Abstract
Catastrophic antiphospholipid syndrome (CAPS) is a rare and potentially life-threatening variant of the antiphospholipid syndrome which is characterised by multiple small vessel thrombosis which can lead to multiorgan failure. CAPS is a clinical emergency which all clinicians need to be aware of because early diagnosis and treatment may improve maternal and fetal outcome. Here, we report a case of CAPS in pregnancy in a 31-year-old female patient who presented at 28 weeks of gestation. A literature review of CAPS in pregnancy and the puerperium is also included.
Keywords: Rheumatology, Pregnancy, Drugs And Medicines
Background
Antiphospholipid syndrome (APS) is a state of hypercoagulation that is characterised by recurrent vascular thrombosis, thrombocytopaenia and recurrent fetal loss.1 APS is an autoantibody-mediated acquired thrombophilia characterised by the presence of three main antiphospholipid antibodies namely; anticardiolipin, lupus anticoagulant and antibeta2-glycoprotein.2
Catastrophic antiphospholipid syndrome (CAPS) was first described in 1992 as a potential life-threatening variant of the APS, characterised by multiple small vessel thrombosis leading to multiorgan failure.3 CAPS is rare and develops in about 1% of cases of APS.4 CAPS is defined as multiorgan thrombosis, affecting a minimum of three different organs, requiring histopathological confirmation of small vessel occlusion in at least one organ or tissue and presence of antiphospholipid antibodies on two separate occasions, 6 weeks apart.4 5
Here, we report a case of a 31-year-old female patient who developed CAPS during pregnancy.
Case presentation
A 31-year-old woman, gravida 3 para 0+2 presented at 28+5 weeks gestation complaining of epigastric pain, worsening bilateral limb oedema and blurred vision. Physical examination revealed high blood pressure of 180/114 mm Hg, bilateral lower limb oedema and brisk reflexes 4+. Blood investigations showed low platelets (67×109 L) and high uric acid of 442 µmol/L (normal range 142.8–339.2 µmol/L). The 24-hour urine protein test was more than 6000 mg (normal range 1–150 mg/24 hours) in keeping with severe pre-eclampsia. The patient was started on intravenous labetalol, and magnesium sulfate and was given two doses of 12 mg of dexamethasone intramuscularly. The baby was delivered via an emergency caesarean section at 28+6 weeks gestation and transferred to the neonatal paediatric intensive care unit (NPICU). The patient received one dose of co-amoxiclav intravenous and was started on 40 mg/day of enoxaparin subcutaneously.
Day 3 postpartum, the patient developed severely reduced visual acuity, abdominal pain, headaches and started spiking a temperature up to 39.4°C. On examination, the patient was found to have abdominal tenderness in the right upper quadrant, and Murphy’s sign was positive. Review by ophthalmologist showed bilateral exudative retinal detachment. Blood tests showed a white cell count of 13.74×109/L, platelet count dropped to less than 50×109 L, and haemoglobin level dropped to 8.3 g/dL. Blood picture showed normochromic, anisoctyic erythrocytes. Her renal function also deteriorated (estimated glomerular filtration rate dropping from 107 to 35 mL/min/1.73 m2), while the activated partial thromboplastin time (APTT) ratio increased to 1.61 and prothrombin time to 14.7 s. Ultrasound abdomen showed acalculous cholecystitis, and CT scan of the abdomen and pelvis showed mild ureterohydronephrosis with enhancing ureteric walls suspicious of bilateral pyelonephritis, bilateral bibasal consolidations and suspected liver abscesses. MR liver showed very small early liver abscesses which were not amenable to drainage. A transthoracic cardiogram revealed a poor left ventricular ejection fraction of 43% and a pericardial effusion, while cardiac MR showed myopericarditis and peripartum cardiomyopathy with global left ventricular wall hypokinesia (figure 1). MR head revealed multiple ischaemic foci in both cerebral hemispheres (figure 2). Urine and blood cultures were negative, but both were taken after the administration of antibiotics.
Figure 1.
