Abstract
Multiple initiatives at the national and international level support natural drug discovery. Psychiatrists and patients are not well informed about natural psychotropics in general. Existing antidepressant and antipsychotic drugs were developed from atropine, a natural product. Subsequent drug developments were largely based on extension and modification of earlier molecular scaffolds. This limits their mechanisms of action to similar neuropathways. Natural psychotropic substances, particularly those with hallucinogenic and psychedelic properties and different chemical structures, may serve as new paths to novel psychotropic drug development.
Keywords: natural psychoactive substances, botanical psychotropics, designer drugs, psychotropic designs
Introduction
Natural psychotropic research has not been at the forefront of psychopharmacology until recently. In 2019, the World Health Organization will include traditional medicine (TM) into its global compendium. The US National Cancer Institute (NCI) announced a new initiative for drug discovery from natural products (Thornburg et al., 2018). The NCI’s natural product repository consists of over 230 000 natural product extracts collected from biodiverse areas in different parts of the world. The US National Center for Complementary and Integrative Health of the National Institutes of Health announced a similar program on natural product drug discovery (NCCIH, 2016). All of these initiatives have generated much interest and discussion regarding medicines and psychotropics derived from plants, animals, and minerals.
The recent increase in interest regarding natural psychotropics has been fueled by the emerging threat of new psychoactive substances worldwide, as alerted by the United Nations Office on Drugs and Crime (UNODC, 2019). New psychoactive substances are mostly available as natural products, such as cacti and animal venom or from modification of their active ingredients, such as synthetic cannabinoids.
Health supplements containing TM are a big business. The absence of strict regulation and the adulteration of some of these products has triggered repeated warnings from the US Food and Drug Administration (FDA, 2019a) and the implementation of stricter oversight this year (FDA, 2019b). Yet based on the sales figures of these supplements, it is clear that TM is highly popular. One of the reasons why patients have resorted to TM may be related to the belief that TM products are “natural” and therefore “safe.” As a result, patient concerns about the safety of manmade drugs may lead to noncompliance, especially during long-term drug maintenance. A significant number of patients suffering from mood disorders failed to respond to existing antidepressant drugs according to large-scale studies such as Star* D (Trivedi et al., 2006). Desperate patients understandably might resort to other forms of medicine that claim efficacy. The success of TM has shown that there is an unmet need for new psychiatric drugs to serve many patients who are not satisfied with current psychiatric drugs. The US National Institute of Mental Health psychoactive drug screening program (NIMH, 2017) represents an effort to stimulate innovative research to discover novel tools and potential therapeutic agents for the treatment of psychiatric disorders, complementary to the NCI and National Institutes of Health initiatives.
Many patients and clinicians alike, however, are not well-informed about TM and may not know that existing antidepressant and antipsychotic drugs in fact are based on plant molecules. Later generations of antidepressant and antipsychotics were mostly synthesized from drug molecules modified from earlier scaffolds. Discovery of psychotropic molecules, natural or synthetic, has mostly been serendipitous. New molecules with targets different from the current are urgently needed. We review in this manuscript the evolution of psychiatric drugs from natural molecules, the merits of this approach, and future opportunities in designer drugs.
Methods
We searched English and other language literature with English abstracts listed in PubMed on the website www.ncbi.nlm.nih.gov up to June 15, 2019, crossing the keywords “traditional medicine, herbs, animal products, minerals” with the words “psychotropics, psychedelics, new psychoactive substances, molecular structure, drug design” respectively. The keywords “psychotropics,” “drug design,” and “psychedelics” were further crossed with the words “traditional medicine,” “herbs,” “insects,” and “animals” and searched on Google from May 1 to June 15, 2019. Research and research news regarding the above areas obtained through Google search but missed in the first round of the PubMed search were back searched in PubMed. Foreign language publications with English translations or relevant abstracts in English were also included in the search. This review focuses on psychotropic drug design and is not a review on the efficacy of natural medicine. Therefore, we do not intend to cover all existing natural psychotropics and will use only selected examples for the discussion of drug design opportunities.
Natural Psychotropics
Early medicine came from the environment. Through the consumption of certain plants, animals, and minerals, medicinal properties such as antiinflammatory, analgesic, and psychotropic effects were noticed by people. Those with marked hallucinogenic and anesthetic properties would serve well for religious, ceremonial, or recreational purposes in primitive communities. In these societies, the role of a priest and a healer frequently overlapped, though the accumulated knowledge would be an early form of pharmacology and psychopharmacology.
