Abstract
Background
Psychotropic drugs are the cornerstone of schizophrenia treatment, often requiring lifelong treatment. Data on pharmacotherapy in inpatient settings are lacking.
Methods
Prescription data of schizophrenic inpatients within the time period 2000–2015 were obtained from the database of the Drug Safety Program in Psychiatry (AMSP). Data were collected at 2 index dates per year; the prescription patterns and changes over time were analyzed.
Results
Among 30 908 inpatients (mean age 41.6 years, 57.8% males), the drug classes administered most often were antipsychotics (94.8%), tranquilizers (32%), antidepressants (16.5%), antiparkinsonians (16%), anticonvulsants (14.1%), hypnotics (8.1%), and lithium (2.1%).
The use of second-generation antipsychotics significantly increased from 62.8% in 2000 to 88.9% in 2015 (P < .001), whereas the prescription of first-generation antipsychotics decreased from 46.6% in 2000 to 24.7% in 2015 (P < .001). The administration of long-acting injectable antipsychotics decreased from 15.2% in 2000 to 11.7% in 2015 (P = .006). Clopazine was the most often used antipsychotic, having been used for 21.3% of all patients. Polypharmacy rates (≥5 drugs) increased from 19% in 2000 to 26.5% in 2015. Psychiatric polypharmacy (≥3 psychotropic drugs) was present in 44.7% of patients.
Conclusions
Combinations of antipsychotics and augmentation therapies with other drug classes are frequently prescribed for schizophrenic patients. Though treatment resistance and unsatisfactory functional outcomes reflect clinical necessity, further prospective studies are needed on real-world prescription patterns in schizophrenia to evaluate the efficacy and safety of this common practice.
Keywords: AMSP, antipsychotics, pharmacotherapy, prescription, schizophrenia
Significance Statement.
Antipsychotic medication is the primary treatment for schizophrenia. Despite clinicians’ common use of antipsychotic combination treatment, the evidence for the efficacy and tolerability of this strategy is weak and guidelines reserve it as a last‐stage treatment option. There is also a debate about the choice of an antipsychotic agent for schizophrenia, with most consensus panel recommendations preferring second-generation antipsychotics (SGAs) to first-generation antipsychotics (FGAs). The prescription patterns of 30 908 schizophrenic inpatients analyzed here illustrate the clinical significance of (psychiatric) polypharmacy. This real-world analysis also presents a characterization of FGAs vs SGAs in the treatment of schizophrenia confirming the preference of SGAs, with clozapine being the antipsychotic substance most often used. However, FGAs still remain an important treatment strategy, used for at least one-quarter of patients in our large psychiatric inpatient population.
Introduction
Although the lifetime prevalence of schizophrenia is only about 0.7 to 1% of the general population in industrialized countries (Bromet and Fennig, 1999), it is ranked among the top 25 leading causes of disability worldwide (Global Burden of Disease Study 2013 Collaborators, 2015). This highlights the importance of appropriate treatment of this disabling and stigmatizing chronic disease.
Since the early 1950s, the pillar of schizophrenia treatment has been the first-generation or “typical” antipsychotics such as chlorpromazine or haloperidol. The market introduction of clozapine then shaped the term “atypical,” second-generation antipsychotic (SGA) due to the revelation that antipsychotic substances with a low potential for causing extrapyramidal symptoms are effective in schizophrenia treatment. Despite the remarkable chemical, pharmacological, and clinical heterogeneity, this term has since been broadly applied to further antipsychotic drugs (Möller, 2000). For the past 20 to 25 years, SGAs have gradually substituted first-generation antipsychotics (FGAs) in daily clinical routine (Leslie and Rosenheck, 2002; Gill et al., 2005). This transition from “typical” to “atypical” antipsychotics is mostly attributed to many psychiatrists’ view and hope that SGAs are more efficacious and safer than FGAs.
More than 15 years ago, doubts arose concerning the better efficacy and higher tolerability of the SGAs. Geddes et al. (2000) conducted a systematic review and meta-regression analysis of 52 randomized controlled trials (RCTs) with a total of 12 649 patients comparing SGAs (clozapine, risperidone, olanzapine, quetiapine, amisulpride, and sertindole) with FGAs (mostly haloperidol). The authors suggested that the initial view of slightly superior efficacy and better tolerability and a lower risk of causing extrapyramidal symptoms for SGAs was too simplistic. Many of the included trials compared moderate doses of an SGA with a higher dose than the recommended dose of a potent FGA such as haloperidol. When compared with a subgroup of patients receiving 12 mg/d or less of haloperidol (or equivalent), the authors found no difference in efficacy or overall tolerability between SGAs and FGAs. Only a modest benefit in terms of causing fewer extrapyramidal symptoms for SGAs remained.
Conducted a decade ago, in 2005 and 2006, 2 pragmatic clinical trials with no sponsorship by the pharmaceutical industry compared the efficacy of SGAs and FGAs: The UK Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia study (CUtLASS; Jones et al., 2006) suggested that SGAs and FGAs are equally effective in the treatment of schizophrenia, although SGAs cost more. The US Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE; Lieberman et al., 2005) was a double-blind trial in which 1493 chronic schizophrenic patients were randomized to one of the SGAs (olanzapine, quetiapine, risperidone, and ziprasidone) or the medium-potency FGA perphenazine. Even though the CATIE trial suggested that olanzapine was superior in terms of effectiveness compared with other nonclozapine SGAs, this was not considered a class effect and accompanied by more adverse drug reactions like increased weight gain and adverse metabolic events.
The Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia (CUtLASS) 2 study and the phase 2E CATIE study indicated that in schizophrenic patients with poor treatment response, clozapine rather than other SGAs leads to significant improvement in symptoms (Lewis et al., 2006; McEvoy et al., 2006; Stroup et al., 2009). A decade later, there is an ongoing discussion on patients with poor treatment response and strategies involving antipsychotic combinations.
Most recently, Galling et al. (2017) performed a systematic literature search and a random effects meta-analysis of randomized trials comparing combination with a second antipsychotic vs continued antipsychotic monotherapy in schizophrenia. The analysis of their data implies that the common practice of antipsychotic combination in schizophrenia lacks double-blind/high-quality evidence for efficacy, except for negative symptom reduction with aripiprazole combination.
The available methodologically sound guidelines show a high degree of accordance for most recommendations regarding monotherapy, core treatment decisions, including selection criteria for the first antipsychotic (i.e., effect/side-effect profile, patients’ preference, and concomitant conditions), drug dosages, maintenance treatment, definition of nonresponse or treatment resistance, and the importance of psychosocial interventions (American Psychiatric Association: Lehman et al., 2004; Schizophrenia Patient Outcomes Research Team [PORT]: Kreyenbuhl et al., 2010; National Institute for Clinical Excellence: NICE, 2014; Royal Australian and New Zealand College of Psychiatry: Galletly et al., 2016; German Society of Psychiatry, Psychotherapy and Nervous Diseases: DGPPN, 2019). Differences include the duration of maintenance drug treatment and first-line drug choice. The older guidelines—APA and also the update to the 2005 Royal Australian and New Zealand College of Psychiatry guidelines—mostly prefer SGAs (except for clozapine), especially in first-episode patients, whereas the newer guidelines—NICE, DGPPN, and PORT—recommend antipsychotics in general (except for clozapine; and olanzapine in PORT) considering the emerging clinical data and evidence.
Even though meta-analyses and guidelines provide orientation for the treatment of schizophrenia, clinical prescription patterns often differ from the guidelines. For example, in a recent international study Bachmann et al. (2017) assessed trends in clozapine prescription from 2005 to 2014 in 17 countries worldwide and concluded that while clozapine use has increased in most studied countries over recent years, clozapine is still underutilized in many populations, with prescription rates differing by a factor of over 300 between countries.
Another international study by Hálfdánarson et al. (2017) investigated international trends in antipsychotic use in 16 countries from 2005 to 2014 and detected that SGA use increased in all study populations, but the patterns of antipsychotic use varied strikingly between countries.
