Abstract
Primary open angle glaucoma (POAG) is a complex disease with a major genetic contribution. Its prevalence varies greatly among ethnic groups, and is up to five times more frequent in black African populations compared to Europeans. So far, worldwide efforts to elucidate the genetic complexity of POAG in African populations has been limited. We conducted a genome-wide association study in 1113 POAG cases and 1826 controls from Tanzanian, South African and African American study samples. Apart from confirming evidence of association at TXNRD2 (rs16984299; OR[T] 1.20; P = 0.003), we found that a genetic risk score combining the effects of the 15 previously reported POAG loci was significantly associated with POAG in our samples (OR 1.56; 95% CI 1.26–1.93; P = 4.79 × 10−5). By genome-wide association testing we identified a novel candidate locus, rs141186647, harboring EXOC4 (OR[A] 0.48; P = 3.75 × 10−8), a gene transcribing a component of the exocyst complex involved in vesicle transport. The low frequency and high degree of genetic heterogeneity at this region hampered validation of this finding in predominantly West-African replication sets. Our results suggest that established genetic risk factors play a role in African POAG, however, they do not explain the higher disease load. The high heterogeneity within Africans remains a challenge to identify the genetic commonalities for POAG in this ethnicity, and demands studies of extremely large size.
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide (Tham et al. 2014). The disease is an optic neuropathy characterized by loss of retinal ganglion cells resulting in peripheral visual field defects. Later in the disease process, the visual field defects may involve central vision leading to blindness. Primary open-angle glaucoma (POAG) is the commonest subtype of glaucoma. Intraocular pressure (IOP), family history, age, and ancestry are established risk factors. In particular persons of African ancestry have 3–5 × increased risk of POAG, and have a more severe course of disease with a higher risk of blindness (Cook 2009; Kyari et al. 2013). This ethnic predilection along with the familial nature strongly suggests that genetic factors contribute to the pathogenesis of POAG.
Recently, progress has been made in the identification of associated variants using linkage analysis and genome-wide association studies (GWAS). Rare variants with large effects have been identified in MYOC and OPTN, common variants with smaller effect have been reported in genomic regions that include CAV1-CAV2, CDC7-TGFRB3, TMCO1, CDKN2B-AS1, ABCA1, AFAP1, GMDS, TXNRD2, ATXN2, FOXC1, GAS7, ARHGEF12, SIX6, 8q22 and PMM2 (Bailey et al. 2016; Burdon et al. 2011; Chen et al. 2014; Liu et al. 2013; Hysi et al. 2014; Li et al. 2015; Springelkamp et al. 2015; Thorleifsson et al. 2010; Wiggs et al. 2012). However, these loci explain only 5–10% of cases, leaving the heritability of POAG largely unexplained. Most genetic studies were predominantly conducted in European and Asian populations, leaving African ancestry underrepresented up to now. Recent studies in Africans or in cohorts of African descent (i.e., Ghana, South Africa and in African Americans) could not replicate most of the loci previously identified in GWAS of European and Asian populations (Cao et al. 2012; Liu et al. 2013; Williams et al. 2015).
Gene finding can be more effective in study populations where the disease is more common, of earlier onset and more severe. Therefore, in this study, we conducted a genome-wide meta-analysis using African black and South African colored POAG cases and controls, from the Genetics In Glaucoma patients from African descent study (GIGA) recruited at hospitals from South Africa and Tanzania and African Americans enrolled in the BioMe (2018) biobank.
Results
The GIGA dataset consisted of 444 participants from South Africa (NPOAG = 297; Ncontrol = 147) and 695 participants from Tanzania (NPOAG = 366; Ncontrol = 329). The Tanzanian participants were all from the black African origin, 38% of South African participants were also from black African origin while the remaining 62% were self-reported South African Coloured (European, African, Asian admixed). The BioMe dataset consisted of POAG cases (N = 450) and controls (N = 1350) and were all African American. The clinical and demographic characteristics of the GIGA and BioMe participants have been summarized in Table 1.
Table 1.
Demographic and clinical characteristics GIGA study and BioMe
| Clinical and demographic characteristics | POAG cases | Controls |
|---|---|---|
| GIGA | 663 | 476 |
| Median age, years (IQR) | 65.0 (18) | 65.0 (12) |
| Female, n (%) | 281 (42) | 266 (56) |
| Self-reported ethnicity/race, n (%) | ||
| South African coloured | 179 (27) | 96 (20) |
| African black | 484 (73) | 380 (80) |
| Median proportion GAA, % (IQR) | ||
| South African colored | 33.86 (37.26) | 33.12 (28.18) |
| African black | 97.90 (7.80) | 97.28 (7.81) |
| BioMe | 450 | 1350 |
| Median age, years (IQR) | 64.0 (16) | 64.0 (16) |
| Female, n (%) | 290 (64) | 870 (64) |
| Median proportion GAA, % (IQR) | 86.84 (12.84) | 86.74 (12.99) |
GAA genetic African ancestry, IQR interquartile range, POAG primary open-angle glaucoma, SD standard deviation
Association of previously reported POAG loci in African populations
First, we tested the association of the 15 previously established POAG SNPs identified in GWAS of European and Asian populations in the GIGA and BioMe datasets (Bailey et al. 2016; Burdon et al. 2011; Chen et al. 2014; Liu et al. 2013; Hysi et al. 2014; Li et al. 2015; Springelkamp et al. 2015; Thorleifsson et al. 2010; Wiggs et al. 2012). None of these SNPs replicated at a nominal significance level (P < 0.05) in any single ethnicity (Supplementary Table 1), nor in a combined analysis (Table 2 exact replication). Because linkage disequilibrium (LD) patterns may differ between the study populations of the reported GWAS and the current African study participants, we also searched for evidence of transferability of the SNPs. Locuszoom plots were made using the LD pattern of Europeans and Asians (1000Genomes) to investigate whether SNPs in high LD (r2 > 0.8) with the original lead SNP showed evidence of association in our study (Supplementary Fig. 1). This “local” replication strategy queried a 500 kb window centered on the lead SNP, and yielded a total of 246 SNPs in LD (r2 > 0.8) with the 15 lead SNPs. Of these 246 SNPs, three SNPs in the TXNRD2, CDKN2B-AS1, and TMCO1 loci were significantly associated with POAG in our study (P < 0.05) (Table 2, local replication), rs16984299 in TXNRD2 had similar effect size as reported by Cook Bailey et al.(Bailey et al. 2016) and survived multiple testing (OR[T] 1.20; 95% CI 1.06–1.35; PBonferonni = 0.049) when the association was corrected for the effective number of SNPs (N = 16) in the queried 500 kb window. In addition, we also analyzed three independent POAG variants found in African Americans from the Women Health Initiative (Hoffmann et al. 2014). We found rs192917960 at the RBFOX1 locus associated with POAG in BioMe (P = 0.02, Supplementary Table 2), but this association did not withstand correction for multiple testing.
Table 2.
Lookup of known POAG SNPs in GIGA BioMe
| Genomic region | Exact replication | Local replication | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lead SNP | A1 | OR (95% CI) | P value | EUR Proxy variant | Correlated allele | r2 between Lead SNP and Proxy | OR | P value | Number of effec tive SNPs in LD | P adjust | |
| CDC-TGRFB3 | rs1192415 | G | 1.05 (0.88–1.26) | 0.5811 | - | - | - | - | - | - | - |
| TMCO1 | rs4656461 | G | 0.98 (0.85–1.12) | 0.7305 | rs28504591 | A | 0.99 | 1.31 (1.07–1.6) | 0.0094 | 8.3 | 0.07827 |
| AFAP1 | rs4619890 | G | 1.08 (0.93–1.25) | 0.3018 | rs28605661 | G | 0.99 | 1.10 (0.95–1.27) | 0.2219 | 2.98 | 0.66144 |
| FOXC1 | rs2745572 | A | 0.98 (0.85–1.14) | 0.8033 | - | - | - | - | - | - | - |
| GMDS | rs11969985 | G | 1.05 (0.92–1.21) | 0.4471 | rs12530211 | T | 0.98 | 1.09 (0.94–1.24) | 0.2266 | 10.65 | 1 |
| CAV1-CAV2 | rs4236601 | A | 1.04 (0.89–1.19) | 0.6349 | rs10270569 | T | 0.83 | 1.08 (0.92–1.28) | 0.3411 | 3.69 | 1 |
| 8q22 | rs284489 | G | 0.99 (0.87–1.12) | 0.8401 | rs10106029 | G | 0.97 | 1.09 (0.97–1.24) | 0.1428 | 4.69 | 0.66926 |
| CDKN2B-AS1 | rs4977756 | A | 1.06 (0.94–1.19) | 0.3473 | rs10712703 | d | 0.84 | 1.20 (1.04–1.39) | 0.0145 | 6.15 | 0.08907 |
| ABCA1 | rs2472493 | G | 1.11 (0.98–1.25) | 0.09345 | rs2437812 | C | 0.90 | 1.12 (1–1.26) | 0.0560 | 2.17 | 0.12131 |
| ARHGEF12 | rs58073046 | G | 1.19 (0.71–2) | 0.5113 | - | - | - | - | - | - | - |
| ATXN2 | rs7137828 | T | 0.82 (0.91–1.16) | 0.1047 | rs7310615 | G | 0.97 | 0.79 (0.62–1.01) | 0.05864 | 1.19 | 0.06968 |
| SIX6 | rs10483727 | T | 1.11 (0.9–1.37) | 0.3212 | rs6573307 | G | 0.96 | 1.10 (0.94–1.28) | 0.2355 | 9.26 | 1 |
| PMM2 | rs3785176 | C | 1.08 (0.91–1.3) | 0.3746 | - | - | - | - | - | - | - |
| GAS7 | rs9897123 | T | 1.04 (0.92–1.16) | 0.544 | - | - | - | - | - | - | - |
| TXNRD2 | rs35934224 | T | 1.06 (0.93–1.21) | 0.3692 | rs16984299 | C | 0.90 | 0.83 (0.74–0.94) | 0.0032 | 15.63 | 0.04984 |
Lead SNP: SNP reported in European or Asian GWAS associated with primary open-angle glaucoma; A1: effect allele reported in European or Asian GWAS; EUR proxy variant: variant in strong LD (r2 > 0.8 EUR 1000Genomes) with the lead SNP that has a smaller P value in GIGA BioMe than the exact replication of the lead SNP. Cells are left empty (−) when no variant in the queried LD region had a smaller P value; correlated allele: proxy variant allele correlated with lead SNP A1 allele [LDlink (Machiela and Chanock 2015)]; effective number of SNPs: number of effective SNPs within the queried 500 kb region calculated by Genetic Type 1 Error Calculator using the 1000 Genomes African samples as a reference(Li et al. 2012); P adjust: corrected P value for number of effective SNPs; d: deletion
d deletion
Next, we compared effect sizes from the combined analysis of GIGA and BioMe with the effect sizes from published GWAS reports. In total, 12 out of the 15 known lead SNPs had a consistent direction of effect (Supplementary Fig. 2). Allele frequencies for most SNPs were very similar in South African blacks, Tanzanian, and African American datasets, but markedly different compared with the European and Asian studies (Supplementary Fig. 3). In eight of the 15 SNPs, the effect allele had a considerable higher frequency in Africans while five were clearly less frequent compared to Europeans.
