Abstract
A 26-year-old Caucasian man with no medical history, except years of oral and intravenous drug abuse, presented with fatigue, shortness of breath, epistaxis and uncontrolled hypertension. He was pale with skin ecchymosis over his thighs and was anaemic, with severe renal failure and metabolic acidosis. Following initial clinical stabilisation of the patient, a renal biopsy was obtained, which showed vascular and glomerular changes consistent with thrombotic microangiopathic injury and advanced glomerulosclerosis. He was treated with antihypertensives and required haemodialysis. He admitted using ‘crystal meth’ regularly for many years, which is likely responsible for his renal failure. We present the case to illustrate methamphetamine-induced renal disease leading to end-stage renal disease and to bring awareness among practising clinicians, ancillary healthcare workers and public health professionals of this often undervalued cause of renal failure, which can be prevented.
Keywords: drug misuse (including addiction), unwanted effects / adverse reactions, public health, chronic renal failure
Background
Recreational drug abuse is a significant public health problem across the globe, with considerable morbidity, mortality and public health burden. A staggering 24.6 million American teenage (above age 12) and young adult population has recently used illicit drugs per survey in 2013.1 The study reported almost 595 000 (aged 12 or above) current users of methamphetamine (METH) and related products. Drugs for recreation have been associated with chronic kidney disease (CKD) but remains a frequently neglected risk factor for end-stage renal disease (ESRD).
METH, a synthetic drug, is an indirect sympathomimetic amine and lacks direct adrenergic stimulation, but inhibits presynaptic epinephrine and dopamine reuptake-mediated ATP-dependent channels resulting in a surge of both alpha-adrenergic and beta-adrenergic effects. METH’s adverse effects include neuropsychosis, systemic and pulmonary hypertension, rhabdomyolysis, and liver and renal failure, along with cardiomyopathy. Among these inconsiderate risk factors, METH-induced thrombotic microangiopathy (TMA) and ESRD are indeed a rare entity. We report an unfortunate case of a young man who developed ESRD from METH-induced TMA and stress the importance of recognising METH as the causative factor for ESRD. We have reviewed the relevant literature on the subject.
Case presentation
A 26-year-old-Caucasian unemployed man with no relevant medical history, except ongoing drug abuse over many years, presented to the emergency department with fatigue, dyspnoea, and epistaxis of 2 weeks' duration. Additionally, he complained of easy bruisability for the past 8–10 days with multiple skin lesions on the lower extremity. Family history was not relevant.
On evaluation, his vitals were notable for tachycardia (heart rate of 104 beats/min) with a blood pressure of 210/124 mm Hg. Clinically, the pale young man was conscious and oriented with skin ecchymosis over his thighs and extensive tattoos on his chest wall.
Initial laboratory evaluation revealed a creatinine of 21 mg/dL, blood urea nitrogen of 168 mg/dL, bicarbonate of 10 mEq/L with an anion gap of 26 and a phosphate of 10.6 mg/dL.
No baseline creatinine values were available. Urine analysis was positive for 3+ protein and 2+ blood with negative red blood cells (RBCs). His complete blood panel was notable for haemoglobin level of 5.5 g/L and platelets of 114/109/L. Coagulation workup was within normal limits. Creatine kinase levels were at 600. The initial urine drug screen was negative. Serum alcohol levels were undetectable.
The initial differential was for rapidly progressive glomerulonephritis (RPGN) with lupus nephritis, anti-neutrophil cytoplasmic antibody (ANCA) or antiglomerular basement membrane (anti-GBM), GN, thrombotic thrombocytopenic purpura (TTP), acute interstitial nephritis and HIV-associated nephropathy. The patient was started on gentle intravenous isotonic hydration and antihypertensives and was given two units of packed RBCs. Further evaluation with peripheral smear revealed significant schistocytes, reticulocyte count 3.3%, haptoglobin 93 mg/dL (37–184), Von W protease 51% (normal >61), lactate dehydrogenase (LDH) 464 U/L (100–250). Stool occult test was negative. His liver function tests and complements were within normal limits. Echocardiogram showed severe left ventricular hypertrophy, with an ejection fraction of 45% with mild global hypokinesis of the left ventricle. The renal sonogram showed medical renal disease but no hydronephrosis. At this point, his labs were consistent with microangiopathic haemolysis yet did not favour haemolytic uremic syndrome–TTP due to minimally elevated LDH, normal haptoglobin, bilirubin and ADAMTS13 activity.
