Abstract
Background
The hallmark characteristics of the murine model of drug addiction include the escalation of cocaine consumption and compulsive punishment-resistant drug seeking. In this study, we evaluated the motivation for drug seeking in cocaine self-administering rats exposed to an escalated dosing regimen that endeavored to mimic the characteristic of escalating drug intake in human addicts. Tropisetron is a 5-HT3 receptor antagonist and α7-nicotinic receptor partial agonist. Utilizing rats trained on the escalated-dosing regimen, we examined the effects of tropisetron on control over compulsive drug-seeking behavior that was defined as footshock-resistant lever pressing.
Methods
Rats were trained to self-administer cocaine with incremental-infusion doses (from 0.6 to 2.4 mg/kg/infusion) across training sessions (3 h/session) or with a long-access paradigm (i.e., 0.6 mg/kg/infusion, 6 h/d training session). The drug-seeking motivations of 2 groups were estimated by the patterns of drug intake and progressive-ratio schedule. The compulsivity for drug seeking of the group with an escalated dose was further evaluated using the footshock-associated seeking-taking chain task.
Results
The rats trained on the dose-escalated protocol achieved the same levels of motivated drug seeking as those subjected to a long-access paradigm, as indicated by cocaine intake per training session and breakpoints on a progressive ratio schedule. Tropisetron attenuated compulsive behavior of rats when pressing of the seeking lever potentially led to footshock. Intriguingly, tropisetron did not change the motivation to seek cocaine when footshock was absent. Tropisetron had no effect on locomotor activities or saccharin self-administration.
Conclusions
These results demonstrate that tropisetron restored control over compulsive cocaine seeking, and they indicate that 5-HT3/α7-nicotinic receptors may be potential therapeutic targets for relieving compulsive drug seeking.
Keywords: drug addiction, self-administration, compulsive cocaine seeking, footshock, tropisetron
Significance Statement.
The present study developed an alternative model for compulsive drug-seeking behavior via training animals to self-administer ascending drug dose across training sessions. The benefits of using this model as a substitute for the long access paradigm are to improve the experimental efficiency since this model involved 50% less self-administration training time and to screen pharmacotherapeutic targets for drug addiction. Furthermore, we found that tropisetron restored control over compulsive cocaine seeking, indicating that 5-HT3/α7-nicotinic receptors may be potential therapeutic targets for relieving compulsive drug seeking.
Introduction
As described in the DSM-V, drug addiction is characterized in part by using larger quantities of a drug or using a drug over a longer period than was originally intended in the face of adverse consequences. In accordance with these characteristics, the escalation of drug consumption and compulsive drug seeking that resists punishment are phenotypic hallmarks in some animal models of drug addiction. Typically, long access to a drug (i.e., 6 h/d training session) allows escalated intake and exacerbates compulsivity of drug seeking (Ahmed and Koob, 1998). Moreover, an intermittent-access procedure during a 6-hour self-administration session increases incentive motivation to take a drug (Allain et al., 2018). In addition to extending access duration to 6 hours, ascending drug dosages across training sessions might also mimic excessive drug consumption. For example, switching to a higher cocaine dose from 0.6 to 1.2 mg/kg/infusion results in escalated intake on a fixed ration = 1 (FR1) schedule of reinforcement during 2-hour sessions (Mandt et al., 2012), although it remains unknown whether self-administration training with ascending drug dose triggers the compulsive drug seeking.
Deficient inhibitory control, such as that of compulsive drug seeking, may reflect impaired cognitive flexibility in drug addicts (Ornstein et al., 2000; Dalley et al., 2011; Ersche et al., 2012; Solbakk and Lovstad, 2014; Yan et al., 2014). It is noteworthy that both serotonergic (5-HT) and cholinergic (Ach) transmission are tightly associated with cognitive function via modulating prefrontal pyramidal and interneuron activity (Meneses, 1999; Parikh and Sarter, 2008; Celada et al., 2013). Specifically, α7 nicotinic acetylcholine receptors (nAchRs) and 5-HT3 receptors belong to the Cys-loop ligand-gated ion channel receptor superfamily and are distributed abundantly in the cerebral cortex, limbic system, and hippocampus. Numerous studies show that the ionotropic α7 nAchR- or 5-HT3 receptor-modulated neuronal activities are involved in cognitive functions (Thompson and Lummis, 2007; Lendvai et al., 2013; Beinat et al., 2015; Svob Strac et al., 2016). Tropisetron, a 5-HT3 receptor antagonist and partial agonist of α7 nAchRs, has recently been used to improve cognitive deficits in schizophrenia and Alzheimer’s disease (Zhang et al., 2012; Hashimoto, 2015). Also, one preclinical study reported that tropisetron ameliorates phencyclidine-induced cognitive deficits in mice (Hashimoto et al., 2006). However, it is still unknown whether the multi-functional drug tropisetron ameliorates cognitive flexibility to drug seeking-related punishment.
In the present study, we trained rats to self-administer cocaine with incremental infusion doses (from 0.6 to 2.4 mg/kg/infusion) across training sessions (3 h/session) to model an escalation in cocaine consumption. These dose-escalated (DE) rats exhibited similar progressive ratio (PR) breakpoints as that of rats trained with long access (LgA) self-administration (0.6 mg/kg/infusion, 6 h/session) and higher PR breakpoints than those of rats initially trained with a constant dose (0.6 mg) with short access (3 hours). Here we examined whether tropisetron alleviates compulsive cocaine-seeking behavior in a DE model of self-administration.
Methods
Animals
All of the procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Animal Care and Use, and Committees of Peking University or Ningbo University, China. Male Sprague Dawley rats (250 g on arrival; Beijing Vital River Laboratory Animal Technology Co. Ltd.) were housed 4 per cage in polycarbonate cages in a temperature- and humidity-controlled environment with a 12-h-dark/-light cycle (lights on at 8:00 pm). Experiments were conducted between 8:00 am and 8:00 pm. during the rats’ dark cycle. All of the rats received unlimited access to food and drink prior to food restriction. Subsequently, food restriction (20 g/d of rat chow) was introduced 1 day before behavioral training and was maintained throughout the entire training procedure.
Jugular Vein Catheter Implantation
The procedure we employed for jugular-vein catheter implantation has been described thoroughly in previous studies (Shen et al., 2011, 2014). In brief, rats were deeply anesthetized through separate injections of sodium pentobarbital (50 mg/kg, i.m.) and xylazine hydrochloride (5 mg/kg, i.m.). Custom-made catheters consisted of a 3.5-cm silicone tube (SILATIC, Dow Coming Corporation) and a 10-cm polyethylene tube (PE 20, Becton Dickinson). After the silicone tip of the catheter was implanted into the jugular vein, the polyethylene compartment of the catheter was embedded subcutaneously and secured with VELCRO straps in the back area. To prevent clogging, heparin (2 mg/mL/kg) dissolved in normal saline was injected through the catheter twice a day after the surgery.
Cocaine Self-Administration Training
Figure 1A illustrates the cocaine self-administration training protocol. Cocaine self-administration occurred in standard operant chambers with a house light, 2 cue-lights, and a tone generator (AniLab Software & Instruments Co., Ltd., Ningbo, China). Custom-made retractable levers and grid floors connected to electric stimulators (Beijing Jingheqi Technology Co., Ltd. Beijing, China) were equipped in the chambers. After 7 days of recovery from the surgery, the rats received cocaine self-administration training with a consistent dose (0.6 mg/kg/infusion, 0.6 mg/mL delivered over 3–5 seconds) of cocaine infusion for 10 days (i.e., regular training phase). The infusion amount for each rat depended on its body weight and was adjusted by changing the delivered time accordingly. During the regular training procedure, one press on the active lever resulted in a single delivery of cocaine (i.e., FR1) through the catheter, which was coupled with illumination of a cue light above the active lever and a discrete tone for 5 seconds followed by a 20-second timeout period. Cocaine self-administration sessions were terminated either after either 50 cocaine infusions or 3 hours of training time, depending on which criterion was reached first.