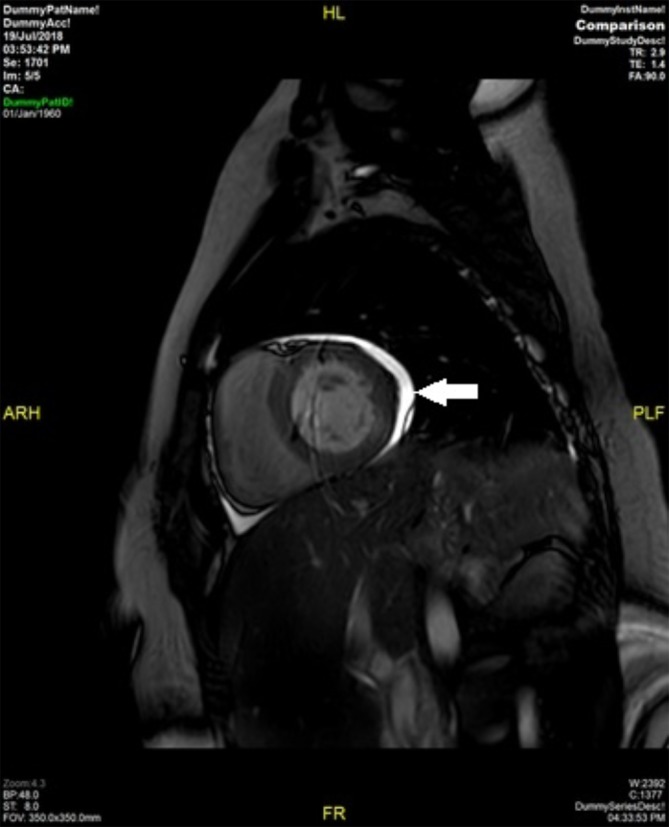
MR cardiac—T2 short axis view through the heart showing small pericardial effusion (arrow).
Figure 2.
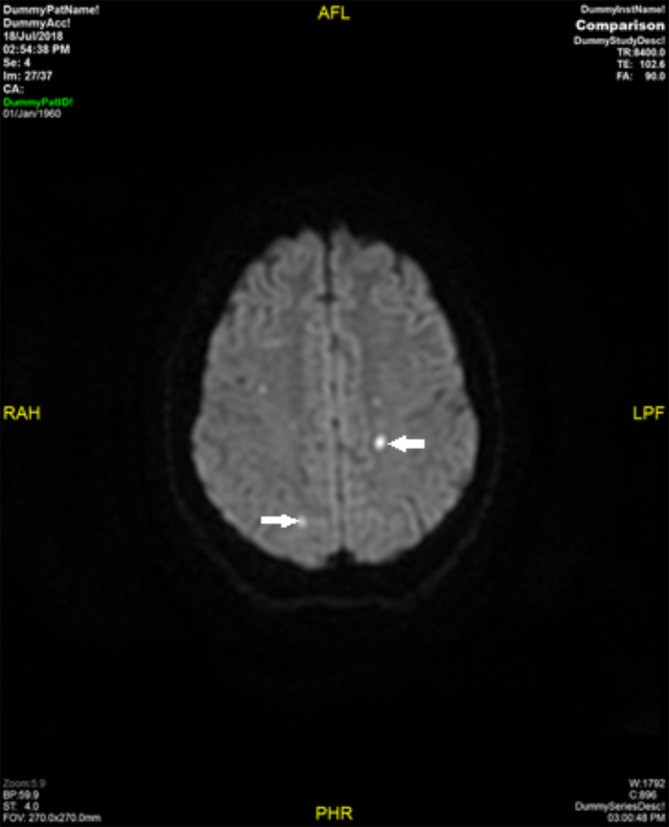
MR brain—diffusion-weighted axial image showing foci of restricted diffusion in periventricular white matter consistent with acute ischaemia (arrows).
