Abstract
Myelodysplastic syndrome (MDS) is frequently complicated by pulmonary disease. Here, we describe secondary pulmonary alveolar proteinosis (sPAP) that developed during corticosteroid therapy for organising pneumonia (OP) associated with MDS. A 75-year-old woman with MDS complained of cough for 2 weeks. Chest CT showed bilateral patchy consolidations with reversed halo sign. Bronchoalveolar lavage (BAL) examination showed remarkably increased cell density with an increased lymphocyte proportion. Abnormal radiological findings improved rapidly on administration of systemic corticosteroid under the diagnosis of OP; however, they relapsed a few weeks later. Transbronchial lung biopsy showed periodic acid-Schiff stain-positive amorphous materials. Autoantibodies against granulocyte-macrophage colony-stimulating factor (GM-CSF) in serum and BAL fluid (BALF) were both negative, while GM-CSF level in BALF was elevated. The patient was diagnosed with sPAP. When chest radiological findings show exacerbation during corticosteroid therapy for OP in a patient with MDS, physicians should consider sPAP complication as a differential diagnosis.
Keywords: interstitial lung disease, haematology (incl blood transfusion)
Background
Myelodysplastic syndrome (MDS) is associated with several pulmonary complications, including infectious disease, interstitial pneumonia, organising pneumonia (OP) and secondary pulmonary alveolar proteinosis (sPAP).1–4 Notably, OP is a relatively rare complication that generally responds to steroid therapy, whereas sPAP is an extremely rare complication that is resistant to steroid therapy and shows a poor prognosis.1 5 6 Here, we describe a patient with MDS who developed sPAP during corticosteroid therapy for OP.
Case presentation
A 75-year-old Japanese woman was diagnosed with MDS on the basis of bone marrow aspiration findings in December 2012. The WHO classification of MDS was ‘unclassifiable’. The chromosome was a normal karyotype and the International Prognostic Scoring System (IPSS) score was 0, which constitutes a low-risk category. She never smoked and had no previous lung diseases. Chest X-ray showed no abnormal shadow. The patient was followed up without any medications. In September 2013, the patient was admitted to our hospital with the chief complaint of non-productive cough for prior 2 weeks.
Investigations
The patient demonstrated normal body temperature and normopnea (18 breaths per minute), and her oxygen saturation was 97% on room air. Laboratory examinations showed increased serum level of Krebs von den Lungen-6 (KL-6), a marker of interstitial lung disease (table 1).7 Chest X-ray showed bilateral lung infiltration. Chest CT showed bilateral patchy consolidations with reversed halo sign which is defined as a central ground-glass opacity surrounded by denser consolidation in the shape of a crescent or a ring (figure 1). Bronchoalveolar lavage (BAL) examination showed a turbid appearance of BAL fluid (BALF) with a remarkably increased cell density, including an increased lymphocyte proportion (table 1). Transbronchial lung biopsy (TBLB) was performed, but the specimen size was insufficient for definitive pathological diagnosis. BALF culture was negative for pathogenic microorganisms. Bone marrow aspiration showed a slight increase in blasts, although they remained below 5%. The WHO classification of MDS at that time was ‘refractory cytopenia with multi-lineage dysplasia’.
Table 1.
Laboratory findings on admission and bronchoalveolar lavage (BAL) examination
| Laboratory findings on admission | BAL examination | |||
| Variable | Variable | First time | Second time | |
| Total leucocytes (/mL) | 500 | Appearance | Typically turbid | Milky |
| Neutrophils (%) | 44.4 | Cell count (×104/mL) | 525 | 8 |
| Lymphocytes (%) | 53.7 | Differential count (%) | ||
| Monocytes (%) | 0 | Macrophage | 10.2 | 50.2 |
| Eosinophils (%) | 0 | Lymphocytes | 86.0 | 40.0 |
| Basophils (%) | 1.9 | Neutrophils | 3.7 | 7.9 |
| Platelets (×104/μL) | 85.2 | Eosinophils | 0.1 | 1.9 |
| Haemoglobin (g/L) | 98 | CD4/CD8 ratio | Not examined | 0.47 |
| Lactate dehydrogenase (U/L) | 234 | |||
| C reactive protein (mg/dL) | 0.42 | |||
| KL-6 (U/mL) | 1069.1 | |||
| SP-D (ng/mL) | 51.9 | |||
| PR3-ANCA (U/mL) | <1.0 | |||
| MPO-ANCA (U/mL) | <1.0 | |||
| RF (IU/mL) | <10 | |||
KL-6, Krebs von den Lungen-6; MPO-ANCA, myeloperoxidase-antineutrophil cytoplasmic antibodies; PR3-ANCA, proteinase-3-antineutrophil cytoplasmic antibodies; RF, rheumatoid factor; SP-D, surfactant protein D.