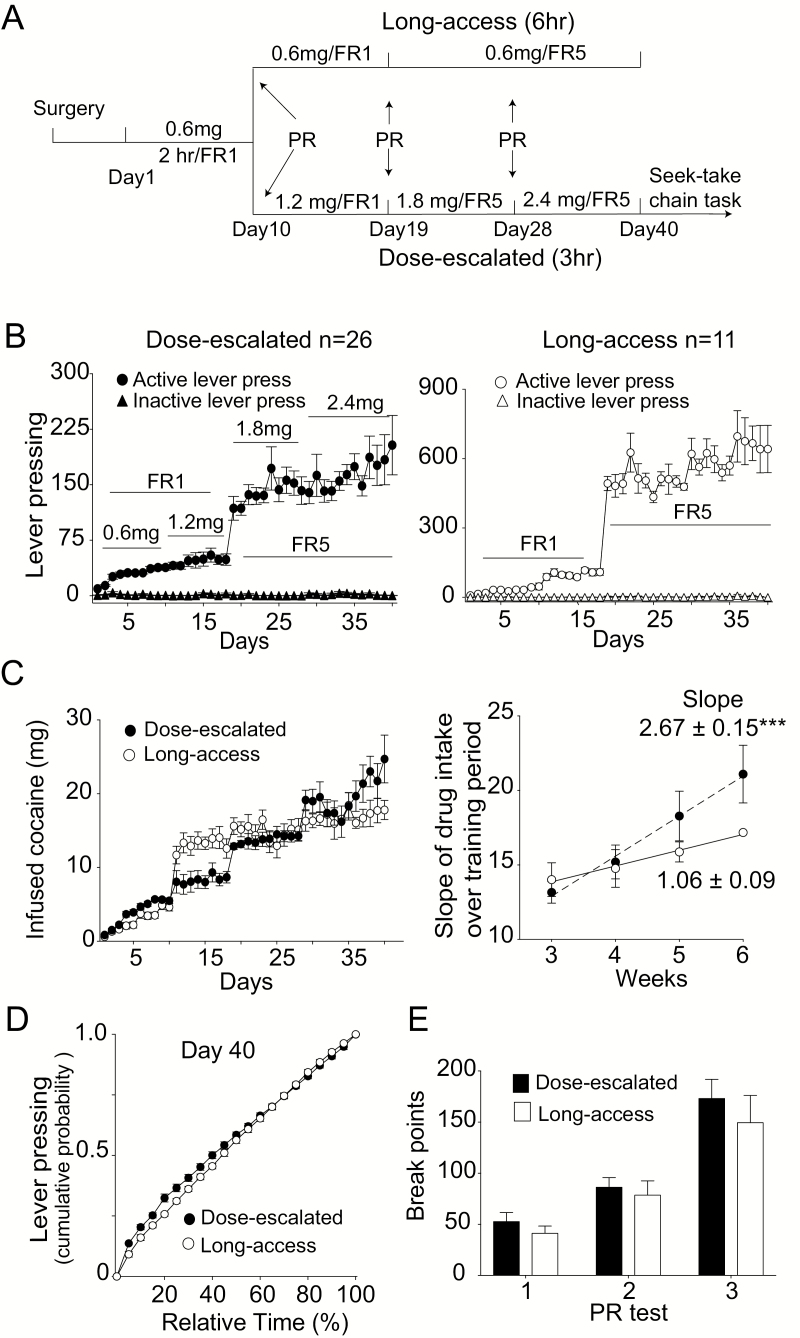
Figure 1.
Cocaine self-administration training in 3-hour sessions with escalated dosing achieved the same level of cocaine seeking as that of long access (LgA) training (6-hour sessions). (A) The paradigm of cocaine self-administration with escalated dosing or LgA. The arrows indicate the time for progressive ratio (PR) testing. (B) Active and inactive lever pressings during self-administration with escalated dosing (B1, n = 26) or LgA (B2, n = 11). (C) Left: cocaine intake per session during self-administration with escalated dosing or LgA. Right: the slope of drug intake over dose-escalated (DE) or LgA training period (i.e., from weeks 3 to 6). DE-trained rats show a greater slope of voluntary intake than LgA-trained rats (***P < .001 compared with LgA group). (D) The temporal pattern of drug-seeking behavior during the training. Both groups showed linear distributions, indicating invariable lever pressings during the training. (E) The breakpoint under PR testing. The first PR test represented the data from rats initially trained with a constant dose (0.6 mg/kg) and short access (2 hours) for 10 days. Then, these rats were randomly divided into LgA or DE groups. The breakpoints by the second and third PR tests represented the data from the LgA or DE group. Two-way ANOVA analysis indicated training history (escalated dosing vs LgA) had the same effect on the breakpoint. Data are represented as the mean values ± SEM.
After the regular training phase, the cocaine self-administering rats were divided into DE and LgA groups (Figure 1A). In the DE group, the cocaine infusion dose was doubled (1.2 mg/kg/infusion, 0.05 mL delivered over 3–5 seconds), tripled (1.8 mg/kg/infusion), and quadrupled (2.4 mg/kg/infusion). Typically, 7 to 10 sessions with each dose were needed to reach a stable level of consumption (<20% variation in 3 consecutive days) prior to switching to the next dose. To prevent overdosing, each session ceased after 40 cocaine infusions or 3 hours, depending on which criterion was achieved first. To increase the response rate (Ferster and Skinner, 1957), a fixed-ratio5 schedule was introduced during the triple- and quadruple-training doses.
Animals in the LgA group received the same dose of cocaine as in regular training (0.6 mg/kg/infusion), and the LgA training sessions were terminated after either after 120 cocaine infusions or 6 hours of training time depending on which criterion was reached first. Rats in the LgA training group received a PR test every 10 to 12 days. After the second PR test, a fixed-ratio5 was introduced into the LgA training procedure (Figure 1A).
The slope of average daily intake per week over the DE or LgA training period (i.e., week 3–6 from Figure 1C left) was used to evaluate the escalation of voluntary intake over time. The cumulative distribution of active lever-pressing was calculated by the accumulated numbers of lever-pressing with 5-minute bin-crossing training times per session, which was used to compare drug-seeking patterns between the DE and LgA training groups (Wise and Koob, 2014).
PR Test
During the PR test, the number of active lever presses required to acquire 1 cocaine infusion (0.6 mg/kg/infusion with 20-second timeout) increased as follows: 1, 3, 6, 9, 12, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, 268, 346, 445, 574, and 737 lever presses (Richardson and Roberts, 1996). The PR test would be terminated once 60 minutes had elapsed without a reward. The breakpoint was defined as the last completed response during the PR test. After the first PR test, rats were separated into the DE group (n = 26) and LgA (n = 11) group, and both groups initially had the same breakpoint score. Thus, the animals assigned to the DE and LgA groups experienced 2 more PR tests.
