Abstract
Prostaglandins are widely used in aortic coarctation to maintain ductal patency and preserve systemic perfusion until surgical intervention can be performed. Although the short-term use of prostaglandins to ameliorate aortic narrowing in neonates with a closed ductus has been reported, it has not been described as a longer term therapy in extremely preterm neonates. A 27-week gestation baby weighing 560 g presented at 40 days of age with coarctation and a closed ductus arteriosus. He was successfully treated with a 7-week course of prostaglandin E2 therapy because surgical intervention was not deemed feasible in view of his size. Treatment resulted in a relaxation of the aortic constriction and improvement in aortic blood flow velocity profile, highlighting the value of long-term prostaglandin therapy in this population and supporting the hypothesis that the presence of ductal tissue contributes to the development of juxtaductal aortic constriction in some extremely preterm infants.
Keywords: congenital disorders, neonatal and paediatric intensive care
Background
Surgical options for congenital cardiac defects are extremely limited in very preterm babies, and this case demonstrated an alternative management option in such cases.
Case presentation
A boy, the second of dichorionic diamniotic twins, weighing 560 g was born by caesarean section at 27 weeks gestation. His mother had received antenatal steroids for impending preterm delivery but no other medications. He was intubated shortly after delivery and given surfactant therapy. He initially received high-frequency oscillatory ventilation for severe respiratory failure and dopamine and dobutamine for systemic hypotension.
Echocardiography at 5 days of age showed a large patent ductus arteriosus (PDA) with a low velocity, balanced bidirectional flow pattern. The heart was structurally normal except for a small patent foramen ovale. A repeat echocardiogram 3 days later revealed a 2 mm midmuscular ventricular septal defect; the ductus arteriosus was closed, and there was no evidence of residual pulmonary hypertension.
There was gradual progress over the next few days with extubation to nasal continuous positive airway pressure (CPAP) on day 14. Echocardiography was repeated on day 29 because of persistent hypotension; cardiovascular examination at this time was normal. Although the ductus remained closed, there was tapering of the distal descending aorta (narrowest diameter 1.8 mm, baby’s weight 0.86 kg) with an increased flow velocity of 2.1 m/s associated with turbulent flow and significant diastolic decay. Repeat echocardiography on day 40 showed an aortic diameter of 1.6 mm (baby’s weight 0.95 kg) and an increased velocity of 3.8 m/s (figure 1). Following discussion with the specialist cardiology service, intravenous dinoprostone (prostaglandin E2) at 100 ng/kg/min was started, and he was electively intubated and ventilated.
Figure 1.
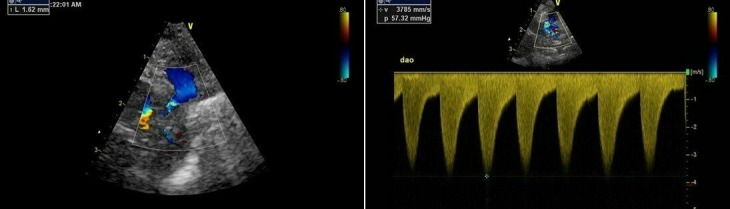
Echocardiographic findings on day 40.
Reassessment 24 hours later showed an aortic diameter of 2.1 mm with a reduced flow velocity of 3.1 m/s. Dinoprostone was reduced to 50 ng/kg/min. The aortic diameter measured in serial assessments over the next 6 days was approximately 2.0 mm (baby’s weight 1.04 kg) with an aortic velocity gradually decreasing to 2.0 m/s. The ductus arteriosus had reopened but was very tiny and tortuous with a high-velocity flow pattern. The baby was extubated on day 43, and prostaglandin therapy was continued while focusing on establishing enteral feeding, maximising nutrition and promoting growth with a view to considering surgical intervention once the baby had achieved a target weight of 2.5 kg. On day 58, the aortic diameter was 2.8 mm with a peak velocity of 1.6 m/s (figure 2). Dinoprostone was reduced to 5 ng/kg/min by day 78, and he was changed to oral dinoprostone (25 µg/kg hourly) on day 80.
Figure 2.
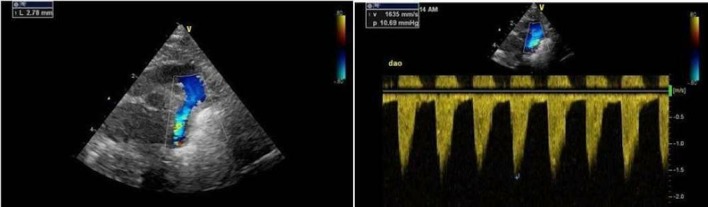
Echocardiographic findings on day 58.
Repeat echocardiography showed a descending aortic diameter consistently above 2.5 mm and a flow velocity below 2 m/s. Dinoprostone was stopped on day 90. Subsequent echocardiograms suggested a slight worsening of the aortic narrowing (diameter 2.0 mm) accompanied by an increased flow velocity (2.9 m/s), but the baby remained clinically well. He was repatriated to his local neonatal unit on day 104 weighing 2.52 kg and in 5 L/min of high-flow oxygen with diuretic therapy for chronic lung disease of prematurity.