It is interesting that although there have been many botanical, animal, and mineral products used in TM over many years, there are few used specifically for mental disorders. It is possible that the recognition of mental disorders as treatable medical conditions is only a recent development. The understanding of the heterogeneity of mental disorders, in terms of etiology, symptomatology, and clinical course and in modern neurotransmitter and neuropathway terms did not occur until the last century. On the other hand, natural psychotropics were convenient tools for religious and ceremonial purposes. A recent study of 126 psychoactive botanicals used in different cultures determined that more than one-half were used for hallucinogenic purposes, followed by plants used for sedative and narcotic properties. Plants with anxiolytic and antidepressant effects were the least popular across different cultures (Alrashedy and Molina, 2016). Even until recently, few hallucinogenic molecules have been used successfully for the development of new drugs. The serendipitous observation of ketamine’s rapid antidepressant effect appears to have triggered new interest in drugs of abuse as leads to develop new psychotropics. In this regard, natural psychoactive plants and animals may offer new drug development opportunities (Gray et al., 2016; Uthaug et al., 2018, 2019).
Botanical Medicine
Plants are remarkable pharmaceutical synthesis factories. A wealth of molecules, from simple to highly complex, are synthesized by plants. Many of the complex molecules that plants synthesize, such as paclitaxel (Taxol), have proven to be a difficult task for synthetic chemists to replicate but are valuable scaffolds for new drug molecule construction. Some of these compounds are essential for a plant’s physiological function, whereas others are secondary metabolites. A large number of compounds synthesized by plants became important for their survival in evolution, helping to defend the plant against predators, enemies, assaults, and other competing plants or to protect them from harsh environments against biotic and abiotic factors. Thus, it is not surprising there are molecules with antiinflammatory, antioxidative, antiviral, antibacterial, antifungal, and antineoplastic action. Other molecules could be poisonous, stimulatory, or sedating to animals or a combination of these properties. Humans became aware of the medicinal properties and health benefits through experience and utilized these plants in a raw or semiprepared form as raw medicine. Examples include salicylic acid from willow tree bark as an antiinflammatory antipyretic agent (salicylic acid) and cinchona tree (quinine) for malaria. Even today, people have continued to harvest useful molecules from plants, such as paclitaxel (Taxol) from the Pacific Yew for cancer and artemisinin from Artemisia annua (sweet wormwood herb) for resistant malaria. It is foreseeable that even more useful phytochemicals will continue to be discovered through new initiatives such as the NCI Program for natural product discovery (Thornburg et al., 2018) or through serendipity.
Molecules of plant origin are commonly classified into major categories based on their unique structures and biochemical characteristics. Table 1 lists these major categories and some well-known medicinal and psychotropic compounds in each category.
Table 1.
Categories of botanical compounds and properties
| Category | Plant | Ingredients | Properties/Indications |
|---|---|---|---|
| Saccharides and glycosides (compounds in other categories are also often stored as glycosides) |
Immuno-modulation Antiinflammatory Adaptogens Antidepressant, sedative antiinflammatory |
||
| Panax ginseng | Ginsenosides | Adaptogen | |
| Ganoderma fungi | Polysaccharides | Immuno-modulating Sedating |
|