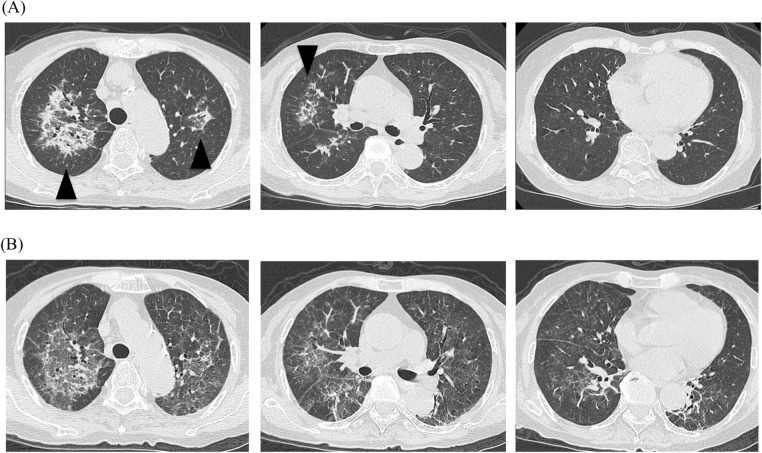
Figure 1.
Chest CT images. (A) On admission. Lung infiltrations were compatible with reversed halo sign (arrow head). (B) Three weeks after the administration of corticosteroid therapy. Pre-existing consolidations had subsided, but a new ground grass opacity was spread throughout the lung field.
Based on a clinical diagnosis of OP associated with MDS, prednisolone (PSL) 20 mg/day (0.5 mg/kg/day) was administered. The patient’s symptoms dramatically improved within a few days; bilateral infiltrations in chest X-ray also disappeared. About 2 weeks after corticosteroid therapy had been initiated, PSL was tapered to 17.5 mg/day, resulting in aggravation of chest X-ray findings within 1 week. Chest CT showed new spreading of bilateral diffuse ground grass attenuation (GGA), although the previous consolidations continued to show improvement (figure 1).
Differential diagnosis
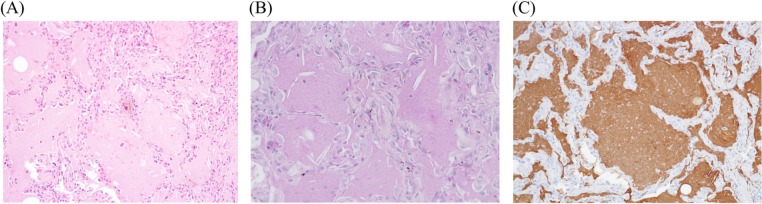
We considered the possibility of Pneumocystis jirovecii pneumonia, cytomegalovirus pneumonia or another microbial infection due to the patient’s immunocompromised status. However, serum 1, 3-β-D-glucan and whole blood cytomegalovirus antigen levels were not elevated. Sputum culture was negative for pathogenic microorganisms. Considering the relapse of OP due to high MDS disease activity, we increased PSL to 45 mg/day (1 mg/kg/day) and began to administer azacytidine to control MDS. However, chest X-ray findings continued to worsen. We performed an additional bronchoscopy analysis. BALF was milky in appearance and showed remarkable reduction in cell density, compared with the findings of the first bronchoscopy (table 1); moreover, we observed amorphous materials that were positive for periodic acid-Schiff (PAS) stain. An additional BALF culture was negative for pathogenic microorganisms. TBLB specimens revealed eosinophilic amorphous materials in the alveoli, which were positive for PAS stain and surfactant apoprotein A (figure 2). Granulocyte-macrophage colony-stimulating factor (GM-CSF) level in BALF was elevated to 10.85 pg/mL (normal range: 0–2.5 pg/mL). Autoantibodies against GM-CSF in serum and BAL fluid were both negative. Thus, the patient was diagnosed with sPAP associated with MDS.5–8
Figure 2.
Transbronchial lung biopsy (TBLB) specimen obtained during the second bronchofiberscopy. TBLB specimen stained with haematoxylin and eosin showed eosinophilic amorphous materials in the alveoli (A), which were positive for periodic acid-Schiff stain (B) and surfactant apoprotein A (C).