Serological workup for lupus, ANCA and anti-GBM vasculitis was unrevealing, along with negative HIV and non-reactive hepatitis profile. Serum cryoglobulins were negative.
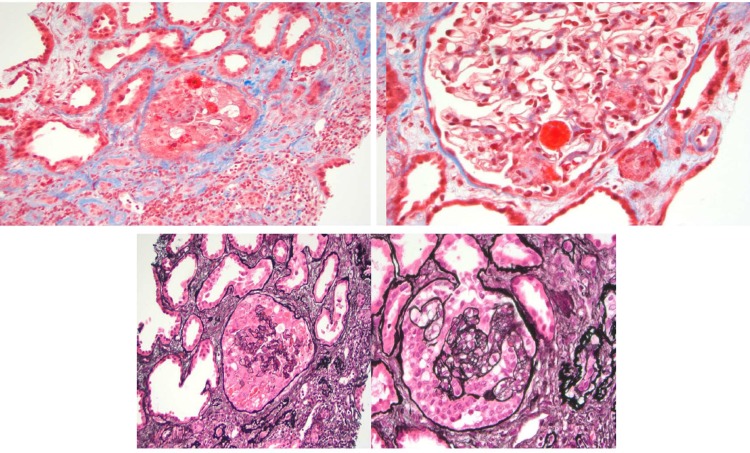
As the patient’s renal function has not improved with significant uremic azotemia, he was started on haemodialysis. A renal biopsy (figures 1, 2) was done on day 4 for the definitive diagnosis, and it showed vascular and glomerular changes most consistent with subacute thrombotic microangiopathic injury, advanced glomerulosclerosis (9/19 glomeruli=47%), prominent mucoid intimal hyperplasia involving afferent arterioles, cellular crescent formation (2/19 glomeruli) and 40% tubular atrophy/interstitial fibrosis. Immunofluorescence staining was normal.
Figure 1.
Light microscopy of glomeruli with thrombotic microangiopathic process and mesangial cell proliferation. Tuft necrosis, rare crescents and mesangiolysis. Forty per cent tubular atrophy and interstitial fibrosis. Immunofluorescence: C3 1+ (focal and granular) and IgM 1+ (focal and granular).
Figure 2.
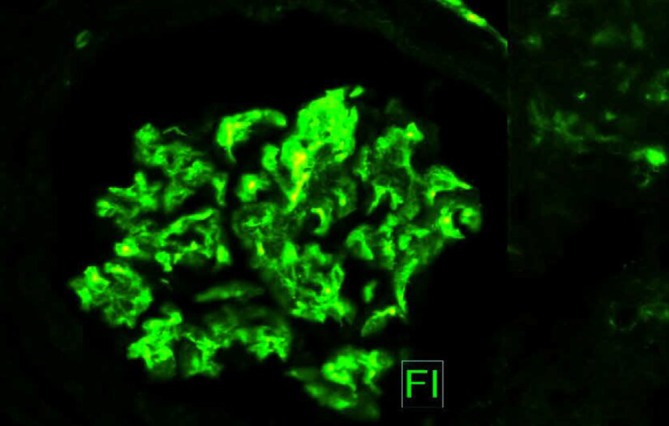
Immunofluorescence: C3 1+ (focal and granular) and IgM 1+ (focal and granular).