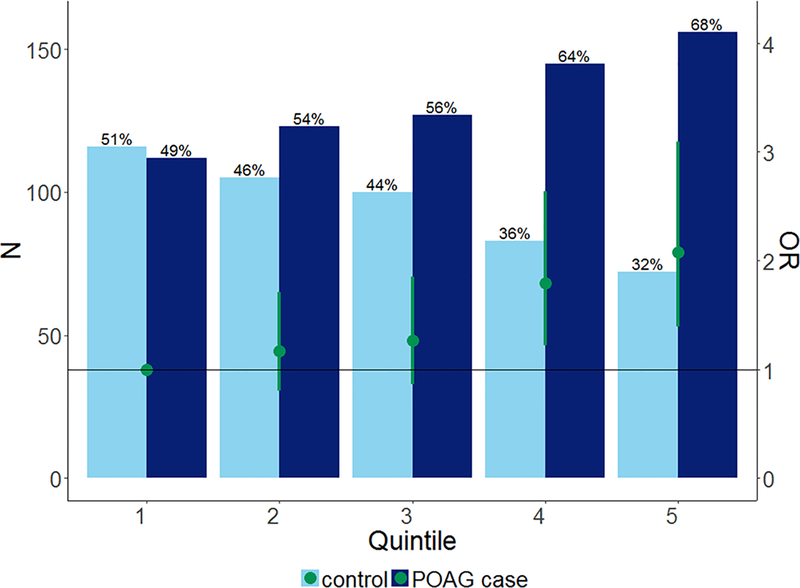
To study the contribution of the known SNPs to the risk of POAG in GIGA, we calculated a multilocus Genetic Risk Score (GRS) based on 15 known SNPs. Three known SNPs for TXNRD2, CDKN2B-AS1, and TMCO1 were replaced by the proxies that were identified by the local replication approach described above. Scores were weighted based on the effect sizes found in the GWAS meta-analysis of European populations. The GRS, adjusted for age, sex, and first five principal components were associated with POAG in the GIGA sample (OR 1.56; 95% CI 1.26–1.93; P = 4.85 × 10−5). We then stratified the GRS in quintiles, and estimated the risk of POAG for each quintile relative to the lowest one (Fig. 1). Trend analysis showed a significant stepwise increase in the risk of POAG per quintile (Ptrend = 2.81 × 10−5), with a twofold increase in POAG risk for the highest quintile compared to the lowest. The risk attributed to genetics was calculated in reference to the mean genetic risk score in the controls. We found that these 15 known variants taken all together attributed 4% (95% CI 2–6%) to the overall POAG risk in this study population when we adjusted for age, sex and principal components.
Fig. 1.
Genetic Risk Score. Genetic risk score based on the 15 known POAG-loci identified in Europeans and Asians GWAS (rs1192415, rs28504591, rs4619890, rs2745572, rs11969985, rs4236601, rs284489, rs10712703, rs2472493, rs58073046, rs7137828, rs10483727, rs3785176, rs9897123, rs16984299). Participants were grouped into quintiles of the genetic risk scores. Green circles represent the POAG odds ratio (adjusted for age, sex and principal components) when comparing each quintile to the lowest quintile (Q1 = reference line). The green-capped lines represent 95% CI of the POAG odds ratios. Bars represent the percentage of POAG cases (dark blue) and controls (light blue) per quintile.
Discovery (stage 1)
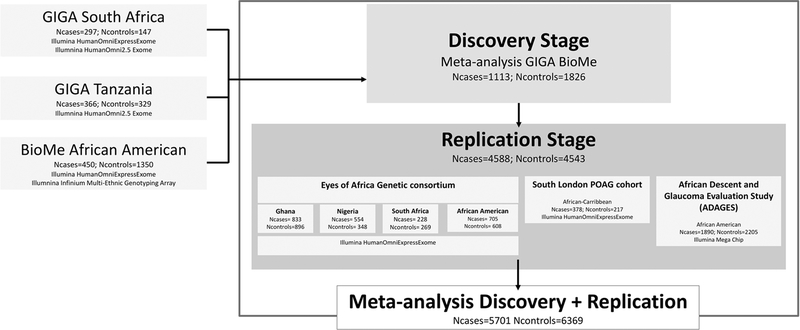
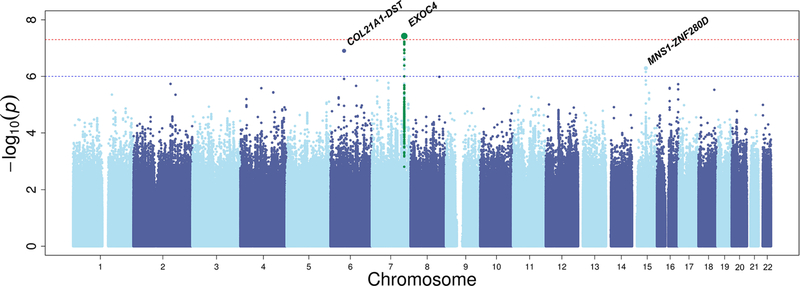
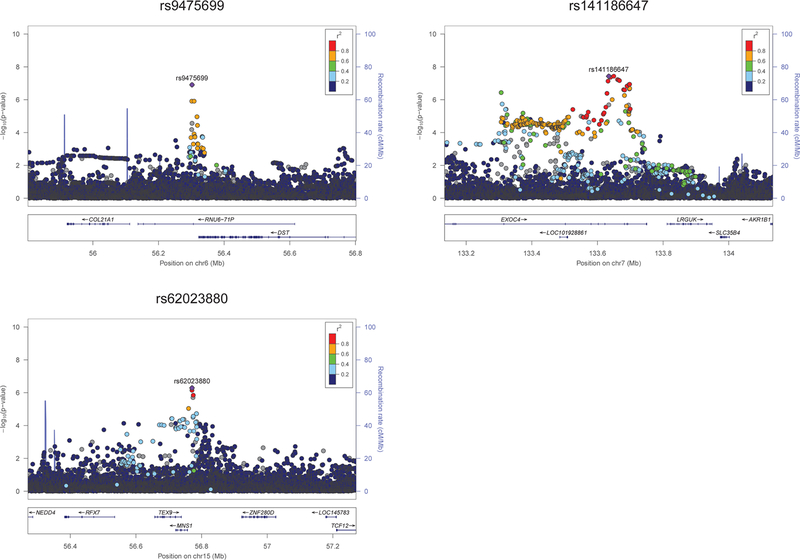
To identify new loci associated with POAG in African populations, we performed GWAS using our African ancestry datasets. The scheme of the study design is depicted in Fig. 2. In the discovery stage, we meta-analyzed GWAS results from the GIGA study (South Africa and Tanzania) and BioMe (African American) including in total 1113 POAG cases and 1826 controls. A total of 13.8 million SNPs were available after applying our QC and filtering criteria (see “Methods” section). The genomic inflation factor was 0.94 (SE 1.49 × 10−6) and the quantile–quantile plot did not show any systemic inflation in the association results, suggesting that confounding by cryptic population stratification was unlikely (Supplementary Fig. 4). The discovery association results across the whole genome are shown in Fig. 3. We identified one novel region reaching genome-wide significance (P < 5 × 10−8) in the discovery stage, and two suggestive regions (P < 1 × 10−6) (Table 3). The top newly associated SNPs were rs141186647[A] an intronic variant in EXOC4 on chromosome 7 (OR 0.48; P = 3.15 × 10−8), rs9475699[A] downstream of DST on chromosome 6 (OR 1.65; P = 1.25 × 10−7), and rs62023880[A] upstream of MNS1 on chromosome 15 (OR 1.39; P = 5.12 × 10−7). The regional association plots for these three SNPs are shown in Fig. 4. We did not observe any significant heterogeneity for these SNPs in the meta-analysis of GIGA and BioMe. The association results per ethnic group are provided in Supplementary Table 2, showing similar effects in Tanzanians, South Africans, and African Americans. Conditional and joint analyses did not identify any additional independent signals within the set of SNPs reaching P < 1 × 10−6. Additionally, we explored if haplotypes encompassing any of the three top SNPs were associated with POAG in GIGA BioMe, the results for this haplotype association analysis are provided in Supplementary Table 3.
