Abstract
Background
This retrospective, historically controlled investigative study examined the benefit of a nutritional support pathway that included nutritional education before the start of conditioning and emphasized oral nutrition in response to nutrition-related adverse events in patients undergoing hematopoietic stem cell transplantation (HSCT).
Material/Methods
Participants were patients undergoing allogeneic HSCT; 46 were in the control group (i.e., did not follow our nutritional pathway) and 36 were in the group that underwent nutritional intervention (enhanced nutrition group). We compared the following parameters between groups from the day before the start of conditioning to the day after completion of parenteral nutrition (PN): percent loss of body weight (%LBW), percent loss of skeletal muscle mass (%LSMM), and estimated basal energy expenditure (EBEE) sufficiency rate. The relationship between each parameter and %LBW was also examined. We also compared nutritional indices, gastrointestinal graft versus host disease (GvHD) grade, oral energy intake, and %LBW between groups.
Results
There was a relationship between %LBW, %LSMM, and EBEE sufficiency rate in both groups. Compared with the control group, the enhanced nutrition group had significantly improved energy intake amount, EBEE sufficiency rate, PN duration, and oral energy intake over time. The enhanced nutrition group also had increased oral energy intake, no difference in gastrointestinal GvHD grade, and improved %LBW compared with the control group.
Conclusions
Use of our nutritional support pathway in patients undergoing HSCT may be beneficial for %LBW and gastrointestinal GvHD grade, enabling early enhanced nutritional intervention after HSCT.
MeSH Keywords: Graft vs. Host Disease; Nutrition Assessment; Nutritional Support; Transplantation, Homologous
Background
Hematopoietic stem cell transplantation (HSCT) can lead to nutrition-related adverse events as a result of reduced body weight [1,2]. In particular, during the period from before the start of conditioning to the marrow suppression period, high levels of chemotherapy and total body irradiation can cause adverse events including vomiting and nausea, low appetite, mucosal defect, and taste defect, and oral intake can become difficult if graft versus host disease (GvHD) develops [3,4]. These adverse events and GvHD may lead to the need for total parenteral nutrition (TPN) [5–7]. Therefore, nutritional support including a discussion on adverse events and gastrointestinal GvHD is needed [8–12].
Improving oral nutritional interventions in patients with cancer has been shown to be associated with a reduction in weight loss and maintenance of quality of life [13,14]. We previously used a nutritional pathway and showed the association between gastrointestinal GvHD and nutrition-related adverse events in patients undergoing HSCT [3,4]. However, the association with weight loss or nutritional adjustments has not yet been adequately evaluated and managing weight loss reduction remains challenging.
We hypothesized that implementing a nutritional pathway for patients undergoing HSCT that included nutritional education before the start of conditioning could reduce weight loss. We then examined the association between nutritional intake and various body composition factors. The purpose of this study was to compare nutrition-related clinical indices between patients who participated in a pilot study (historical control cases) and patients who received a nutritional intervention to increase oral intake, and to evaluate the benefit of our nutritional support pathway in patients undergoing HSCT.
Material and Methods
Patients
The Shizuoka Cancer Center Ethics Committee approved this retrospective, historically controlled investigative study (approval number: T24-32). Participants were patients aged 16 to 70 years old with pretreatment performance status (PS) 0–1, who underwent initial HSCT at the Department of Hematology and Stem Cell Transplantation, Shizuoka Cancer Center [15]. Exclusion criteria were parenteral nutrition (PN) period exceeding Day 100 (with the transplantation day as Day 0), organ function not maintained, body mass index (BMI) outside the range 18.5–24.9 kg/m2, previous transplantation, patient refusal of nutritional intervention using the nutritional pathway, and participation deemed inappropriate by the physician [16–18].
A total of 166 patients underwent HSCT from January 2007 through October 2016: the historical control group comprised 101 patients who were involved in the pilot study investigating factors related to weight loss from January 2007 through March 2012. The group that underwent nutritional intervention (enhanced nutrition group) comprised 65 patients who were treated from April 2012 through October 2016 and used our nutritional pathway, which included pretreatment education of patients by a nutritionist (Supplementary Figure 1), monitoring of weight loss and nutritional intake during the hospital course, and enhanced oral intake [19,20]. For pretreatment of HSCT, we used myeloablative conditioning (MAC) and reduced-intensity conditioning (RIC) [21]. In all patients, the transplantation source included allogenic peripheral blood stem cell transplantation, cord blood transplantation, and unrelated donor bone marrow transplantation. All participants provided written informed consent.
Material and Methods
All patients undergoing bone marrow transplantation at the Shizuoka Cancer Center routinely receive any nutritional counseling. The nutritional pathway used by the hematology/stem cell transplant team at the Shizuoka Cancer Center follows the food hygiene protocol in the Hematopoietic Stem Cell Transplant Society Guideline (Supplementary Figure 2) [22–24]. Our nutritional support is composed of regular cuisine and food items and does not use enhanced nutrition or immunonutrition such as glutamine and arginine [25]. In our HSCT nutritional pathway, a nutritionist visits the patient at the bedside every day from before the start of conditioning to completion of PN and inquires about the patient’s preferences and adverse events, reports to the physician the energy and protein intakes from both PN and oral nutrition, confirms the physician’s orders for dietary adjustment, and applies dietary changes in real time (Supplementary Figures 1, 3) [3,4,26].