When 195 individuals with schizophrenia and psychosis on the Severe Mental Illness Register in Cheshire, UK, were followed up between 2004 and 2012 (Heald et al., 2017), an increase in psychiatric polypharmacy over the follow-up period was determined. Clozapine and long-acting injectable antipsychotics (LAIs) were often not documented in the general practitioner records.
Given the challenging controversy concerning real-world clinical practice and recommendations for pharmacotherapy of schizophrenia, it is particularly important to reflect on actual prescription patterns, including trends for recent changes over time. In this study, we, therefore, investigated pharmacotherapy of schizophrenia in a large psychiatric inpatient population between 2000 and 2015.
Methods
Data Source
The prescription data analyzed for the present study were gathered from the International Drug Safety Program in Psychiatry (Arzneimittelsicherheit in der Psychiatrie [AMSP]). AMSP is an ongoing international multicenter drug safety program collecting data on psychopharmacotherapy and severe adverse drug reactions from psychiatric hospitals in a naturalistic setting since 1993. Its methods have been described in detail elsewhere (Grohmann et al., 2004, 2014). Briefly, AMSP consists of 2 principal data collections (prescription data and severe adverse drug reactions) from 116 hospitals so far in Germany, Switzerland, and Austria, and temporarily from 1 hospital each in Belgium and Hungary. The number of participating hospitals increased from 9 in 1994 to 63 in 2015. In a cross-sectional approach, all participating hospitals record drug prescriptions for all inpatients under surveillance on 2 reference days per year. All drugs administered on these days are assessed along with the patient’s age, gender, and leading psychiatric diagnosis.
Furthermore, severe adverse drug reactions that are experienced at these hospitals in association with psychopharmacological treatment are continuously reported and analyzed. For the present study, only the cross-sectional AMSP dataset with prescriptions from 133 777 patients surveyed between 2000 and 2015 was used. In this time period, 116 hospitals provided data on schizophrenic inpatients.
Study Population and Design
Within the AMSP dataset all patients with a current diagnosis of schizophrenia based on ICD-10 diagnostic codes F20.0, F20.1, F20.2, F20.3, F20.4, F20.5, F20.6, F20.8, F20.9, and F20.*, that is, when a subclassification was missing, were selected. For the study population, the demographic information and prescriptions were analyzed on the day of data collection.
Psychiatric medication was classified into the following subgroups: antipsychotics, antidepressants, tranquilizers (benzodiazepines), hypnotics (benzodiazepine-like drugs), lithium salts, anticonvulsants, and antiparkinsonian medication (almost exclusively biperiden). Antipsychotic drugs were further divided into FGAs, SGAs, and LAIs. FGAs were additionally classified according to their potency into high-potency and low-potency FGAs. As low-potency FGAs are mostly used for sedative effects and not for treatment of psychosis, they are dealt with in the co-medication section.
Psychiatric polypharmacy was defined as an intake of more than 2 psychotropic drugs of different classes and polypharmacy in general as intake of ≥5 drugs (Masnoon et al., 2017). Data were obtained from an anonymized data bank. Evaluations based on the AMSP data bank have been approved by the Ethics Committee of the University of Munich and the Ethics Committee of the Hannover Medical School (Nr. 8100_BO_S_2018). This study adheres to the Declaration of Helsinki and its later amendments. The AMSP program is a continuous observational postmarketing drug surveillance program and does not interfere with the ongoing clinical treatment of patients under surveillance.
Data Analysis
The analysis was mainly descriptive. Tables and figures demonstrate the mode and development of prescription practice during the observation period. One exception to the descriptive approach was the use of linear regression to detect time trends in the development of the relative frequencies related to the base population of n = 30 908. Furthermore, 1-way ANOVA was calculated to compare dosages of the most commonly used antipsychotics over 4 aggregated time periods (2000–2003, 2004–2007, 2008–2011, and 2012–2015). A Wilcoxon Signed-Ranks Test was conducted to compare the number of prescribed drugs in the years 2000 and 2015. To visualize the distribution of patients with polypharmacy, violin plots were created. Level of significance was set at P < .05. All statistical analyses were performed using R version 3.0.2., and graphs were created with R and Microsoft Excel.
Results
Characteristics of the Study Population
Characteristics of the study population are presented in Table 1. A total of 30 908 patients with a leading admission diagnosis of schizophrenia based on ICD-10 codes was identified. There were more male inpatients (57.8%) under surveillance than females. The different subtypes of schizophrenia presented here are not analyzed separately in our study.
Table 1.
Demographical Data of the Study Population
| Characteristics | Patients, n = 30 908 (100%) |
|---|---|
| F20.0 paranoid subtype | 26 674 (86.3) |
| F20.1 disorganized (hebephrenic) subtype | 1282 (4.2) |
| F20.2 catatonic subtype | 649 (2.1) |
| F20.3 undifferentiated subtype | 514 (1.7) |
| F20.4 postschizophrenic depression | 94 (0.3) |
| F20.5 residual subtype | 1220 (3.9) |
| F20.6 simple-type schizophrenia | 157 (0.5) |
| F20.** schizophrenia, not specified | 318 (1.0) |
| Male | 17 865 (57.8) |
| Female | 13 043 (42.2) |
| Age, ≤30 years | 8222 (26.6) |
| Age, 31–60 years | 19 256 (62.3) |
| Age, >60 years | 3431 (11.1) |
| Mean age, years | 41.60 |
Psychiatric Medication Over Time
Over the entire observed time period from 2000 to 2015, the majority of patients took at least 1 psychotropic drug (98.5%; n = 30 449). Linear regression analysis did not show a significant change in the percentage of patients receiving psychopharmacological treatment over the observed time period ((F 1,14) = 0.03, P = .87).
The most frequently prescribed psychiatric drugs from 2000 to 2015 were antipsychotics given in 94.8% (n = 29 313) of all patients, followed by tranquilizers (32%, n = 9900). Antidepressants were used in 16.5% (n = 5111), antiparkinsonian medication in 16% (n = 4931), anticonvulsants in 14.1% (n = 4351), hypnotics in 8.1% (n = 2505), and lithium in 2.1% (n = 639).
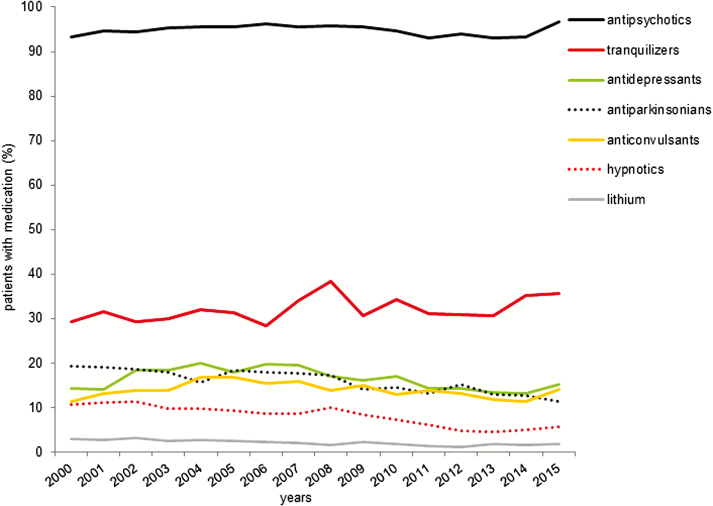
The prescription rate of antipsychotics remained stable over time [regression analysis: (F 1,14) = 0.15, P = .71; Figure 1]. There was also no difference in prescription of antidepressants [(F 1,14) = 3.03, P = .10] or anticonvulsants [(F 1,14) = 0.736, P = .41]. The trend for an increase of prescription of tranquilizers from 29.3% in 2000 to 35.6% in 2015 [(F 1,14) = 3.93, P = .07] did not reach statistical significance. The administration of lithium declined slightly [b = –0.10, R2 = 0.72, (F 1,14) = 35.65, P < .001] during the observation period from 3% in 2000 to 1.9% in 2015.