| Polygala tenuifolia | Tenuifoliside a | Antidepressant Sedative |
|
| Phenylpropanoids | UV protection, antimicrobes, pigments, scents |
||
| a.Simple phenylpropanoids |
Illicium verum
(star anise) |
Shikimic acid | Precursor for synthesis of oseltamivir (Tamiflu) |
| Illicium anisatum (Japanese star anise) | Anisatin | Toxic Neurotoxin |
|
| Cinnamon tree bark | Cinnamaldehyde | Anti-Tau protein, insecticide | |
| b.Flavonoids | Ginkgo biloba | Ginkgetin | Dementia |
| Green tea | Catechin | Antioxidant, neuroprotection | |
|
Albizia julibrissin
(mimosa, silk tree) Other Albizia species |
Quercetrin Albiota |
Antidepressant Sedative Antiinflammatory Antibacterial, antioxidant Anticancer |
|
|
Pueraria lobata, Rhododendron dauricum |
Daidzein, Daidzin Puerarin, Genistein Hyperoside, Apigenin |
Angiogenesis Anticancer Estrogenic |
|
| c.Coumarins | Cassia Cinnamon Tonka beans | Cinnamaldehyde Coumarin |
Antioxidant Analgesic Antiinflammatory, and Antimutagenic |
| d.Lignans |
Schisandra chinesis
Sesame seeds Soybeans |
Schisanhenol Schizandrol Isotaxiresinol Podophyllotoxin |
Hepatoprotection Neuromodulation Phytoestrogens Antioxidant Antiinflammatory Anticancer |
| Quinones and quinonoids |
Antibacterial Antiparasitic Anticancer Purgative |
||
| St John’s wort | Hypericin | Depression | |
| Polygonum multiflorum | Emodin Chrysophanol |
Antiaging, antiatherosclerotic Immunomodulation |
|
| Salvia miltiorrhiza | Cryptotanshinone Tanshinone |
Antioxidant, antiaging Antimicrobes |
|
| Terpenes/triterpenoids/ saponins/steroids |
Antiinflammatory Immuno-modulation Antistress Cardiovascular actions Hormonal actions |
||
| Ziziphus jujube | Jujuboside a | Sedative, antidepressant Neuroprotective |
|
|
Digitalis purpurea
(common foxglove) |
Digoxin | Congestive heart failure Atrial fibrillation |
|
|
Artemisia annua
(sweet wormwood) |
Artemisinin | Antimalarial | |
| Peony root | Paeoniflorin | Antiinflammatory Antibacterial, antiviral Anticancer |
|
| Panax ginseng | Panaxadiol | Adaptogen | |
| St John’s wort | Hyperforin, Adhyperforin | Antidepressant | |
| Alkaloids | Opium poppy (Papaver somniferum) |
Morphine | Sedation Analgesic |
| Atropa belladonna | Atropine, scopolamine | Anticholinergic | |
| Coffee beans | Caffeine | Wakefulness | |
| Tea | Caffeine | Wakefulness |
From the Natural Compound Atropine to Modern Synthetic Antidepressants and Antipsychotics
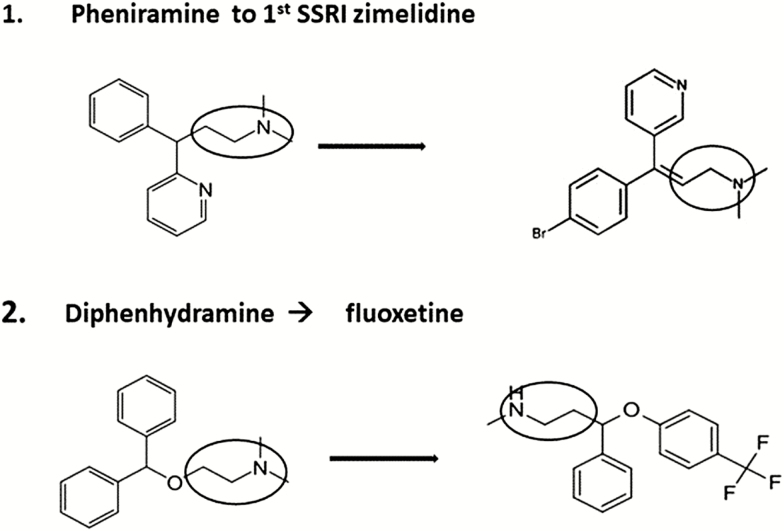
Serendipity has characterized the history of psychiatric drug discoveries. This began when researchers searched not for psychiatric drugs but for better antihistamines after the discovery of the natural molecule atropine in plants of the nightshade family (Solanaceae). Diphenhydramine, a potent antihistamine, was synthesized based on the backbone of atropine (Figure 1). The search for more antihistamine molecules led to the synthesis of the phenothiazines, and their unexpected antipsychotic properties were observed by clinicians. In modifying the phenothiazine molecule, scientists synthesized tricyclic antidepressants (TCAs), and again astute clinicians recognized their antidepressant properties. The subsequent development of the new generations of specific serotonin (5HT) re-uptake inhibitors (SSRIs) could be seen as a discovery of new antidepressant molecules through modifying existing antihistamine molecules, as pheniramine was used to make zimelidine and diphenhydramine to make fluoxetine (Wong et al., 1974, 2005) (Figure 1). Continuous modification of earlier phenothiazines in the search for better antipsychotic molecules led to making the potent dopamine antagonist haloperidol and then the atypical antipsychotics. The discovery of lithium’s antimanic action and ketamine’s unexpected fast antidepressant effect, on the other hand, are both other examples of serendipity in cooperation with careful clinical observations.