Fig. 2.
Study design
Fig. 3.
Manhattan plot for the association of genome-wide SNPs with primary open-angle glaucoma in GIGA BioMe meta-analysis. Manhattan plot of the GWAS meta-analysis of GIGA and BioMe (N=1113cases/N = 1826 controls). The figure shows −log10-transformed P values for all SNPs. The upper dotted horizontal line represents the genome-wide significance threshold of P < 5.0 × 10−8; the lower dotted line indicates a P value of 1 × 10−6. Green dots represents variants in that are in linkage disequilibrium (r2 > 0.6 1000 Genomes African ancestry) with the top SNP rs141186647.
Table 3.
Association results for the top SNPs in previously unreported regions with P < 1 × 10−6 in the discovery phase (GIGA + BioMe)
| SNP | CHR | POS | Nearest gene | A1 | Meta-analysis GIGA BioMe | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ncases/Ncontrols | Frequency A1 cases/controls | OR (95% CI) | P value | I 2 | Phet | |||||
| rs9475699 | 6 | 56302054 | COL21A1-DST | A | 858/1061 | 0.22/0.17 | 1.65 (1.37–1.98) | 1.25E-07 | 41.4 | 0.1632 |
| rs141186647 | 7 | 133634202 | EXOC4 | A | 1113/1826 | 0.04/0.06 | 0.48 (0.37–0.62) | 3.75E-08 | 29.2 | 0.2271 |
| rs62023880 | 15 | 56770871 | MNS1-ZNF280D | A | 1113/1826 | 0.30/0.26 | 1.39 (1.22–1.58) | 5.12E-07 | 30.4 | 0.2192 |
SNP rsID, CHR chromosome, POS base pair; nearest gene (reference NCBI build37) is given as locus label, A1 effect allele, OR odds ratio on POAG based on allele A1; Phet P value for heterogeneity
Fig. 4.
Regional Plots for SNPs P < 1 × 10−6 in the discovery stage (stage 1)
Replication of associated variants in African populations (stage 2 and stage 3)
All SNPs reaching P < 1 × 10−6 in stage 1 were followed-up in a replication (stage 2) comprising four independent African ancestral studies from South Africa, Ghana, Nigeria and African Americans (Eyes of Africa Genetic Consortium; Ncases = 2320; Ncontrols = 2121), the South London POAG case–control cohort comprising individuals from West African origin (Ncases = 378; Ncontrols = 217) and The African Descent and Glaucoma Evaluation Study (ADAGES) including African Americans (Ncases = 1890; Ncontrols = 2205). In total, 22 SNPs at the three independent loci were brought forward for replication. Variant rs9475699 (downstream of DST) reached a nominal level of statistical significance (OR 1.19, P = 0.032) in the Ghanaian study population (Supplementary Table 4). We then performed a meta-analysis of all six replication datasets (stage 2), first using a fixed effects model, and found no statistical significant replication (Table 4). Subsequent meta-analysis by means of the Han and Eskin random-effects model for SNPs with significant (P < 0.05) heterogeneity, also did not identify any SNPs with significant association. In stage 3, we performed a meta-analysis of all studies (stage 1 + stage 2), totaling 5701 POAG cases and 6369 controls. Given the high degree of heterogeneity observed in the fixed effect meta-analysis at this stage, we performed Han and Eskin random-effects model. Neither fixed effects nor Han and Eskin random-effect meta-analysis resulted in genome-wide significant signals (Table 4, Supplementary Table 4 and Supplementary Fig. 5).
Table 4.
Association and meta-analysis of the discovery and replication studies for the top-ranked loci
| SNP | CHR | POS | Nearest gene | A1 | GIGA BioMe (discovery) | Meta-analysis replication studies | Meta-analysis discovery and replication | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency A1 cases/controls | Ncases/Ncontrols | OR | P value | Frequency A1 cases/controls | Ncases/Ncontrols | OR | P value | I 2 | Phet | Frequency A1 cases/controls | ORa | P value* | I 2 | Phet | |||||
| rs9475699 | 6 | 56302054 | COL21A1-DST | A | 0.22/0.17 | 858/1061 | 1.65 (1.37–1.99) | 1.25E-07 | 0.22/0.22 | 2698/2338 | 0.95a (0.81–1.11) | 0.141 * | 59.6 | 0.042 | 0.22/0.21 | 1.04 (0.82–1.31) | 3.49E-06 | 85.06 | 3.03E-06 |
| rs141186647 | 7 | 133634202 | EXOC4 | A | 0.04/0.06 | 1113/1826 | 0.48 (0.37–0.62) | 3.75E-08 | 0.04/0.04 | 4588/4543 | 0.97 (0.92–1.02) | 0.278 | 0 | 0.723 | 0.04/0.05 | 0.90 (0.71–1.16) | 5.91E-05 | 79.86 | 4.30E-05 |
| rs62023880 | 15 | 56770871 | MNS1-ZNF280D | A | 0.30/0.26 | 1113/1826 | 1.39 (1.22–1.58) | 5.12E-07 | 0.28/0.26 | 4588/4543 | 1.02 (0.997–1.04) | 0.086 | 0 | 0.575 | 0.28/0.26 | 1.08 (0.97–1.21) | 1.17E-04 | 76.31 | 2.97E-04 |
SNP rsID, CHR chromosome, POS base pair; nearest gene (reference NCBI build37) is given as locus label, A1 effect allele, OR odds ratio on POAG based on allele A1, Phet P value for heterogeneity
P value’s in italics represent Han and Eskin’s random effect meta-analysis P values
OR from a random effect meta-analysis
Cross-ethnic validation
We further investigated to what extent loci found in our African ancestry GWAS confer a risk of POAG in Europeans. We investigated the top three ranked loci from the discovery stage in two independent European ancestry studies from the National Eye Institute Glaucoma Human Genetics Collaboration (NEIGHBOR) and the Massachusetts Eye and Ear Infirmary (MEEI) (totaling 2606 POAG cases and 2606 controls) with imputed genotype data using the Haplotype Reference Consortium (HRC) (McCarthy et al. 2016). rs141186647 (EXOC4) and rs9475699 (COL21A1-DST) are rare in the European cohorts but had excellent imputation scores (MAF 0.00019, r2 0.987; and MAF 0.0023, r2 0.963, respectively). However, neither SNP demonstrated significant association in the European datasets (rs141186647 OR[A] 5.09; P = 039; rs9475699 OR[A] 1.27; P = 0.59). SNP rs62023880 (MNS1-ZNF280D) on chromosome 15, which was a common variant in the NEIGHBOR/MEEI sample (MAF = 0.15,) also did not show statistically significant replication (OR 0.947; P = 0.34).
Bioinformatical lookup of functional and regulatory effects and expression of POAG-associated SNPs
We explored the functional and regulatory annotations of the three lead SNPs found in the discovery stage, including proxy-SNPs within high LD (r2 > 0.8 in 1000G AFR). The significant top hit rs141186647 at 7q33 represented an intronic variant within the Exocyst Complex Component 4 gene (EXOC4). The locus contains a set of SNPs in high LD that reside within the introns and within exon 15 (rs34608222; synonymous) of EXOC4 of which only rs79198429 (r2 = 0.92 with rs141186647) is annotated as possibly disrupting; transcription factor binding (Regu-lomeDB score 3a; Supplementary Table 5). This variant is located inside a region annotated as an enhancer histone mark in multiple tissues by the RoadMap Epigenomics project, which is predicted to bind the transcriptional coactivator protein P300, and to alter five binding motifs including AP-1 transcription factor (Roadmap Epigenomics et al. 2015). None of the explored SNPs in this region were associated with eQTL’s.
In silico analyses of SNPs correlated with rs9475699 located 21 kb downstream the DST gene and rs62023880 neighboring MNS1 gene did not identify any markers with evidence for gene regulatory effects.