We compared the following parameters between the control group and enhanced nutrition group (Supplementary Figures 1, 3) from the day before the start of conditioning (T1: baseline) to the day after PN completion (T2). At T1, we evaluated BMI and percentage of the ideal body weight (%IBW). From T1 to T2, we compared the following variables between groups: %LBW; number of cases with high %LBW (≥7.5%) over 3 months; skeletal muscle mass (whole body and trunk and limb mass); and total body fat. In addition, the percent loss of skeletal muscle mass (%LSMM) and percent loss of fat mass (%LFM) were calculated and their relationships to %LBW were compared in each group [27]. Measurements were taken 2 hours after breakfast, from 10 am to 12 pm. We set the reference extracellular fluid to total body fluid ratio as 0.35, and extracellular water to total body water ratio as 0.40. We set the upper limits of extracellular fluid/total body fluid ratio and extracellular water/body water ratio, indicating mild edema, as 0.35–0.38 and 0.40–0.43, respectively. If edema was noted, the measurements were performed again the next day, as edema may influence lean body mass. All variables were measured using the high-precision body composition analyzer In Body S20® (InBody Co., Ltd., Seoul, South Korea), which uses bioelectrical impedance analysis. The reduction rate for each variable was calculated [28,29]. The energy and protein amounts for daily total nutritional intake, PN intake, and oral intake were calculated. The energy intake sufficiency rate was calculated using the following equation: [(PN calories, orally ingested calories, or enhanced nutrition calories)/total energy intake]×100. We compared the estimated basal energy expenditure (EBEE) sufficiency rate per ideal body weight (IBW) as determined by the Harris-Benedict method for total energy intake during the study period and evaluated the relation to %LBW in both groups [30,31]. The EBEE sufficiency rate was calculated using the following equation: [total energy intake sufficiency/EBEE IBW]×100. IBW was calculated using the following equation: height2×22. Oral energy intake as a percentage of EBEE per day at T2 was compared, and the relation to %LBW was evaluated in both groups. We compared the oral energy intake/IBW/day over time in the 2 groups at T2.
To evaluate toxicity during the study period, we examined the relationship over time of the nutrition-related adverse event severity score and PS. The nutrition-related adverse event severity score was defined as the Common Terminology Criteria for Adverse Events (CTCAE) v3.0 and 4.0 total score, in which 1 point is defined as grade ≥1 vomiting and grade ≥2 nausea, low appetite, mucosal defect, and taste defect, divided by the number of cases for that day using information from medical records [32]. PS was defined as the PS divided by the number of cases for all patients each day. To examine symptoms that resolved over time, we divided the total daily severity score for each nutrition-related adverse event by the number of subjects and presented this as a cumulative graph, then added PS to assess as a time-series graph. We compared the incidence of acute GvHD (grade ≥1) and grade of skin, liver, and gastrointestinal GvHD during the study period using the Transplant Registry Unified Management Program [33]. We divided the gastrointestinal GvHD cases in both groups into grade 0 and grade ≥1, and compared their relation to EBEE sufficiency rate and %LBW. We evaluated the relation between gastrointestinal GvHD (grades 0–4) and oral energy intake/IBW/day in the 2 groups. We then compared the duration of PN administration during the study period and oral energy intake/IBW/day in each group. In addition to the nutrition-related indices, we also investigated the exact day of neutrophil engraftment, defined as the first of 3 consecutive days when an absolute neutrophil count ≥500/cm3 was achieved with no decline thereafter. We also examined serum albumin (Alb) and C-reactive protein (CRP) levels prior to pretreatment (during the 2 weeks before T1), as well as the day of their maximum values of these parameters during the clinical course, and evaluated the relationship between these 2 laboratory values. We compared the nutritional status of these 2 groups: MAC and RIC [34].
Statistical analysis
All data are reported as medians (range). The Wilcoxon signed-rank test was used to compare the 2 groups. Spearman’s rank-order correlation was used to analyze the relationship between nutrition-related adverse event severity score over time and PS. JMP version 12.0 for Windows (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis, and statistical significance was defined as P<0.05.
Results
From January 2007 through October 2016, 166 patients underwent HSCT. Of these patients, 101 were in the control group and 65 were in the enhanced nutrition group. In the control group, 22 patients had a BMI outside of the range 18.5–24.9 kg/m2 at T1, and 33 patients deviated from the nutritional pathway (29 transplantation-related deaths and 4 receiving PN exceeding Day 100 due to GvHD or other reasons. In the enhanced nutrition group, 17 patients had a BMI outside the range 18.5–24.9 kg/m2 at T1, and 12 patients deviated from the nutritional pathway (5 transplantation-related deaths and 7 receiving PN exceeding Day 100 due to GvHD or other reasons). The remaining 46 patients in the control group and 36 patients in the enhanced nutrition group were included in the analysis.
Baseline characteristics are shown in Table 1. No differences were found in sex, median age, pretreatment regimen, or transplant source between the 2 groups. Table 1 shows the characteristics of patients in the 2 groups during the assessment period. No differences were found in BMI or %IBW between the 2 groups at T1. A significant difference was found between the groups in duration of PN. Compared with the control group, the enhanced nutrition group had significantly improved %LBW and %LSMM as well as significantly fewer cases of high %LBW of ≥7.5% over 3 months. The %LBW and %LSMM were related in both groups, but not %LFM (Figure 1A, 1B).
Table 1.
