEIN OCCLUSIONS:
Mean (6 SD) ΔA of DH (6.9 ± 7.1) was similar to ΔA of MA (5.3 ± 5.9; P = .49) and both were significantly smaller than mean ΔA of RVO (14.6 ± 7.7; P < .0001) (Table 1). Mean (6 SD) ΔV of DH (−4.7 ± 8.0) and MA (−6.0 ± 4.6) demonstrate that their pixel intensities are on average brighter than those of venules and significantly different from those of RVO (6.4 ± 6.3; P < .0001). There were no significant differences among the groups in the relative densitometry values for adjacent arterioles and venules (data not shown).
Compared to RVO patients, subject type (either DH or MA) remained a significant predictor of ΔA and ΔV after adjusting for age, sex, hypertension, diabetes, and history of blood thinner use (Table 2). Specifically, we found that DH was associated with a 6.6 (2.0) pixel intensity unit (standard error [SE]) decrease in ΔA and 10.3 (2.0) pixel intensity unit decrease in ΔV (“more negative”) compared to RVO in the multivariable model (P = .0013 and P < .0001, respectively). DH was not associated with a significant change in either ΔA or ΔV compared to MA in the multivariable model (P ≥ 0.43), while MA was associated with a 7.5 (2.6) pixel intensity unit decrease in ΔA and 11.3 (2.2) pixel intensity unit decrease in ΔV compared to RVO (P = .0077 and P < .0001, respectively). The multivariable models also revealed that diabetes was a predictor of smaller ΔA (beta estimate [SE] = −6.8 [2.6]; P = .011) and ΔV (beta estimate [SE] = −6.9 [2.6]; P = .012) for DH compared to RVO and for DH compared to MA (beta estimate [SE] to predict ΔA = −6.4 [2.8], P = .026; beta estimate [SE] to predict ΔV = −7.7 [2.7], P = .0066).
TABLE 2.
Multiple Linear Regression of Densitometry Differences Between the Hemorrhage and Adjacent Arteriole (ΔA) and Venule (ΔV) in Patients With Disc Hemorrhages, Retinal Vein Occlusions, and Retinal Arterial Macroaneurysms
| DH (N = 40) vs RVO (N = 25) |
DH (N = 40) vs MA (N = 14) |
MA (N = 14) vs RVO (N = 25) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted R2 | Beta Estimate (Standard Error) |
P Value | Adjusted R2 |
Beta Estimate (Standard Error) |
P
Value |
Adjusted R2 |
Beta Estimate (Standard Error) |
P Value | |
| ΔA Predictors | |||||||||
| Age | 0.29 | −0.2 (0.09) | .12 | 0.033 | −0.001 (0.09) | .99 | 0.30 | 0.04 (0.1) | .71 |
| Sex (male as referent) | −2.8 (2.1) | .19 | −0.4 (2.9) | .89 | −5.6 (2.6) | .041 | |||
| Hypertension (no as referent) | 0.7 (2.1) | .75 | 3.2 (2.4) | .19 | 1.4 (2.6) | .60 | |||
| Diabetes mellitus (no as referent) | −6.8 (2.6) | .011 | −6.4 (2.8) | .026 | −3.5 (3.0) | .26 | |||
| Using blood thinning medication (no as referent) | 1.6 (2.1) | .46 | 0.2 (2.2) | .92 | 0.7 (2.8) | .81 | |||
| Subject typea | −6.6 (2.0) | .0013 | 1.9 (2.4) | .43 | −7.5 (2.6) | .0077 | |||
| ΔV Predictors | |||||||||
| Age | 0.39 | 0.04 (0.09) | .70 | 0.10 | 0.003 (0.08) | .97 | 0.50 | 0.07 (0.08) | .39 |
| Sex (male as referent) | −2.5 (2.1) | .25 | −2.2 (2.8) | .43 | −3.3 (2.2) | .14 | |||
| Hypertension (no as referent) | 0.9 (2.1) | .67 | 3.4 (2.3) | .15 | −1.3 (2.2) | .55 | |||
| Diabetes mellitus (no as referent) | −6.9 (2.6) | .012 | −7.7 (2.7) | .0066 | −2.7 (2.5) | .29 | |||
| Using blood thinning medication (no as referent) | 0.3 (2.1) | .89 | −0.4 (2.1) | .84 | −1.2 (2.4) | .63 | |||
| Subject typea | −10.3 (2.0) | <.0001 | 1.3 (2.4) | .60 | −11.3(2.2) | <.0001 | |||
DH = disc hemorrhage; MA = macroaneurysm; RVO = retinal vein occlusion.