Seeking-Taking Chain Task
After cocaine DE or saccharin self-administration, rats advanced into the seeking-taking chain task (STCT) (Vanderschuren and Everitt, 2004; Pelloux et al., 2007; Chen et al., 2013). Each cycle of the STCT initiated with an insertion of the seeking lever only. Seeking-lever presses resulted in taking-lever insertion and seeking-lever retraction simultaneously. Taking lever presses triggered a single cocaine infusion (1.2 mg/kg/infusion) or delivery of 1 drop of saccharin solution (0.2 mL, 0.1% in dH2O) accompanied by both cue-light illumination and a discrete tone for 5 seconds. Once the rewards were delivered, the taking lever was retracted. The interval between each cycle was 20 seconds. During STCTs, rats underwent 4 schedules of training from FR1, FR10, FR30 (RI15), to FR60 (RI30). The STCT training on each schedule took 5 to 7 days. Once a rat earned 10 cocaine infusions or saccharin deliveries at over 3 consecutive days with <20% variation, the rats were moved to a schedule of FR30 (RI15) or FR60 (RI30). With these schedules, seeking lever pressing after random intervals (0.1–30 seconds, average 15 seconds or 0.1–60 seconds, average 30 seconds) following the completion of FR30 or 60, triggered taking-lever insertion and seeking-lever retraction. One taking-lever press yielded 1 cocaine infusion.
Punishment
According to a previous study, punishment and reward must be scheduled separately within each trial during STCT (Pelloux et al., 2007). During punishment sessions, a mild footshock (0.4 mA, 0.5 seconds) was added to the FR60 (RI30) STCT, with the 60th press of the seeking-lever triggering a mild footshock with 50% probability after a random interval from 0.1 to 60 seconds. Then, the taking-lever was inserted and the seeking lever was retracted. One press on the taking lever would lead to 1 infusion of cocaine (1.2 mg/kg/infusion) or 1 drop of 0.1% saccharin solution. Fifteen minutes before STCT with footshock conditioning, systemic tropisetron (1.0 or 3.0 mg/kg, i.p.) was administrated to the footshock-resistant rats to examine its effect on compulsive seeking behavior. The dose of tropisetron was identified as effective in a rat model of phencyclidine-induced cognitive deficits (Hashimoto et al., 2006) and in memory-related task performance in rats (Callahan et al., 2017; Poddar et al., 2018). Pharmacokinetic analysis revealed the half‐life of tropisetron in the plasma was approximately 6 hours after i.v. and oral administration (Kees et al., 2001; Roila et al., 2010). In the present study, only the footshock-resistant group that exhibited compulsive behavior was considered to be drug-addicted rats, while the footshock-sensitive group was considered to be drug-liking rats. We were concerned about whether tropisetron improves addictive behavior, and thus the effect of tropisetron was tested only on the footshock-resistant group.
Saccharin Self-Administration Training
In this study, saccharin was used as “natural rewards” to train self-administrated rats (Choi et al., 2002). Reportedly, SD rats consumed more saccharin water when the concentration is between 0.1% and 0.3% and the preference peak of saccharine concentration is 0.1% (Sclafani et al., 2010). There were 2 separated groups trained with saccharin self-administration. One group was used for testing the effect of footshock in rats with saccharin STCT. These rats underwent regular saccharin self-administration training around 10 sessions before entering STCT in an operant chamber equipped with retractable levers. One press on the active lever resulted in delivery of a drop of 0.1% saccharin solution paired with a tone and cue light. After the 20-second time-out period, a new trial was initiated with the illumination of the house light. The session was terminated after rats earned 50 deliveries of saccharin or after 3 hours, depending on which criterion was achieved first. Once advancing into the STCT, 20 hours of food deprivation and 4 hours of water deprivation were conducted prior to each training session.
Another group was used for testing whether tropisetron altered natural reward in cocaine-addicted rats. The rats from this group had experienced cocaine DE self-administration and exhibited footshock-resistant behavior. Since a similar context or cue might provoke seeking behavior in cocaine-trained rats, the retractable levers were replaced by 2 nose-poking holes that were mounted 3 cm above the floor on same side of the training chamber. Additionally, the grid floor used for cocaine self-administration was replaced by an aluminum plate with circular holes (circle diameter = 1.5 cm, distance between hole centers = 3.0 cm). Once triggers in the active poke were activated, the pump would deliver a drop of saccharin solution (0.1%, 0.1 mL, 6 seconds) as well as coincident activation of a cue light and tone over 5 seconds, followed with 20-second time-out periods. Rats underwent limited water access for 6 hours before training. Saccharin self-administration training continued around 5 days until rats established stable nose-poke numbers (more than 40 in 3 consecutive days) and saccharin consumption (more than 4 mL in total). To test the effect of tropisetron on natural reward, rats received systemic delivery of tropisetron (3.0 mg/kg/mL, i.p., MedchemExpress China) or saline (1 mL/kg, i.p.) 15 minutes before saccharin self-administration training on the sixth day.
Open Field Test
After habituation for 3 days, rats were placed in open-field chambers (45 × 45 cm, AniLab), and locomotor activity was recorded through night vision cameras for 1 hour. Then rats were injected with tropisetron (3 mg/kg, i.p.) or saline (1 mL/kg, i.p.) and were returned to the same chamber for 2 hours of locomotor testing.
Data Analysis and Statistics
All of the data were analyzed using 1- or 2-way ANOVAs followed by Bonferroni post hoc tests. Values of P < .05 were considered statistically significant. All of the statistical tests were conducted using Prism software (Graphpad, La Jolla, CA).
Result
Cocaine Self-Administration Training With Escalated Dosing Exhibits Similar Drug-Seeking As That Induced by the Long-Access Training Paradigm
To test whether DE training potentially increased the motivation to seek cocaine, we compared our DE training procedure with traditional LgA training. The detailed training procedures are shown in Figure 1A–B. Before either DE or LgA training, all of the rats pre-experienced 10 to 12 sessions of regular cocaine self-administration training and PR testing. Figure 1B shows the active and inactive lever-pressing for each session in the DE group (left) and LgA group (right). Although the duration of LgA training sessions was twice as long as those of DE-training sessions, training history had no significant effect on the amount of cocaine intake over all of the sessions (Figure 1C left; 2-way ANOVA, factor A days: F (39, 1069) = 35.61, P < .0001; factor B training paradigm: F (1, 1069) = 0.1125, P = .7374; days × training paradigm interaction: F (39, 1069) = 2.555, P < .0001). There are 2 reasons that may cause more lever presses by the LgA group compared with those of the DE group. First, the LgA group was trained for 6 hours per session, while the DE group was only trained for 3 hours. Second, the DE group received a higher dose of cocaine (1.8–2.4 mg/kg) than did the LgA group. The slope of drug intake over the DE- or LgA-training period from week 3 to week 6 (Figure 1C left) was used as an indicator of escalating voluntary intake (McNamara et al., 2010; Ducret et al., 2016). DE-trained rats demonstrated a greater slope of drug intake than that of LgA-trained rats, indicating greater escalation of voluntary intake in the DE group (Figure 1C right; F (1, 4) = 84.94, P < .001).