Characteristics of patients.
| Control group | Enhanced nutrition group | P | |
|---|---|---|---|
| Study period | 2007.04–2012.12 | 2013.01–2016.12 | |
|
| |||
| Sample size, n (Male/Female) | 46 (34/12) | 36 (20/16) | 0.0819 |
|
| |||
| Age, mean (range) | 54 (17–68) | 60 (27–70) | 0.0394 |
|
| |||
| Disease | 0.4412 | ||
| Acute myeloid leukemia | 14 | 11 | |
| Myelodysplastic syndrome | 13 | 6 | |
| Chronic myeloid leukemia | 3 | 1 | |
| Acute lymphoblastic leukemia | 10 | 7 | |
| Malignant lymphoma | 5 | 9 | |
| Multiple myeloma | 1 | 2 | |
|
| |||
| Conditioning regimen | 0.741 | ||
| MAC | 33 | 27 | |
| Busulfan (>6.4 mg/kg) | 17 | 16 | |
| TBI ≤5Gy in a single fraction, 8Gy in multiple fractions) | 13 | 9 | |
| Melphalan (>140 mg/m2) | 3 | 2 | |
| RIC | 13 | 9 | |
| Busulfan (≤6.4 mg/kg) | 2 | 0 | |
| TBI (<5Gy in a single fraction, <8Gy in multiple fractions) | 0 | 3 | |
| Melphalan (≤140 mg/m2) | 11 | 6 | |
|
| |||
| Transplant source | 0.2677 | ||
| Peripheral blood stem cell transplantation | 13 | 5 | |
| Unrelated bone marrow transplantation | 28 | 25 | |
| Cord blood transplantation | 5 | 6 | |
|
| |||
| Cases of %LBW ≥7.5% (n) | 15 | 3 | 0.0141 |
|
| |||
| Days on parental nutrition, n (range) | 62 (29–100) | 53 (34–92) | 0.0372 |
MAC – meloablative conditioning; TBI – total body irradiation; RIC – reduced-intensity conditioning; %LBW – percent loss of body weight. P value is before versus after assessment period (Mann-Whitney test or chi-square test).
Figure 1.
Relationship between percent loss of body weight, percent loss of skeletal muscle mass, and percent loss of body fat in the control group and the enhanced nutrition group (A, B). Relationship between percent loss of body weight and basal energy expenditure sufficiency rate (C, D). LBW – loss body weight; LSMM – loss of skeletal muscle mass; LFM – loss of fat mass; EBEE – estimated basal energy expenditure; IBW – ideal body weight.
In terms of the nutrition-related indices, a significant difference was found between the control group and enhanced nutrition group in total calorie intake. No significant differences between groups were seen in PN calorie intake and PN protein intake (Table 2). No difference was found in the oral intake initiation day or PN calorie intake ratio between the two groups. The enhanced nutrition group had a significantly increased EBEE sufficiency rate. %LBW and EBEE sufficiency rate were related in both groups (Figure 1C, 1D). Compared with the control group, the enhanced nutrition group had a significantly improved daily oral energy intake EBEE sufficiency rate at T2 (Table 2) and significantly improved oral energy intake/IBW/day over time (Figure 2). PN duration (the assessment duration) and oral energy intake/IBW/day were related in both groups (Figure 3A, 3B). The nutrition-related adverse event severity score and PS showed a direct relationship over time in both groups (Figure 4).
Table 2.
Assessment results in the control group and enhanced nutrition group.
| Control group | Enhanced nutrition group | P | |
|---|---|---|---|
| Total calorie intake (range) | 24 kcal/IBW/day (17–36) | 26 kcal/IBW/day (19–38) | 0.0386 |
| PN calorie intake (range) | 13 kcal/IBW/day (0–32) | 13 kcal/IBW/day (0–18) | 0.8611 |
| Orally ingested calories (range) | 10 kcal/IBW/day (4–28) | 13 kcal/IBW/day (5–29) | 0.0977 |
| Total protein intake (range) | 0.7 g/IBW/day (0.5–1.3) | 0.9 g/IBW/day (0.6–1.3) | 0.0062 |
| PN protein intake (range) | 0.4 g/IBW/day (0.0–1.0) | 0.5 g/IBW/day (0.0–0.6) | 0.1865 |
| Orally ingested protein intake (range) | 0.3 g/IBW/day (0.1–1.1) | 0.4 g/IBW/day (0.2–1.1) | 0.0699 |
| Oral intake initiation day (range) | Day 14 (0–53) | Day 16 (0–41) | 0.7974 |
| PN energy rate (range) | 57% (0–88) | 51% (0–78) | 0.1959 |
| EBEE rate/IBW% (range) | 104% (73–167) | 115% (79–175) | 0.021 |
| Daily oral energy intake EBEE sufficiency rate at T2 (range) | 94% (30–140) | 106% (55–183) | 0.0159 |
EBEE – estimated basal energy expenditure; IBW – ideal body weight; PN – parenteral nutrition; T2 – the day after PN completion. P value is before versus after assessment period (Mann-Whitney test or chi-square test).
Figure 2.