Broad spectrum antibiotics (intravenous piperacillin/tazobactam 4.5 g three times per day and levofloaxcin 500 mg/day orally) were started to cover pulmonary and abdominal sepsis, enoxaparin dose was reduced from 40 mg to 20 mg/day in view of the low platelets, and the patient was transferred to intensive care for closer monitoring. After 4 days, headaches and abdominal pain improved, fever settled and the blood pressure was controlled by carvedilol 3.125 mg two times per day and nifedipine 20 mg three times per day.
After 7 days of broad-spectrum antibiotics, the patient started spiking a temperature again and reported left-sided pleuritic chest pain. On examination, the patient was found to have a temperature of 38.8°C, pulse rate of 115 beats/min and oxygen saturation was 93% on room air. No infectious source was identified. CT pulmonary angiogram revealed left lower lobar pulmonary embolism and intrasplenic vessel thrombosis. At this point, the patient was found to have positive anticardiolipin antibodies, positive antibeta2-glycoprotein antibodies and positive lupus anticoagulant. Histology of the placenta showed placental infraction. Antinuclear antibody, extractable nuclear antigen antibody and antidouble-stranded DNA antibody were negative.
A diagnosis of CAPS was made on the basis of positive serology, multiorgan thrombosis (lung, brain, spleen) and histologically proven placental infarction.
Treatment
In addition to broad-spectrum antibiotics, the patient was given three pulses of 1 g intravenous methylprednisolone followed by prednisolone 60 mg/day, 4 days of 0.5 g/kg/day of intravenous IGs, 1 mg/kg two times per day enoxaparin and aspirin 75 mg/day. Clinical improvement was noted within a few days. The patient was initiated on long-term warfarin. Prednisolone dose was tapered slowly over 4 months.
Outcome and follow-up
The patient was discharged home after 4 weeks. Repeat antiphospholipid antibodies 6 weeks later remained positive. The platelet count and renal function improved to normal levels. Her visual acuity also improved significantly, and funduscopy was essentially normal except for a few areas of retinal pigmentation. An echocardiogram 8 months later showed improved left ventricular systolic function, and the ejection fraction was 55%. The baby was discharged from NPICU after 10 weeks with normal growth and development.
Discussion
CAPS is a life-threatening condition which is rarely seen in pregnancy making early diagnosis difficult. It presents with very non-specific symptoms which may mimic many other conditions such as sepsis, infective endocarditis, vasculitis and other autoinflammatory conditions. Timely diagnosis and aggressive management is critical for a good outcome. Here, we report a 31-year-old previously healthy woman who developed multiple clinical manifestations at 28 weeks of gestation which initially were attributed to pre-eclampsia and sepsis. Partial response to antibiotics and development of multiorgan thrombosis eventually led to the diagnosis of CAPS.
Gόmez-Puerta et al reported in 2007 that CAPS during pregnancy or puerperium represents only 6% of all cases of CAPS.6 A PubMed literature search was carried out using the search term ‘CAPS’, ‘CAPS in pregnancy’ ‘CAPS and puerperium’, and 35 different cases were identified from 1994 to 2018 (table 1). Table 1 summarises the clinical manifestations, treatment given and maternal and fetal outcomes of each case. The combination of treatment given is further summarised in table 2.
Table 1.