Outcome and follow-up
Subsequent cardiology follow-up at 7 months of age confirmed mild juxtaductal coarctation with an increased flow velocity of 3.4 m/s and some diastolic decay. Femoral pulses were palpable but weak. CT angiography performed at 8 months confirmed a short-segment coarctation in the descending aorta just distal to the origin of the left subclavian artery with a coarctation index of 0.36. The aortic diameter was 5×5.5 mm. The baby remained asymptomatic, and a decision was made to manage conservatively. At the age of 20 months, the narrowing had improved spontaneously with an aortic diameter of 8 mm (baby’s weight 7.95 kg) and an aortic velocity of 1.8 m/s with no diastolic decay. There was spontaneous closure of the ventricular septal defect (VSD). There are currently no plans for surgical intervention.
Discussion
Our case highlights an alternative management strategy in coarctation in extremely preterm neonates when surgical intervention is not feasible or available. Prostaglandins are commonly used to maintain ductal patency in cases of duct-dependent coarctation to preserve or improve lower body systemic blood flow. However, our case demonstrates that, even when the ductus arteriosus has closed spontaneously, prostaglandins might still be effective in relaxing the periductal aortic constriction. While this has been described previously as short-term therapy in term neonates, this is the first report of longer term treatment in a baby born weighing <1000 g.1
Coarctation of the aorta is the third most common congenital cardiac lesion in preterm infants accounting for approximately 7% of all cardiac defects.2 However, in a recently reported cohort of 197 very low birthweight babies with coarctation, only 16% underwent surgery, and the operative mortality was 21%.2 The authors speculated that the low rates of surgical intervention reflected reluctance on the part of clinicians or families for surgery because of anticipated poor outcomes. Birth weight <1000 g is independently predictive of increased mortality (adjusted OR 7.0) with few extremely preterm babies being considered for surgical intervention.2 Our case demonstrates that long-term prostaglandin therapy is potentially safe and effective in this population.
One of the postulated pathological mechanisms leading to coarctation of the aorta is that ectopic ductal tissue is present at the level of the isthmus. Ho and Anderson demonstrated a sling of ductal tissue located around the aortic isthmal orifice in 12 patients with coarctation and a discrete diaphragm of ductal tissue in six cases.3 In our case, use of prostaglandin E2 at a dose of 100 ng/kg/min successfully resulted in an improvement of the aortic narrowing with a widening of the aortic isthmal diameter and a decrease in aortic flow velocity. This supports the view that there was some relaxation of the previously constricted ductal tissue within the aorta. The benefit persisted despite a gradual decrease in the dose of prostaglandin over a 6-week period indicating gradual growth of the aortic isthmus. Stopping prostaglandin at 90 days of age led to a slight worsening of the aortic constriction, but this was not clinically significant, and the baby did not require further intervention.
To date, the potential benefit of prostaglandin therapy in coarctation with a closed ductus arteriosus has mostly been shown in larger, more mature infants but the principle is arguably even more relevant in preterm extremely low birthweight neonates in whom surgery is rarely offered. Liberman reported three term neonates with coarctation of the aorta and a closed ductus who were treated with prostaglandin E1 infusions; prostaglandin therapy was only used for short periods since each infant underwent early corrective surgery.1 Callahan et al also reported the short-term use of prostaglandin E1 in a near-term baby in whom a trial of cessation of prostaglandin therapy resulted in a worsening of the aortic constriction.4
The natural history of coarctation and the effect of prostaglandins are different in extremely preterm infants whose clinical course is complicated by the effects of surfactant-deficient lung disease, concurrent infection, myocardial insufficiency and haemodynamic instability. Differences in pharmacokinetics and pharmacodynamics associated with preterm birth are also likely to impact on the efficacy of prostaglandin therapy in this population.
Patient’s perspective.
We lived several hundred miles away from the neonatal intensive care unit where our baby was cared for having been transferred antenatally because of extremely preterm delivery. This treatment meant our baby could be transferred to a neonatal unit closer to home alongside his twin brother and meant that the family could stay together.
Learning points.
We report the first case of an extremely low birthweight baby who received long-term treatment with prostaglandin E2 for the management of aortic coarctation.
Prostaglandin E2 is effective in coarctation because it causes relaxation of the previously constricted ductal tissue within the aorta.
Long-term prostaglandin E2 therapy offers an alternative management strategy in coarctation in extremely preterm neonates when surgical intervention is not feasible or available.
The efficacy and safety profile of prostaglandin therapy in extremely immature infants differs from that in term babies.
Footnotes
Contributors: NVS was responsible for conceiving the idea behind the case report. NVS and BK collated the clinical and echocardiographic information. NVS, BK and AC were all jointly involved in writing up the case report and approving the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Parental/guardian consent obtained.
References
- 1. Liberman L, Gersony WM, Flynn PA, et al. Effectiveness of prostaglandin E1 in relieving obstruction in coarctation of the aorta without opening the ductus arteriosus. Pediatr Cardiol 2004;25:49–52. 10.1007/s00246-003-0549-5 [DOI] [PubMed] [Google Scholar]
- 2. Desai J, Aggarwal S, Lipshultz S, et al. Surgical Interventions in Infants Born Preterm with Congenital Heart Defects: An Analysis of the Kids' Inpatient Database. J Pediatr 2017;191:103–9. 10.1016/j.jpeds.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 3. Ho SY, Anderson RH. Coarctation, tubular hypoplasia, and the ductus arteriosus. Histological study of 35 specimens. Br Heart J 1979;41:268–74. 10.1136/hrt.41.3.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Callahan PF, Quivers ES, Bradley LM, et al. Echocardiographic evidence for a ductal tissue sling causing discrete coarctation of the aorta in the neonate: case report. Pediatr Cardiol 1998;19:182–4. 10.1007/s002469900276 [DOI] [PubMed] [Google Scholar]