To assess the expression of the annotated genes in human eye tissues, we examined the online Ocular Tissue Database (https://genome.uiowa.edu/otdb/) (Wagner et al. 2013). Expression of EXOC4, DST and MNS1 was observed in tissues relevant to POAG, such as the trabecular meshwork, optic nerve head and optic nerve. Supplementary Table 6 depicts the differences in expression levels of these three genes across tissue types. In the optic nerve head, the highest level of expression was found for DST gene (PLIER 632.5).
Gene-based tests
We performed gene-based tests using VEGAS2 (2018) on the GIGA BioMe meta-analysis results, and first investigated the 15 known POAG genes. None of these were significant at a nominal statistical level, the smallest P value was found for FOXC1 (P = 0.103, nSNPs = 573) (Supplementary Table 7). We subsequently explored the gene-based test results of a total of 25,590 autosomal genes, using a Bonferroni corrected gene-based significance threshold of Pgene–based< 1.95 × 10−6 (0.05/25590). The EXOC4 gene (P = 3.10 × 10−5) did not withstand Bonferroni correction.
Discussion
To date, only European and Asian ancestry GWA studies have contributed to the 15 currently known genetic loci for POAG. Although the frequency of POAG in persons from African descent is high compared to those of European or Asian descent, studies of individuals of African descent are missing so far. The current study focuses on filling this gap. In this case–control study consisting of Africans from the African continent as well as of African Americans, we confirmed three POAG loci (CDKN2B-AS1, TMCO1, TXNRD2) at nominal significance that were previously found in Europeans, and report one novel candidate locus (EXOC4). A variant (rs1063192) near CDKNB2-AS1 has previously been shown in the Afro-Caribbean population of Barbados, although this study could not replicate other known putative loci (Cao et al. 2012). Another insight gained from the current study was that the “local approach” rather than exact replication yielded these replicable findings in Africans. Interestingly, these proxy SNPs in Africans have a very similar effect size compared to the lead SNP in European GWAS.
This study has strengths and limitations. Of particular strength was the Pan-African origin of the study participants. Previous studies from the African continent were smaller and they all focused mainly on West Africans. Our study is the first genetic analysis which included East Africans. A probable disadvantage of applying a Pan-African approach must also be considered. The high genetic diversity present across African populations, even when they are geographically close, may reduce the likelihood of reproducing associations in multi-center studies. Other strengths were the careful diagnosis of cases, the strict criteria for controls, and the application of local replication. Optic discs were graded in an objective manner from fundus photographs by glaucoma experts using internationally accepted standards (Foster et al. 2002). Controls underwent the same review process as cases and had to be over 50 years of age to increase the diagnostic certainty of non-disease status. The limitations of our study include the relatively low power to detect genome-wide significance for small effect sizes, as reflected by the genomic inflation factor < 1.0, and the lack of a replication set from East Africa.
As the genome of African populations is much older, genetic diversity is increased, and LD across loci is decreased. Rather than focusing only on the lead SNPs from European/Asian GWAS, we considered all variants that were in strong European/Asian LD with the lead SNPs. We analyzed these variants in our African samples, and found evidence for nominal replication of three SNPs in TMCO1 (rs28504591), CDKN2B-AS1 (rs10712703), and TXNRD2 (rs16984299), of which the latter withstood Bonferroni correction for the number of effective SNPs. The most significant SNP identified in GWAS is often not the causal variant (McCarthy and Hirschhorn 2008). We found similar effect sizes compared to the European GWAS for the three SNPs identified by the local replication approach. The overall weaker LD structure in Africans favors proximity of these proxy SNPs to the true causal variant. This makes it more likely that these proxies are functional. We, therefore, recommend candidate gene studies in African populations that failed to replicate known disease loci found in European or Asian populations to use this local approach.
Although this study found evidence that at least one known POAG gene plays a role in African glaucoma, we could not significantly replicate the remaining 14 associated SNPs even when we applied the local approach. Yet our GRS that was based on known European and Asian POAG SNPs showed a significant trend (P = 2.81 × 10−5) and a twofold increase in POAG risk comparing extreme risk groups. Of note, the allele frequency distributions for these SNPs differed markedly between our African study and the original European/Asian studies. This points towards differences in genetic architecture, and makes it difficult to estimate statistical power.
This study identified a novel candidate variant within the EXOC4 gene in the meta-analysis of GIGA and BioMe. Recent reports provide evidence that this gene is implicated in cognitive traits as intelligence and educational attainment, and is also associated with the neurodegenerative Alzheimer’s disease (Okbay et al. 2016; Sherva et al. 2014; Sniekers et al. 2017). The EXOC4 gene is ubiquitously expressed, and is particularly abundant in the brain. EXOC4 encodes the SEC-8 protein, a component of a complex which is essential for exocytosis; it directs Golgi-derived secretory vesicles to specific docking sites on the plasma membrane. Exocyst proteins are needed for rapid membrane expansion, which happens during outgrowth of neurons and synaptogenesis. So far, the exocyst complex has not been studied in connection with glaucomatous optic neuropathy, however, it is expressed in the trabecular meshwork. In this tissue, it plays a role in the formation of invadopodia, protrusions that are important for releasing matrix metalloproteinase into the extracellular matrix to decrease trabecular outflow resistance (Han et al. 2013). Strikingly, our African POAG cases had high IOP, and it is intriguing to speculate that EXOC4 contributes to POAG by interfering with matrix metalloproteinase release and trabecular outflow.
Replication of our genome-wide significant finding from the discovery set in our other African studies was challenging for this relatively rare variant. Meta-analysis of the discovery and replication stage showed considerable variation in effect size and direction of effect between the discovery and the replication set, indicating substantial heterogeneity. This heterogeneity is likely to be caused by differences in genetic ancestry as most of the replication studies were from the West-African origin, while the population GIGA BioMe included a substantial proportion of persons from East Africa. Differences in LD pattern between causal variants and identified markers as shown in Supplementary Fig. 7 can cause this directionally inconsistent association across studies more commonly known as the flip-flop phenomenon (Lin et al. 2007).
In conclusion, we conducted the first GWAS of POAG comprising continental Africans. We verified the European glaucoma gene TXNRD2 and identified a novel candidate locus encompassing EXOC4 that requires further follow up in large African studies. A GRS combining the effects of the known POAG SNPs indicated that these SNPs are implicated to play a role in African POAG. Future studies on POAG in Africa should take the substantial genetic heterogeneity into account by ascertaining large discovery and replication sets from the same geographic area.
Methods
Study population
The Genetics In Glaucoma patients from African descent study (GIGA) is a multicenter case–control study comprising POAG patients and controls from South Africa and Tanzania. Participants from Black African and South African Coloured ancestry were ascertained from the ophthalmology outpatient department of the Groote Schuur Hospital in Cape Town, South Africa (Ncases = 327; Ncontrols= 194), and from hospitals in Tanzania: Muhimbili National Hospital and CCBRT Disability Hospital in Dar es Salaam (Ncases = 395; Ncontrols = 382). The study was conducted according to the guidelines for human research by the National Institute for Medical Research in Tanzania. Ethical approval was obtained from the institutional review boards at each study site, and written informed consent was provided by each participant.
The Charles Bronfman Institute for Personalized Medicine BioMe BioBank is an electronic medical record (EMR)-linked clinical care biobank that integrates research data and clinical care information of patients at The Mount Sinai Medical Center New York. This center serves diverse local communities of upper Manhattan with broad health disparities including POAG. The current analysis includes participants who self-reported to be of African ancestry (Ncases = 450; Ncontrols= 1350) who were enrolled between September 2007 and October 2014. Ethical approval was obtained from the institutional review boards at Mount Sinai, and written informed consent was obtained from all participants.
Phenotype definition
In GIGA, POAG cases met category 1 or 2 of the ISGEO classification for open-angle glaucoma (Foster et al. 2002). In brief, cases had either a definite visual field defect and Vertical Cup Disc Ratio (VCDR) ≥ 0.7, or VCDR > 0.8 in the absence of a visual field test. Other inclusion criteria were an open angle on gonioscopy and age of onset older than 35 years. Glaucoma patients diagnosed with secondary causes were excluded from this study. Controls were recruited at the same ophthalmology clinics and underwent identical examinations as the POAG cases. Inclusion criteria were: no signs of glaucoma, IOP ≤ 21 mmHg; VCDR< 0.5, and a VCDR inter-eye asymmetry < 0.2, no family history of glaucoma, and age > 55 years. Case and control status was adjudicated by two experienced ophthalmologists (AT and HL).
In BioMe information on POAG status, sex, age was derived from patients’ EMR. POAG patients were considered cases if they had ≥ 1 diagnoses for POAG (ICD-9 codes 365.01, 365.05, 365.11, 365.12 or ICD-10 code H40.11). Participants with pre-glaucoma (ICD-9 code 365), ocular hypertension only (ICD-9 code 365.04), unspecified glaucoma (ICD-9 code 365.10) or with secondary glaucoma (Supplementary Table 8) were excluded from the analyses. Details of the ICD-9 or ICD-10 codes used can be found in Supplementary Table 9. Controls were those of African ancestry over 40 years of age not being diagnosed with any type of glaucoma.