Figure 1.
Conversion of antihistamines to specific serotonin re-uptake inhibitors (SSRIs), with ethylamine backbone with (CH3CH2NH2) circled.
It is fair to say that it was the continuous modification of the natural alkaloid atropine molecule that resulted in the making of the 2 major existing groups of modern psychotropic drugs: the antidepressant and antipsychotic drugs. Atropine thus could be viewed as the mother of current antidepressants and antipsychotics. However, these drug molecules share the same ethylamine backbone (CH3CH2NH2) of the atropine and antihistamine molecules from which they were derived.
Antidepressants and antipsychotics with a totally new mechanism of action might not come from further modification of these molecules. Indeed, other botanical molecules have not been used for the development of new psychotropics with a new mechanism of action. Although many of the TM antidepressants and sedatives were found to act on the same monoamine, indoleamine, or gamma-aminobutyric acid targets as the manmade antidepressants and sedatives (reviewed by Tang et al., 2017a), this does not mean that psychotropic discovery should be limited to molecules targeting the same neurotransmitter targets. An example of interest is the root of 3 types of peony (Paeonia suffruticosa, Paeoniae alba, Paeonia ruba), which are often ingredients in TM formula for mood disorders. The active ingredients have been identified as paeoniflorin, albiflorin, and related molecules, all of which possess interesting antiinflammatory, immunoregulatory, cardiac-regulatory, and neuroprotective actions. This herb has not been reported to possess significant activity on common neurotransmitter targets itself. It is possible that inhibition of inflammation is an important part of treatment for some patients suffering from mood disorders. This may be linked to the Inflammatory Theory of Depression or a toxic brain hypothesis of depression (reviewed by Tang et al., 2017b).
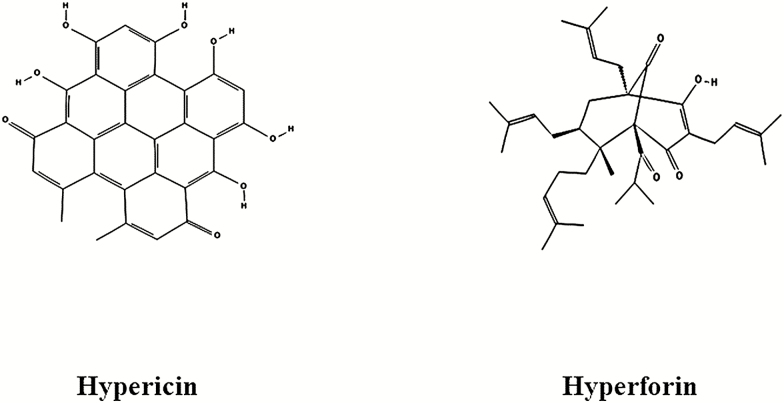
Another interesting example of novel molecular structure is St John’s wort (Hypericum perforatum), a popular TM used for antidepressant purposes in Europe and Asia. One of the most extensively studied TMs (Linde et al., 2008; Apaydin et al., 2016), it has been found to be as effective as SSRIs. Hypericin, with a very interesting and unusual structure (Figure 2), was suspected to be the main molecule responsible for the antidepressant action. However, it was difficult to explain its antidepressant action judging from the almost planar shape of the hypericin molecule when monoamine reuptake inhibition by the raw plant extract was demonstrated (Calapai et al., 2001). The discovery of 2 phloroglucinol derivatives (cyclic terpenes), hyperforin (Cervo et al., 2002) and adhyperforin (Tian et al., 2014), with monoamine reuptake inhibition property (Jensen et al., 2001) may explain the antidepressant action of St John’s wort (Kaehler et al., 1999). A unique mechanism of monoamine reuptake inhibition through protonophore is also a possibility (Sell et al.,2014). The discovery of hyperforin’s antioxidative and antiinflammatory action (Jiang et al., 2018) suggests its potential use in neurodegenerative disorders, such as Alzheimer’s disease (Griffith et al., 2010). As hyperforin is an extremely unstable molecule (Gray et al., 2000), other unknown metabolites may be responsible for its antidepressant action. Hyperforin activates the Pregnane-X receptor (Taneja et al., 2018) and is a potent inducer of CYP 2C9 and CYP 3A4 (Chen et al., 2004). This may cause undesirable drug-drug interactions when used with other psychotropics. In any case, the multi-target multi-dimensional property makes hyperforin a very interesting scaffold for future psychotropic drug design.