Figure 1.
Psychopharmacological medication over time. Shown are the yearly prescription rates of psychopharmacological drug classes. The prescription rates of lithium, antiparkinsonian medications, and hypnotics decreased over time (linear regression analysis: all P < .01).
Furthermore, a significant decrease was found for the use of antiparkinsonian medication from 19.2% in 2000 to 11.4% in 2015 [b = –0.01, R2 = 0.82, (F 1,14) = 64.92, P < .001] and hypnotics from 10.7% in 2000 to 5.6% in 2015 [b = –0.01, R2 = 0.88, (F 1,14) = 98.5, P < .001].
Antipsychotics
Antipsychotic Groups
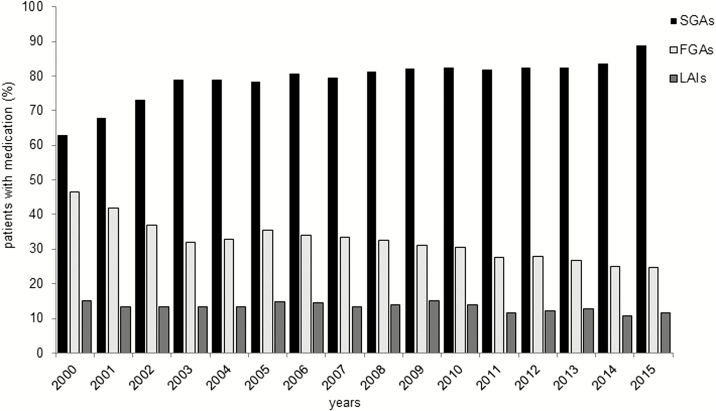
Over the total observation period, the most frequently prescribed subclass of antipsychotics by far were SGAs (n = 24 573, 79.5% of all patients), followed by FGAs (n = 9967, 32.2%) and LAIs (n = 4120, 13.3%).
The probability of receiving an SGA significantly increased [b = 1.13, R2 = 0.74, (F 1,14) = 39.1, P < .001] from 62.8% in 2000 to the maximum prescription rate of 88.9% in 2015, whereas the prescription of FGAs significantly decreased from 46.6% in 2000 to 24.7% in 2015 [b = –1.14, R2 = 0.84, (F 1,14) = 72.17, P < .001; see Figure 2].
Figure 2.
Antipsychotic medication over time. Shown are the time trends of second-generation antipsychotics (SGAs), first-generation antipsychotics (FGAs), and long-acting injectable antipsychotics (LAIs) prescriptions over time. While the use of SGAs increased, the use of FGAs and LAIs decreased over time (linear regression analysis: all P < .001).
From 2000 to 2015 the use of LAIs decreased over time [b = –0.18, R2 = 0.43 (F 1,14) = 10.42, P = .006], with a prescription rate of 15.2% of all schizophrenic patients in 2000 to 11.7% in 2015.
Antipsychotic Drugs
Table 2 summarizes the prescription rates of the most frequently used antipsychotics over time (usage >2% within 1 year of the investigated time period except for aripipsrazole LAI). The most commonly prescribed antipsychotics (defined as usage >10% in 2015) were clozapine (21.3%), olanzapine (20.7%), risperidone (17.7%), quetiapine (14.6%), haloperidol (13.6%), amisulpride (9.4%), and aripiprazole (8.9%).
Table 2.
Prescription Rates of the Most Frequently Prescribed Antipsychotics
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | b | r 2 | F | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | ||||||||||||||||||||
| FGAs | ||||||||||||||||||||
| Benperidol | 4.1 | 3.6 | 3.2 | 2.3 | 1.6 | 2.2 | 1.8 | 2.2 | 2.4 | 1.8 | 1.5 | 1.3 | 2.3 | 1.9 | 1.6 | 1.9 | –0.12 | 0.46 | F(1, 14) = 13.92 | .003 |
| Flupenthixol | 5.7 | 4.5 | 3.8 | 4.9 | 4.9 | 4.9 | 5.6 | 5.8 | 6.2 | 5.5 | 5.6 | 5.3 | 4.8 | 4.7 | 4.9 | 5 | 0.01 | –0.06 | F(1, 14) = 0.20 | .660 |
| Fluphenazine | 4.8 | 2.3 | 2.1 | 1.6 | 1.8 | 1.6 | 0.8 | 0.8 | 0.7 | 1.1 | 0.6 | 0.8 | 0.8 | 0.6 | 0.8 | 0.7 | –0.17 | 0.54 | F(1, 14) = 18.79 | .001 |
| Haloperidol | 18.2 | 15 | 12.5 | 12.4 | 13.3 | 14.7 | 14.7 | 13.5 | 15 | 13.5 | 13.6 | 12.5 | 12.8 | 12.2 | 11.8 | 12 | –0.22 | 0.36 | F(1, 14) = 9.38 | .008 |
| Perazine | 5.4 | 4.5 | 3.1 | 3.3 | 2.9 | 3.3 | 2.8 | 2.1 | 1.5 | 2.3 | 2.1 | 1.7 | 1.2 | 1 | 1 | 1.1 | –0.25 | 0.84 | F(1, 14) = 82.50 | <.001 |
| Zuclopenthixol | 3.3 | 2.6 | 3.1 | 2.3 | 2.6 | 3 | 3.5 | 4.1 | 3.5 | 2.7 | 3.3 | 3.1 | 3 | 1.8 | 2.6 | 3 | –0.02 | –0.05 | F(1, 14) = 0.26 | .573 |
| SGAs | ||||||||||||||||||||
| Amisulpride | 9 | 9.2 | 10.3 | 10.6 | 10.6 | 9.3 | 9.8 | 8.7 | 9.7 | 7.3 | 8.5 | 8.4 | 8 | 8.8 | 11.5 | 11.1 | –0.01 | –0.07 | F(1, 14) = 0.01 | .935 |
| Aripiprazole | 0.3 | 3.9 | 7.4 | 7.5 | 7 | 7.8 | 9.1 | 10.7 | 11.5 | 11.9 | 12.8 | 12 | 15 | 0.96 | 0.89 | F(1, 11) = 93.39 | <.001 | |||
| Clozapine | 22.1 | 21.5 | 21.8 | 22.2 | 22 | 20.6 | 22.3 | 20.8 | 19.8 | 21.7 | 20.8 | 19 | 20.8 | 20.7 | 23.1 | 22.1 | –0.04 | –0.04 | F(1, 14) = 0.46 | .524 |
| Olanzapine | 21.3 | 19.8 | 20.7 | 21.3 | 18 | 19.4 | 18.7 | 20.2 | 21.9 | 18.8 | 19.8 | 20.9 | 22.8 | 23.4 | 21.5 | 23.2 | 0.17 | 0.21 | F(1, 14) = 4.95 | .045 |
| Paliperidone | 2 | 4 | 3.4 | 1.3 | 2.2 | 2.4 | 2 | 1.6 | 1.6 | –0.18 | 0.19 | F(1, 7) = 2.90 | .137 | |||||||
| Quetiapine | 5.2 | 12.4 | 9.6 | 11.5 | 12.1 | 14.3 | 14.4 | 13.4 | 16.4 | 18.2 | 19.9 | 17.4 | 16 | 15.8 | 17.3 | 19.8 | 0.69 | 0.70 | F(1, 14) = 36.56 | <.001 |