Genotyping
In GIGA, 1162 participants were genotyped using either the Illumina HumanOmniExpressExome Beadchip (964,193 variants; Illumina, Inc., San Diego, CA, USA; n = 999) or the Illumina HumanOmni2.5Exome Beadchip (2,406,945 variants; Illumina, Inc., San Diego, CA, USA; n = 163). Extensive quality control (QC) was performed on the genotyped data in PLINK v1.07 (Purcell et al. 2007). Variants with a call rate < 95%, as well as variants showing an extreme deviation from Hardy–Weinberg equilibrium (P < 1 × 10−6), or MAF < 0.01 were excluded. All SNPs were mapped to genome build hg19/GRCh37. Individual level QC was performed by exclusion of individuals with a call rate < 95%, discordant sex in self-report versus genetically determined sex, excess or reduced heterozygosity, relatedness (PI-HAT > 0.25) or duplicative samples based on identity by descent (IBD) sharing calculations. The final dataset consisted of 663 and 476 successfully genotyped POAG cases and controls, respectively.
Participants from BioMe were genotyped on either Illumina HumanOmniExpressExome-8 v1.0 beadchip array or the illumina Multi-Ethnic Genotyping Array (MEGA). As in GIGA, QC was performed following a similar protocol. Exclusion of variants was based on a call rate < 95%, extreme deviation from Hardy–Weinberg equilibrium (P < 1 × 10−5), or MAF < 0.01. Individual level QC excluded samples with a call rate < 95%, gender mismatches, ethnic outliers, excess or reduced heterozygosity and first degree relatives or duplicates.
Imputation
Imputation of unknown genetic variation was performed by means of the “cosmopolitan” approach of using all available populations in a reference panel. The 1000 Genomes Project phase III version 5 was used as an imputation reference panel for GIGA (Genomes Project et al. 2015). The pipeline implemented at the Michigan Imputation Server (https://imputationserver.sph.umich.edu) was used for prephasing and imputation (Minimac) of GIGA genotypes (Das et al. 2016). Imputations of BioMe genotypes were carried out with the program IMPUTE using the 1000 Genomes project phase I version 3 as a reference (Genomes Project et al. 2012; Howie et al. 2009).
Population structure
In GIGA, the population structure was examined by principal-components analysis (PCA) in PLINK v1.9 (Chang et al. 2015); PCA plots for each array and population are displayed in Supplementary Fig. 8. Scree plots were examined to determine the number of principal-components (PC) for adjustment of potential population stratification (shown in Supplementary Fig. 9). The first five PCs were used as covariates for South African samples, the first four for Tanzanian samples.
In BioMe, population structure (Supplementary Fig. 8) was controlled for by means of genetic matching using the first two PCs. Additional matching was performed based on age and sex.
Replication
The Eyes of Africa Genetic Consortium, the South London POAG case–control cohort and The African Descent and Glaucoma Evaluation Study (ADAGES III) served as replication panels. The Eyes of Africa Genetic consortium is a Pan-African study of genetic determinants of POAG, and comprises studies recruited from Ghana, Nigeria, South Africa and the USA, totaling a sample size of 2320 POAG cases and 2121 controls. The methods of ascertaining POAG cases has been described in detail elsewhere (Liu et al. 2013). In brief, POAG cases met the following inclusion criteria: glaucomatous optic neuropathy (VCDR > 0.7 or notch in the neuroretinal rim), and visual field loss (examined by frequency doubling technology or standard automated perimetry) consistent with optic nerve damage in at least one eye. Controls were participants with no known first-degree relative with glaucoma, IOP less than 21 mmHg in both eyes without treatment, and no evidence of glaucomatous optic neuropathy in either eye. Genotyping of cases and controls was performed on the Illumina OmniExpressExome array. Genotype QC is described in Supplementary Appendix 2.
The South London POAG case–control cohort consists of 378 POAG patients and 217 controls of West African ancestry residing in the United Kingdom. Patients were recruited from glaucoma clinics in South London and included if they had visual field loss in at least one eye attributed to glaucoma by a glaucoma specialist, had a VCDR of more than 0.6, were receiving intraocular-lowering medication (or had previous surgery), and had open drainage angles on gonioscopy. Controls were recruited from other eye clinics and were included if the examining ophthalmologist deemed there was no sign of POAG, had healthy optic discs (VCDR < 0.6), and normal intraocular pressure without any IOP-lowering therapy (< 20 mmHg). The majority of controls did not have formal visual field testing. Genotyping was performed in a single batch using Illumina’s OmniExpress array. Genotype QC has been described in the Supplementary Appendix 2.
The African Descent and Glaucoma Evaluation Study (ADAGESIII) is a large collection of African American POAG patients and healthy controls recruited at five eye centers in the US (La Jolla, California; New York, New York; Birmingham, Alabama; Houston, Texas; Atlanta, Georgia). The methods of recruitment and selection of POAG cases have been described previously (Zangwill et al. 2018). In brief, eligibility for inclusion as a POAG case required glaucomatous visual field damage defined as a pattern standard deviation or glaucoma hemifield test results outside normal limits. If good-quality visual fields were not available glaucomatous optic disc damage defined as evidence of excavation, neuroretinal rim thinning or notching, localized or diffuse retinal nerve fiber layer defect, or an inter-eye asymmetry of the vertical cup-to-disc ratio of more than 0.2 was required. Controls were ascertained at Wake Forest School of Medicine. Details on genotyping and QC are summarized in the Supplementary Appendix 2.
Statistical analysis
We conducted a three-stage GWAS. Stage 1 was aimed at the discovery and consisted of a meta-analysis on summary data from GIGA and BioMe. Stage 2 included replication of independent and lead SNPs identified at stage 1 reaching a significance level P < 1 × 10−6. Stage 3 combined all results in an overall meta-analysis.
Genome-wide association testing in the GIGA study assumed an additive genetic model adjusting for sex and age and included the aforementioned PCs of the principal-components analysis. Association analyses were carried out in EPACTS (http://www.sph.umich.edu/csg/kang/epacts/index.html) by means of the Firth bias-corrected likelihood-ratio test (Firth 1993). In BioMe SNPTEST was applied (https://mathgen.stats.ox.ac.uk/genetics_software/snptest/snptest.html) using the frequentist association tests implemented in the program, based on an additive model (Marchini et al. 2007). Cases and controls were matched by age, sex and the first two principal components in a 1:2 case/control ratio. To control for genotype uncertainty, we used the missing data likelihood score test (the score method).
Stage 1
A centralized filtering was performed on GIGA and BioMe GWAS results prior to the meta-analysis. Summary result files were assessed and filtered for monomorphic SNPs and SNPs with a minor allele frequency < 0.01. SNP’s that failed or had low-quality imputation, i.e. Minimac r2/SNPTEST INFO < 0.5, were also excluded. The cleaned summary statistics of both studies were then meta-analyzed by means of an inverse variance fixed effects meta-analysis implemented in METAL (Wilier et al. 2010). Summary statistics were corrected using the ‘genomic-control’ option in METAL to eliminate any residual bias. Only variants present in GIGA South Africa, GIGA Tanzania, as well as BioMe were taken for further analysis.
We searched for evidence of replication of the 15 known POAG variants found in European and Asian GWA studies by employing two replication strategies. First, we used the “exact” approach that involves only the lead significant markers. P values at each known POAG SNP in our study were examined and a P < 0.05 was considered as evidence for statistically significant replication. Next, we analyzed the transferability of SNPs by applying the “local” approach. All SNPs in strong LD (r2 > 0.8) with the known POAG SNP in the 1000 Genomes European population were analyzed. For evidence of local transferability, P values were adjusted for the number of effective SNPs within a locus as determined by the Genetic Type I Error Calculator (2018) (Li et al. 2012).
To identify potential additional independent signals nearby the lead SNP in the meta-analysis of GIGA and BioMe, we conducted a conditional analysis implemented in Genome-wide complex trait analysis (GCTA 2018) software, using the cojo method, which performs conditional and joint analyses with model selection (Yang et al. 2011). The genome-wide meta-analysis summary statistics from the discovery stage were used as the input data. Within the GCTA analysis, MAF was restricted to ≥ 1% and P < 1 × 10−6. For this analysis, we used the GIGA Tanzania 1000 Genome phase 3 imputed data to calculate LD between pairwise SNPs. SNPs further than 10 Mb apart were assumed to be in LD.
We next applied haplotype association analysis to identify POAG associated haplotypes that harbor the variants identified in the discovery stage with P < 1 × 10−6. The haplotype association analysis comprised two steps. First, pairwise measures of LD were calculated in Haploview to identify LD blocks (LD) (Gabriel et al. 2002). Second, significant haplotypes were identified using a Chi-squared test implemented in Haploview (Barrett et al. 2005).
Stage 2
SNPs put forward for replication were first assessed in each replication sample. P value thresholds for significance were adjusted for the number of SNPs tested by the Bonferroni method. Results of all five replication studies were subsequently combined in an inverse variance meta-analysis.
Stage 3
Finally, results from stage 1 and 2 were combined in a transethnic meta-analysis. SNPs showing evidence of effect heterogeneity between studies (Phet < 0.05) were analyzed using the Han Eskin random-effects model (Han and Eskin 2011). This analysis implemented in METASOFT software increases the power to detect associations under heterogeneity.