The patient was started on haemodialysis following initial aggressive fluid resuscitation and remained dialysis dependent on his 6 months' follow-up. TMA-induced ESRD is highly likely from his METH abuse, as the patient later admitted to using ‘crystal meth’ every week for several years with possible multiple episodes of hypertensive surges as evident by his severe left ventricular hypertrophy on echocardiogram. We believe that the marked hypertension induced by METH use resulted in the development of macroangiopathic injury in the renal parenchyma.
Investigations
Serum serological workup (anti-nuclear antibody (ANA), ANCA, hepatitis serologies and complements).
Renal ultrasonogram.
Renal biopsy.
Differential diagnosis
RPGN from lupus, ANCA, anti-GBM, cryoglobulinemic vasculitis and TTP-Hemolytic Uremic Syndrome (HUS).
IgA nephritis.
Acute interstitial nephritis.
HIV-associated nephropathy.
Treatment
Intravenous isotonic hydration.
Blood transfusion.
Antihypertensives.
Haemodialysis.
Outcome and follow-up
The patient remained on haemodialysis, dependent on the 6 months' follow-up.
Discussion
METH, a synthetic agent, is a psychostimulant sympathomimetic drug and has become the second most frequently used drug of abuse worldwide, in various forms and products, with approximately 34 million users worldwide in 2013.2 METH is an N-methylated derivative of amphetamine (AMPH), and though both share a nearly identical chemical structure and have similar pharmacokinetic properties, they differ in the rates of abuse3 and in the fact that METH is relatively more potent than AMPH.4 Ecstasy (MDMA-3,4 methylenedioxymethamphetamine), a closely related AMPH, was initially introduced as an appetite suppressant but rapidly transformed into a commonly used recreational drug leading to increased presentations of patients to the emergency departments with hyperthermia, dehydration, rhabdomyolysis and related increased risk of acute kidney injury.5 6 The burgeoning number of clandestine drug laboratories, which led to dramatic increases in METH production, resulted ultimately in significant public health, legal and environmental problems across nations.7 Unfortunately, METH can be synthesised by a simple one-step process by reduction of ephedrine or pseudoephedrine, which are widely available in North America as nonprescription allergy medicines and through methods described in detail on the internet.7
METH and other related substances can cause a variety of adverse effects impacting cardiovascular and cerebrovascular systems, leading to complications that include malignant hypertension, arrhythmias, aortic dissection, myocardial infarction, stroke (both ischaemic and haemorrhagic) and cardiomyopathy.8 There are reports of METH-induced ischaemic colitis and retinal vasculitis in the literature.9 10
The pharmacological and clinical effects of the METH are related to the release of neurotransmitters such as dopamine, serotonin and norepinephrine and its actions through non-exocytic mechanisms.11 Norepinephrine activates the α1 receptors in the arterial vasculature to stimulate the vasoconstriction and increases the cardiac contractility and heart rate via the β1 receptors.12 13 The coronary vasospasm mediated by the catecholamine excess has been proposed to be the cause of the METH-associated cardiomyopathy. Other theories included activation of the reactive oxygen species, myocardial mitochondrial injury and changes in the myocardial metabolism.14 Seo et al demonstrated in a study in mouse models that METH can mediate the release of the endothelin in brain endothelial cells, proposing an additional mechanism of tissue damage from arterial vasoconstriction.15
Tokunaga et al have studied the renal function and oxidative damage in METH-treated rats. The authors have found that METH might induce renal dysfunction with renal tubular impairment from the leaked creatinine phosphokinase (CPK) from muscle. Oxidative DNA damage might also be induced by the repeated administration of METH.16 Zhang et al have found a reduction in oxidative stress in the organs of rats exposed to METH following treatment with N-acetylcysteine amide.17
AMPHs have been incriminated in causing non-traumatic rhabdomyolysis and acute renal failure with earliest reports dating back to 1970s.18 Ginsberg et al reported that a patient presented with acute kidney injury, non-traumatic rhabdomyolysis and disseminated intravascular coagulation following METH consumption.18 METHs and related substances have deleterious effects on the kidney, and while non-traumatic rhabdomyolysis is most commonly reported, there are reports of METH-induced acute cortical necrosis, necrotising vasculitis and mesangiocapillary glomerulonephritis.2 6 19
Richards et al 19 have studied 367 patients who presented to the emergency department over 5 years in Sacramento (California) with rhabdomyolysis and found that 166 patients (43%) were positive for METH on toxicology. The authors concluded that an association between METH abuse and rhabdomyolysis may exist, and CPK should be measured as a screen in this group of patients.