| Risperidone | 14.1 | 11.2 | 15.3 | 16.1 | 17.4 | 16.5 | 19.5 | 18.6 | 17.1 | 19.1 | 20.3 | 17.7 | 18.4 | 17.7 | 21.3 | 22.9 | 0.48 | 0.65 | F(1, 14) = 28.40 | <.001 |
| Ziprasidone | 0.1 | 2.1 | 3.7 | 2.9 | 2.6 | 2.3 | 2 | 1.6 | 2.8 | 2.2 | 1.4 | 1.5 | 1.2 | 1.1 | 1.1 | –0.07 | 0.06 | F(1, 13) = 1.89 | .183 | |
| LAIs | ||||||||||||||||||||
| Aripiprazole LAI | 0.5 | 1.2 | 0.70 | |||||||||||||||||
| Flupenthixol dec. | 4.8 | 5 | 4.2 | 3.2 | 2.8 | 3.3 | 3.5 | 3.4 | 3.2 | 3.3 | 2.9 | 2.9 | 2.6 | 2 | 2 | 1.5 | –0.18 | 0.78 | F(1, 14) = 53.62 | <.001 |
| Fluphenazine dec. | 3.7 | 1.9 | 1.9 | 1.1 | 1.1 | 1.1 | 0.9 | 0.7 | 0.7 | 0.7 | 0.3 | 0.4 | 0.4 | 0.4 | 0.1 | 0.3 | –0.16 | 0.67 | F(1, 14) = 31.56 | <.001 |
| Haloperidol dec. | 4.3 | 4.8 | 4.3 | 2.7 | 2.2 | 2.9 | 1.8 | 2 | 3 | 2.2 | 2.7 | 1.4 | 1.7 | 2.4 | 1.1 | 1.2 | –0.19 | 0.63 | F(1, 14) = 26.55 | <.001 |
| Paliperidone pal. | 1.6 | 4 | 4.7 | 3.9 | 4.2 | 0.51 | 0.27 | F(1, 14) = 2.45 | .207 | |||||||||||
| Risperidone LAI | 0.3 | 4.2 | 5.6 | 5.5 | 6.8 | 5.7 | 6 | 7.3 | 6.7 | 4.8 | 2.6 | 2.1 | 2.3 | 2.2 | –0.12 | –0.03 | F(1, 14) = 0.66 | .426 | ||
| Zuclopenthixol dec. | 2.4 | 1.7 | 2.8 | 2.1 | 1.6 | 2.1 | 1.7 | 1.6 | 1.1 | 1.4 | 1.3 | 0.5 | 0.9 | 1.2 | 0.9 | 1 | –0.11 | 0.68 | F(1, 14) = 32.63 | <.001 |
Abbreviations: b, unstandardized slope; dec., decanoate; FGA, first-generation antipsychotic; LAI, long-acting injectable antipsychotics; pal., palmitate; SGA, second-generation antipsychotic.
Table 2 includes all antipsychotics with a usage >2% within 1 year of the investigated time period (except for aripiprazole LAI). Percentages relate to the total population of n = 30 908 patients. Results from the linear regression analysis are shown.
Interestingly, the prescription of clozapine, available in healthcare since 1971, remained unchanged over time [(F 1,14) = 0.43, P = .524], while the use of the SGAs risperidone, quetiapine, and aripiprazole increased markedly (see Table 2). With the exception of flupenthixol (5.1% of all patients) and zuclopenthixol (3% of all patients), all other high-potency FGAs decreased in their prescription rate over time.
Since the introduction of long-acting risperidone in 2002 and paliperidone palmitate in 2011, there has been a considerable shift in LAIs use: a decreasing use of haloperidol and flupenthixol decanoate was accompanied by increasing use of long-acting risperidone and paliperidone palmitate. Long-acting aripiprazole, introduced in 2014, was used in 1.2% in 2015; the use of olanzapine pamoate never exceeded 0.4% (data not shown).
Antipsychotic Dosages
The prescribed dosages of all antipsychotics with a usage >10% in 2015 are given in Table 3. Mean dosages per day decreased significantly for all substances except olanzapine.
Table 3.
Dosages of Antipsychotics Over Time
| 2000–2003 | 2004–2007 | 2008–2011 | 2012–2015 | ANOVA | P value | |
|---|---|---|---|---|---|---|
| Amisulpride | 599.78 (256.20) | 621.87 (305.37) | 610.67 (303.72) | 549.89 (280.94) | F(3,2868) = 9.07 | <.001 |
| Aripiprazole | 18.58 (8.6) | 16.74 (8.36) | 15.6 (7.88) | F(2,2329) = 92.9 | <.001 | |
| Clozapine | 363.56 (194.38) | 358.51 (214.77) | 345.96 (201.92) | 326.57 (186.06) | F(3,6495) = 10.62 | <.001 |
| Haloperidol | 14.23 (9.02) | 12.73 (8.84) | 11.34 (7.76) | 10.81 (8.10) | F(3,4118) = 30.42 | <.001 |
| Olanzapine | 18.60 (6.95) | 19.08 (8.44) | 18.94 (7.91) | 18.82 (8.35) | F(3,6295) = 0.93 | .424 |
| Quetiapine | 566.19 (262.18) | 645.80 (353.56) | 575.34 (297.53) | 478.56 (263.75) | F(3,4318) = 60.38 | <.001 |
| Risperidone | 4.72 (1.72) | 4.46 (1.94) | 4.51 (2.01) | 4.20 (1.98) | F(3,5434) = 13.57 | <.001 |
Table 3 shows the dosages of all antipsychotics administered in more than 10% of patients in 2015. Mean values and their SD are shown in mg. P values are derived from 1-way ANOVAs.
Polypharmacy
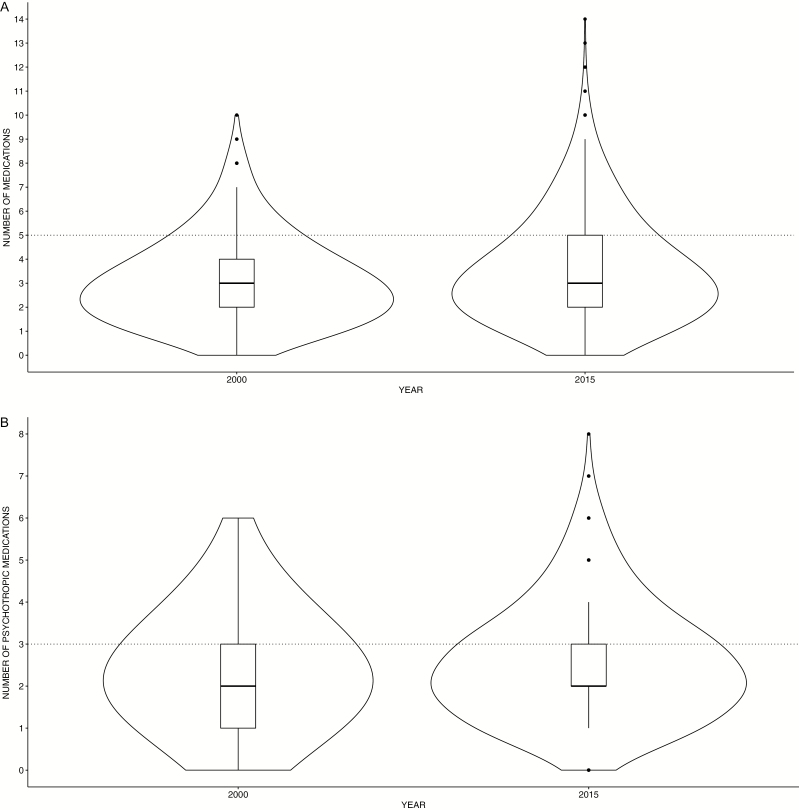
Polypharmacy was common with 23.7% of patients receiving ≥5 drugs. These rates increased from 19% in 2000 to 26.5% in 2015. Furthermore, excessive polypharmacy with ≥8 drugs increased from 2.5% in 2000 to 6% in 2015.
A Wilcoxon Signed-Ranks Test indicated that the number of prescribed drugs in 2015 [Md (median) = 3, M (mean) = 3.56] was significantly higher than in 2000 (Md = 3, M = 3.06) (Wilcoxon test statistic (W) = 983 120, P < .001; see Figure 3A).
Figure 3.