Figure 2.
Structure of hypericin and hyperforin in St John’s Wort.
Compared with mood disorders and anxiety, TMs for psychotic disorders are few. As discussed above, it is possible that modern classifications of mental disorders only happened in the last century. Discovery of useful antipsychotic molecules in TM will require more laboratory work and, more importantly, good-quality randomized controlled clinical trials (Rathbone et al., 2005; Deng and Xu, 2017; Hoenders et al., 2018).
Hallucinogenic or Mind-Altering Botanicals, Toads, and Spider Wasps
Although hallucinogenic botanicals and animals were discovered early in human history (Carod-Artal, 2015) and they played an important role in neuroscience research and our understanding of the neuropathways of the brain, there has been a lack of psychiatric drugs developed from hallucinogenic molecules as yet.
However, there has been a recent surge of interest in the application of hallucinogenic botanicals and animals as psychotherapeutics (Garcia-Romeu et al., 2016). Botanicals with psychotropic properties such as ayahuasca (chacruna), peyote cacti, psilocybin mushrooms, iboga (Bwiti), coca leaves, kava, kratom, and khat (the 3Ks) have attracted much interest as platforms for new psychotropic development (Ujváry, 2014; Mithoefer et al., 2016; Carhart-Harris and Goodwin, 2017; Feng et al., 2017; Johnson and Griffiths, 2017; De Gregorio et al., 2018) and also, interestingly, as adjuncts to psychotherapy (Sherwood and Prisinzano, 2018; Uthaug et al., 2018). It is foreseeable that some hallucinogenic molecules will serve as scaffolds for new psychiatric drug development.
Interestingly, toad venom (Qi et al., 2018) has been suggested as a potential source of psychotropics. Uthaug et al. (2019) argued that the 5-methoxy-N,N-dimethyltryptamine in toad venom could be responsible for its antianxiety and antidepressant properties. The best-known toad venom in TM is from the Chinese toad Bufo bufo gargarizans. A large number of biologically active complex terpene and steroidal ingredients have been identified, including bufotalin, bufotenidine, bufalin, and bufadienolide (McBride, 2000). The toad venom from Bufo bufo gargarizans is the main ingredient in Venum bufonis, an important TM used for the treatment of anxiety, sedation, detoxification, pain, animal bites, and many other health problems (Qi et al., 2018). It is another example of a multi-target multi-dimensional TM. The molecules certainly would serve as good scaffolds for multi-target drug development.
Spider wasp’s venom is another unusual source of psychotropics. Spider wasps have been reported to inoculate spiders with its venom, and these injected ingredients control the spider’s net-weaving behavior. This has been suggested as a potential model for mind control psychotropics (Takasuka et al., 2015; Kloss et al., 2017).
Thus, there is great hope that these unusual psychoactive botanicals (Barsuglia et al., 2018; Griffiths et al., 2019) and animal venoms may open new avenues for the development of novel psychotropics (Takeda, 1994; Uthaug et al., 2018; Dos Santos et al., 2019).
Minerals as Psychotropics Drugs
Oxides of lead and sulfide of arsenic (realgar As2S3) and mercury (HgS) have been used for sedation and cerebral vascular emergency management purposes in TM and are still the ingredients in some over-the-counter TMs. Although the toxic arsenic mineral has been developed as an anticancer agent, the mechanism of action for the sedating property of these minerals is still unclear. Because another mineral, lithium, has a clear psychotropic action, further research on the sedating properties of these minerals may reveal other unknown biology of mood maintenance.
Designer Drugs
It has always been the hope of researchers that rational drug design will follow a better understanding of the pathology of mental disorders and the technical advances in synthetic chemistry. Although it is true that tremendous progress has been made in neuroscience, we are still at only an early stage of understanding the exact mechanism of most psychiatric disorders. The synthesis of the phenothiazines from the natural botanical compound atropine and subsequent synthesis of the atypical antipsychotics, TCAs, and SSRIs was a big step in psychiatric drug development. These drugs opened new research paths into the pathophysiology of mental disorders through the discoveries of their neurotransmitter targets in the brain. Identification of multiple neurotransmitter receptors enabled the synthesis of multi-target 5HT receptor designer antidepressant drugs such as vortioxetine. Further exploitation of specific 5HT receptor target brought about the development of the nondopaminergic antipsychotic primavanserin, an inverse agonist. Understanding of partial agonist action on DA receptor led to the synthesis of aripiprazole. All these examples demonstrated that psychotropic drug design follows our better understanding of the potential targets. Research on natural psychotropic substances may identify more novel targets.