To understand the temporal pattern of drug seeking during the training session, we analyzed the cumulative probability distribution of active lever pressing within the 3-hour training session on day 40. Both groups showed linear distributions (Figure 1D), indicating invariable lever pressings during training. The timing of each PR testing during the self-administration training is shown in Figure 1A. The first PR test represented the data from rats initially trained with a constant dose (0.6 mg/kg) and short access (2 hours) for 10 days. A 2-way repeated-measures ANOVA showed that the timing of PR testing, but not the training protocol, significantly affected breakpoints (Figure 1E; factor A PR training times: F (2, 74) = 30.77, P < .0001; factor B training paradigm: F (1, 74) = 1.433, P = .2351; training times × training paradigm interaction: F (2, 74) = 0.1565, P = .8554). Taken together, these results suggest that DE training resulted in similar effects on cocaine-seeking motivation as long-access training.
Group Response to Footshock Punishment
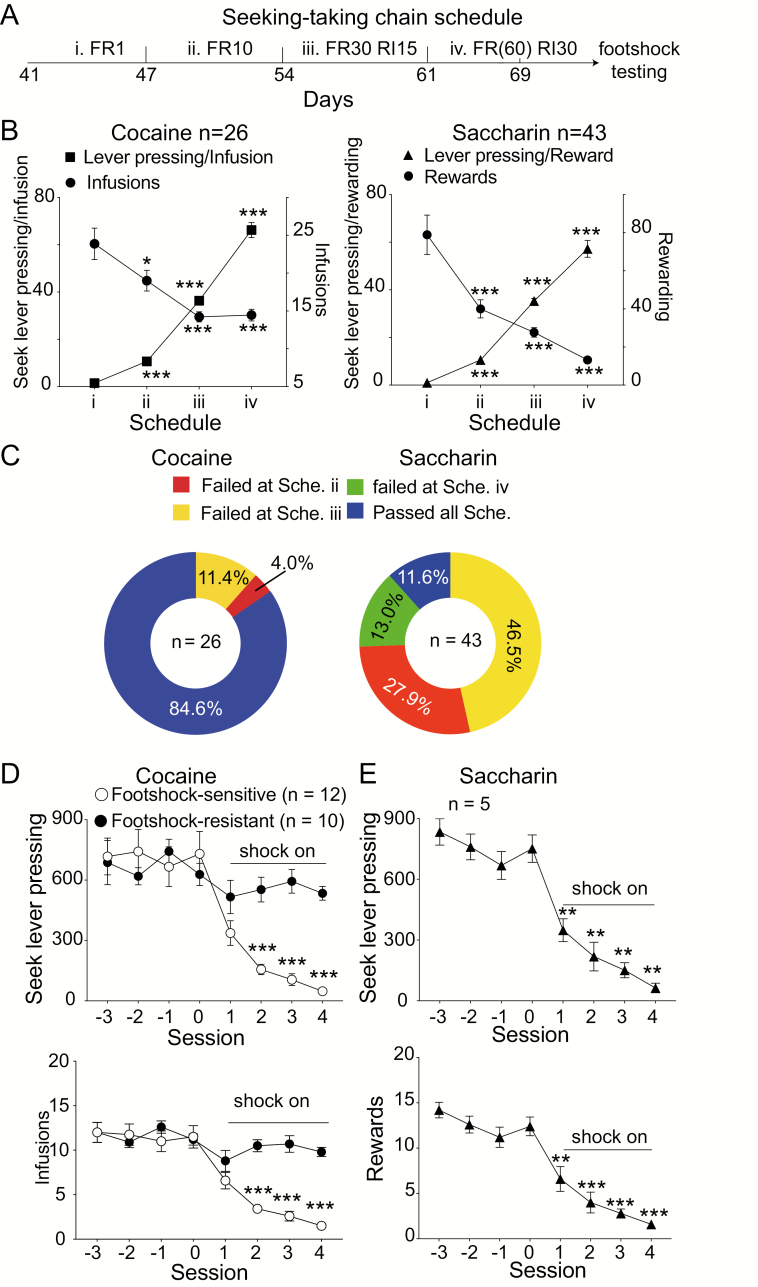
Because the hallmarks for addiction include both the escalation of cocaine consumption and compulsive drug seeking that resists to punishment-resistant drug seeking, we further estimated the compulsivity for drug-seeking in DE rats using footshock-associated STCT. Rats underwent 4 stages of training from FR1, FR10, FR30 (RI15), to FR60 (RI30) during STCTs (Figure 2A). Rats exhibited enhanced drug-seeking motivation indicated by an increased average number of seeking lever presses for 1 infusion (1-way ANOVA analysis, F (3,63) = 433.7, P < .0001), although the infusion numbers diminished correspondingly (1-way ANOVA analysis: F = 9.929, P < .0001, factor training procedure: F (3, 52) = 9.929, P < .0001). Multiple comparisons showed that the number of seeking lever presses for 1 infusion with schedules II, III, or IV was higher than that for schedule I, while the infusion number per session was lower than that for schedule I (Bonferroni’s post hoc tests; Figure 2B, left). During schedules III or IV, the reduction of acquired infusions or rewards was because the FR was set to a higher level (FR = 30 or 60) and because a random interval was induced (Figure 2B, left). Twenty-two of 26 rats passing all schedules of the STCT (Figure 2C, left) would experience punishment procedures in which 50% of seeking lever presses were linked to footshock. Rats conducting >400 seeking lever presses (549.4 ± 16.59) and earning >10 cocaine infusions (average 13.6 ± 0.87, 10 of 22 rats) over at least 3 consecutive days were considered footshock-resistant, while those conducting <200 seeking lever presses (103.1 ± 18.17) and earning <10 cocaine infusions (2.5 ± 0.33; 12 of 22 rats) were considered footshock-sensitive (Figure 2D). Two-way ANOVA showed that the seeking lever presses (2-way ANOVA, factor A footshock: F (7, 140) = 23.03, P < .0001; factor B grouping: F (1, 20) = 5.081, P = .0356; grouping × footshock interaction: F (7, 140) = 11.28, P < .0001) and drug infusions (2-way ANOVA, factor A footshock: F (7, 140) = 28.18, P < .0001; factor B grouping: F (1, 20) = 14.53, P = .0011; grouping × footshock interaction: F (7, 140) = 15.11, P < .0001) in the footshock-sensitive group were significantly diminished when rats faced potential punishments. Multiple comparisons showed that the number of seeking lever presses or infusions in the footshock-sensitive group during sessions of “shock on” was remarkably lower than that of “shock off” (Bonferroni’s post hoc tests; Figure 2D).
Figure 2.
Following training of the seeking-taking chain tasks (STCT), a portion of the cocaine-trained rats with escalated dosing showed resistance to punishment. (A) The paradigm of the STCT with different parameters and punishment schedule. (B) The number of seeking responses and infusion (or rewarding) per session under different training schedules for cocaine (left) or saccharin water (right). *P < .05, ***P < .001 compared with schedule I using a Bonferroni’s post hoc. (C) The pie chart indicates the proportion of cocaine- (left) or saccharin-administered (right) rats failing or passing the stages of the seeking-taking chain schedule. (D) About 45% of cocaine-administering rats that passed all of the stages of the STCT showed resistance to punishment. (E) All of the saccharin-administered rats were sensitive to punishment. **P < .01, ***P < .001 compared with the session “0” using a Bonferroni’s post hoc. All of the data are represented as the mean values ± SEM.