Power analysis
Power analysis was performed using the Power for Genetic Association analyses (PGA) package (Menashe et al. 2008). For replication of known POAG SNPs power analysis showed that for α = 0.05/15 tests, (Supplementary Fig. 6 red line) and minor allele frequencies of 0.05, 0.10, 0.25; minimal OR’s of 1.5, 1.35 and 1.25, respectively, could be detected at statistical significance assuming 80% power. For genome-wide analysis in the discovery stage, we had > 80% power given an alpha of 5 × 10−8 to detect variants with odds ratios of 1.89 and 3.25 for effect allele frequencies of 0.05 and 0.01, respectively (Supplementary Fig. 6 green line). For validation of SNPs in stage 2, we had > 80% power at an alpha of 0.05/3 independent SNPs = 0.017 to detect loci with odds ratios of 1.29 and 1.7 for effect allele frequencies of 0.05 and 0.01, respectively (Supplementary Fig. 6 line blue line).
Bioinformatics analysis
Several bioinformatics tools to assess whether SNPs or their linked genetic variants were associated with a putative function that might affect patient outcomes were consulted. HaploReg v4.1 and the RegulomDB v1.1 that include Genotype-Tissue Expression (GTEx) database from the Encyclopedia of DNA Elements (ENCODE) project were used to identify the regulatory potential on candidate functional variants to examine the particular tracks of interest, such as TF-ChIP signals, DNase peaks, DNase footprints and predicted DNA sequence motifs for transcription factors (Boyle et al. 2012; Ward and Kellis 2012). The GTEx data were used to identify the correlations between SNPs and whole-blood-specific gene expression levels. The Ocular Tissue Database (2018) (IOWA) was checked for expression of associated genes in relevant ocular tissue, in which levels of gene expression are indicated as Affymetrix Probe Logarithmic Intensity Error (PLIER) normalized value [with normalization in PLIER as described in Wagner et al. 2013].
Gene-based tests
We performed a gene-based test in VEGAS2 (Mishra and Macgregor 2015) to confirm known POAG genes and to identify additional loci not reaching genome-wide significance in a single marker-based analysis. VEGAS2 examines the association from all SNPs across a gene and corrects for gene size and LD between SNPs. The 1000 Genomes phase 3 African populations were downloaded from the VEGAS website and used as the reference panel for pairwise LD correlations. SNPs were allocated to one or more autosomal genes using customized gene boundaries of ± 10 kb.
Genetic risk score
To further evaluate to which extent known POAG SNPs confer risk in our study, a genetic risk score (GRS) was calculated in the GIGA dataset. Fifteen well imputed/genotyped (Minimac r2 > 0.5) SNPs that were previously reported in large GWAS were used for constructing the GRS. For each individual, a weighted GRS was computed by multiplying the number of effect alleles with the log (OR) reported in the literature. We assessed the association of the GRS with POAG in a logistic regression model adjusting for sex, age and PCs. An estimation of the attributable genetic risk was calculated using the R package “attribrisk”.
Supplementary Material
Acknowledgements
Supported by Grants from Combined Ophthalmic Research Rotterdam, The Netherlands; BrightFocus Foundation (G2015084), USA; Algemene Nederlandse Vereniging ter Voorkoming van Blindheid, The Netherlands; Landelijke Stichting voor Blinden en Slechtzienden, The Netherlands; Stichting Beheer Het Schild, The Netherlands; Prof. Dr. Henkes stichting, The Netherlands; Rotterdamse Stichting Blindenbelangen, The Netherlands; Stichting Glaucoomfonds, The Netherlands. Ruth Loos is funded by NIDDK (R01DK110113, R01DK101855, R01DK107786). Ruth Loos and Eimear Kenny received from NHGRI (U01HG007417). The Mount Sinai BioMe Biobank Program is supported by The Andrea and Charles Bronfman Philanthropies and, partially by a grant from NHGRI (U01HG007417). The South London Case–Control Study was supported by a Grant from the International Glaucoma Association and genotyping for this cohort and some of the GIGA study by a National Institute for Health Research (NIHR) Senior Research Fellowship to Christopher Hammond. The NEIGHBORHOOD data collection and analysis are supported by NIH/NEI R01EY022305. The ADAGES data collection and analysis are supported by NIH/NEI EY023704, P30EY022589, EY110008, EY019869, EY021818, R01 DK087914, R01 DK066358, R01 DK053591, U01 DK105556, R01 HL56266, R01 DK070941, DRC DK063491, and CTSI UL1TR001881. The authors thank all the GIGA study and BioMe participants for their cooperation. We acknowledge Suzanne van Schaik, Milou van Bruchem, Hannah Hardjosantoso, Katinka Snoek, Chawan Amin, Vicky Hokken, Corina Brussee, Hilda Roothaert, Najaf Amin, and all ophthalmologist, residents and nurses of the Ophthalmology departments from the Groote Schuur Hospital, Muhimbili National Hospital, CCBRT and Mount Sinai for their continuous efforts in the recruitment of participants.
Footnotes
The members of GIGA Study Group, Eyes of Africa Genetics Consortium, NEIGHBORHOOD Consortium are listed in Electronic Supplementary material.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00439-018-1943-7) contains supplementary material, which is available to authorized users.
Conflict of interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
URLs
BioMe (2018) http://icahn.mssm.edu/research/ipm/programs/biome-biobank
EPACTS (2018) https://genome.sph.umich.edu/wiki/EPACTS
GCTA (2018) http://cnsgenomics.com/software/gcta/#Overview
Genetic Type I Error Calculator (2018) http://grass.cgs.hku.hk/gec/index.php
METAL (2018) https://genome.sph.umich.edu/wiki/METAL
METASOFT (2018) http://genetics.cs.ucla.edu/meta/
Michigan Imputation Server (2018) https://imputationserver.sph.umich.edu/start.html
Plink v 1.9.0 (2018) https://www.cog-genomics.org/plink2
Plink v.1.07 (2018) http://zzz.bwh.harvard.edu/plink/index.shtml
SNPTEST (2018) https://mathgen.stats.ox.ac.uk/genetics_software/snptest/snptest.html
The Ocular Tissue Database (2018) https://genome.uiowa.edu/otdb/ VEGAS2 (2018) https://vegas2.qimrberghofer.edu.au/
References
- Bailey JN, Loomis SJ, Kang JH, Allingham RR, Gharahkhani P, Khor CC, Burdon KP, Aschard H, Chasman DI, Igo RP Jr, Hysi PG, Glastonbury CA, Ashley-Koch A, Brilliant M, Brown AA, Budenz DL, Buil A, Cheng CY, Choi H, Christen WG, Curhan G, De Vivo I, Fingert JH, Foster PJ, Fuchs C, Gaasterland D, Gaasterland T, Hewitt AW, Hu F, Hunter DJ, Khawaja AP, Lee RK, Li Z, Lichter PR, Mackey DA, McGuffin P, Mitchell P, Moroi SE, Perera SA, Pepper KW, Qi Q, Realini T, Richards JE, Ridker PM, Rimm E, Ritch R, Ritchie M, Schuman JS, Scott WK, Singh K, Sit AJ, Song YE, Tamimi RM, Topouzis F, Viswanathan AC, Verma SS, Vollrath D, Wang JJ, Weisschuh N, Wissinger B, Wollstein G, Wong TY, Yaspan BL, Zack DJ, Zhang K, Study EN, Consortium A, Weinreb RN, Pericak-Vance MA, Small K, Hammond CJ, Aung T, Liu Y, Vithana EN, MacGregor S, Craig JE, Kraft P, Howell G, Hauser MA, Pasquale LR, Haines JL, Wiggs JL (2016) Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat Genet 48:189–194. 10.1038/ng.3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Mailer J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265. 10.1093/bioinformatics/bth457 [DOI] [PubMed] [Google Scholar]
- Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, Karczewski KJ, Park J, Hitz BC, Weng S, Cherry JM, Snyder M (2012) Annotation of functional variation in personal genomes using RegulomeDB. Genome Res 22:1790–1797. 10.1101/gr.137323.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon KP, Macgregor S, Hewitt AW, Sharma S, Chidlow G, Mills RA, Danoy P, Casson R, Viswanathan AC, Liu JZ, Landers J, Henders AK, Wood J, Souzeau E, Crawford A, Leo P, Wang JJ, Rochtchina E, Nyholt DR, Martin NG, Montgomery GW, Mitchell P, Brown MA, Mackey DA, Craig JE (2011) Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet 43:574–578. 10.1038/ng.824 [DOI] [PubMed] [Google Scholar]