In DH vs RVO comparison, RVO is the reference group. In DH vs MA comparison, MA is the reference group. In MA vs RVO comparison, RVO is the reference group.
HIGHER LENGTH-TO-WIDTH RATIO WAS ASSOCIATED WITH OPTIC DISC HEMORRHAGES HAVING PIXEL INTENSITIES CLOSER TO ARTERIOLES:
The mean (± SD) length-to-width ratio for DH was 4.9 ± 2.5. Each unit increase in hemorrhage length-to-width ratio was associated with 1.2 (0.5) and 1.3 (0.5) pixel intensity unit (SE) decreases in DH ΔA and ΔV (more negative), leading to DH having densitometry closer to the adjacent arteriole (P ≤ .014; Table 3).
TABLE 3.
Linear Regression of Densitometry Differences Between the Hemorrhage and Adjacent Arteriole (ΔA) and Venule (ΔV) Against Length-to-Width Ratio of Hemorrhage in Disc Hemorrhage Patients
| Adjusted R2 |
Beta Estimate (Standard Error) |
P Value |
|
|---|---|---|---|
| Length-to-width ratio as predictora of ΔA | 0.15 | −1.2 (0.5) | .014 |
| Length-to-width ratio as predictora of ΔV | 0.11 | −1.3 (0.5) | .013 |
Analyses were adjusted for age.
HEMORRHAGES IN RETINAL VEIN OCCLUSION AND MACROANEURYSM PATIENTS TENDED TO BECOME DARKER OVER TIME:
The onset from symptoms to documentation of the hemorrhage in RVO and MA ranged from 2.5 days to 24 weeks. There was a trend for blood to appear darker (ΔA and ΔV became more positive) as the time between onset of symptoms and photodocumentation lengthened, although this linear relationship did not reach statistical significance (P = .18 and P = .07, respectively).
NORMAL TENSION GLAUCOMA VERSUS HIGH TENSION GLAUCOMA:
Compared with HTG, patients with NTG were more frequently female (89.3% vs 58.3%, P = .039), were less likely to have hypertension (32.1% vs 75.0%, P = .013), had lower IOP (13.3 mm Hg vs 16.4 mm Hg, P = .0022), and had better mean deviation on visual field (−2.7 vs −6.0, P = .0052) at time of hemorrhage discovery (Table 4). There were no significant differences in ΔA, ΔV, or length-to-width ratio in NTG vs HTG (P ≥ .27).
TABLE 4.