While multi-target designer drugs were once touted as the hope for a next generation of psychotropics, there has been limited success in finding new psychotropic drugs that function through a totally new mechanism and design. The existing drugs in psychiatry so far have come largely from serendipitous discoveries, followed by further modification of existing drug molecules to increase potency and to decrease side effects. It has been a long time since the discovery of phenothiazines from antihistamines, TCAs from phenothiazines, and monoamine oxidase inhibitors from isoniazid. In these cases, which all occurred in the last century, psychotropics were discovered through the careful observation by astute clinicians in the clinic after capable synthetic chemists in the laboratories constructed new molecules. Ketamine is a lone new example in many years, a compound that may act through a different mechanism of action compared with other antidepressant drugs. This development also occurred through the careful observation of clinicians, who noticed the unexpected antidepressant response from the existing molecule, which previously was not indicated for depression. This lends support to the need for collaboration between synthetic chemists designing new molecules according to clinical needs and well-prepared, astute clinicians observing patients for abnormal responses.
Conclusion
Natural medicine is popular, but its true value is found in its active ingredients and novel molecules. Phytochemicals like atropine have proven to be valuable scaffolds or chemical platforms from which new drugs were developed. Our existing antipsychotics and antidepressants originated from atropine through serendipity, but serendipity only occurs with the prepared mind. Many of the important drug discoveries have occurred through careful clinical observation of unexpected or unusual effects of compounds with other indications. The recent surge in neuroscience and neuropharmacology research related to new psychoactive substances may open new paths to novel compounds, with alternate mechanisms of action on psychiatric disorders. Neuropsychopharmacologists and clinicians together will continue to play critical but cooperative roles in future discoveries.
Statement of Interest
Neither author has conflict of interests, financial or nonfinancial, to declare. Neither author has received any form of financial or nonfinancial support in preparing this manuscript.
References
- Agarwal V, Abhijnhan A, Raviraj P (2007) Ayurvedic medicine for schizophrenia. Cochrane Database Syst Rev 4:CD006867. [DOI] [PubMed] [Google Scholar]
- Alrashedy NA, Molina J (2016) The ethnobotany of psychoactive plant use: a phylogenetic perspective. Peerj 4:e2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apaydin EA, Maher AR, Shanman R, Booth MS, Miles JN, Sorbero ME, Hempel S (2016) A systematic review of St. John’s wort for major depressive disorder. Syst Rev 5:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsuglia J, Davis AK, Palmer R, Lancelotta R, Windham-Herman AM, Peterson K, Polanco M, Grant R, Griffiths RR (2018) Intensity of mystical experiences occasioned by 5-MeO-DMT and comparison with a prior psilocybin study. Front Psychol 9:2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calapai G, Crupi A, Firenzuoli F, Inferrera G, Squadrito F, Parisi A, De Sarro G, Caputi A (2001) Serotonin, norepinephrine and dopamine involvement in the antidepressant action of hypericum perforatum. Pharmacopsychiatry 34:45–49. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Goodwin GM (2017) The therapeutic potential of psychedelic drugs: past, present, and future. Neuropsychopharmacology 42:2105–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carod-Artal FJ. (2015) Hallucinogenic drugs in pre-Columbian Mesoamerican cultures. Neurologia 30:42–49. [DOI] [PubMed] [Google Scholar]
- Cervo L, Rozio M, Ekalle-Soppo CB, Guiso G, Morazzoni P, Caccia S (2002) Role of hyperforin in the antidepressant-like activity of Hypericum perforatum extracts. Psychopharmacology 164:423–428. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ferguson SS, Negishi M, Goldstein JA (2004) Induction of human CYP2C9 by rifampicin, hyperforin, and phenobarbital is mediated by the pregnane X receptor. J Pharmacol Exp Ther 308:495–501. [DOI] [PubMed] [Google Scholar]
- De Gregorio D, Enns JP, Nuñez NA, Posa L, Gobbi G (2018) d-Lysergic acid diethylamide, psilocybin, and other classic hallucinogens: mechanism of action and potential therapeutic applications in mood disorders. Prog Brain Res 242:69–96. [DOI] [PubMed] [Google Scholar]
- Deng H, Xu J (2017) Wendan decoction (Traditional Chinese medicine) for schizophrenia. Cochrane Database Syst Rev 6:CD012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos RG, Hallak JEC (2019) Ayahuasca, an ancient substance with traditional and contemporary use in neuropsychiatry and neuroscience. Epilepsy Behav pii: S1525–5050(19)30251–3. doi: 10.1016/j.yebeh.2019.04.053. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- FDA statement (2019a) FDA acts on dietary supplements. Available at: https://www.fda.gov/food/cfsan-constituent-updates/fda-acts-dietary-supplements-containing-dmha-and-phenibut Retrieved May 15, 2019.