Outcome and follow-up
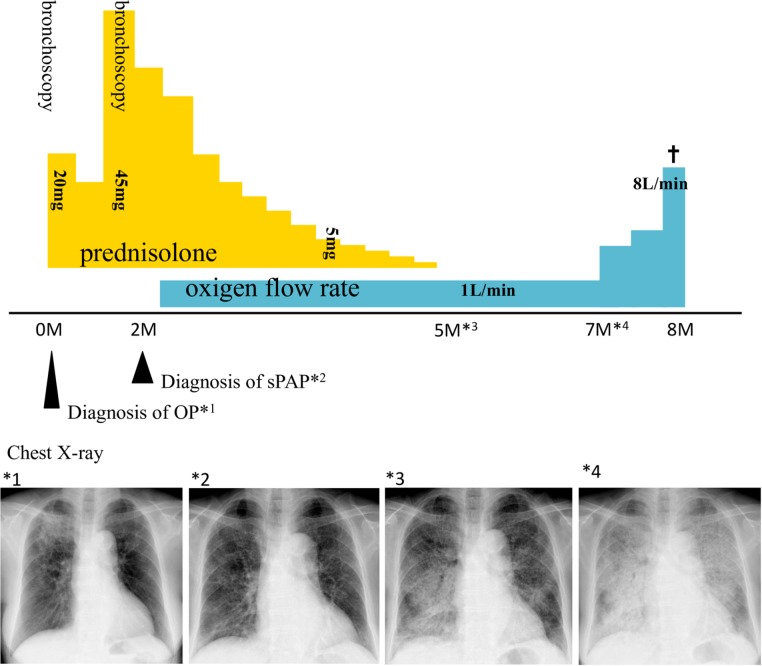
We performed an additional bone marrow aspiration procedure. The specimen showed an increased proportion of blasts, such that they exceeded 5% of the total cell number. The WHO classification of MDS at that time was ‘refractory anaemia with excess blasts-1’. The chromosome remained a normal karyotype and the IPSS score was 0.5, which comprises the intermediate-1 category of the low-risk group. After diagnosis of sPAP, we tapered PSL and discontinued it at 5 months after initiation. However, lung infiltration continued to worsen gradually. The patient died of respiratory failure 8 months after the initiation of corticosteroid therapy (figure 3).
Figure 3.
Clinical course after admission. On admission, there were consolidations in the upper lung fields (*1). After the initiation of prednisolone (PSL), these consolidations subsided, but new infiltrations spread throughout the lung field (*2). After the diagnosis of secondary pulmonary alveolar proteinosis, PSL was tapered and discontinued (*3); the infiltration then gradually worsened (*4). The patient died of respiratory failure 8 months after the initiation of PSL.
Discussion
In the present case, a 75-year-old woman presented with patchy lung consolidations with reversed halo sign on chest CT and was clinically diagnosed with OP associated with MDS. The consolidations improved after the administration of corticosteroid therapy, new bilateral diffuse GGA appeared. We diagnosed the GGA as sPAP associated with MDS based on the presence of PAS stain-positive amorphous materials in BALF and a TBLB specimen. To the best of our knowledge, this is the first case in which sPAP developed during corticosteroid therapy for OP associated with MDS.
Although we could not obtain a sufficient amount of lung tissue in the first bronchoscopy procedure, we made a clinical diagnosis of OP because of the increased lymphocyte proportion of in the initial BALF, as well as on the basis of radiological features on chest CT (eg, bilateral patchy consolidations with reversed halo sign). Notably, patchy consolidations might be regarded as unusual radiological findings preceding sPAP. However, the shadows rapidly improved after administration of corticosteroid and continued to show positive change, while new bilateral GGA emerged. Steroid therapy is reportedly ineffective for PAP; thus, if the patchy consolidations had been caused by sPAP, they would have continued to worsen regardless of corticosteroid therapy.6 9 In addition, the second acquisition of BALF revealed completely different cell populations, relative to those observed in the first acquisition of BALF. These findings suggested that the patchy consolidations and bilateral GGA were each caused by different underlying pathogenesis. Therefore, the patchy consolidations may have constituted OP.
Although the precise number is unknown, the incidence of OP associated with MDS is estimated to be less than 3.4 per million; this is also the reported incidence of OP with haematologic malignancies.1
In addition, PAP is a rare pulmonary disease, and Inoue et al reported that the overall incidence of PAP is 8.7 per million; the incidence of sPAP is thought to represent approximately 10% of all PAP cases.5 Ishii et al reported that haematological disorders are the most common conditions underlying sPAP, and 74% of affected patients have MDS.6 Therefore, the incidence of sPAP associated with MDS may be less than 1 per million. Accordingly, this case represents an extremely rare instance of MDS complicated by both OP and sPAP.