Clinical, Demographic, and Disc Hemorrhage Features In Normal Tension Glaucoma and High Tension Glaucoma
| Clinical and Demographic Features | NTG (N = 28) | HTG (N = 12) | P Value |
|---|---|---|---|
| Age in years, mean (SD) | 65.5 (8.9) | 67.2 (10.9) | .54a |
| Female sex, n (%) | 25 (89.3) | 7 (58.3) | .039c |
| Hypertension, n (%) | 9 (32.1) | 9 (75.0) | .013b |
| Diabetes mellitus, n (%) | 2 (7.1) | 3 (25.0) | .15c |
| Using blood thinning medication, n (%) | 9 (32.1) | 6 (50.0) | .31c |
| LogMAR best-corrected visual acuity, mean (SD) | 0.05 (0.1) | 0.11 (0.2) | .35a |
| Intraocular pressure at time of hemorrhage discovery, in mm Hg, mean (SD) | 13.3 (2.4) | 16.4 (2.7) | .0022a |
| Maximum intraocular pressure in affected eye, in mm Hg, mean (SD) (n = 38) | 17.1 (2.3) | 24.6 (4.6) | <.0001a |
| Cup-to-disc ratio at time of hemorrhage discovery, mean (SD) | 0.7 (0.1) | 0.8 (0.1) | .0016a |
| Mean deviation on reliable visual field closest to time of hemorrhage discovery in affected eye in decibels, mean (SD) (n = 39) | −2.7 (2.2) | −6.0 (3.5) | .0052a |
| Hemorrhage descriptors | |||
| ΔA (arterial-hemorrhage densitometry), mean (SD), pixel intensity units | 7.9 (7.3) | 4.4 (7.6) | .27a |
| ΔV (venous–hemorrhage densitometry), mean (SD), pixel intensity units | −3.9 (7.9) | −6.4 (8.2) | .42a |
| Length-to-width ratio, mean (SD) | 5.1 (2.5) | 4.6 (2.4) | .61a |
HTG = high tension glaucoma; NTG = normal tension glaucoma.
Mann-Whitney U test.
χ2 test.
Fisher exact test.
Overall, there was excellent inter-rater reliability between the 2 raters (ICC > 0.90 for ΔA values, ΔV values, and length-to-width ratios obtained).
DISCUSSION
WE DESCRIBE A NOVEL APPROACH TO DETERMINE THE vascular etiology of DH. By identifying the source of DH, we can learn more about their association with POAG progression and the underlying pathophysiology. Specifically, if DH are primary rather than secondary events in the pathogenesis of POAG, then preventing DH may become an important objective in glaucoma management. To the best of our knowledge, no prior work has formally addressed the question of the source of blood in glaucomatous DH. Theories have been proposed suggesting an arterial source for DH. For example, Lee and associates suggests that gliosis owing to disc damage could disrupt retinal capillaries in a way that arterial bleeding could occur in glaucoma.21 Using our technique, it appears that DH are primarily arterial in origin in that they exhibit smaller ΔA and negative ΔV, like MA.
A significant number of patients with DH (predominantly NTG) and MA in this clinic-based study were female. The etiology underlying this sex differential is unclear, although this trend has been previously reported.4,20,22–24 Nevertheless, sex was not a significant predictor of densitometry in our multivariable model and thus, its role in the development of DH is unclear.
Prior studies have shown DH to be associated with hypertension, diabetes, IOP, and use of aspirin.3,4,25 However, to our knowledge, no study has been performed to see if the characteristics of DH differ in the setting of these risk factors. In our study, although only 5 patients with DH had diabetes, we found that DH with smaller arteriolehemorrhage densitometry differentials (eg, closer to arteriole densitometry) were more likely to be longer (larger length-to-width ratio) and have a history of diabetes. Diabetes is associated with vascular dysregulation and it is possible that this underlying mechanism may have influenced the DH characteristics in these patients.26 Future investigations assessing a larger cohort of POAG patients with DH and diabetes need to be conducted to study this relationship further.
There is strong evidence that dysregulation of vascular resistance is a key component in the pathogenesis of POAG, particularly NTG, where DH are more common.27–31 Although there are many hypotheses, it is not known why DH are more frequent in NTG vs HTG. In order to understand this phenomenon further, we compared the DH characteristics in NTG vs HTG. We found no significant differences in densitometry or geometric profile between the 2 groups. Although this may be secondary to sample size, another explanation is that the 2 entities are not completely unique disease processes, but exist on a spectrum of POAG.