In another group, rats were trained for sweetened water (0.1% saccharin) self-administration with similar STCTs. Along STCT schedules, rats also exhibited enhanced saccharin-seeking motivation that was indicated by an increased average number of seeking lever presses for saccharin reward (1-way ANOVA analysis, F (3, 30) = 324.4, P < .0001), although the infusion numbers diminished correspondingly (1-way ANOVA analysis, F = 16.64, P < .0001, factor training procedure: F (3, 23) = 16.64, P = .037). Multiple comparisons showed that the number of seeking lever presses for 1 drop of 0.1% saccharin significantly increased with schedules II, III, or IV compared with that of schedule I, while the number of saccharin intakes significantly decreased (Bonferroni’s post hoc test; Figure 2B right). Only 5 of 24 rats passed all schedules of STCTs (Figure 2C, right). Rats manifesting >450 seeking lever presses (753.5 ± 33.87) and earning >10 saccharin-water deliveries (12.6 ± 0.6164) over the last 3 days of FR60 (RI30) showed significant decreases in seeking lever presses (Figure 2E; 1-way ANOVA analysis, F = 28.04, P < .0001) and saccharin-water delivery (Figure 2E; 1-way ANOVA analysis, F = 26.8, P < .0001). Multiple comparisons showed that both seeking lever presses and saccharin delivery were remarkably inhibited during sessions of “shock on” compared with that during “shock off” (Bonferroni’s post hoc test; Figure 2E).
Tropisetron Restores the Control Over Compulsive Cocaine Seeking in Footshock-Resistant Rats
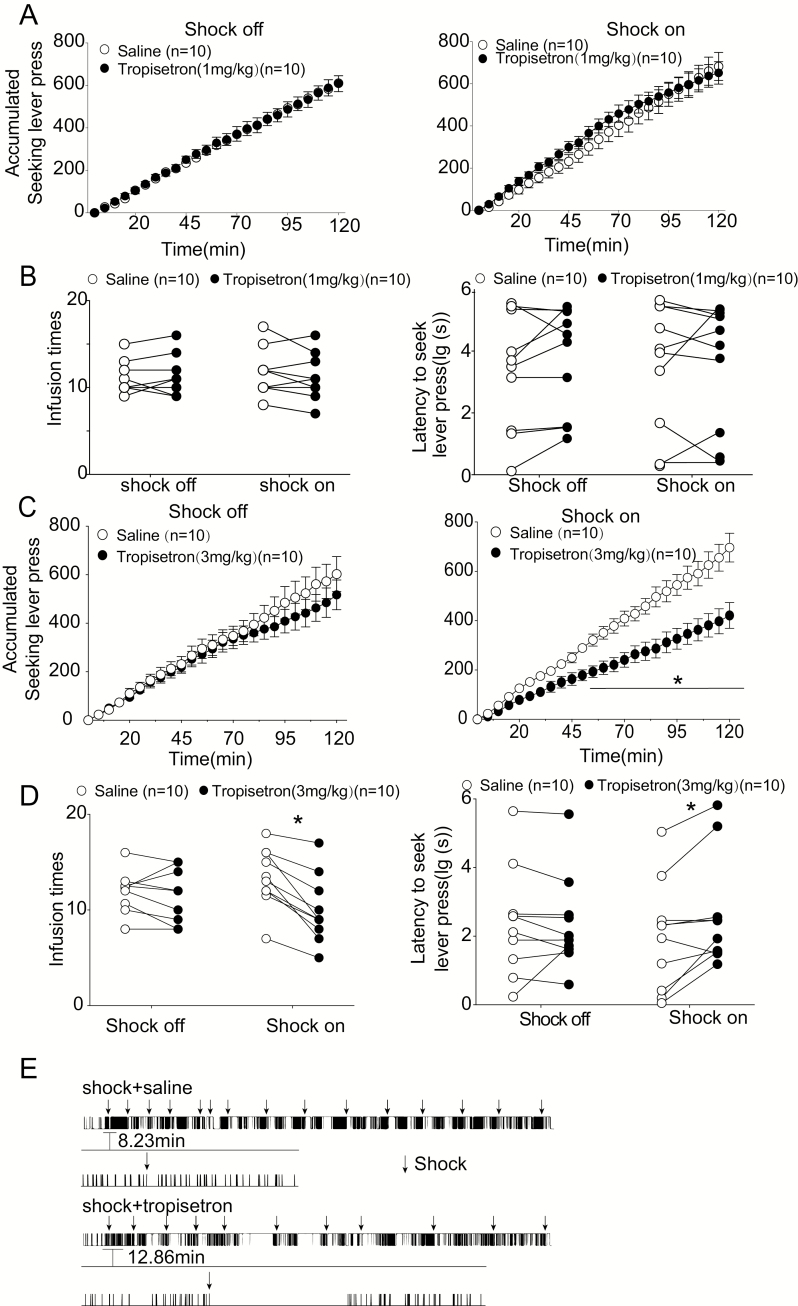
Footshock-resistant rats were used to examine the effect of systemic tropisetron (1.0 and 3.0 mg/kg, i.p.; 15 minutes before testing) on the compulsive cocaine seeking. The dose of tropisetron was identified as effective in a rat model of phencyclidine-induced cognitive deficits (Hashimoto et al., 2006) and in memory-related task performance in rats (Callahan et al., 2017; Poddar et al., 2018). Figure 3A shows accumulative seeking lever presses within 5-minute bins during the testing session. Tropisetron (1.0 mg/kg) did not alter the seeking lever-press behavior, regardless of whether pressing could trigger a footshock (Figure 3A right, for “shock on,” 2-way ANOVA, factor A: cumulative time F (24, 432) = 190.3, P < .0001; factor B: treatment F (1, 18) = 0.4075, P = .5313; interaction: F (24, 432) = 0.8858, P = .6224; Figure 3A left, for “shock off”, factor A: cumulative time F (24, 432) = 342.5, P < .0001; factor B: treatment F (1, 18) = 0.01004, P = .9213; interaction: F (24, 432) = 0.1127, P > .99). Tropisetron (1 mg/kg) did not affect cocaine infusion (Figure 3B left, 2-way ANOVA, factor A: footshock F(1, 9) = 0.2789, P = .6102; factor B: tropisetron F(1, 9) = 0.6164, P = .4525; footshock × tropisetron interaction: F (1, 9) = 3.183, P = .1081) or the latency to press the seeking levers at the second cycle of footshock testing (Figure 3B right, 2-way ANOVA, factor A: footshock F(1, 9) = 0.0002, P = .9892; factor B: tropisetron F(1, 9) = 1.73, P = .2209; footshock × tropisetron interaction: F (1, 9) = 0.5328, P = .4840). However, tropisetron with a higher dose (3.0 mg/kg) significantly inhibited the seeking lever presses if pressing had a potential to trigger footshock (2-way ANOVA, factor A: time F (24, 432) = 159.3, P < .0001; factor B: treatment F (1, 18) = 13.22, P = .0019; time × treatment interaction: F (24, 432) = 10.15, P < .0001). Multiple comparisons showed that tropisetron remarkably inhibited seeking lever presses from the 55th minute when footshock was available (Bonferroni’s post hoc test; Figure 3C right). When footshock was turned off, tropisetron (3.0 mg/kg, i.p.; 15 minutes before testing) had no effect on seeking responses (Figure 3C left, 2-way ANOVA, factor A: cumulative time F (24, 432) = 122.8, P < .0001; factor B: treatment F (1, 18) = 0.4937, P = .4913; cumulative time × treatment interaction: F (24, 432) = 1.352, P = .1249). Moreover, tropisetron (3 mg/kg) significantly suppressed cocaine intake when the seeking lever triggered footshock conditioning (Figure 3D left, 2-way ANOVA, factor A: tropisetron F (1, 9) = 0.4584, P = .5154; factor B: footshock F (1, 9) = 97.55, P < .0001; footshock × tropisetron interaction: F (1, 9) = 8.205, P = .0186). Interestingly, we observed that tropisetron extended the latency to press the seeking lever at the second cycle of footshock testing, indicating more hesitation to press the lever in rats previously undergoing punishment training (Figure 3D right, 2-way ANOVA, factor A: tropisetron F (1, 9) = 0.2459, P = .6318; factor B: footshock F (1, 9) = 4.889, P = .0541; footshock × tropisetron interaction: F (1, 9) = 5.263, P = .0475). Multiple comparisons showed that, compared with the saline-treated group, tropisetron significantly decreased cocaine intake and increased the latency to press the seeking levers when footshock was available (Bonferroni’s post hoc test; Figure 3D). These results revealed that tropisetron might potentiate the inhibitory effect of punishment on compulsive drug seeking. Representative samples of seeking lever-pressing under footshock conditioning with an FR60 (RI30) schedule from a footshock-resistant rat in the saline- or tropisetron-treated group are shown in Figure 3E.