Polypharmacy. (A) Violin plots of the total number of simultaneously prescribed drugs in 2000 and 2015. The boxplot in the center of the violin plot shows the median concurrently prescribed drug and the interquartile range, the thin black line represents the 95% confidence interval. In addition, the displayed area represents a kernel density estimate to indicate the distribution form of the prescription data. The thin dotted line shows the threshold of 5 drugs, from which polypharmacy starts. (B) Psychiatric polypharmacy. Violin plots of the total number of simultaneously prescribed psychotropic drugs in 2000 and 2015. The white boxplot in the center of the violin plot shows the median simultaneously prescribed psychotropic drugs. The thick grey bar in the middle represents the interquartile range, the thin black line represents the 95% confidence interval. In addition, the displayed area represents a kernel density estimate to indicate the distribution form of the prescription data. The thin dotted line shows the threshold of 3 psychopharmacological drugs, indicating psychiatric polypharmacy.
Most patients (74.6%) received more than 1 psychotropic drug. Psychiatric polypharmacy (≥3 psychotropic drugs) was present in 44.7% of patients. Furthermore, 21.4% received ≥4 psychotropic drugs with prescriptions of up to 10 psychotropic drugs per patient. As with the total number of medications, a Wilcoxon Signed-Ranks Test indicated that the number of prescribed psychotropic drugs in 2015 (Md = 2, M = 2.57) was significantly higher than in 2000 (Md = 2, M = 2.37) (W = 1 035 100, P = .01; see Figure 3B).
Combination and Augmentation Therapy
Combination and Augmentation of Single Antipsychotics
We have classified combination and augmentation therapy as follows: the concomitant prescription of 2 antipsychotics is defined as combination therapy and the additional prescription of other psychotropic drugs as augmentation therapy.
Augmentation therapy of the most common single antipsychotic agent (all above 10% usage in 2015) with antidepressants, tranquilizers/hypnotics, anticonvulsants, and combination therapy are presented in Table 4 for the first and last years of this study, 2000 and 2015.
Table 4.
Combination and Augmentation Therapy of Single Antipsychotics
| and AP | and TR | and AD | and AC | and HYP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2015 | 2000 | 2015 | 2000 | 2015 | 2000 | 2015 | 2000 | 2015 | |
| Amisulpride | 52.1 | 71.9 | 29.4 | 37.1 | 17.6 | 15,6 | 8.4 | 16.2 | 14.3 | 5.4 |
| Aripiprazole | / | 76.2 | / | 26.9 | / | 16,6 | / | 13.9 | / | 5.8 |
| Clozapine | 50.3 | 72.3 | 22.9 | 34.5 | 17.8 | 22.6 | 9.6 | 23.2 | 6.7 | 4.6 |
| Haloperidol | 72.4 | 83.1 | 45.7 | 58.2 | 6.2 | 13.0 | 13.6 | 17.5 | 9.9 | 7.9 |
| Olanzapine | 47.8 | 54.5 | 31.8 | 37.3 | 15.2 | 11.7 | 9.7 | 10.5 | 11.8 | 5.2 |
| Quetiapine | 49.2 | 73.5 | 28.6 | 33.1 | 20.6 | 14.6 | 11.1 | 16.2 | 12.7 | 7.3 |
| Risperidone | 39.9 | 64.3 | 33.5 | 35.4 | 14.9 | 16.2 | 14.4 | 12.4 | 16.5 | 5.3 |
Abbreviations: AC, anticonvulsants; AD, antidepressants; AP, antipsychotics; HYP = hypnotics; TR, tranquilizers.
Table 4 shows the combination and augmentation therapies in 2000 compared with 2015 in percent of patients for all antipsychotics administered in more than 10% of patients in 2015.
Most frequently the concomitant prescription of 2 antipsychotics was observed (49.0% of all patients treated with psychotropic drugs). Haloperidol was most frequently combined with another antipsychotic agent in both years; all other antipsychotics with the exception of olanzapine and risperidone were also combined in more than 70% with other antipsychotics in 2015.
In particular, haloperidol was mostly administered in combination with clozapine (24.5%) followed by quetiapine (19.7%) and olanzapine (19%). Concerning the combinations with clozapine, most frequently amisulpride (21.1%) and aripiprazole (19.8%) were included. Risperidone was preferentially combined with quetiapine (19.3%) and clozapine (17%), whereas olanzapine’s combination partners were mainly aripiprazole (20.9%) and risperidone (16.6%; data not shown). Furthermore, the analysis of the most frequently chosen antipsychotic combination pairs in 2000 and 2015 showed a shift in combination strategies over time (Table 5).
Table 5.
Most Frequent Antipsychotic Combination Pairs
| 2000 | % | 2015 | % | |
|---|---|---|---|---|
| 1 | Haloperidol + Olanzapine | 3.8 | Quetiapine + Risperidone | 10.9 |
| 2 | Haloperidol + Clozapine | 3.7 | Quetiapine + Aripiprazole | 9.8 |
| 3 | Haloperidol + Risperidone | 2.6 | Olanzapine + Aripiprazole | 6.5 |
| 4 | Clozapine + Amisulpride | 2.2 | Olanzapine + Risperidone | 5 |
| 5 | Benperidol + Clozapine | 2.2 | Clozapine + Aripiprazole | 5 |
| 6 | Clozapine + Olanzapine | 1.7 | Haloperidol + Quetiapine | 4.5 |
| 7 | Haloperidol + Quetiapine | 1.3 | Olanzapine + Quetiapine | 4.5 |
| 8 | Haloperidol + Perazine | 1.3 | Clozapine + Amisulpride | 4.3 |
| 9 | Clozapine + Risperidone | 1.1 | Haloperidol + Olanzapine | 3.5 |
| 10 | Flupenthixol + Olanzapine | 1.1 | Haloperidol + Clozapine | 3.5 |
Table 5 shows the most frequent antipsychotic combination pairs in 2000 compared with 2015 in percent of patients. Combinations of more than 2 antipsychotics were counted accordingly for all possible combinations of 2 drugs. In grey shade, the combination pairs which were present in 2000 and 2015 are highlighted.
As 96.3% of all patients received an antipsychotic medication, co-prescriptions usually included antipsychotics. Therefore, when the use of other drug groups is summarized in the following, it has to be borne in mind that this almost always means their concomitant use with antipsychotics.
Tranquilizers, Hypnotics, and Low-Potency FGAs
Table 6 summarizes all co-medications with usage >2% within 1 year of the investigated time period. At least 28.3% of all schizophrenic patients and 38.3% at the maximum received tranquilizers. Notably, the usage of lorazepam increased over time with a maximum of 24.5% of patients receiving the drug in 2015.
Table 6.