There are several limitations to this project. First, this was a retrospective cross-sectional study. Although DH are strongly associated with POAG, they have also been attributed to diabetes, hypertension, and posterior vitreous detachments (PVD).5,32–34 However, none of our POAG patients had documented evidence of diabetic retinopathy or hypertensive retinopathy and no patients had symptoms suggestive of an acute PVD. Next, background illumination was variable among the fundus photographs owing to natural differences in exposure as well as the use of different imaging modalities; Optos ultrawide-field scanning laser ophthalmoscopy was mainly used to acquire images of RVO and MA, while a flash-based Topcon fundus camera was used to acquire images of DH. This prevented us from comparing the raw pixel intensity values for hemorrhages across different images. To overcome this challenge, we compared the hemorrhages to the adjacent retinal arterioles and venules on similar backgrounds. This allowed the calculation of ΔA and ΔV that could be compared between hemorrhages of different images. Future studies can further investigate the role that background variability may have on assessing pixel intensity of the hemorrhages by using a single imaging modality.
A third limitation is that unlike MA and RVO, DH are typically asymptomatic and thus, photodocumentation of them can occur several weeks after their formation. Usually, they are found during a routine ophthalmology examination.35 As the hemorrhage evolves, it becomes more deoxygenated and may appear darker. This was seen in this study, as hemorrhages from MA and RVO trended toward becoming darker over time. As a result, there would tend to be an inherent bias for all hemorrhages to appear more venous in origin (less oxygen), especially DH, as their photodocumentation may occur at a later date. However, we compared densitometry between groups and found that the densitometry of DH aligned with that of MA. Thus, if this limitation and subsequent darkening of blood were true, it is likely that DH images captured closer to their date of formation would appear brighter, supporting the hypothesis that they are likely arterial in origin.
Finally, the pixel intensity of hemorrhages can potentially vary depending on the location for MA and RVO (eg, preretinal, intraretinal, subretinal). It is unclear what significance this plays, as on average MA tend to be brighter than RVO. Owing to the retrospective nature of this project, routine optical coherence tomography (OCT) tests were not obtained through the MA and RVO hemorrhages. Future prospective studies could combine our approach with OCT to determine if the location of the hemorrhage within the retina has any significant effect on retinal architecture or visual function. We emphasize that the densitometry calculated was an average pixel intensity. Consistent with Figure 2, the pixel intensities in hemorrhages tended to produce normal distributions, and simply using the average of the pixel intensities in the hemorrhage (as opposed to the entire distribution of pixel intensities in a hemorrhage) allowed us to pursue multivariable linear regression analyses. We acknowledge that the range of the hemorrhage’s histogram can overlap with those of the adjacent vasculature. Thus, although the mean densitometry suggested that disc hemorrhages were primarily arterial in nature, a mixed origin cannot be ruled out. Furthermore, we limited our analysis to POAG patients and as a result, these findings may not be generalizable to angle-closure glaucoma or secondary open-angle glaucomas.
Our approach to assess the origin of DH has several strengths. The decision to compare the densitometry of the retinal hemorrhage to nearby arterioles and venules overcomes the issue of variable exposure that exists from one image to the next. Also, we took great care to maintain the same background when making these comparisons. The analyses of DH in internet stock images and Massachusetts Eye & Ear patients remained consistent, further validating the technique. The addition of RVO and MA provides accepted controls for known sources of arterial and venous blood, respectively. Providing a time stamp for onset of symptoms to onset of photodocumentation in the instances of RVO and MA allowed us to estimate how time influences densitometry readings, and we have strong reason to believe that time from event to photodocumentation does not confound our results.
Our novel quantitative approach suggests that DH are produced by arterial sources. If vascular dysregulation is implicated in DH, then it is likely that the underlying ischemia-reperfusion injury or focal compression by the blood itself leads directly to retinal ganglion cell loss, producing changes such as notching and optic nerve pits, regardless of the prevailing IOP. Furthermore, if DH result from vascular dysregulation, targeted therapies to improve regulatory tone in posterior segment vessels, especially in patients who are prone to repeated DH, may prove to be beneficial. One such treatment may be the early use of brimonidine in these patients, which has been shown to improve blood flow autoregulation and potentially reduce visual field progression.36–38 Ultimately, by understanding the pathophysiology of DH and its association with POAG progression, we can begin developing more individualized treatments for patients with glaucoma.