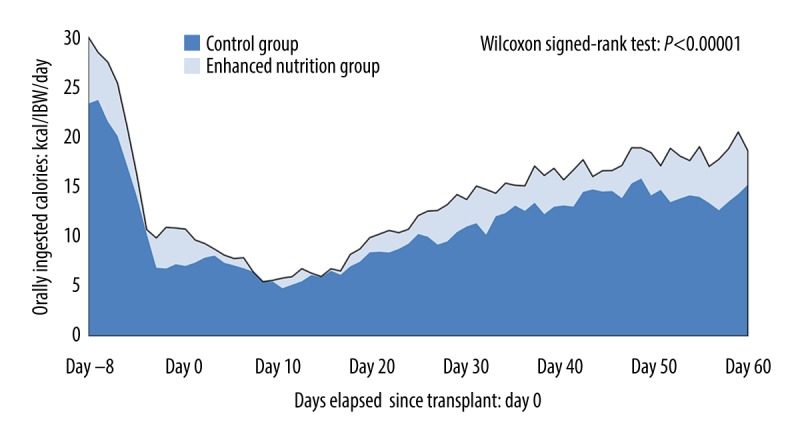
Oral energy intake over time in each group.
Figure 3.
Relationship between total parenteral nutrition (TPN) period and oral energy intake in each group (A, B). Relationship between gastrointestinal graft versus host disease (GvHD) and oral energy intake in each group (C, D).
Figure 4.
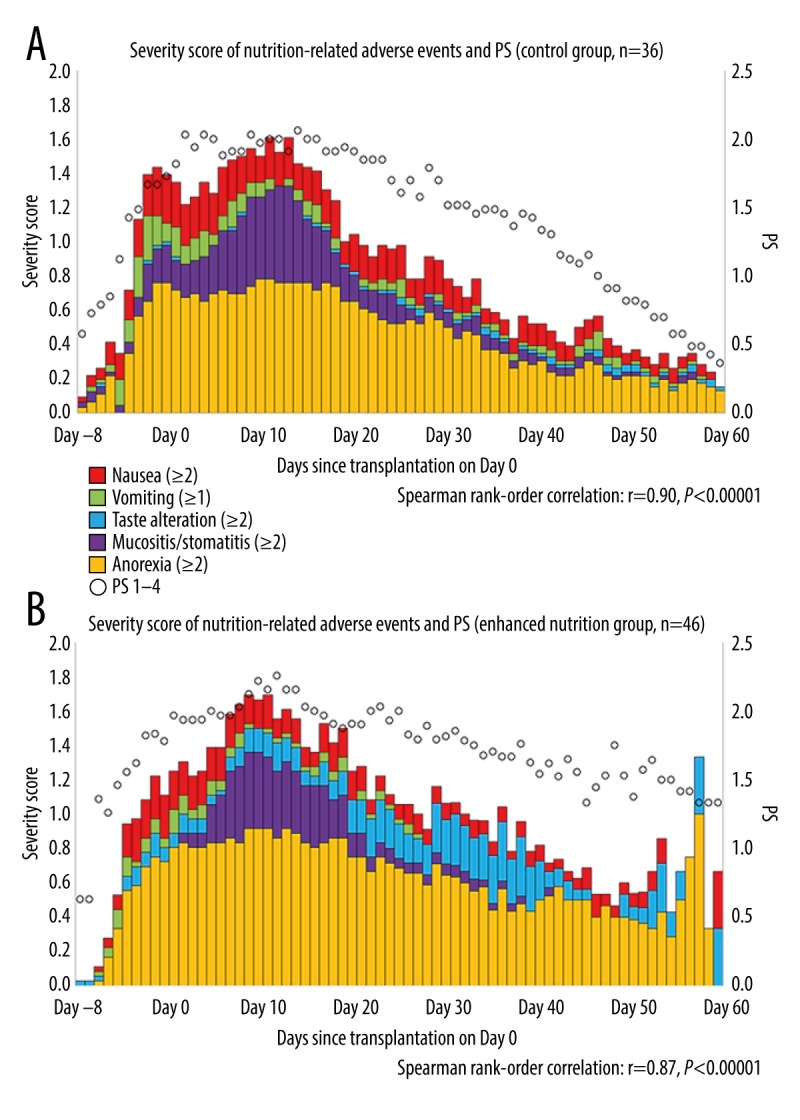
(A, B) Severity score of nutrition-related adverse events and performance status (PS) over time in both groups.
No significant differences were found between groups in the incidence rate of GvHD grade ≥1. Compared with the control group, the enhanced nutrition group had a significantly higher skin GvHD grade, but no difference in grade was found in liver or gastrointestinal GvHD. No difference was found in gastrointestinal GvHD grade between the control group (grade 0, 29; grade 1, 15; grade 2, 1; grade 3, 1) and the enhanced nutrition group (grade 0, 26; grade 1, 9; grade 2, 1; grade 3, 0; P=0.6902). After dividing the gastrointestinal GvHD cases in each group by grade 0 and grade ≥1, %LBW was significantly lower in grade ≥1 patients than in grade 0 patients in the control group, but no such difference in %LBW was found in the enhanced nutrition group (Table 3). A relationship was found between gastrointestinal GvHD grade (grades 0–3) and oral energy intake/IBW/day in the control group but not in the enhanced nutrition group (Figure 3C, 3D). Engraftment day was significantly earlier in the control group than in the enhanced nutrition group (Day 17 versus Day 19; P=0.0438). Both groups showed a significant difference in both Alb and CRP levels prior to pretreatment and peri-engraftment (control group: Alb Day 14, CRP Day 12; enhanced nutrition group: Alb Day 15, CRP Day 12), but no relationship was found between the differences in the 2 groups (Figure 5). No significant differences were found between groups in the nutritional status of MAC and RIC (Table 4).
Table 3.