Figure 3.
Tropisetron restored control over compulsive cocaine seeking. (A) Tropisetron (1.0 mg/kg) did not alter the seeking lever-press behavior, regardless of whether pressing could trigger a footshock. (B) Tropisetron (1 mg/kg) did not affect cocaine infusion or the latency to press the seeking levers at the second cycle of footshock testing. (C) Tropisetron (3.0 mg/kg) attenuated seeking responses when seeking lever pressing could potentially deliver footshock (*P < .05 from the 55th minute compared with the saline-pretreated group when footshock was available, Bonferroni’s post hoc test; right). (D) Tropisetron (3.0 mg/kg) reduced cocaine intake and extended the latency to seek lever press at the second cycle of footshock testing. *P < .05 compared with saline-pretreated group using a Bonferroni’s post hoc. All data are mean values ± SEM. (E) Examples of seeking lever presses from 1 rat during footshock session (2 hours) with vehicle without tropisetron (3.0 mg/kg, i.p.) treatment. Sticks and arrows represent seeking lever presses and footshock, respectively.
Tropisetron Had No Effect on Locomotor Activities and Natural Reward
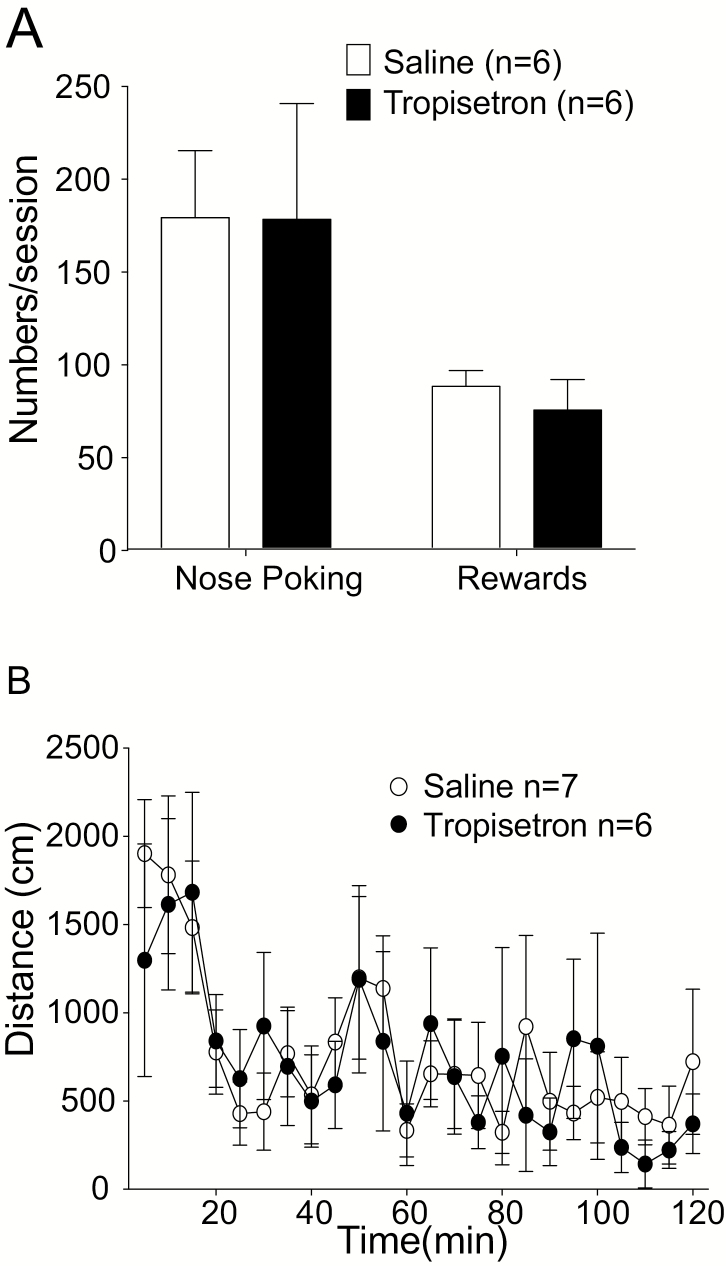
To test whether tropisetron altered natural reward, footshock-resistant rats were trained for sweetened water self-administration, in which nose poking caused the delivery of a drop of sweetened water. Additionally, we investigated the effect of tropisetron on locomotor activity. No effect of tropisetron was observed on natural reward or locomotor activity (Figure 4A, paired t test, t = 0.035, df = 5, P = .9733 for nose poking, t = 1.581, df = 5, P = .1747 for rewards; Figure 4B, 2-way ANOVA, time lapse × treatment interaction: F (23, 258) = 0.3869, P = .9956).
Figure 4.
Tropisetron did not affect locomotor activity or the motivation to seek natural reward and locomotor activity. (A) The number of nose-pokes for sweetened water and sweetened water intake after systemic tropisetron was the same as without tropisetron. (B) No difference in locomotor activity was measured between tropisetron- (n = 6) and saline-treated (n = 7) groups. Tropisetron or saline was administered 5 minutes before placing the animal into an open field chamber and locomotor activity was monitored. Data are represented as the mean values ± SEM.
Discussion
The goal of the present study was to develop an alternative model for compulsive drug-seeking behavior via training animals to take higher doses of cocaine and to investigate the effect of tropisetron on compulsive behavior of punishment-resistant rats by using this model. We validated that the DE model of cocaine self-administration resulted in the same levels of drug-seeking as rats trained using the LgA-training paradigm, as confirmed by cocaine intake per training session and breakpoints during PR tests. Importantly, the DE model escalated drug intake in a manner that was more robust than that of LgA training. In our view, one of the benefits of using the DE model as a substitute for LgA is to improve the experimental efficiency since the DE model involved 50% less self-administration training time. Eventually, 40% of rats that successfully passed through all STCT schedules showed footshock-resistant cocaine seeking.
It had been reported that a subpopulation of rats exhibited resistance to punishment of drug seeking only after self-administration with LgA (Pelloux et al., 2007; Chen et al., 2013; Pelloux et al., 2013). Our results showed that about 38% rats (10 of 26) exhibit footshock-resistance after the training of STCT, which is consistent with the above reports. Thus, it indicates that self-administration with the DE protocol also potentiates the compulsivity of drug seeking. In our present study, rats underwent STCT with a gradually ascending fixed-ratio and random interval crossing sessions, which separates the seeking and taking components (Everitt, 2014). We found that 83.3% of all DE-trained rats passed through STCT training, and 40% of STCT-trained rats showed no change in drug-seeking behavior under footshock conditioning. In contrast, only 11.6% of saccharin rats passed through STCT, and none overcame the footshock conditioning. Most addictive drugs distinctively impaired neural plasticity and synaptic homeostasis enduringly, which links compulsive drug-seeking behavior and relapse (Jones and Bonci, 2005; Kalivas, 2009). It explains that 40% rats from the cocaine group were resistant to footshock, while all rats from the saccharin group failed in this study. Taken together, these results show that DE-trained rats exhibited unmanageable motivation for drug seeking and could be utilized as an appropriate model to screen the pharmacotherapeutic target of drug addiction.