Prescription Rates of the Most Frequently Prescribed Co-Medications
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | b | r 2 | F | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | ||||||||||||||||||||
| Tranquilizers | ||||||||||||||||||||
| Diazepam | 7.7 | 9.2 | 6.9 | 7.4 | 9.9 | 10 | 8 | 10.9 | 12.5 | 8.3 | 9.6 | 8 | 8.5 | 8.1 | 10.1 | 10.1 | 0.09 | 0.01 | F(1, 14) = 1.15 | .309 |
| Lorazepam | 19.6 | 20.7 | 20.7 | 21.2 | 19.3 | 19.2 | 18.8 | 20.8 | 23.6 | 20.9 | 22.3 | 21.2 | 20.8 | 21.2 | 23.4 | 24.5 | 0.22 | 0.37 | F(1, 14) = 9.74 | .007 |
| Oxazepam | 1 | 1.2 | 1.3 | 1.1 | 2.4 | 1.7 | 1.4 | 2.1 | 2 | 1.5 | 1.7 | 1.3 | 1.2 | 1.1 | 1.5 | 1.1 | –0.01 | –0.07 | F(1, 14) = 0.05 | .774 |
| Hypnotics | ||||||||||||||||||||
| Zolpidem | 2.1 | 1.2 | 2.5 | 1.6 | 2.1 | 2 | 1.9 | 2.1 | 2.3 | 1.7 | 1.3 | 1.7 | 1 | 1.2 | 1.2 | 1.8 | –0.05 | 0.18 | F(1, 14) = 4.25 | .071 |
| Zopiclone | 4.6 | 5.8 | 5.2 | 5.1 | 4.4 | 4.2 | 4.2 | 4.1 | 5.2 | 4 | 3.8 | 2.5 | 2.5 | 2.2 | 2.7 | 2.8 | –0.20 | 0.73 | F(1, 14) = 41.88 | <.001 |
| Low-pot. FGAs | ||||||||||||||||||||
| Chlorprothixene | 9.5 | 7.6 | 6.1 | 7.2 | 6.9 | 6.3 | 5.1 | 5.7 | 4.4 | 3.8 | 2.9 | 2.6 | 2.6 | 2.4 | 3.1 | 2.4 | –0.44 | 0.89 | F(1, 14) = 120.07 | <.001 |
| Levomepromazine | 6 | 4.1 | 3.7 | 4.3 | 5 | 6.4 | 4.6 | 3.8 | 3.8 | 3.1 | 4.6 | 2.7 | 2.6 | 2 | 2.3 | 1.9 | –0.22 | 0.57 | F(1, 14) = 21.23 | <.001 |
| Melperone | 2.6 | 2.6 | 2.1 | 2 | 1.8 | 1.6 | 1.9 | 1.8 | 2.5 | 2.1 | 1.7 | 2.2 | 2.5 | 1.2 | 1.7 | 1.7 | –0.04 | 0.15 | F(1, 14) = 3.58 | .067 |
| Pipamperone | 2.9 | 2.3 | 3.3 | 3.5 | 2.8 | 3.6 | 2.6 | 4.9 | 2.7 | 4.4 | 3.7 | 4 | 4.7 | 4.7 | 3.7 | 5.9 | 0.15 | 0.48 | F(1, 14) = 14.94 | .002 |
| Promethazine | 3.4 | 3.4 | 3 | 3 | 1.9 | 2.8 | 2.7 | 3.5 | 3.9 | 4.1 | 3.7 | 4.3 | 4.4 | 3.7 | 3.2 | 1.2 | 0.02 | –0.06 | F(1, 14) = 0.11 | .762 |
| Prothipendyl | 0.7 | 0.7 | 1.1 | 1.5 | 2.9 | 2.2 | 2.5 | 2.5 | 3.4 | 3.4 | 3.2 | 2.9 | 2.2 | 3 | 2.6 | 4.8 | 0.18 | 0.60 | F(1, 14) = 23.05 | <.001 |
| Antidepressants | ||||||||||||||||||||
| Other AD | 1.7 | 1.9 | 2.1 | 2.4 | 4.8 | 4.2 | 4.2 | 6.4 | 3.3 | 2.9 | 3.8 | 3.5 | 3.7 | 3.4 | 3.3 | 4.2 | 0.09 | 0.08 | F(1, 14) = 2.30 | .149 |
| SSRI | 7.6 | 7.4 | 11.7 | 11.3 | 11.3 | 10.6 | 11.9 | 3.9 | 9.8 | 9.9 | 9.7 | 7.8 | 7.2 | 7.1 | 7.1 | 8.4 | –0.16 | 0.05 | F(1, 14) = 1.87 | .187 |
| SNRI | 0.5 | 1.3 | 1.5 | 2 | 2.1 | 1.7 | 2.4 | 2.9 | 3.3 | 2.8 | 2.9 | 2.3 | 3 | 2.7 | 2.8 | 2.6 | 0.12 | 0.57 | F(1, 14) = 20.63 | <.001 |
| TCA | 4.4 | 3.4 | 2.8 | 3.2 | 2.4 | 2.0 | 2.3 | 5.9 | 1.6 | 1.3 | 1.5 | 1.2 | 1.2 | 1.6 | 1.0 | 1.3 | –0.18 | 0.43 | F(1, 14) = 10.46 | .006 |
| Anticonvulsants | ||||||||||||||||||||
| Carbamazepine | 6.6 | 5.6 | 4.7 | 3 | 2.2 | 2.9 | 1.6 | 2.4 | 1.4 | 1.3 | 1 | 0.9 | 0.7 | 0.5 | 1 | 0.5 | –0.35 | 0.78 | F(1, 14) = 54.41 | <.001 |
| Pregabalin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.3 | 0.5 | 0.7 | 1.6 | 1.9 | 1.4 | 2 | 1.9 | 2.6 | 0.18 | 0.83 | F(1, 14) = 76.36 | <.001 |
| Valproate | 4.4 | 6.1 | 7.9 | 8.5 | 6.3 | 9.3 | 11.6 | 10.3 | 8.2 | 10.2 | 8.7 | 8.8 | 8.5 | 7.6 | 6.5 | 6.9 | 0.07 | –0.04 | F(1, 14) = 0.49 | .499 |
Abbreviations: AD, antidepressant; b, unstandardized slope; FGA, first-generation antipsychotic; Low-pot. FGAs, low-potency first-generation antipsychotics; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Table 6 includes all psychopharmacological co-medications with a usage >2% within 1 year of the investigated time period. Percentages relate to the total population of n = 30 908 patients. Results from the linear regression analysis are shown. Other AD include the drugs agomelatine, bupropion, mirtazapine, tianeptine, trazodone, and vortioxetine.
Co-medication with tranquilizers was the second most common co-prescription. Haloperidol was most often simultaneously administered with tranquilizers and aripiprazole least frequently.
Hypnotics were a comparatively rare augmentation partner for all antipsychotics in question in 2015: the use of this augmentation medication ranged only between 4% and 7% in 2015 (Table 4).
Apart from aripiprazole, all SGAs were augmented with tranquilizers in 2015 in about one-third of patients, and this treatment strategy showed a mildly increasing tendency over time, whereas the use of tranquilizers together with clozapine increased markedly (Table 4).
Low-potency FGAs were administered to 14.8% of patients. The percentage of patients receiving low-potency FGAs decreased over time [b = –0.34, R2 = 0.65 (F 1,14) = 26.11, P < .001] from 21.7% in 2000 to 17.1% in 2015 (single substances are shown in Table 6). Remarkably, the “dirty drug” levomepromazine with prominent anticholinergic effects was significantly less prescribed over time, whereas pipamperone and prothipendyl, having no relevant pharmacokinetic drug-drug interactions, were more commonly administered low-potency FGAs.
Antidepressants
Over the observation period from 2000 to 2015, antidepressants, in general, were administered to 13.2% to 20% of all schizophrenic patients (Figure 1; Table 6). Selective serotonin reuptake inhibitors were by far the most frequently used type of antidepressants (8.9%). In 2015 sertraline (3.5%), mirtazapine (2.4%), escitalopram (2%) and venlafaxine (1.8%) were the most commonly prescribed antidepressants (data not shown).
Antidepressants were most commonly administered as augmentation agents to clozapine (22.6% in 2015) and least frequently to olanzapine (11.7% in 2015; see Table 4).
Anticonvulsants
Anticonvulsants were given in 11.3% to 16.7% of all schizophrenic patients over the last 15 years (Figure 1). The most commonly used anticonvulsant in schizophrenia by far was valproate (6.9% of patients in 2015). The second most frequently prescribed antiepileptic drug in 2015 was pregabalin (2.6%) followed by lamotrigine (1.9%), clonazepam (0.9%), and carbamazepine (0.5%, not all data shown; Table 6).
Augmentation with anticonvulsants was most common again for clozapine in 2015 (23.2%), whereas amisulpride and olanzapine were least often co-prescribed with anticonvulsants in 2015 (Table 4).
Discussion
To the best of our knowledge, the present study is the first to examine pharmacotherapy for schizophrenia in a large population of 30 908 hospitalized patients suffering from schizophrenia in German-speaking countries. The study provides a real-life picture of schizophrenia treatment with data from 116 psychiatric hospitals and their medication records over time.