Thirty-five cases of CAPS in pregnancy and puerperium
| Author (reference no.) | Maternal age | Time of onset | CAPS features | Treatment received | Maternal outcome | Fetal outcome |
| Khizroeva et al 14 | n/a | 28 weeks of gestation | Multiorgan failure | n/a | n/a | n/a |
| Hanouna et al
15
(Case 1) |
30 years | 15th day of puerperium | Renal, hepatic, cutaneous, haemolytic anaemia, placenta | Heparin, aspirin, glucocorticoids | n/a | Healthy child |
| Hanouna et al
15
(Case 2) |
32 years | Eighth day of puerperium | Cardiac, renal, hepatic, cutaneous, haemolytic anaemia, thrombocytopaenia | Heparin, aspirin, glucocorticoids, IVIG | n/a | Healthy twins |
| Hanouna et al
15
(Case 3) |
26 years | 25 weeks of gestation | Cardiac, neurological, renal, cutaneous, haemolytic anaemia | Heparin, glucocorticoids, IVIG, plasma exchange, dialysis | n/a | Fetal death |
| Hanouna et al
15
(Case 4) |
31 years | Third day of puerperium | Cardiac, renal, splenic, cutaneous, hepatic, thrombocytopaenia, haemolytic anaemia | Heparin, aspirin, glucocorticoids, IVIG, dialysis | n/a | Fetal death |
| Hanouna et al
15
(Case 5) |
37 years | Fifth day of puerperium | Neurological, cutaneous, hepatic, thrombocytopaenia, haemolytic anaemia, placenta | Heparin | Venous thrombosis at 2 months, PE at 3 months | Fetal death |
| Hanouna et al
15
(Case 6) |
33 years | On the day of the delivery at 36.5 weeks gestation | Adrenal, cutaneous, hepatic, thrombocytopaenia, haemolytic anaemia | Heparin, glucocorticoids | Adrenal insufficiency | Healthy child |
| Hanouna et al
15
(Case 7) |
32 years | Fourth week of puerperium | Cardiac, neurological, pulmonary, renal, hepatic, pancreatic, splenic, ocular, thrombocytopaenia | Heparin, glucocorticoids, IVIG, plasma exchange, dialysis | Mild retinal sequelae | Fetal death |
| Hanouna et al
15
(Case 8) |
29 years | 15th day of puerperium | Cardiac, neurological, renal, cutaneous, hepatic, pancreatic, gastric, ocular, thrombocytopaenia, haemolytic anaemia | Heparin, glucocorticoids, plasma exchange, dialysis | Renal insufficiency, stroke at 4 years, sudden death at 6 years | Fetal death |
| Hanouna et al
15
(Case 9) |
32 years | Second day of puerperium | Adrenal, renal, hepatic, thrombocytopaenia | Heparin, aspirin, glucocorticoids | Adrenal insufficiency | Healthy child |
| Hanouna et al
15
(Case 10) |
36 years | Third day of puerperium | Cardiac, neurological, renal, hepatic, pancreatic, cutaneous, thrombocytopaenia, haemolytic anaemia | Heparin, glucocorticoids | n/a | Healthy child |
| Hanouna et al
15
(Case 11) |
32 years | On the day of the delivery at 17 weeks gestation | Cutaneous, hepatic, renal, adrenal, thrombocytopaenia, gallbladder | Heparin, glucocorticoids, plasma exchange, dialysis | Renal insufficiency with proteinuria | Fetal death at 17 weeks |
| Hanouna et al
15
(Case 12) |
27 years | On the day of the delivery at 13 weeks gestation | Cardiac, cutaneous, hepatic, placenta, thrombocytopaenia | Heparin, glucocorticoids, IVIG | n/a | Fetal death at 13 weeks |
| Hanouna et al
15
(Case 13) |
23 years | 31 weeks of gestation | Cardiac, renal, cutaneous, thrombocytopaenia, haemolytic anaemia | Heparin, aspirin, glucocorticoids, plasma exchange | Sudden death at 2.5 years | Child with developmental delay |
| Derks et al
16
(Case 1) |
32 years | n/a | Multiple infarcts in liver and placenta | Intensive medical treatment including anticoagulation | Died of massive PE | n/a |
| Derks et al
16
(Case 2) |
27 years | n/a | Thrombocytopaenia, Disturbances in hepatic function, epigastric pain | Glucocorticoids | n/a | Fetal death |
| Derks et al