In the present study, only the footshock-resistant group that exhibited compulsive behavior was considered to comprise drug-addicted rats, while the footshock-sensitive group was considered to comprise drug-liking rats. Since we were concerned that tropisetron could simply remit addictive behavior (i.e., compulsive seeking), the effect of tropisetron was tested only on the footshock-resistant group. Tropisetron attenuated both seeking-and taking-lever pressings with footshock conditioning. Although we did not confirm the effect of tropisetron on the PR breakpoint, it seems that tropisetron does not directly impact the drug-seeking because this drug failed to inhibit the seeking lever presses during STCTs when footshock was offline.
Nicotinic AChRs and serotonergic 5-HTRs are widely distributed in layers 3 to 5 of the prefrontal cortex (PFC). In particular, α7 nAChRs and 5-HT3Rs are ligand-gated ion channels mediating cation influx that are localized on both pyramidal neurons and interneurons. While 5-HT3 receptor activation facilitates the activity of cortical GABAergic interneurons and in turn increases inhibitory input to pyramidal neurons (Celada et al., 2013; Leiser et al., 2015), α7 nAChRs increase both excitatory and inhibitory transmission in the PFC (Udakis et al., 2016). Thus, both receptors play a critical role in modulating excitatory-inhibitory balance and cognitive function (Shimizu et al., 2013; Pehrson and Sanchez, 2014). However, few studies have investigated their roles in drug-related addictive behavior or addiction-related cognitive impairment. It has been reported that dihydro-β-erythroidine, a selective α4-nAChR antagonist, prevents the induction of behavioral sensitization to cocaine (Champtiaux et al., 2006). Dihydro-β-erythroidine or the nonselective nicotinic- receptor antagonist mecamylamine disrupts amphetamine-induced behavioral sensitization (Schoffelmeer et al., 2002). A study reported that co-infusion of tropisetron blocked the self-infusion of cocaine in the ventral tegmental area (Rodd et al., 2005). Given the importance of α7 nAchRs and the 5-HT3Rs on cognitive function, and the evidence of tropisetron’s efficacy on improving cognitive deficits in psychiatric disorders (Zhang et al., 2012; Hashimoto, 2015; Callahan et al., 2017; Poddar et al., 2018), the effect of tropisetron on compulsive behavior in footshock-resistant rats using STCTs implies that activating these receptors might restore impaired cognitive flexibility in drug-addicted rats (Dalley et al., 2007; Eagle et al., 2008; Gould, 2010).
It is worth noting that tropisetron might have anxiolytic or analgesic properties due to blocking 5-HT3R (Faerber et al., 2007; Tiippana et al., 2013). Also, tropisetron inhibits footshock-induced reinstatement of alcohol seeking in alcohol-extinguished rats, indicating its therapeutic effect on stress responses (Lê et al., 2006). However, in the present study, if tropisetron attenuated pain perception or stress caused by footshock, it would promote seeking behavior because the impact of punishment triggered by lever-pressing would have been weakened. Alternatively, it cannot be excluded that tropisetron might enhance the shock sensitivity that freezes rats and inhibits drug-seeking behavior. Regardless, our results suggest that tropisetron might reestablish punishment recognition and, thus, restore control over compulsive cocaine seeking. Notably, tropisetron probably did not reduce drug craving because it had no effect on seeking behavior when footshock was absent. To our knowledge, this is the first study to investigate the effects of dual actions to α7 nAchRs and the 5-HT3Rs on compulsive behavior of addicted animals.
It is generally agreed that the PFC exerts top-down inhibitory control, and compulsive behavior results from medial PFC-associated cognitive deficits (Kalivas and Volkow, 2005; Dalley et al., 2011; Munakata et al., 2011). Several studies have reported that drug-addicted animals suffer from cognitive inflexibility when engaged in attentional set-shifting tasks or reversal learning tests (Jentsch et al., 2002; Parsegian et al., 2011). These disrupted cognitive functions in addicted animals may be related to impaired excitatory-inhibitory balance in the PFC and in PFC output (Chen et al., 2013), in which both nicotinic and 5-HT receptors play crucial roles. Interestingly, a partial agonist at α4β2-nicotinic receptors, varenicline, or a selective α7-nAchR agonist, PNU282987, significantly improves performance on reversal learning in both cocaine-naive and cocaine-experienced monkeys (Gould et al., 2013). Therefore, the impact of tropisetron on both α7 nAchRs and 5-HT3Rs might contribute to its therapeutic effect on compulsive cocaine seeking; however, further studies are needed to elucidate the neurocircuitry involved.
In summary, the present study found that an escalated dose model of cocaine use was able to trigger escalation of voluntary cocaine intake and strong drug-seeking. The effect of tropisetron on drug-related compulsivity was tested on rats that experienced DE training that exhibited footshock-resistant cocaine-seeking behavior. Tropisetron augmented the capacity of footshock to attenuate both seeking and taking lever pressings in rats where seeking behavior had proven resistant to punishment-induced reductions. Collectively, these data suggest that DE training provides an efficient and practical model of compulsive drug seeking that may be beneficial for screening pharmacotherapeutic targets for drug addiction.
Acknowledgments
The authors acknowledge Dr Peter Kalivas for providing insightful suggestions on the manuscript and Dr Fu-Qiang Zhang for technical assistance.
This work was supported by the Natural Science Foundation of China (grant nos. 81271472 and 31571094 to H.-W.S).
Statement of Interest
None.