- Cao D, Jiao X, Liu X, Hennis A, Leske MC, Nemesure B, Hejtmancik JF (2012) CDKN2B polymorphism is associated with primary open-angle glaucoma (POAG) in the Afro-Caribbean population of Barbados, West Indies. PLoS One 7:e39278 10.1371/journal.pone.0039278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4:7 10.1186/S13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lin Y, Vithana EN, Jia L, Zuo X, Wong TY, Chen LJ, Zhu X, Tarn PO, Gong B, Qian S, Li Z, Liu X, Mani B, Luo Q, Guzman C, Leung CK, Li X, Cao W, Yang Q, Tham CC, Cheng Y, Zhang X, Wang N, Aung T, Khor CC, Pang CP, Sun X, Yang Z (2014) Common variants near ABCA1 and in PMM2 are associated with primary open-angle glaucoma. Nat Genet 46:1115–1119. 10.1038/ng.3078 [DOI] [PubMed] [Google Scholar]
- Cook C (2009) Glaucoma in Africa: size of the problem and possible solutions. J Glaucoma 18:124–128. 10.1097/IJG.0b013e318189158c [DOI] [PubMed] [Google Scholar]
- Das S, Forer L, Schonherr S, Sidore C, Locke AE, Kwong A, Vrieze SI, Chew EY, Levy S, McGue M, Schlessinger D, Stambolian D, Loh PR, Iacono WG, Swaroop A, Scott LJ, Cucca F, Kronenberg F, Boehnke M, Abecasis GR, Fuchsberger C (2016) Next-generation genotype imputation service and methods. Nat Genet 48:1284–1287. 10.1038/ng.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth D (1993) Bias reduction of maximum likelihood estimates. Biometrika 80:27–38 [Google Scholar]
- Foster PJ, Buhrmann R, Quigley HA, Johnson GJ (2002) The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 86:238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D (2002) The structure of haplotype blocks in the human genome. Science 296:2225–2229. 10.1126/science.1069424 [DOI] [PubMed] [Google Scholar]
- Genomes Project C, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA (2012) An integrated map of genetic variation from 1092 human genomes. Nature 491:56–65. 10.1038/nature11632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR (2015) A global reference for human genetic variation. Nature 526:68–74. 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Eskin E (2011) Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am J Hum Genet 88:586–598. 10.1016/j.ajhg.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Kampik D, Grehn F, Schlunck G (2013) TGF-beta2-induced invadosomes in human trabecular meshwork cells. PLoS One 8:e70595. 10.1371/journal.pone.0070595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann TJ, Tang H, Thornton TA, Caan B, Haan M, Millen AE, Thomas F, Risch N (2014) Genome-wide association and admixture analysis of glaucoma in the Women’s Health Initiative. Hum Mol Genet 23:6634–6643. 10.1093/hmg/ddu364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J (2009) A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 5:e1000529 10.1371/journal.pgen.1000529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysi PG, Cheng CY, Springelkamp H, Macgregor S, Bailey JN, Wojciechowski R, Vitart V, Nag A, Hewitt AW, Hohn R, Venturini C, Mirshahi A, Ramdas WD, Thorleifsson G, Vithana E, Khor CC, Stefansson AB, Liao J, Haines JL, Amin N, Wang YX, Wild PS, Ozel AB, Li JZ, Fleck BW, Zeller T, Staffieri SE, Teo YY, Cuellar-Partida G, Luo X, Allingham RR, Richards JE, Senft A, Karssen LC, Zheng Y, Bellenguez C, Xu L, Iglesias AI, Wilson JF, Kang JH, van Leeuwen EM, Jonsson V, Thorsteinsdottir U, Despriet DD, Ennis S, Moroi SE, Martin NG, Jansonius NM, Yazar S, Tai ES, Amouyel P, Kirwan J, van Koolwijk LM, Hauser MA, Jonasson F, Leo P, Loomis SJ, Fogarty R, Rivadeneira F, Kearns L, Lackner KJ, de Jong PT, Simpson CL, Pennell CE, Oostra BA, Uitterlinden AG, Saw SM, Lotery AJ, Bailey-Wilson JE, Hofman A, Vingerling JR, Maubaret C, Pfeiffer N, Wolfs RC, Lemij HG, Young TL, Pasquale LR, Delcourt C, Spec-tor TD, Klaver CC, Small KS, Burdon KP, Stefansson K, Wong TY, Group BG, Consortium N, Wellcome Trust Case Control, Viswanathan C, Mackey A, Craig DA, Wiggs JE, van Duijn JL, Hammond CM, Aung CJT (2014) Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat Genet 46:1126–1130. 10.1038/ng.3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyari F, Abdull MM, Bastawrous A, Gilbert CE, Faal H (2013) Epidemiology of glaucoma in sub-saharan Africa: prevalence, incidence and risk factors. Middle East Afr J Ophthalmol 20:111–125. 10.4103/0974-9233.110605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MX, Yeung JM, Cherny SS, Sham PC (2012) Evaluating the effective numbers of independent tests and significant p value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum Genet 131:747–756. 10.1007/S00439-011-1118-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Allingham RR, Nakano M, Jia L, Chen Y, Ikeda Y, Mani B, Chen LJ, Kee C, Garway-Heath DF, Sripriya S, Fuse N, Abu-Amero KK, Huang C, Namburi P, Burdon K, Perera SA, Gharahkhani P, Lin Y, Ueno M, Ozaki M, Mizoguchi T, Krishnadas SR, Osman EA, Lee MC, Chan AS, Tajudin LS, Do T, Goncalves A, Reynier P, Zhang H, Bourne R, Goh D, Broadway D, Husain R, Negi AK, Su DH, Ho CL, Blanco AA, Leung CK, Wong TT, Yakub A, Liu Y, Nongpiur ME, Han JC, Hon DN, Shantha B, Zhao B, Sang J, Zhang N, Sato R, Yoshii K, Panda-Jonas S, Ashley Koch AE, Herndon LW, Moroi SE, Challa P, Foo JN, Bei JX, Zeng YX, Simmons CP, Bich Chau TN, Sharmila PF, Chew M, Lim B, Tarn PO, Chua E, Ng XY, Yong VH, Chong YF, Meah WY, Vijayan S, Seongsoo S, Xu W, Teo YY, Cooke Bailey JN, Kang JH, Haines JL, Cheng CY, Saw SM, Tai ES, Consortium IC-G, Consortium N, Richards JE, Ritch R, Gaasterland DE, Pasquale LR, Liu J, Jonas JB, Milea D, George R, Al-Obeidan SA, Mori K, Macgregor S, Hewitt AW, Girkin CA, Zhang M, Sundaresan P, Vijaya L, Mackey DA et al. (2015) A common variant near TGFBR3 is associated with primary open angle glaucoma. Hum Mol Genet 24:3880–3892. 10.1093/hmg/ddv128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PI, Vance JM, Pericak-Vance MA, Martin ER (2007) No gene is an island: the flip-flop phenomenon. Am J Hum Genet 80:531–538. 10.1086/512133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hauser MA, Akafo SK, Qin X, Miura S, Gibson JR, Wheeler J, Gaasterland DE, Challa P, Herndon LW, International Consortium of African Ancestry RiG, Ritch R, Moroi SE, Pasquale LR, Girkin CA, Budenz DL, Wiggs JL, Richards JE, Ashley-Koch AE, Allingham RR (2013) Investigation of known genetic risk factors for primary open angle glaucoma in two populations of African ancestry. Investig Ophthalmol Vis Sci 54:6248–6254. 10.1167/iovs.13-12779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiela MJ, Chanock SJ (2015) LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 31:3555–3557. 10.1093/bioinformatics/btv402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini J, Howie B, Myers S, McVean G, Donnelly P (2007) A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 39:906–913. 10.1038/ng2088 [DOI] [PubMed] [Google Scholar]
- McCarthy MI, Hirschhorn JN (2008) Genome-wide association studies: potential next steps on a genetic journey. Hum Mol Genet 17:R156–R165. 10.1093/hmg/ddn289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A, Kang HM, Fuchsberger C, Danecek P, Sharp K, Luo Y, Sidore C, Kwong A, Timpson N, Koskinen S, Vrieze S, Scott LJ, Zhang H, Mahajan A, Veldink J, Peters U, Pato C, van Duijn CM, Gillies CE, Gandin I, Mezzavilla M, Gilly A, Cocca M, Traglia M, Angius A, Barrett JC, Boomsma D, Branham K, Breen G, Brummett CM, Busonero F, Campbell H, Chan A, Chen S, Chew E, Collins FS, Corbin LJ, Smith GD, Dedoussis G, Dorr M, Farmaki AE, Ferrucci L, Forer L, Fraser RM, Gabriel S, Levy S, Groop L, Harrison T, Hattersley A, Holmen OL, Hveem K, Kretzler M, Lee JC, McGue M, Meitinger T, Melzer D, Min JL, Mohlke KL, Vincent JB, Nauck M, Nickerson D, Palotie A, Pato M, Pirastu N, McInnis M, Richards JB, Sala C, Salomaa V, Schlessinger D, Schoenherr S, Slagboom PE, Small K, Spector T, Stambolian D, Tuke M, Tuomilehto J, Van den Berg LH, Van Rheenen W, Volker U, Wijmenga C, Toniolo D, Zeggini E, Gasparini P, Sampson MG, Wilson JF, Frayling T, de Bakker PI, Swertz MA, McCa-rroll S, Kooperberg C, Dekker A, Altshuler D, Wilier C, Iacono W, Ripatti S et al. (2016) A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet 48:1279–1283. 10.1038/ng.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menashe I, Rosenberg PS, Chen BE (2008) PGA: power calculator for case–control genetic association analyses. BMC Genet 9:36 10.1186/1471-2156-9-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Macgregor S (2015) VEGAS2: software for more flexible gene-based testing. Twin Res Hum Genet 18:86–91. 10.1017/thg.2014.79 [DOI] [PubMed] [Google Scholar]