16
(Case 3) |
36 years | n/a | Hepatic infarcts, petechiae | Glucocorticoids, IVIG, plasmapheresis | n/a | Healthy Child |
| Marson et al 17 | 33 years | 23 weeks of gestation | HELLP, thrombocytopaenia, anaemia, acalculous cholecystitis, Cutaneous | Therapeutic plasma exchange | Recovery | Intrauterine death at 23 weeks |
| Bendon et al 18 | 22 years | 30 weeks of gestation | Placental infarctions myocardium, renal, gastrointestinal and myometrium TMA | Death | Intrauterine fetal death | |
| Hochfeld et al 19 | 37 years | Second day after fetal death | Renal failure, cerebral, cardiac, pulmonary, splenic, and adrenal infarcts, cerebral haemorrhage | Glucocorticoids, cyclophosphamide, plasma exchange | Death | Intrauterine fetal death |
| Kupferminic et al 20 | 17 years | Fifth day of puerperium | HELLP, ARDS, placental infarcts, renal failure | Glucocorticoids, plasma exchange, dialysis | Recovery | Prematurity |
| Kitchens21 | 38 years | 38 weeks of gestation | HELLP, portal vein, inferior vena cava, mesenteric vein thrombosis | Anticoagulation | Recovery | |
| Wislowska et al 22 | 26 years | 25 weeks of gestation | ARDs, encephalopathy, nephritis, Skin ulcers | LMWH, glucocorticoids, cyclophosphamide | Recovery | Miscarriage |
| Sinha et al 23 | 22 years | 25 weeks of gestation | HELLP, placental infarcts, cerebral infarcts, Bone marrow necrosis | Glucocorticoids, plasma exchange, IVIG | Death | Death |
| Asherson et al 24 | 22 years | 20 weeks of gestation | HELLP, ARDS, cerebral infarcts | Glucocorticoids, intravenous heparin, cyclophosphamide | Recovery | Death |
| Asherson et al 24 | 27 years | Postfetal loss | PE, digital necrosis, hepatic, renal, intestinal, mesenteric thrombosis | Glucocorticoids, anticoagulation | Death | Death |
| Ortiz at al25 | 32 years | Second day of puerperium | Renal, multiple cerebral infarcts | Glucocorticoids, FFP, LMWH | Recovery | n/a |
| Koenig et al 26 | 19 years | 17 weeks of gestation | HELLP, hepatic infarctions, bone necrosis | LMWH, FFP | Recovery | Death |
| Coward et al 27 | 30 years | Third week of puerperium | TIA, status epilepticus, renal failure, adrenal haemorrhage | Anticoagulants, dialysis | Death | Healthy child |
| Weiser M6 | 33 years | Fifth day of puerperium | HELLP, ARDS, renal failure, cerebral infarctions and haemorrhage | Glucocorticoids, IVIG, anticoagulation, dialysis | Death | Healthy child |
| Gomes-Puerta et al 6 (case 1) | 29 years | 28 weeks of gestation | HELLP, bone marrow hypoplasia, renal failure, DVT, respiratory failure, livedo reticularis | Glucocorticoids, LMWH | Death | Prematurity |
| Gomes-Puerta et al 6 (case 2) | 26 years | Third day of puerperium | DVT, PE, respiratory failure | LMWH, glucocorticoids, IVIG | Recovery | Healthy child |
| Gomes-Puerta et al 6 (Case 3) | 28 years | Sixth day of puerperium | Placental infarctions, Respiratory failure, Renal failure, Encephalopathy | LMWH, plasma exchange | Recovery | Healthy child |
| Elchalal et al 28 | 37 years | 16 weeks of gestation | Thrombocytopaenia, Bilateral pleural effusions | LMWH, IVIG, Glucocorticoids | Recovery | Fetal death |
| Ciolkiewicz et al 7 | 24 years | Postfetal loss | PE, Myocardial infarction, multiorgan failure | n/a | Death | Fetal death |
ARDS, acute respiratory distress syndrome; CAPS, catastrophic antiphospholipid syndrome; DVT, deep vein thrombosis; FFP, fresh frozen plasma; HELLP, haemolysis, elevated liver enzymes, low platelet count; IVIG, intravenous immunoglobulin; LMWH, low molecular weight heparin; PE, pulmonary embolism; TMA, thrombotic microangiopathy; n/a, not available.
Table 2.