One of our main findings was the expected increase of SGAs with an accompanied decrease in FGAs. Surprisingly, the use of LAIs also decreased in our cohort (13.3%), even though naturalistic studies clearly show their superiority when compared with oral nonclozapine antipsychotics for relapse prevention and risk of rehospitalization (Tiihonen et al., 2017).
Clozapine was the most frequently prescribed antipsychotic substance for inpatients in the observation period of 15 years (21.3%). Considering that inpatients nowadays are often severely/chronically ill and/or treatment resistant, this finding cannot be generalized to all schizophrenic patients.
Nevertheless, treatment resistance is one of the most important clinical issues in schizophrenia treatment. Most guidelines define schizophrenic patients as treatment resistant if failure of at least 2 antipsychotic trials with different compounds, including at least 1 SGA, in an adequate dose during a period between 2 and 8 weeks occurs (Lehman et al., 2004; Buchanan et al., 2010; National Institute for Health and Clinical Excellence, 2014). Approximately 20% to 30% of all patients with schizophrenia do not respond adequately to the first antipsychotic agent prescribed (Hasan et al., 2017).
Because clozapine is the only antipsychotic agent with proven efficacy that is licensed for treatment-resistant schizophrenia, it is the gold standard for patients who have not responded to 2 antipsychotic monotherapy trials (Conley et al., 2001; Moore et al., 2007; McIlwain et al., 2011).
Clozapine has been reported to have lower rates of readmittance to hospital compared with other antipsychotics (Taylor et al., 2009; Tiihonen et al., 2011). Therefore, clinical guidelines from NICE and other evidence-based treatment guidelines recommend that treatment-resistant schizophrenic patients should be offered clozapine at the earliest opportunity (National Institute for Health and Clinical Excellence, 2014). However, the initiation of clozapine in routine clinical practice is often delayed (Wheeler et al., 2008).
In our study, clozapine evolved into the antipsychotic substance with the highest rates of co-prescription with antidepressants and anticonvulsants in 2015. Furthermore, tranquilizers were given in 34.5% of clozapine-medicated patients in 2015. This finding is critical due to the additive sedative effects of clozapine and tranquilizers and the resulting high medical burden of chronically ill patients.
Combination and augmentation therapies were common in our study cohort with only one-quarter of patients receiving monotherapy with 1 psychotropic drug. The most frequently chosen treatment strategy was the combination of 2 antipsychotics in 49% of all patients though antipsychotic monotherapy is preferred by the guidelines (Lehman et al., 2004; Buchanan et al., 2010; DGPPN, 2019). Antipsychotic combination treatment is only recommended as a last-line treatment when clozapine has not succeeded (Conley et al., 2001; Moore et al., 2007).
Despite the recommendations, adjunctive treatment strategies are commonly used in schizophrenia (Buchanan et al., 2002). Two different drug co-prescription approaches need to be differentiated (Falkai et al., 2005): antipsychotic combination treatment (combination of different antipsychotics) and augmentation therapy (simultaneous application of antipsychotics with agents of another drug class, e.g., mood stabilizer).
According to the review by Stahl et al. (2004), antipsychotic combination treatment is applied to 10% to 20% of schizophrenic outpatients, whereas even 50% of schizophrenic inpatients receive more than 2 antipsychotics. Another study reported rates of 42.5% for antipsychotic combination treatment and 70% for augmentation therapy (Pickar et al., 2008).
The “real world” co-prescription approach in schizophrenia possibly aims at intensifying antipsychotic potency by exploiting different receptor-binding profiles and reducing adverse effects.
The most common antipsychotic combinations studied so far include olanzapine (Zink, 2005) or clozapine (Chan and Sweeting, 2007) and constitute the neurobiological approach of facilitating an additive and complementary dopamine receptor blockade by sulpiride (Shiloh et al., 1997) or amisulpride (Pani et al., 2008). Other strategies support differential agonistic and antagonistic effects in the dopaminergic system (aripiprazole; Chang et al., 2008) or agonistic effects in the serotonergic and adrenergic system (ziprasidone; Zink et al., 2009). The neurobiological rationale for using the combination of FGAs with SGAs on a large scale aims at implementing additional antidopaminergic receptor occupancy but results in higher risks of serious side-effects and is not verified by controlled clinical trials.
The analysis of our data showed that all antipsychotics were frequently combined with other antipsychotics, mostly with (other) SGAs, possibly because of an insufficient monotherapy. Keeping the AMSP methodology in mind, the underlying reasons for the common drug co-prescriptions cannot be determined on the basis of the present data. However, our observations are in line with those in the literature.
Clozapine was most frequently combined with amisulpride and aripiprazole over the whole time period as recommended by trials. An open retrospective study (Zink et al., 2004) showed that amisulpride could have beneficial effects in ameliorating positive and negative symptoms in patients with clozapine resistance. The addition of aripiprazole to clozapine vs clozapine monotherapy in a randomized, double-blind, placebo-controlled trial in patients with schizophrenia resulted in significant improvements in terms of body weight and lipid parameters (Fleischhacker et al., 2010).
Being structurally and pharmacologically similar to clozapine, the frequently prescribed combination of olanzapine with risperidone or aripiprazole in our study has also been associated with improvements of positive and/or negative symptoms (Zink et al., 2005). Interestingly, the second most often observed combination in 2015 was quetiapine with aripiprazole, possibly following a similar approach.
Haloperidol was mostly prescribed in combination with clozapine, quetiapine, and olanzapine. Since 2002, the rates of haloperidol use as a single substance remained fairly constant; it still represents a mainstay in the management of emergencies such as severe acute psychosis or agitation. Its low affinity for D2 receptors is the pharmacological rationale for the combination of clozapine with FGAs like haloperidol. The addition of haloperidol to schiozophrenic patients with an ongoing clozapine treatment has significantly elevated D2 receptor occupancy from 55% to 79%, and this change has been associated with significant prolactin elevation (Kapur et al., 2001). Additionally, the possible anti-aggression properties due to the relative inhibition of the dopamine D4 receptor relative to D2, one of the prominent features of clozapine, is lost by the concomitant significant blockade of D2 when combined with haloperidol (El-Mallakh et al., 2013).
Overall, antipsychotic combination treatment vs monotherapy remains a controversial approach. Antipsychotic co-treatment is barely evidence-based regarding superior efficacy (Freudenreich and Goff, 2002; Stahl, 2002) and is associated with concerns about long-term safety due to the risk of cumulative side-effects (Correll et al., 2007) or pharmacokinetic interactions, mortality (Waddington et al., 1998; Joukamaa et al., 2006), and results in an additional increase of expenses (Stahl, 2002).
Concerning possible advantages of an antipsychotic combination treatment, the outcomes of a meta-analysis by Correll et al. (2009) suggested that antipsychotic combination treatment may be superior to standard (non-clozapine) monotherapy in nonresponsive patients. While the overall results may be supportive for antipsychotic combinations, the positive findings were mainly based on studies conducted in China and the study database was too heterogeneous so the generalizability of these findings is questionable. Furthermore, a more recent meta-analysis by Correll et al. (2017) compared the efficacy of antipsychotic drugs co-prescribed with other antipsychotic or nonantipsychotic medications vs placebo or monotherapy among adults with schizophrenia. None of the 42 pharmacological co-treatment strategies showed superiority over monotherapy.
With the introduction of SGAs, the rate of antipsychotic combination treatment has increased (Ganguly et al., 2004). This striking trend may be attributed to the unique pharmacodynamic profile of each individual SGA and their lower affinity for dopamine receptors compared with FGAs. In our sample, the increase in SGA prescription was also accompanied by higher rates of polypharmacy and decreasing rates in dosages of most SGAs.