- FDA statement (2019b) Available at: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-new-efforts-strengthen-regulation-dietary Retrieved May 15, 2019.
- Feng LY, Battulga A, Han E, Chung H, Li JH (2017) New psychoactive substances of natural origin: a brief review. J Food Drug Anal 25:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romeu A, Kersgaard B, Addy PH (2016) Clinical applications of hallucinogens: a review. Exp Clin Psychopharmacol 24:229–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DE, Rottinghaus GE, Garrett HE, Pallardy SG, Gray DE, Rottinghaus GE, Garrett HE, Pallardy SG (2000) Simultaneous determination of the predominant hyperforins and hypericins in St. John’s Wort (Hypericum perforatum L.) by liquid chromatography. J AOAC Int 83:944–949. [PubMed] [Google Scholar]
- Gray R, Bressington D, Hughes E, Ivanecka A (2016) A systematic review of the effects of novel psychoactive substances ‘legal highs’ on people with severe mental illness. J Psychiatr Ment Health Nurs 23:267–281. [DOI] [PubMed] [Google Scholar]
- Griffith TN, Varela-Nallar L, Dinamarca MC, Inestrosa NC (2010) Neurobiological effects of Hyperforin and its potential in Alzheimer’s disease therapy. Curr Med Chem 17:391–406. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Hurwitz ES, Davis AK, Johnson MW, Jesse R (2019) Survey of subjective “God encounter experiences”: comparisons among naturally occurring experiences and those occasioned by the classic psychedelics psilocybin, LSD, ayahuasca, or DMT. Plos One 14:e0214377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenders HJR, Bartels-Velthuis AA, Vollbehr NK, Bruggeman R, Knegtering H, de Jong JTVM (2018) Natural medicines for psychotic disorders: a systematic review. J Nerv Ment Dis 206:81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AG, Hansen SH, Nielsen EO (2001) Adhyperforin as a contributor to the effect of Hypericum perforatum L. in biochemical models of antidepressant activity. Life Sci 68:1593–1605. [DOI] [PubMed] [Google Scholar]
- Jiang X, Kumar M, Zhu Y (2018) Protective effect of Hyperforin on β amyloid protein induced apoptosis in PC12 cells and colchicine induced Alzheimer’s disease: an anti-oxidant and anti-inflammatory therapy. J Oleo Sci 67:1443–1453. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Griffiths RR (2017) Potential therapeutic effects of psilocybin. Neurotherapeutics 14:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaehler ST, Sinner C, Chatterjee SS, Philippu A (1999) Hyperforin enhances the extracellular concentrations of catecholamines, serotonin and glutamate in the rat locus coeruleus. Neurosci Lett 262:199–202. [DOI] [PubMed] [Google Scholar]
- Kloss TG, Gonzaga MO, de Oliveira LL, Sperber CF (2017) Proximate mechanism of behavioral manipulation of an orb-weaver spider host by a parasitoid wasp. Plos One 12: e0171336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde K, Berner MM, Kriston L (2008) St John’s wort for major depression. Cochrane Database Syst Rev 2008:CD000448. doi: 10.1002/14651858.CD000448.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride MC. (2000) Bufotenine: toward an understanding of possible psychoactive mechanisms. J Psychoactive Drugs 32:321–331. [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Grob CS, Brewerton TD (2016) Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA. Lancet Psychiatry 3:481–488. [DOI] [PubMed] [Google Scholar]
- NCCIH: National Center for Complementary and Integrative Health (NCCIH) (2016) Available at: https://nccih.nih.gov/grants/naturalproducts. Retrieved June 26, 2019.
- NIMH: National Institute of Mental Health (NIMH) (2017) Available at: https://www.nimh.nih.gov/funding/grant-writing-and-application-process/concept-clearances/2017/the-nimh-psychoactive-drug-screening-program-pdsp.shtml. Retrieved June 26, 2019.