Supplementary Material
Acknowledgments
FUNDING/SUPPORT: HARVARD GLAUCOMA CENTER OF EXCELLENCE, BOSTON, MASSACHUSETTS, USA. B.FINANCIAL DISCLOsures: Unrelated to this work, Lucy Q. Shen is a consultant for Genentech and receives research funding from Topcon. Unrelated to this work, Louis R. Pasquale is a consultant for Bausch & Lomb and Eyenovia. The following authors have no financial disclosures: Jonathan C. Chou, Clara C. Cousins, John B. Miller, Brian J. Song, Michael A. Kass, and Janey L. Wiggs. All authors attest that they meet the current ICMJE criteria for authorship.
Footnotes
Supplementary Material available at AJO.com
REFERENCES
- 1.Razeghinejad MR, Nowroozzadeh MH. Optic disc hemorrhage in health and disease. Surv Ophthalmol 2017;62(6):784–802. [DOI] [PubMed] [Google Scholar]
- 2.Drance SM. Disc hemorrhages in the glaucomas. Surv Ophthalmol 1989;33(5):331–337. [DOI] [PubMed] [Google Scholar]
- 3.Soares AS, Artes PH, Andreou P, Leblanc RP, Chauhan BC, Nicolela MT. Factors associated with optic disc hemorrhages in glaucoma. Ophthalmology 2004;111(9):1653–1657. [DOI] [PubMed] [Google Scholar]
- 4.Healey PR, Mitchell P, Smith W, Wang JJ. Optic disc hemorrhages in a population with and without signs of glaucoma. Ophthalmology 1998;105(2):216–223. [DOI] [PubMed] [Google Scholar]
- 5.Budenz DL, Anderson DR, Feuer WJ, et al. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology 2006;113(12):2137–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drance S, Anderson DR, Schulzer M, Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol 2001;131(6):699–708. [DOI] [PubMed] [Google Scholar]
- 7.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 2003;121(1):48–56. [DOI] [PubMed] [Google Scholar]
- 8.Budenz DL, Huecker JB, Gedde SJ, Gordon M, Kass M, Ocular Hypertension Treatment Study Group. Thirteen-year follow-up of optic disc hemorrhages in the Ocular Hypertension Treatment Study. Am J Ophthalmol 2017;174: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begg IS, Drance SM, Sweeney VP. Ischaemic optic neuropathy in chronic simple glaucoma. Br J Ophthalmol 1971;55(2):73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grieshaber MC, Terhorst T, Flammer J. The pathogenesis of optic disc splinter haemorrhages: a new hypothesis. Acta Ophthalmol Scand 2006;84(1):62–68. [DOI] [PubMed] [Google Scholar]
- 11.Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol 1981;99(4):635–649. [DOI] [PubMed] [Google Scholar]
- 12.Fuchsjager-Mayrl G, Wally B, Georgopoulos M, et al. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci 2004;45(3):834–839. [DOI] [PubMed] [Google Scholar]
- 13.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 2002;21(4):359–393. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura Y, Okamura T, Kani K, Toda N. Nitric oxidemediated retinal arteriolar and arterial dilatation induced by substance P. Invest Ophthalmol Vis Sci 1993;34(10):2859–2865. [PubMed] [Google Scholar]
- 15.Albon J, Purslow PP, Karwatowski WS, Easty DL. Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol 2000;84(3):318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy NS, Crapps EE, Bonney RC. Displacement of the optic nerve head. Response to acute intraocular pressure elevation in primate eyes. Arch Ophthalmol 1981;99(12):2166–2174. [DOI] [PubMed] [Google Scholar]
- 17.Lee EJ, Kim TW, Weinreb RN, Kim H. Reversal of lamina cribrosa displacement after intraocular pressure reduction in open-angle glaucoma. Ophthalmology 2013;120(3):553–559. [DOI] [PubMed] [Google Scholar]
- 18.dela Paz NG, D’Amore PA. Arterial versus venous endothelial cells. Cell Tissue Res 2009;335(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green WR, Chan CC, Hutchins GM, Terry JM. Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases. Retina 1981;1(1):27–55. [PubMed] [Google Scholar]