EBEE rate, %LBW, and oral ingestion in gastrointestinal GvHD (grade 0 versus ≥grade 1) in each group.
| Control group | P | ||
|---|---|---|---|
| GvHD intestinal grade | Grade 0: 29 | Grade 1: 15, Grade 2: 1, Grade 3: 1 | |
| EBEE rate/IBW% (range) | 107 (73–144) | 96 (74–167) | 0.1716 |
| %LBW | −3.3 (−9.8–7.4) | −9.0 (−16.7–0) | 0.0006 |
| Oral calorie intake | 13 (5–28) | 8 (4–14) | 0.0003 |
| Enhanced nutrition group | P | ||
| GvHD intestinal grade | Grade 0: 26 | Grade 1: 9, Grade 2: 1, Grade 3: 0 | |
| EBEE rate/IBW% (range) | 112 (79–175) | 116 (96–159) | 0.4744 |
| %LBW | −1.7 (−10.5–4.6) | −1.7 (−9.5–2.4) | 0.9803 |
| Oral calorie intake | 13 (5–29) | 13 (10–18) | 0.6633 |
EBEE – estimated basal energy expenditure; GvHD – graft versus host disease; IBW – ideal body weight; LBW – loss of body weight. P value is before versus after assessment period (Mann-Whitney test).
Figure 5.
Changes in C-reactive protein (CRP) and albumin (Alb) levels before the start of conditioning and around engraftment.
Table 4.
Nutritional status of MAC and RIC in each group.
| Control group | MAC (n: 33) | RIC (n: 13) | P |
|---|---|---|---|
| Age, mean (range) | 45 (17–68) | 60 (26–65) | <0.001 |
| Preoperative BMI (range) | 21.4 kg/m2 (18.7–24.7) | 21.1 kg/m2 (18.5–24.6) | 0.6455 |
| %LBW (range) | −4.8 (−12.3–7.4) | −6.3 (−16.7–2.9) | 0.3486 |
| %LSMM (range) | −5.5 (−17.2–38.0) | −8.6 (−27.0–2.6) | 0.3094 |
| EBEE rate/IBW% (range) | 105% (74–167) | 103% (34–131) | 0.8046 |
| Daily oral energy intake EBEE sufficiency rate at T2 (range) | 94% (30–140) | 102% (34–131) | 0.8434 |
| Enhanced nutrition group | MAC (n: 27) | RIC (n: 9) | P |
| Age, mean (range) | 56 (27–70) | 63 46–69) | 0.0263 |
| Preoperative BMI (range) | 21.2 kg/m2 (19.1–24.6) | 21.6 kg/m2 (19.5–24.5) | 0.9079 |
| %LBW (range) | −0.9 (−12.3–7.4) | −3.4 (−10.5–1.0) | 0.4957 |
| %LSMM (range) | −2.0 (−16.0–12.6) | −4.1 (−20.2–3.5) | 0.1919 |
| EBEE rate/IBW% (range) | 112% (79–166) | 132% (91–175) | 0.1923 |
| Daily oral energy intake EBEE sufficiency rate at T2 (range) | 106% (70–159) | 102% (55–183) | 0.6061 |
MAC – myeloablative conditioning; RIC – reduced-intensity conditioning; BMI – body mass index; LBW – loss of body weight; LSMM – loss skeletal muscle mass; IBW – ideal body weight; EBEE – estimated basal energy expenditure. The P value before versus after assessment period (Mann-Whitney test).
Discussion
This study used various nutritional indices to investigate the reduction in body weight loss in patients who underwent HSCT with or without an enhanced nutritional intervention. In both groups, %LBW, %LSMM, and EBEE sufficiency rate were related, and oral energy intake and PN duration were negatively related, suggesting the need for early nutritional intervention using a nutritional pathway. Compared with the control group, the enhanced nutrition group had significantly improved nutritional indices of energy intake, EBEE sufficiency rate, and oral energy intake over time. These results support our hypothesis that by introducing new nutritional education using the HSCT nutritional pathway for patients before the start of conditioning, energy intake administered throughout the nutritional pathway is increased and body weight loss is reduced. Given that no difference was found between the 2 groups in the amount of PN administered during the assessment period, the improvement in percentage of oral energy intake in the EBEE after PN completion and the significantly shorter PN duration in the enhanced nutrition group implies that the enhanced nutritional pathway may potentially reduce the use of medical resources. Furthermore, given the positive relationship noted between nutrition-related adverse event severity score and PS over time in both groups, we believe a reduction in PS led to a decrease in total energy expenditure (TEE) [35]. Also, it seems that nutrition-related adverse event severity score led to decreased oral intake and reduced the diet-induced energy production [36]. These results, as well as the fact that the EBEE sufficiency rate (control group: 104%, enhanced nutrition group: 115%) was related to %LBW, are consistent with previous studies [37].
No difference in GvHD incidence was found between the control group and the enhanced nutrition group, but skin GvHD grade was significantly different between groups. The chief complaint in skin GvHD is rash, and although GvHD depends on the donor, GvHD prophylaxis, infections, and so on may have been related [22]. Resting energy expenditure was not measured in the present study, so this relationship remains unclear. Compared with the control group, the enhanced nutrition group had a higher oral energy intake but no difference in gastrointestinal GvHD grade, whereas %LBW, which was related to EBEE sufficiency rate, was improved. One reason may be that even in grade 1 GvHD, in which the amount of diarrhea – the chief complaint in gastrointestinal GvHD – exceeded 500 mL/day averaged over 3 days, the nutritionist supported oral intake by providing a low-residue (low fat, low fiber) diet, thereby increasing oral nutritional intake without stressing the gastrointestinal tract.