The clinical course in this case suggests that sPAP developed rapidly after the administration of corticosteroid therapy for OP. Notably, OP in patients with MDS has been reported to show good response to corticosteroid therapy, which can be attributed to favourable respiratory-related prognosis.1 Conversely, in patients with sPAP associated with MDS, corticosteroid therapy has been identified as a risk factor for poor prognosis.6 Corticosteroid inhibits the phagocytic activity of macrophages, which is reportedly a major factor in the pathogenesis of PAP.10 In the present case, sPAP might have developed rapidly due to the inhibition of phagocytic activity in alveolar macrophages during corticosteroid therapy for OP. Further investigation is needed to determine whether corticosteroid therapy could induce sPAP in patients with MDS.
Ishii et al reported that treatment of sPAP associated with MDS should be designed to restore haematopoietic function in MDS.6 However, in elderly patients, such as the patient in the present case, it is often difficult to treat MDS directly. In such situations, even those in which sPAP develops (regardless of steroid use), there is no established treatment other than symptomatic care. The indication of corticosteroid therapy, which may be a risk factor for poor sPAP prognosis, should be carefully considered with respect to pulmonary lesions in MDS patients.6
Here, we report the first case in which sPAP developed during corticosteroid therapy for OP associated with MDS. When chest radiological findings show exacerbation during corticosteroid therapy for OP in a patient with MDS, physicians should consider sPAP complication as a differential diagnosis.
Patient’s perspective.
By reporting on my mother’s disease progression in this way, she will be happy if it helps in the development of treatment for similar diseases and future medicine.
Learning points.
We report the first case in which secondary pulmonary alveolar proteinosis (sPAP) developed during corticosteroid therapy for organising pneumonia (OP) associated with myelodysplastic syndrome (MDS).
When chest radiological findings show exacerbation during corticosteroid therapy for OP in a patient with MDS, physicians should consider sPAP complication as a differential diagnosis.
Further investigation is needed to determine whether corticosteroid therapy could induce sPAP in patients with MDS.
Acknowledgments
The authors are grateful to Dr A Iwasaki for diagnosis and treatment of MDS of the present case.
Footnotes
Contributors: DI treated the patient consulting to HI and MF. DI wrote the manuscript. SM and MF corrected the manuscript. HI advised on the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Next of kin consent obtained.
References
- 1. Daniels CE, Myers JL, Utz JP, et al. Organizing pneumonia in patients with hematologic malignancies: a steroid-responsive lesion. Respir Med 2007;101:162–8. 10.1016/j.rmed.2006.03.035 [DOI] [PubMed] [Google Scholar]
- 2. Ohnishi T, Yamada G, Shijubo N, et al. Secondary pulmonary alveolar proteinosis associated with myelodysplastic syndrome. Intern Med 2003;42:187–90. 10.2169/internalmedicine.42.187 [DOI] [PubMed] [Google Scholar]
- 3. Pomeroy C, Oken MM, Rydell RE, et al. Infection in the myelodysplastic syndromes. Am J Med 1991;90:338–44. 10.1016/0002-9343(91)90574-H [DOI] [PubMed] [Google Scholar]
- 4. Tamaki Y, Seyama K, Takahashi H, et al. Progressive interstitial pneumonia associated with myelodysplastic syndrome: implication of superoxide hyperproduction by neutrophils. Respirology 1997;2:295–8. 10.1111/j.1440-1843.1997.tb00092.x [DOI] [PubMed] [Google Scholar]
- 5. Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med 2008;177:752–62. 10.1164/rccm.200708-1271OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ishii H, Seymour JF, Tazawa R, et al. Secondary pulmonary alveolar proteinosis complicating myelodysplastic syndrome results in worsening of prognosis: a retrospective cohort study in Japan. BMC Pulm Med 2014;14:37 10.1186/1471-2466-14-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kohno N, Kyoizumi S, Awaya Y, et al. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6. Chest 1989;96:68–73. 10.1378/chest.96.1.68 [DOI] [PubMed] [Google Scholar]
- 8. Uchida K, Nakata K, Carey B, et al. Standardized serum GM-CSF autoantibody testing for the routine clinical diagnosis of autoimmune pulmonary alveolar proteinosis. J Immunol Methods 2014;402:57–70. 10.1016/j.jim.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akasaka K, Tanaka T, Kitamura N, et al. Outcome of corticosteroid administration in autoimmune pulmonary alveolar proteinosis: a retrospective cohort study. BMC Pulm Med 2015;15:88 10.1186/s12890-015-0085-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boumpas DT, Chrousos GP, Wilder RL, et al. Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Ann Intern Med 1993;119:1198–208. 10.7326/0003-4819-119-12-199312150-00007 [DOI] [PubMed] [Google Scholar]