Treatment combinations given to patients with CAPS
| Treatment | Number of cases of CAPS |
| Individual treatment | |
| Anticoagulation | 5 |
| Glucocorticoids | 1 |
| Plasma exchange | 1 |
| Treatment combinations | |
| Anticoagulation and glucocorticoids | 7 |
| Anticoagulation and plasma exchange | 1 |
| Anticoagulation, glucocorticoids and IVIG | 5 |
| Anticoagulation, glucocorticoids and plasma exchange | 2 |
| Anticoagulation, glucocorticoids and cyclophosphamide | 2 |
| Glucocorticoids, plasma exchange and cyclophosphamide | 1 |
| Glucocorticoids, IVIG and plasma exchange | 3 |
| Anticoagulation, glucocorticoids, IVIG and plasma exchange | 3 |
CAPS, catastrophic antiphospholipid syndrome; IVIG, intravenous immunoglobulin.
CAPS in pregnancy presents a complex clinical scenario both in terms of diagnosis as well as treatment. The acute manifestations of CAPS are usually the result of an acute thrombotic microangiopathy. The differential diagnosis of CAPS in pregnancy and puerperium includes: disseminated intravascular coagulation (DIC), thrombotic thrombocytopaenic purpura (TTP), haemolytic-uraemic syndrome (HUS), heparin-induced thrombocytopaenia (HIT)7 and HELLP (haemolysis, elevated liver enzymes, low platelet count) syndrome, all of which can form part of the manifestations of CAPS. TTP and HUS are both characterised by thrombocytopaenia, macroangiopathic haemolytic anaemia and ischaemic organ damage. Neurological manifestations and fever dominate the clinical picture in TTP, while patients with HUS tend to suffer more from progressive renal disease.8 Nevertheless, the differentiation between CAPS and TTP/HUS might be difficult. The thrombocytopaenia and schistocytosis tend to be marked in TTP/HUS and mild in CAPS. In TTP/HUS, APTT is normal. On the other hand, elevated APTT, the presence of antiphospholipid antibodies and lupus anticoagulant support CAPS.8 DIC is characterised by thrombocytopaenia, prolonged clotting times, reduced plasma fibrinogen levels and elevated fibrin degradation products. It is also known that DIC can complicate CAPS in one-third of patients.9 HIT is another differential diagnosis of CAPS which is caused by autoantibodies against platelet factor 4-heparin complex.10 HIT is characterised by thrombocytopaenia and vascular thrombosis, and it usually follows the administration of unfractionated heparin and less commonly the administration of low molecular weight heparin.10
The following therapeutic strategy of CAPS during pregnancy and puerperium is proposed. First, it is essential to prevent and treat any triggering factors such as infection. Second, fetal maturation should be evaluated, and the fetus delivered once fetal pulmonary maturation has been optimised. Third, a combination of anticoagulation and immunosuppression is essential despite the increased risk of bleeding in view of low platelet count, elevated APTT ratio and potentially ongoing sepsis. CAPS is a highly thrombotic state despite prolonged bleeding time and low platelets. In those cases with HELLP syndrome or other microangiopathic features, plasma exchange is strongly indicated. High doses of glucorticoids and intravenous immunoglobulin (IVIG) have been shown to improve survival.6 11 12 In fact, triple therapy consisting of a combination of anticoagulation, high-dose glucocorticoids, plasma exchange and/or IVIG has been proposed as the gold standard of care.11 Our patient made an immediate recovery after the administration of anticoagulation, high-dose glucocorticoid therapy and IVIG. Recently, new therapeutic modalities have emerged for the treatment of refractory CAPS including rituximab, defibrotide and eculizumab.12
In conclusion, CAPS is a rare life-threatening disease which requires prompt diagnosis, a multidisciplinary approach and immediate aggressive treatment to avoid irreversible complications and decrease mortality. CAPS has a high mortality rate of up to 30% with the main causes of death being reported to be infections, stroke, cardiac failure and multiorgan failure.13 CAPS is a clinical emergency which all clinicians need to be aware of because timely diagnosis and treatment may improve both the maternal and fetal outcome.
Patient ’s perspective.
I would like to ensure that all the medical professionals are aware of this life-threatening syndrome.