A study by Jones and Rayner (South Africa) in 49 patients referred for CKD in the clinic showed that 44.7% of the METH users had malignant hypertension and 95.7% had CKD.6 Renal biopsy was performed in 24 patients in the study population. Twelve renal biopsies showed hypertensive changes (50%) with six (25%) showing malignant changes. Six (25%) biopsies showed findings in line with ESRD. Mesangiocapillary glomerulonephritis (MCGN) type 1 was found in 14 biopsy cases (58.3%), and all were positive for IgM and C3 complement. Also, nine biopsy cases (37.5%) showed staining for IgG, and seven (29%) for IgA. One patient was positive for HIV and had hypertensive changes on the biopsy. The MCGN in the biopsy findings was thought to result from chronic antigenemia and not from the use of METH.6 Though the patients with hypertensive changes and MCGN were almost equal in the biopsied study subjects, the latter progressed to ESRD faster in the following 5 years. The authors have concluded that METH use appears to be associated with severe hypertension and MCGN, both of which can lead to ESRD and death.6
It should be noted that the direct mechanisms that underlie the toxic actions of METHs are challenging to elucidate as is not the only drug involved in the majority of the cases, and it may be adulterated with other compounds and impurities.
Some of the impurities reported in the literature include N-isopropyl benzylamine, cocaine, phenethylamine and acetic acid.20 21
The index case is possibly one of the first reports of METH-associated ESRD most possibly given his long history of METH use with potential recurrent acute renal injuries, compounded by uncontrolled, untreated hypertension, ultimately leading to the patient’s presentation with TMA-mediated ESRD and subsequent dialysis dependence. Our case illustrates the need for considering METH as a potential cause of ESRD, as the early cessation of METH use could prevent irreversible renal damage. Given the exponential rise in METH use both in the first-world to third-world nations, poor to rich, and celebrities to more impoverished populations, public health officials, clinicians, law enforcement and healthcare workers should educate and bring more awareness to prevent ESRD.
Learning points.
Methamphetamine (METH), a psychostimulant agent, is the second most frequently used drug of abuse worldwide with approximately 34 million users worldwide in 2013.
METH and other related substances of abuse are emerging as an important cause of renal failure with potential to develop end-stage renal disease.
There is an urgent need for public health officials, clinicians, law enforcement and healthcare workers to educate and bring awareness to prevent this preventable cause of renal failure.
Footnotes
Contributors: KMB was involved in the planning; conception; acquisition of data, including images; and patient consent. KMB, NRA, SP and SB helped with the literature search and final drafting of the case report. KMB and NRA are the first authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. 2013. https://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.htm (cited 10 Mar 2019).