The diet before the start of conditioning in patients undergoing HSCT is a normal diet (solid food). In our nutritional pathway (Supplementary Figures 1, 3), as shown in Figures 2 and 4, after passing through the overlapping nutrition-related adverse event severity score and initiating oral intake in patients whose appetite has recovered, the chief complaints (e.g., nausea and poor appetite) were managed by providing gelatin (which does not expand the upper gastrointestinal tract) or sorbet, ice cubes, and hot soup (which have an oral phase). The nutritional pathway is characterized by accounting for the meal completion percentage and meal preferences as the nutrition-related adverse event severity score decreases, and then, with the patient’s consent, transitioning the diet to a regular diet (solid food) with meals adjusted to ensure adequate energy intake [3,4,26].
We do not use enhanced nutrition in the nutritional pathway at our facility for the following reasons: enhanced nutrition has a high osmolality and thus possibly induces diarrhea, which may confound the diagnosis of gastrointestinal GvHD [38,39]. Enhanced nutrition has also been suggested to carry a risk of saliva aspiration in the perioperative period [40]. Additionally, after the introduction of enhanced nutrition, a transition period to a regular diet is needed, and this may potentially prolong the hospital course [41–43]. In addition, we did not use glutamine, which is useful in mucosal repair, in this study, because its use was not appropriate, it does not lead to early engraftment, and evidence of its utility in gastrointestinal GvHD in human studies is lacking [44].
The nutritionist can respond to patient preference and nutrition-related adverse event severity score using various means. With enhanced oral intake, oral energy intake was improved, suggesting the possibility of maintaining quality of life [3,4,26].
We detected no differences in impact on the nutritional status of the MAC compared to the RIC [21,34]. The nutritional pathway can correspond to nutritional status MAC and RIC conditioning regimens similarly. The effectiveness of these preparative treatments can be evaluated objectively by establishing a nutritional pathway with nutritional intervention that continuously achieves the primary endpoint of prevention of loss of body weight [26].
Our study design did not allow for a comparable incidence of GvHD between the 2 groups, which would necessitate randomizing the 2 groups while keeping the human leukocyte antigen allele consistent. In fact, such manipulation of GvHD incidence and pre-transplantation risk intervention are not applicable to the practical clinical use of the nutritional pathway and was neither practical nor explicable to patients [45]. As such, a limitation of this study was that the effect of immunosuppressive agents and steroids on GvHD was not evaluated [46]. In addition, our study did not include data on infections during the HCT period and any viral or fungal stomatitis [47].
In the present study, we considered nutrition-related adverse events (severity score) and gastrointestinal GvHD in patients undergoing HSCT using the nutritional pathway and used the EBEE sufficiency rate to monitor nutritional intake and factors associated with body composition. Our results suggest that initiation of nutritional intervention to improve prevention of high %LBW to <7.5% over 3 months was necessary. The results of this study are important and may help to improve the quality of nutritional care for bone marrow transplant patients.
Conclusions
In conclusion, our nutritional pathway for patients undergoing HSCT includes nutritional education before the start of conditioning and provides nutritional support that takes gastrointestinal GvHD and nutrition-related adverse events into account. This nutritional pathway was shown to enhance oral intake and may help reduce weight loss.
Supplementary Data
Nutritional pathway for patients undergoing hematopoietic stem cell transplantation: Every day a nutritionist visits the patient at the bedside from before the start of conditioning to completion of parenteral nutrition (PN) and inquires about the patient’s preferences and adverse events, reports to the physician the energy and protein intakes from both PN and oral nutrition, confirms the physician’s orders for dietary adjustment, and applies dietary changes in real time.
The food hygiene protocol for Hematopoietic Stem Cell Transplant in the Dietary Department Shizuoka Cancer Center.
The nutritional interventions protocol for Hematopoietic Stem Cell Transplant of Shizuoka Cancer Center: Aoyama formula.