Learning points.
Catastrophic antiphospholipid syndrome (CAPS) is a rare but potentially life-threatening form of APS defined as multiorgan thrombosis affecting at least three organs and histological confirmation of small vessel occlusion in at least one organ or tissue.
CAPS presents with non-specific symptoms which may mimic other conditions such as sepsis, infective endocarditis, vasculitis and other autoinflammatory conditions.
Triple therapy consisting of anticoagulation, high-dose intravenous glucocorticoids, plasma exchange and/or intravenous IG has been associated with better outcomes.
Our patient made a very good recovery thanks to the teamwork and contribution of 14 different consultants in different specialities.
Increased awareness of CAPS is essential for early diagnosis and treatment to improve maternal and fetal outcome.
Footnotes
Contributors: MC wrote this case report and literature review. WSB helped in the write-up of the case and preparation of images. CM did the corrections of the paper and helped in the discussion. JT was involved in the corrections of this case report and the final approval.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Garcia D, Erkan D. Diagnosis and Management of the Antiphospholipid Syndrome. N Engl J Med Overseas Ed 2018;378:2010–21. 10.1056/NEJMra1705454 [DOI] [PubMed] [Google Scholar]
- 2. Linnemann B. Antiphospholipid syndrome - an update. Vasa 2018;47:451–64. 10.1024/0301-1526/a000723 [DOI] [PubMed] [Google Scholar]
- 3. Asherson RA. The catastrophic antiphospholipid syndrome. J Rheumatol 1992;19:508–12. [PubMed] [Google Scholar]
- 4. Cervera R, Piette JC, Font J, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum 2002;46:1019–27. 10.1002/art.10187 [DOI] [PubMed] [Google Scholar]
- 5. Hoayek JG, Moussa HN, Rehman HA, et al. Catastrophic antiphospholipid syndrome in pregnancy, a diagnosis that should not be missed. J Matern Fetal Neonatal Med 2016;29:3950–5. 10.3109/14767058.2016.1160047 [DOI] [PubMed] [Google Scholar]
- 6. Gómez-Puerta JA, Cervera R, Espinosa G, et al. Catastrophic antiphospholipid syndrome during pregnancy and puerperium: maternal and fetal characteristics of 15 cases. Ann Rheum Dis 2007;66:740–6. 10.1136/ard.2006.061671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodríguez-Pintó I, Espinosa G, Erkan D, et al. The effect of triple therapy on the mortality of catastrophic anti-phospholipid syndrome patients. Rheumatology 2018:1264–70. 10.1093/rheumatology/key082 [DOI] [PubMed] [Google Scholar]
- 8. Nayer A, Ortega LM. Catastrophic antiphospholipid syndrome: a clinical review. J Nephropathol 2014;3:9–17. 10.12860/jnp.2014.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asherson RA, Espinosa G, Cervera R, et al. Catastrophic antiphospholipid syndrome: proposed guidelines for diagnosis and treatment. J Clin Rheumatol 2002;8:157–65. [PubMed] [Google Scholar]
- 10. Arepally GM, Ortel TL. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med 2006;355:809–17. 10.1056/NEJMcp052967 [DOI] [PubMed] [Google Scholar]
- 11. Asherson RA, Cervera R, de Groot PG, et al. Catastrophic Antiphospholipid Syndrome Registry Project Group. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus 2003;12:530–4. [DOI] [PubMed] [Google Scholar]
- 12. Espinosa G, Berman H, Cervera R. Management of refractory cases of catastrophic antiphospholipid syndrome. Autoimmun Rev 2011;10:664–8. 10.1016/j.autrev.2011.04.031 [DOI] [PubMed] [Google Scholar]