- Qi J, Zulfiker A, Li C, Good D, Wei MQ (2018) The development of toad toxins as potential therapeutic agents. Toxins 10:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbone J, Zhang L, Zhang M, Xia J, Liu X, Yang Y (2005) Chinese herbal medicine for schizophrenia. Cochrane Database Syst Rev. 4:CD003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell TS, Belkacemi T, Flockerzi V, Beck A (2014) Protonophore properties of hyperforin are essential for its pharmacological activity. Sci Rep 4:7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood AM, Prisinzano TE (2018) Novel psychotherapeutics - a cautiously optimistic focus on Hallucinogens. Expert Rev Clin Pharmacol 11:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasuka K, Yasui T, Ishigami T, Nakata K, Matsumoto R, Ikeda K, Maeto K (2015) Host manipulation by an ichneumonid spider ectoparasitoid that takes advantage of preprogrammed web-building behaviour for its cocoon protection. J Exp Biol 218:2326–2332. [DOI] [PubMed] [Google Scholar]
- Takeda N. (1994) Serotonin-degradative pathways in the toad (Bufo japonicus) brain: clues to the pharmacological analysis of human psychiatric disorders. Comp Biochem Physiol Pharmacol Toxicol Endocrinol 107:275–281. [DOI] [PubMed] [Google Scholar]
- Taneja G, Chu C, Maturu P, Moorthy B, Ghose R (2018) Role of c-Jun-N-terminal kinase in pregnane X receptor-mediated induction of human cytochrome P4503A4 In Vitro. Drug Metab Dispos 46:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SW, Tang WH, Leonard BE (2017a) Herbal medicine for psychiatric disorders: psychopharmacology and neuroscience-based nomenclature. World J Biol Psychiatry 11:1–19. [DOI] [PubMed] [Google Scholar]
- Tang SW, Tang WH, Leonard BE (2017b) Multitarget botanical pharmacotherapy in major depression: a toxic brain hypothesis. Int Clin Psychopharmacol 32:299–308. [DOI] [PubMed] [Google Scholar]
- Tian J, Zhang F, Cheng J, Guo S, Liu P, Wang H (2014) Antidepressant-like activity of adhyperforin, a novel constituent of Hypericum perforatum L. Sci Rep 4:5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg CC, Britt JR, Evans JR, Akee RK, Whitt JA, Trinh SK, Harris MJ, Thompson JR, Ewing TL, Shipley SM, Grothaus PG, Newman DJ, Schneider JP, Grkovic T, O’Keefe BR (2018) NCI program for natural product discovery: a publicly-accessible library of natural product fractions for high-throughput screening. ACS Chem Biol 13:2484–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M; STAR*D Study Team (2006) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163:28–40. [DOI] [PubMed] [Google Scholar]
- Ujváry I. (2014) Psychoactive natural products: overview of recent developments. Ann Ist Super Sanita 50:12–27. [DOI] [PubMed] [Google Scholar]
- UNODC early warning advisory (EWA) on new psychoactive substances (NPS) (2019) United Nations Office on Drugs and Crime. Available at: https://www.unodc.org/LSS/Home/NPS. Retrieved on June 26, 2019.
- Uthaug MV, van Oorsouw K, Kuypers KPC, van Boxtel M, Broers NJ, Mason NL, Toennes SW, Riba J, Ramaekers JG (2018) Sub-acute and long-term effects of ayahuasca on affect and cognitive thinking style and their association with ego dissolution. Psychopharmacology (Berl) 235:2979–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthaug MV, Lancelotta R, van Oorsouw K, Kuypers KPC, Mason N, Rak J, Šuláková A, Jurok R, Maryška M, Kuchař M, Páleníček T, Riba J, Ramaekers JG (2019) A single inhalation of vapor from dried toad secretion containing 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in a naturalistic setting is related to sustained enhancement of satisfaction with life, mindfulness-related capacities, and a decrement of psychopathological symptoms. Psychopharmacology (Berl) doi: 10.1007/s00213-019-05236-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DT, Horng JS, Bymaster FP, Hauser KL, Molloy BB (1974) A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine. Life Sci 15:471–479. [DOI] [PubMed] [Google Scholar]
- Wong DT, Perry KW, Bymaster FP (2005) Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov 4:764–774. [DOI] [PubMed] [Google Scholar]