- Okbay A, Beauchamp JP, Fontana MA, Lee JJ, Pers TH, Rietveld CA, Turley P, Chen GB, Emilsson V, Meddens SF, Oskarsson S, Pickrell JK, Thorn K, Timshel P, de Vlaming R, Abdellaoui A, Ahlu-walia TS, Bacelis J, Baumbach C, Bjornsdottir G, Brandsma JH, Pina Concas M, Derringer J, Furlotte NA, Galesloot TE, Girotto G, Gupta R, Hall LM, Harris SE, Hofer E, Horikoshi M, Huffman JE, Kaasik K, Kalafati IP, Karlsson R, Kong A, Lahti J, van der Lee SJ, deLeeuw C, Lind PA, Lindgren KO, Liu T, Mangino M, Marten J, Mihailov E, Miller MB, van der Most PJ, Oldmeadow C, Payton A, Pervjakova N, Peyrot WJ, Qian Y, Raitakari O, Rueedi R, Salvi E, Schmidt B, Schraut KE, Shi J, Smith AV, Poot RA, St Pourcain B, Teumer A, Thorleifsson G, Verweij N, Vuckovic D, Wellmann J, Westra HJ, Yang J, Zhao W, Zhu Z, Alizadeh BZ, Amin N, Bakshi A, Baumeister SE, Biino G, Bonnelykke K, Boyle PA, Campbell H, Cappuccio FP, Davies G, De Neve JE, Deloukas P, Demuth I, Ding J, Eibich P, Eisele L, Eklund N, Evans DM, Faul JD, Feitosa MF, Forstner AJ, Gandin I, Gunnarsson B, Halldorsson BV, Harris TB, Heath AC, Hocking LJ, Holliday EG, Homuth G, Horan MA et al. (2016) Genome-wide association study identifies 74 loci associated with educational attainment. Nature 533:539–542. 10.1038/nature17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Mailer J, Sklar P, de Bakker PI, Daly MJ, Sham PC (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roadmap Epigenomics C, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, Ziller MJ, Amin V, Whitaker JW, Schultz MD, Ward LD, Sarkar A, Quon G, Sandstrom RS, Eaton ML, Wu YC, Pfenning AR, Wang X, Claussnitzer M, Liu Y, Coarfa C, Harris RA, Shoresh N, Epstein CB, Gjoneska E, Leung D, Xie W, Hawkins RD, Lister R, Hong C, Gascard P, Mungall AJ, Moore R, Chuah E, Tam A, Canfield TK, Hansen RS, Kaul R, Sabo PJ, Bansal MS, Carles A, Dixon JR, Farh KH, Feizi S, Karlic R, Kim AR, Kulkarni A, Li D, Lowdon R, Elliott G, Mercer TR, Neph SJ, Onuchic V, Polak P, Rajagopal N, Ray P, Sallari RC, Siebenthall KT, Sinnott-Armstrong NA, Stevens M, Thurman RE, Wu J, Zhang B, Zhou X, Beaudet AE, Boyer LA, De Jager PL, Farnham PJ, Fisher SJ, Haussler D, Jones SJ, Li W, Marra MA, McManus MT, Sunyaev S, Thomson JA, Tlsty TD, Tsai LH, Wang W, Waterland RA, Zhang MQ, Chadwick LH, Bernstein BE, Costello JF, Ecker JR, Hirst M, Meissner A, Milosavljevic A, Ren B, Stamatoyannopoulos JA, Wang T, Kellis M (2015) Integrative analysis of 111 reference human epigenomes. Nature 518:317–330. 10.1038/nature14248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherva R, Tripodis Y, Bennett DA, Chibnik LB, Crane PK, de Jager PL, Farrer LA, Saykin AJ, Shulman JM, Naj A, Green RC, Consortium G (2014) Alzheimer’s Disease neuroimaging I, Alzheimer’s disease genetics. Genome-wide association study of the rate of cognitive decline in Alzheimer’s disease. Alzheimers Dement 10:45–52. 10.1016/j.jalz.2013.01.008 C). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniekers S, Stringer S, Watanabe K, Jansen PR, Coleman JRI, Krapohl E, Taskesen E, Hammerschlag AR, Okbay A, Zabaneh D, Amin N, Breen G, Cesarini D, Chabris CF, Iacono WG, Ikram MA, Johannesson M, Koellinger P, Lee JJ, Magnusson PKE, McGue M, Miller MB, Oilier WER, Payton A, Pendleton N, Plomin R, Rietveld CA, Tiemeier H, van Duijn CM, Posthuma D (2017) Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat Genet 49:1107–1112. 10.1038/ng.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springelkamp H, Iglesias AI, Cuellar-Partida G, Amin N, Burdon KP, van Leeuwen EM, Gharahkhani P, Mishra A, van der Lee SJ, Hewitt AW, Rivadeneira F, Viswanathan AC, Wolfs RC, Martin NG, Ramdas WD, van Koolwijk LM, Pennell CE, Vingerling JR, Mountain JE, Uitterlinden AG, Hofman A, Mitchell P, Lemij HG, Wang JJ, Klaver CC, Mackey DA, Craig JE, van Duijn CM, MacGregor S (2015) ARHGEF12 influences the risk of glaucoma by increasing intraocular pressure. Hum Mol Genet 24:2689–2699. 10.1093/hmg/ddv027 [DOI] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121:2081–2090. 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, DeWan A, Sigurdsson A, Jonasdottir A, Gudjonsson SA, Magnusson KP, Stefansson H, Lam DS, Tarn PO, Gudmundsdottir GJ, Southgate L, Burdon KP, Gottfredsdottir MS, Aldred MA, Mitchell P, St Clair D, Collier DA, Tang N, Sveinsson O, Macgregor S, Martin NG, Cree AJ, Gibson J, Macleod A, Jacob A, Ennis S, Young TL, Chan JC, Karwatowski WS, Hammond CJ, Thordarson K, Zhang M, Wadelius C, Lotery AJ, Trembath RC, Pang CP, Hoh J, Craig JE, Kong A, Mackey DA, Jonasson F, Thorsteinsdottir U, Stefansson K (2010) Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet 42:906–909. 10.1038/ng.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AH, Anand VN, Wang WH, Chatterton JE, Sun D, Shepard AR, Jacobson N, Pang IH, Deluca AP, Casavant TL, Scheetz TE, Mullins RF, Braun TA, Clark AF (2013) Exon-level expression profiling of ocular tissues. Exp Eye Res 111:105–111. 10.1016/j.exer.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward LD, Kellis M (2012) HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 40:D930–D934. 10.1093/nar/gkr917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Yaspan BL, Hauser MA, Kang JH, Allingham RR, Olson LM, Abdrabou W, Fan BJ, Wang DY, Brodeur W, Budenz DL, Caprioli J, Crenshaw A, Crooks K, Delbono E, Doheny KF, Friedman DS, Gaasterland D, Gaasterland T, Laurie C, Lee RK, Lichter PR, Loomis S, Liu Y, Medeiros FA, McCarty C, Mirel D, Moroi SE, Musch DC, Realini A, Rozsa FW, Schuman JS, Scott K, Singh K, Stein JD, Trager EH, Vanveldhuisen P, Vollrath D, Wollstein G, Yoneyama S, Zhang K, Weinreb RN, Ernst J, Kellis M, Masuda T, Zack D, Richards JE, Pericak-Vance M, Pasquale LR, Haines JL (2012) Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet 8:el002654 10.1371/journal.pgen.1002654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilier CJ, Li Y, Abecasis GR (2010) METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26:2190–2191. 10.1093/bioinformatics/btq340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Carmichael TR, Allingham RR, Hauser M, Ramsay M (2015) The genetics of POAG in black South Africans: a candidate gene association study. Sci Rep 5:8378 10.1038/srep08378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM (2011) GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet 88:76–82. 10.1016/j.ajhg.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangwill LM, Ayyagari R, Liebmann JM, Girkin CA, Feldman R, Dubiner H, Dirkes KA, Holmann M, Williams-Steppe E, Hammel N, Saunders LJ, Vega S, Sandow K, Roll K, Slight R, Auerbach D, Samuels BC, Panarelli JF, Mitchell JP, Al-Aswad LA, Park SC, Tello C, Cotliar J, Bansal R, Sidoti PA, Cioffi GA, Blumberg D, Ritch R, Bell NP, Blieden LS, Davis G, Medeiros FA, Ng MCY, Das SK, Palmer ND, Divers J, Langefeld CD, Freedman BI, Bowden DW, Christopher MA, Chen YI, Guo X, Taylor KD, Rotter JI, Weinreb RN, African D, Glaucoma Evaluation Study IIIGSG (2018) The African descent and glaucoma evaluation study (ADAGES) III: contribution of genotype to glaucoma phenotype in African Americans: study design and baseline data. Ophthalmology. 10.1016/j.ophtha.2017.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.