- 13. Cervera R, Rodríguez-Pintó I, Espinosa G. The diagnosis and clinical management of the catastrophic antiphospholipid syndrome: A comprehensive review. J Autoimmun 2018;92:1–11. 10.1016/j.jaut.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 14. Khizroeva J, Bitsadze V, Makatsariya A. Catastrophic antiphospholipid syndrome and pregnancy. Clinical report. J Matern Fetal Neonatal Med 2018;8:1–4. [DOI] [PubMed] [Google Scholar]
- 15. Hanouna G, Morel N, Le Thi Huong D, et al. Catastrophic antiphospholipid syndrome and pregnancy: an experience of 13 cases. Rheumatology 2013;52:1635–41. 10.1093/rheumatology/ket167 [DOI] [PubMed] [Google Scholar]
- 16. Derks M, Oudijk MA, van der Made F, et al. [Catastrophic antiphospholipid syndrome during pregnancy]. Ned Tijdschr Geneeskd 2011;155:A3263. [PubMed] [Google Scholar]
- 17. Marson P, Bagatella P, Bortolati M, et al. Plasma exchange for the management of the catastrophic antiphospholipid syndrome: importance of the type of fluid replacement. J Intern Med 2008;264:201–3. 10.1111/j.1365-2796.2008.01942.x [DOI] [PubMed] [Google Scholar]
- 18. Bendon RW, Wilson J, Getahun B, et al. A maternal death due to thrombotic disease associated with anticardiolipin antibody. Arch Pathol Lab Med 1987;111:370–2. [PubMed] [Google Scholar]
- 19. Hochfeld M, Druzin ML, Maia D, et al. Pregnancy complicated by primary antiphospholipid antibody syndrome. Obstet Gynecol 1994;83:804–5. [PubMed] [Google Scholar]
- 20. Kupferminc MJ, Lee MJ, Green D, et al. Severe postpartum pulmonary, cardiac, and renal syndrome associated with antiphospholipid antibodies. Obstet Gynecol 1994;83:806–17. [PubMed] [Google Scholar]
- 21. Kitchens CS. Thrombotic storm: when thrombosis begets thrombosis. Am J Med 1998;104:381–5. 10.1016/S0002-9343(98)00061-8 [DOI] [PubMed] [Google Scholar]
- 22. Wislowska M. Successful treatment of catastrophic antiphospholipid syndrome in a pregnant woman. Clin Exp Rheumatol 1999;17:261. [PubMed] [Google Scholar]
- 23. Sinha J, Chowdhry I, Sedan S, et al. Bone marrow necrosis and refractory HELLP syndrome in a patient with catastrophic antiphospholipid antibody syndrome. J Rheumatol 2002;29:195–7. [PubMed] [Google Scholar]
- 24. Asherson RA, Cervera R, Piette JC, et al. Catastrophic antiphospholipid syndrome: clues to the pathogenesis from a series of 80 patients. Medicine 2001;80:355–77. 10.1097/00005792-200111000-00002 [DOI] [PubMed] [Google Scholar]
- 25. Ortiz P, Castro A, Vallés M, et al. [Catastrophic antiphospholipid syndrome in the immediate puerperium]. Nefrologia 2003;23:459–62. [PubMed] [Google Scholar]
- 26. Koenig M, Roy M, Baccot S, et al. Thrombotic microangiopathy with liver, gut, and bone infarction (catastrophic antiphospholipid syndrome) associated with HELLP syndrome. Clin Rheumatol 2005;24:166–8. Coward LJ, Kullmann DM, Hirsch NP, Howard RS, Lucas SB. Catastrophic primary antiphospholipid syndrome presenting as status epilepticus. J Neurol Neurosurg Psychiatry 2005;76:1607–8.16227567 [Google Scholar]
- 27. Elchalal U, Gabbay E, Nadjari M, et al. Catastrophic antiphospholipid syndrome in the second trimester of pregnancy. Isr Med Assoc J 2006;8:856–7. [PubMed] [Google Scholar]
- 28. Ciołkiewicz M, Domysławska I, Kita K, et al. [Secondary lethal catastrophic antiphospholipid syndrome in 24-years old female patient with overlap syndrome (systemic sclerosis and systemic lupus erythematosus)]. Pol Merkur Lekarski 2006;20:337–40. [PubMed] [Google Scholar]