- 2. Gupta A, Kuperman M, Shah S. N-methylamphetamine (“Crystal Meth”)−Associated Acute Renal Cortical Necrosis. Kidney Int Rep 2018;3:1473–6. 10.1016/j.ekir.2018.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melega WP, Williams AE, Schmitz DA, et al. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther 1995;274:90–6. [PubMed] [Google Scholar]
- 4. Hall DA, Stanis JJ, Marquez Avila H, et al. A comparison of amphetamine- and methamphetamine-induced locomotor activity in rats: evidence for qualitative differences in behavior. Psychopharmacology 2008;195:469–78. 10.1007/s00213-007-0923-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fahal IH, Sallomi DF, Yaqoob M, et al. Acute renal failure after ecstasy. BMJ 1992;305:29 10.1136/bmj.305.6844.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones ES, Rayner BL. Hypertension, end-stage renal disease and mesangiocapillary glomerulonephritis in methamphetamine users. S Afr Med J 2015;105:199–201. 10.7196/SAMJ.8731 [DOI] [PubMed] [Google Scholar]
- 7. Barr AM, Panenka WJ, MacEwan GW, et al. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci 2006;31:301–13. [PMC free article] [PubMed] [Google Scholar]
- 8. Schürer S, Klingel K, Sandri M, et al. Clinical characteristics, histopathological features, and clinical outcome of methamphetamine-associated cardiomyopathy. JACC Heart Fail 2017;5:435–45. 10.1016/j.jchf.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 9. Johnson TD, Berenson MM. Methamphetamine-induced ischemic colitis. J Clin Gastroenterol 1991;13:687–9. 10.1097/00004836-199112000-00015 [DOI] [PubMed] [Google Scholar]
- 10. Shaw HE, Lawson JG, Stulting RD. Amaurosis fugax and retinal vasculitis associated with methamphetamine inhalation. J Clin Neuroophthalmol 1985;5:169–76. [DOI] [PubMed] [Google Scholar]
- 11. Sulzer D, Sonders MS, Poulsen NW, et al. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 2005;75:406–33. 10.1016/j.pneurobio.2005.04.003 [DOI] [PubMed] [Google Scholar]
- 12. Kish SJ. Pharmacologic mechanisms of crystal meth. CMAJ 2008;178:1679–82. 10.1503/cmaj.071675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dünser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med 2009;24:293–316. 10.1177/0885066609340519 [DOI] [PubMed] [Google Scholar]
- 14. Lord KC, Shenouda SK, McIlwain E, et al. Oxidative stress contributes to methamphetamine-induced left ventricular dysfunction. Cardiovasc Res 2010;87:111–8. 10.1093/cvr/cvq043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seo J-W, Jones SM, Hostetter TA, et al. Methamphetamine induces the release of endothelin. J Neurosci Res 2016;94:170–8. 10.1002/jnr.23697 [DOI] [PubMed] [Google Scholar]
- 16. Tokunaga I, Kubo S, Ishigami A, et al. Changes in renal function and oxidative damage in methamphetamine-treated rat. Leg Med 2006;8:16–21. 10.1016/j.legalmed.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 17. Zhang X, Tobwala S, Ercal N. N-acetylcysteine amide protects against methamphetamine-induced tissue damage in CD-1 mice. Hum Exp Toxicol 2012;31:931–44. 10.1177/0960327112438287 [DOI] [PubMed] [Google Scholar]
- 18. Ginsberg MD, Hertzman M, Schmidt-Nowara WW. Amphetamine intoxication with coagulopathy, hyperthermia, and reversible renal failure. A syndrome resembling heatstroke. Ann Intern Med 1970;73:81–5. 10.7326/0003-4819-73-1-81 [DOI] [PubMed] [Google Scholar]
- 19. Richards JR, Johnson EB, Stark RW, et al. Methamphetamine abuse and rhabdomyolysis in the ED: a 5-year study. Am J Emerg Med 1999;17:681–5. 10.1016/S0735-6757(99)90159-6 [DOI] [PubMed] [Google Scholar]
- 20. Peck Y, Clough AR, Culshaw PN, et al. Multi-drug cocktails: Impurities in commonly used illicit drugs seized by police in Queensland, Australia. Drug Alcohol Depend 2019;201:49–57. 10.1016/j.drugalcdep.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 21. Shekari N, Vosough M, Tabar Heidar K. Chemometrics-assisted chromatographic fingerprinting: An illicit methamphetamine case study. J Sep Sci 2017;40:1318–26. 10.1002/jssc.201601313 [DOI] [PubMed] [Google Scholar]