Footnotes
Source of support: This research project was sponsored by The Foundation for Promotion of Cancer Research and The Japan Dietetic Association under 2015 and 2016 Grants for Nutritional Guidance-related Research
References
- 1.Deeg HJ, Seidel K, Bruemmer B, et al. Impact of patient weight on non-relapse mortality after marrow transplantation. Bone Marrow Transplant. 1995;15:461–68. [PubMed] [Google Scholar]
- 2.Dickson TM, Kusnierz-Glaz CR, Blume KG, et al. Impact of admission body weight and chemotherapy dose adjustment on the outcome of autologous bone marrow transplantation. Biol Blood Marrow Transplant. 1995;5:299–305. doi: 10.1016/s1083-8791(99)70005-4. [DOI] [PubMed] [Google Scholar]
- 3.Aoyama T, Imataki O, Mori K, et al. Nutritional risk in allogeneic stem cell transplantation: Rationale for a tailored nutritional pathway. Ann Hematol. 2017;96:617–25. doi: 10.1007/s00277-016-2910-9. [DOI] [PubMed] [Google Scholar]
- 4.Aoyama T, Arai H, Imataki O, et al. Factors influencing loss of body weight in cord blood transplantation with nutritional support for hematopoietic stem cell transplantation. Asian Journal of Clinical Nutrition. 2017;9:137–46. [Google Scholar]
- 5.Mousavi M, Hayatshahi A, Sarayani A, et al. Impact of clinical pharmacist-based parenteral nutrition service for bone marrow transplantation patients: A randomized clinical trial. Support Care Cancer. 2013;21:3441–48. doi: 10.1007/s00520-013-1920-6. [DOI] [PubMed] [Google Scholar]
- 6.Weisdorf S, Hofland C, Sharp HL, et al. Total parenteral nutrition in bone marrow transplantation: A clinical evaluation. J Pediatr Gastroenterol Nutr. 1984;3:95–100. doi: 10.1097/00005176-198401000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Weisdorf DJ, Snover DC, Haake R, et al. Acute upper gastrointestinal graft-versus-host disease: Clinical significance and response to immunosuppressive therapy. Blood. 1990;76:624–29. [PubMed] [Google Scholar]
- 8.Mattsson J, Westin S, Edlund S, et al. Poor oral nutrition after allogeneic stem cell transplantation correlates significantly with severe graft-versus-host disease. Bone Marrow Transplant. 2006;38:629–33. doi: 10.1038/sj.bmt.1705493. [DOI] [PubMed] [Google Scholar]
- 9.van der Meij BS, de Graaf P, Wierdsma NJ, et al. Nutritional support in patients with GvHD of the digestive tract: State of the art. Bone Marrow Transplant. 2013;48:474–82. doi: 10.1038/bmt.2012.124. [DOI] [PubMed] [Google Scholar]
- 10.Lipkin AC, Lenssen P, Dickson BJ. Nutrition issues in hematopoietic stem cell transplantation: State of the art. Nutr Clin Pract. 2005;20:423–39. doi: 10.1177/0115426505020004423. [DOI] [PubMed] [Google Scholar]
- 11.Fuji S, Einsele H, Savani BN, et al. Systematic nutritional support in allogeneic hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2015;21:1707–13. doi: 10.1016/j.bbmt.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Botti S, Liptrott SJ, Gargiulo G, et al. Nutritional support in patients undergoing haematopoietic stem cell transplantation: A multicentre survey of the Gruppo Italiano Trapianto Midollo Osseo (GITMO) transplant programmes. Ecancermedicalscience. 2015;9:545. doi: 10.3332/ecancer.2015.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2012;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin C, Spiro A, Ahern R, et al. Oral nutritional interventions in malnourished patients with cancer: A systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:371–85. doi: 10.1093/jnci/djr556. [DOI] [PubMed] [Google Scholar]
- 15.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 16.Yamamoto W, Ogusa E, Matsumoto K, et al. Lymphocyte recovery on day 100 after allogeneic hematopoietic stem cell transplant predicts non-relapse mortality in patients with acute leukemia or myelodysplastic syndrome. Leuk Lymphoma. 2013;55:1113–18. doi: 10.3109/10428194.2013.823491. [DOI] [PubMed] [Google Scholar]
- 17.Kim HT, Armand P. Clinical endpoints in allogeneic hematopoietic stem cell transplantation studies: The cost of freedom. Biol Blood Marrow Transplant. 2013;19:860–66. doi: 10.1016/j.bbmt.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. BMI classification. Available at http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 19.Kanda K. effects of the physiological responses on gargling with soda water containing lemon for cancer chemotherapy patients with dysgeusia. Available at https://kaken.nii.ac.jp/grant/KAKENHI-PROJECT-15592254/
- 20.Keohane PP, Attrill H, Love M, et al. Relation between osmolality of diet and gastrointestinal side effects in enteral nutrition. Br Med J (Clin Res Ed) 1984;288:678–80. doi: 10.1136/bmj.288.6418.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Japan Society for Hematopoietic Stem Cell Transplantation. Hematopoietic cell transplant society guidelines II, pretransplant treatment. Available at http://www.jshct.com/guideline/pdf/06n_zenshochi.pdf.
- 22.Japan Society for Hematopoietic Stem Cell Transplantation. Hematopoietic cell transplant society guidelines I, Infection management after hematopoietic cell transplant. Available at https://www.jshct.com/guideline/guidelines_list.shtml.
- 23.World Health Organization. Five keys to safer food manual. Available at http://www.who.int/foodsafety/publications/5keysmanual/en/
- 24.Ministry of Health, Labour and Welfare. Consideration of mandatory HACCP implementation. Available at http://www.mhlw.go.jp/english/policy/health-medical/food/index.html.