- 20.Rabb MF, Gagliano DA, Teske MP. Retinal arterial macroaneurysms. Surv Ophthalmol 1988;33(2):73–96. [DOI] [PubMed] [Google Scholar]
- 21.Lee EJ, Han JC, Kee C. A novel hypothesis for the pathogenesis of glaucomatous disc hemorrhage. Prog Retin Eye Res 2017;60:20–43. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Iwase A, Kawase K, Sawada A, Ishida K. Optic disc hemorrhages detected in a large-scale eye disease screening project. J Glaucoma 2004;13(5):356–360. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama K, Tomita G, Kawase K, et al. Disc hemorrhage and peripapillary atrophy in apparently healthy subjects. Acta Ophthalmol Scand 1999;77(2):139–142. [DOI] [PubMed] [Google Scholar]
- 24.Panton RW, Goldberg MF, Farber MD. Retinal arterial macroaneurysms: risk factors and natural history. Br J Ophthalmol 1990;74(10):595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YD, Han SB, Park KH, et al. Risk factors associated with optic disc haemorrhage in patients with normal tension glaucoma. Eye (Lond) 2010;24(4):567–572. [DOI] [PubMed] [Google Scholar]
- 26.Chou J, Rollins S, Fawzi AA. Role of endothelial cell and pericyte dysfunction in diabetic retinopathy: review of techniques in rodent models. Adv Exp Med Biol 2014;801: 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasquale L, Jonas J, Anderson D. Anatomy and physiology In: Weinreb RN, Harris A, eds. Ocular Blood Flow in Glaucoma: The 6th Consensus Report of the World Glucoma Association. Amsterdam, Netherlands: Kugler Publications; 2009:3–13. [Google Scholar]
- 28.Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J 2013;4(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan N, Wang P, Tang L, Liu X. Ocular blood flow and normal tension glaucoma. Biomed Res Int 2015;2015:308505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitazawa Y, Shirato S, Yamamoto T. Optic disc hemorrhage in low-tension glaucoma. Ophthalmology 1986;93(6): 853–857. [DOI] [PubMed] [Google Scholar]
- 31.Pasquale LR, Hanyuda A, Ren A, et al. Nailfold capillary abnormalities in primary open-angle glaucoma: a multisite study. Invest Ophthalmol Vis Sci 2015;56(12):7021–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhler TA, Piltz-Seymour J. Optic disc hemorrhages in glaucoma and ocular hypertension: implications and recommendations. Curr Opin Ophthalmol 2008;19(2):89–94. [DOI] [PubMed] [Google Scholar]
- 33.Jonas JB, lester M. Disc hemorrhage and glaucoma. Ophthalmology 1995;102(3):365–366. [DOI] [PubMed] [Google Scholar]
- 34.Roberts TV, Gregory-Roberts JC. Optic disc haemorrhages in posterior vitreous detachment. Aust N Z J Ophthalmol 1991; 19(1):61–63. [DOI] [PubMed] [Google Scholar]
- 35.Heijl A Frequent disc photography and computerized perimetry in eyes with optic disc haemorrhage. A pilot study. Acta Ophthalmol (Copenh) 1986;64(3):27–281. [DOI] [PubMed] [Google Scholar]
- 36.Feke GT, Hazin R, Grosskreutz CL, Pasquale LR. Effect of brimonidine on retinal blood flow autoregulation in primary open-angle glaucoma. J Ocul Pharmacol Ther 2011;27(4): 347–352. [DOI] [PubMed] [Google Scholar]
- 37.Feke GT, Bex PJ, Taylor CP, et al. Effect of brimonidine on retinal vascular autoregulation and short-term visual function in normal tension glaucoma. Am J Ophthalmol 2014;158(1): 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furlanetto RL, De Moraes CG, Teng CC, et al. Risk factors for optic disc hemorrhage in the low-pressure glaucoma treatment study. Am J Ophthalmol 2014;157(5):945–952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