References
- Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300. [DOI] [PubMed] [Google Scholar]
- Allain F, Bouayad-Gervais K, Samaha AN (2018) High and escalating levels of cocaine intake are dissociable from subsequent incentive motivation for the drug in rats. Psychopharmacology (Berl) 235:317–328. [DOI] [PubMed] [Google Scholar]
- Beinat C, Banister SD, Herrera M, Law V, Kassiou M (2015) The therapeutic potential of α7 nicotinic acetylcholine receptor (α7 nAChR) agonists for the treatment of the cognitive deficits associated with schizophrenia. CNS Drugs 29:529–542. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Bertrand D, Bertrand S, Plagenhoef MR, Terry AV Jr (2017) Tropisetron sensitizes α7 containing nicotinic receptors to low levels of acetylcholine in vitro and improves memory-related task performance in young and aged animals. Neuropharmacology 117:422–433. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Artigas F (2013) Serotonin modulation of cortical neurons and networks. Front Integr Neurosci 7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Kalivas PW, Bardo MT (2006) Contribution of dihydro-beta-erythroidine sensitive nicotinic acetylcholine receptors in the ventral tegmental area to cocaine-induced behavioral sensitization in rats. Behav Brain Res 168:120–126. [DOI] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A (2013) Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496:359–362. [DOI] [PubMed] [Google Scholar]
- Choi DS, Wang D, Dadgar J, Chang WS, Messing RO (2002) Conditional rescue of protein kinase C epsilon regulates ethanol preference and hypnotic sensitivity in adult mice. J Neurosci 22:9905–9911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315:1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW (2011) Impulsivity, compulsivity, and top-down cognitive control. Neuron 69:680–694. [DOI] [PubMed] [Google Scholar]
- Ducret E, Puaud M, Lacoste J, Belin-Rauscent A, Fouyssac M, Dugast E, Murray JE, Everitt BJ, Houeto JL, Belin D (2016) N-acetylcysteine facilitates self-imposed abstinence after escalation of cocaine intake. Biol Psychiatry 80:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW (2008) The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 199:439–456. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Chamberlain SR, Müller U, Bullmore ET, Robbins TW (2012) Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry 169:926–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ. (2014) Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories–indications for novel treatments of addiction. Eur J Neurosci 40:2163–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faerber L, Drechsler S, Ladenburger S, Gschaidmeier H, Fischer W (2007) The neuronal 5-HT3 receptor network after 20 years of research–evolving concepts in management of pain and inflammation. Eur J Pharmacol 560:1–8. [DOI] [PubMed] [Google Scholar]
- Ferster CB, Skinner BF (1957) Schedules of reinforcement. East Norwalk, CT: Appleton-Century-Crofts. [Google Scholar]
- Gould RW, Garg PK, Garg S, Nader MA (2013) Effects of nicotinic acetylcholine receptor agonists on cognition in rhesus monkeys with a chronic cocaine self-administration history. Neuropharmacology 64:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ. (2010) Addiction and cognition. Addict Sci Clin Pract 5:4–14. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. (2015) Tropisetron and its targets in Alzheimer’s disease. Expert Opin Ther Targets 19:1–5. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Ishima T, Hagiwara H, Iyo M (2006) Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of tropisetron: role of alpha7 nicotinic receptors. Eur J Pharmacol 553:191–195. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R 2nd, Taylor JR (2002) Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology 26:183–190. [DOI] [PubMed] [Google Scholar]
- Jones S, Bonci A (2005) Synaptic plasticity and drug addiction. Curr Opin Pharmacol 5:20–25. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413. [DOI] [PubMed] [Google Scholar]
- Kees F, Färber L, Bucher M, Mair G, Mörike K, Grobecker H (2001) Pharmacokinetics of therapeutic doses of tropisetron in healthy volunteers. Br J Clin Pharmacol 52:705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Funk D, Harding S, Juzytsch W, Fletcher PJ, Shaham Y (2006) Effects of dexfenfluramine and 5-HT3 receptor antagonists on stress-induced reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 186:82–92. [DOI] [PubMed] [Google Scholar]
- Leiser SC, Li Y, Pehrson AL, Dale E, Smagin G, Sanchez C (2015) Serotonergic regulation of prefrontal cortical circuitries involved in cognitive processing: A review of individual 5-HT receptor mechanisms and concerted effects of 5-HT receptors exemplified by the multimodal antidepressant vortioxetine. ACS Chem Neurosci 6:970–986. [DOI] [PubMed] [Google Scholar]
- Lendvai B, Kassai F, Szájli A, Némethy Z (2013) Α7 nicotinic acetylcholine receptors and their role in cognition. Brain Res Bull 93:86–96. [DOI] [PubMed] [Google Scholar]
- Mandt BH, Gomez E, Johnston NL, Zahniser NR, Allen RM (2012) Cocaine dose and self-administration history, but not initial cocaine locomotor responsiveness, affects sensitization to the motivational effects of cocaine in rats. J Pharmacol Exp Ther 342:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara R, Dalley JW, Robbins TW, Everitt BJ, Belin D (2010) Trait-like impulsivity does not predict escalation of heroin self-administration in the rat. Psychopharmacology (Berl) 212:453–464. [DOI] [PubMed] [Google Scholar]
- Meneses A. (1999) 5-HT system and cognition. Neurosci Biobehav Rev 23:1111–1125. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O’Reilly RC (2011) A unified framework for inhibitory control. Trends Cogn Sci 15:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW (2000) Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology 23:113–126. [DOI] [PubMed] [Google Scholar]
- Parikh V, Sarter M (2008) Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci 1129:225–235. [DOI] [PubMed] [Google Scholar]
- Parsegian A, Glen WB Jr, Lavin A, See RE (2011) Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry 69:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson AL, Sanchez C (2014) Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction. CNS Spectr 19:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A (2007) Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology (Berl) 194:127–137. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Murray JE, Everitt BJ (2013) Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Eur J Neurosci 38:3018–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar I, Callahan PM, Hernandez CM, Yang X, Bartlett MG, Terry AV Jr (2018) Tropisetron enhances recognition memory in rats chronically treated with risperidone or quetiapine. Biochem Pharmacol 151:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ (2005) Intracranial self-administration of cocaine within the posterior ventral tegmental area of Wistar rats: evidence for involvement of serotonin-3 receptors and dopamine neurons. J Pharmacol Exp Ther 313:134–145. [DOI] [PubMed] [Google Scholar]
- Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn L, Ballatori E, Bria E, Clark-Snow R, Espersen B, Feyer P (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy-and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21:v232–v243. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, De Vries TJ, Wardeh G, van de Ven HW, Vanderschuren LJ (2002) Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci 22:3269–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Bahrani M, Zukerman S, Ackroff K (2010) Stevia and saccharin preferences in rats and mice. Chem Senses 35:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW (2011) Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2B-containing receptors. Proc Natl Acad Sci U S A 108:19407–19412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Scofield MD, Boger H, Hensley M, Kalivas PW (2014) Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci 34:5649–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Mizuguchi Y, Ohno Y (2013) Improving the treatment of schizophrenia: role of 5-HT receptors in modulating cognitive and extrapyramidal motor functions. CNS Neurol Disord Drug Targets 12:861–869. [DOI] [PubMed] [Google Scholar]
- Solbakk AK, Løvstad M (2014) Effects of focal prefrontal cortex lesions on electrophysiological indices of executive attention and action control. Scand J Psychol 55:233–243. [DOI] [PubMed] [Google Scholar]
- Švob Štrac D, Pivac N, Mück-Šeler D (2016) The serotonergic system and cognitive function. Transl Neurosci 7:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Lummis SC (2007) The 5-HT3 receptor as a therapeutic target. Expert Opin Ther Targets 11:527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiippana E, Hamunen K, Kontinen V, Kalso E (2013) The effect of paracetamol and tropisetron on pain: experimental studies and a review of published data. Basic Clin Pharmacol Toxicol 112:124–131. [DOI] [PubMed] [Google Scholar]
- Udakis M, Wright VL, Wonnacott S, Bailey CP (2016) Integration of inhibitory and excitatory effects of α7 nicotinic acetylcholine receptor activation in the prelimbic cortex regulates network activity and plasticity. Neuropharmacology 105:618–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ (2004) Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305:1017–1019. [DOI] [PubMed] [Google Scholar]
- Wise RA, Koob GF (2014) The development and maintenance of drug addiction. Neuropsychopharmacology 39:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan WS, Li YH, Xiao L, Zhu N, Bechara A, Sui N (2014) Working memory and affective decision-making in addiction: a neurocognitive comparison between heroin addicts, pathological gamblers and healthy controls. Drug Alcohol Depend 134:194–200. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Liu L, Liu S, Hong X, Chen DC, Xiu MH, Yang FD, Zhang Z, Zhang X, Kosten TA, Kosten TR (2012) Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am J Psychiatry 169:974–981. [DOI] [PubMed] [Google Scholar]