- 25.Gardner A, Mattiuzzi G, Faderl S, et al. Randomized comparison of cooked and noncooked diets in patients undergoing remission induction therapy for acute myeloid leukemia. J Clin Oncol. 2008;26:5684–88. doi: 10.1200/JCO.2008.16.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoyama T, Imataki O, Arai H, et al. Comparison of nutrition-related adverse events and clinical outcomes between ICE (ifosfamide, carboplatin, and etoposide) and MCEC (ranimustine, carboplatin, etoposide, and cyclophosphamide) therapies as pretreatment for autologous peripheral blood stem cell transplantation in patients with malignant lymphoma. Med Sci Monit Basic Res. 2018;24:31–39. doi: 10.12659/MSMBR.908113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White JV, Guenter P, Jensen G, et al. Academy Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) JPEN J Parenter Enteral Nutr. 2012;36:275–83. doi: 10.1177/0148607112440285. [DOI] [PubMed] [Google Scholar]
- 28.Shafer K, Siders WA, Jhonson LKMS, et al. Validity of segmental multiple-frequency bioelectrical impedance analysis to estimate body composition of adults across a range of body mass index. Nutrition. 2009;25:25–32. doi: 10.1016/j.nut.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham JJ. Body composition as a determinant of energy expenditure: A synthetic review and a proposed general prediction equation. Am J Clin Nutr. 1991;54:963–69. doi: 10.1093/ajcn/54.6.963. [DOI] [PubMed] [Google Scholar]
- 30.Singhal S, Gordon LI, Tallman MS, et al. Ideal rather than actual body weight should be used to calculate cell dose in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;37:553–57. doi: 10.1038/sj.bmt.1705282. [DOI] [PubMed] [Google Scholar]
- 31.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA. 1918;4:370–73. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saijo N, Fukuda H, Shimoyama M. [Japanese translation of common terminology criteria for adverse events (CTCAE), and instructions and guidelines]. Int J Clin Oncol. 2004;(Suppl 3):1–82. [in Japanese] [PubMed] [Google Scholar]
- 33.Japanese Data Center for Hematopoietic Cell Transplantation. Transplant registry unified management program (trump) Available at http://www.jdchct.or.jp/en/outline/
- 34.Falda M, Busca A, Baldi I, et al. Nonmyeloablative allogeneic stem cell transplantation in elderly patients with hematological malignancies: results from the GITMO (Gruppo Italiano Trapianto Midollo Osseo) multicenter prospective clinical trial. Am J Hematol. 2007;82:863–66. doi: 10.1002/ajh.20990. [DOI] [PubMed] [Google Scholar]
- 35.Black AE, Coward WA, Cole TJ, et al. Human energy expenditure in affluent societies: An analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr. 1996;50:72–92. [PubMed] [Google Scholar]
- 36.Tappy L. Thermic effect of food and sympathetic nervous system activity in humans. Reprod Nutr Dev. 1996;36:391–97. doi: 10.1051/rnd:19960405. [DOI] [PubMed] [Google Scholar]
- 37.Seattle Cancer Care Alliance. Hematopoietic stem cell transplantation nutrition care criteria. 2nd ed. Seattle (WA): Cancer Care Alliance; 2002. [Google Scholar]
- 38.Japan Society for Hematopoietic Cell Transplantation. Act for appropriate provision of hematopoietic stem cells to be used in transplantations. Available at https://www.jshct.com/modules/en/index.php?content_id=1.
- 39.Whelan K, Schneider SM. Mechanisms, prevention, and management of diarrhea in enteral nutrition. Curr Opin Gastroenterol. 2011;27:152–59. doi: 10.1097/MOG.0b013e32834353cb. [DOI] [PubMed] [Google Scholar]
- 40.Gomes CA, Jr, Andriolo RB, Bennett C, et al. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst Rev. 2015;22:CD008096. doi: 10.1002/14651858.CD008096.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagiwara S, Mori T, Tuchiya H, et al. Multidisciplinary nutritional support for autologous hematopoietic stem cell transplantation: A cost-benefit analysis. Nutrition. 2011;27:1112–147. doi: 10.1016/j.nut.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Andersen S, Brown T, Kennedy G, et al. Implementation of an evidenced based nutrition support pathway for haematopoietic progenitor cell transplant patients. Clin Nutr. 2015;34:536–40. doi: 10.1016/j.clnu.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Arends J, Bachmann P, Baracos V, et al. 2016 ESPEN Guideline ESPEN guidelines on nutrition in cancer patients. Available at http://www.elsevier.com/locate/clnu. [DOI] [PubMed]
- 44.Noth R, Häsler R, Stüber E, et al. Oral glutamine supplementation improves intestinal permeability dysfunction in a murine acute graft-vs.-host disease model. Am J Physiol Gastrointest Liver Physiol. 2013;304:646–54. doi: 10.1152/ajpgi.00246.2012. [DOI] [PubMed] [Google Scholar]
- 45.Kota H, Chamberlain RS. Immunonutrition is associated with a decreased incidence of graft-versus-host disease in bone marrow transplant recipients: A meta-analysis. JPEN J Parenter Enteral Nutr. 2017;41:1286–92. doi: 10.1177/0148607116663278. [DOI] [PubMed] [Google Scholar]
- 46.Wolf D, von Lilienfeld-Toal M, Wolf AM, et al. Novel treatment concepts for graft-versus-host disease. Blood. 2012;119:16–25. doi: 10.1182/blood-2011-08-339465. [DOI] [PubMed] [Google Scholar]
- 47.Krawczyk J, Kraj L, Korta T, et al. Nutritional status of hematological patients before hematopoietic stem cell transplantation and in early post transplantation period. Nutr Cancer. 2017;69:1205–10. doi: 10.1080/01635581.2017.1367937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nutritional pathway for patients undergoing hematopoietic stem cell transplantation: Every day a nutritionist visits the patient at the bedside from before the start of conditioning to completion of parenteral nutrition (PN) and inquires about the patient’s preferences and adverse events, reports to the physician the energy and protein intakes from both PN and oral nutrition, confirms the physician’s orders for dietary adjustment, and applies dietary changes in real time.
The food hygiene protocol for Hematopoietic Stem Cell Transplant in the Dietary Department Shizuoka Cancer Center.
The nutritional interventions protocol for Hematopoietic Stem Cell Transplant of Shizuoka Cancer Center: Aoyama formula.