Further findings of our study concern the co-prescriptions of other psychopharmaceuticals. During our total observation period, the second most commonly prescribed psychiatric drugs were tranquilizers, given to 32% of all patients. A significant increase in the prescription—especially for lorazepam—was detected. Due to our study design, we were not able to distinguish between substances used for acute treatment or maintenance purposes, thus possibly reflecting different treatment approaches. We also only recorded primary diagnosis and cannot exclude prescription for comorbidities.
The guidelines for the treatment of schizophrenia recommend benzodiazepines, alone or as augmentation agents to antipsychotics, for acute agitation in schizophrenic patients (Lehman et al., 2004; Kreyenbuhl et al., 2010; DGPPN, 2019). While rates of additional benzodiazepine medication are likely to vary worldwide, a study from Taiwan showed that nearly 80% of schizophrenia patients were prescribed benzodiazepines over the course of 1 year (Wu et al., 2011).
A recent systematic review has not verified the efficacy of the augmentative use of benzodiazepines to antipsychotics in the treatment of schizophrenic patients (Sim et al., 2015). Additionally, a current meta-analysis of 16 RCTs (Dold et al., 2013) confirmed this finding. Correspondingly, benzodiazepines were repeatedly recommended for ultra short-term sedation of acutely agitated patients but not for augmentation of antipsychotics in the medium- and long-term pharmacotherapy of schizophrenia and related disorders.
Co-treatment with benzodiazepines in patients with schizophrenia has been associated with a marked increase in the mortality hazard ratio, both for suicidal and nonsuicidal deaths. Of particular interest is that a similar increase in the mortality hazard ratio has not been observed in schizophrenic patients treated with 2 or more concomitant antipsychotics or with an antidepressant-antipsychotic co-prescription (Tiihonen et al., 2012). However, antipsychotic combination and augmentation treatment is associated with an increased risk of adverse drug reactions such as grand mal seizures (Druschky et al., 2018).
Antidepressants added to antipsychotics for the treatment of schizophrenia are rather routinely prescribed in clinical practice (Zink et al., 2010), although the evidence regarding their efficacy is still limited and inconsistent (Hinkelmann et al., 2013). Terevnikov et al. (2015) therefore conducted a meta-analysis including 36 RCTs assessing the efficacy of add-on antidepressants for the treatment of negative, positive, cognitive, depressive, and antipsychotic-induced extrapyramidal symptoms in schizophrenia. Mirtazapine and mianserin were associated with a rather consistent efficacy for negative symptoms, and both appeared to enhance neurocognition. Trazodone and nefazodone seemed to improve the antipsychotic-induced extrapyramidal symptoms. Imipramine and duloxetine tended to alleviate depressive symptoms. No clear evidence supported the efficacy of selective serotonin reuptake inhibitors on any clinical domain of schizophrenia.
Another meta-analysis (Sepehry et al., 2007) including 11 studies also showed no global support for an improvement in negative symptoms after selective serotonin reuptake inhibitor augmentation but demonstrated a moderate effect in chronically ill schizophrenia patients.
On the other hand, a recent comparative effectiveness study (Stroup et al., 2019) using US national Medicaid data found a reduced risk of psychiatric hospitalization and emergency department visits in schizophrenic outpatients with adjunctive antidepressant prescriptions.
The analysis of antidepressant-antipsychotic co-prescriptions in the present study demonstrated that not mirtazapine but selective serotonin reuptake inhibitors were the most commonly prescribed antidepressants (8.9%). In addition, the frequency of antidepressant augmentation therapy remained fairly constant over time.
Next to augmentation treatments with benzodiazepines and antidepressants, anticonvulsants were administered in 14% of patients. The efficacy of anticonvulsants for schizophrenia treatment has a low level of evidence (DGPPN, 2019). Prescriptions could possibly be explained by diagnostic ambiguities between affective and psychotic disorders or interpreted as an attempt that is not evidence based to control aggressive behavior. Of note, 23% of clozapine-treated patients received anticonvulsants and especially valproate in 2015, which is another critical finding for this group of severely affected and pharmacoresistant patients (Wang et al., 2016).
Several limitations of the presented study need to be addressed when interpreting the results. First, the data source used is limited by its retrospective design with cross-sectional data. Therefore, the study was not designed to follow-up on the course and changes of different therapies over time in individual patients. Correspondingly, medication changes including cross-tapering strategies may have influenced co-medication therapy results and possibly resulted in an overestimation of polypharmacy. Considering the large sample size, this bias may be reduced in comparison with many other pharmacoepidemiological studies for schizophrenia. Second, the results presented here only refer to rather severely ill and/or treatment-resistant inpatients from hospitals in German-speaking countries. Hence, the derived data cannot be generalized for international treatment practices or outpatient settings for schizophrenic patients. Third, only data on the primary diagnosis were assessed, and the prescription of psychopharmacological substances for comorbidities may have influenced the pharmacological treatment.
In conclusion, this observational study presents an update on pharmacotherapy of schizophrenia regarding daily clinical practice in German-speaking countries. Most inpatients are treated with combination and augmentation therapies often associated with low levels of evidence and high rates of off-label use, though a diagnosis- and guideline-oriented pharmacotherapy of schizophrenia strongly emphasizes antipsychotic monotherapy. Therefore, there is a necessity for more detailed recommendations and guidance in clinical practice guidelines. Real-world evidence on efficacy and safety of combination and augmentation therapies in the heterogenic group of schizophrenia patients could be obtained by large pragmatic trials focussing on distinct drug constellations. Furthermore, high-quality, prospective patient registries that include a structural assessment of long-term outcomes and intensified adverse event monitoring systems are of paramount importance to evaluate common practice.
Acknowledgments
The authors are grateful to all participating hospitals and drug monitors for their voluntary and careful collection of data. We also thank Mr Pietro Nickl for copyediting the manuscript.
The research was supported by in-house funding from the Hannover Medical School. The grant provider had no role in study design, collection, interpretation, or analysis of data nor in the writing of the report or the decision to submit the paper for publication.
Statement of Interest
Since 1993 educational and research grants have been given by the following pharmaceutical companies to the 3 local nonprofit associations of the AMSP:
(1) Austrian companies: AESCA Pharma GmbH, AstraZeneca Österreich GmbH, Boehringer Ingelheim Austria, Bristol–Myers Squibb GmbH, CSC Pharmaceuticals GmbH, Eli Lilly GmbH, Germania Pharma GmbH, GlaxoSmithKline Pharma GmbH, Janssen-Cilag Pharma GmbH, Lundbeck GmbH, Novartis Pharma GmbH, Pfizer Med Inform, Servier Austria GmbH, and Wyeth Lederle Pharma GmbH; (2) German companies: Abbott GmbH & Co. KG, AstraZeneca GmbH, Aventis Pharma Deutschland GmbH GE-O/R/N, Bayer Vital GmbH & Co. KG, Boehringer Mannheim GmbH, Bristol-Myers-Squibb, Ciba Geigy GmbH, Desitin Arzneimittel GmbH, Duphar Pharma GmbH & Co. KG, Eisai GmbH, esparma GmbH Arzneimittel, GlaxoSmithKline Pharma GmbH & Co. KG, Hoffmann-La Roche AG Medical Affairs, Janssen-Cilag GmbH, Janssen Research Foundation, Knoll Deutschland GmbH, Lilly Deutschland GmbH Niederlassung Bad Homburg, Lundbeck GmbH & Co. KG, Novartis Pharma GmbH, Nordmark Arzneimittel GmbH, Organon GmbH, Otsuka-Pharma Frankfurt, Pfizer GmbH, Pharmacia & Upjohn GmbH, Promonta Lundbeck Arzneimittel, Rhone Poulenc Rohrer, Sanofi-Synthelabo GmbH, Sanofi-Aventis Deutschland, Schering AG, SmithKlineBeecham Pharma GmbH, Solvay Arzneimittel GmbH, Synthelabo Arzneimittel GmbH, Dr Wilmar Schwabe GmbH & Co., Thiemann Arzneimittel GmbH, Troponwerke GmbH & Co. KG, Upjohn GmbH, Wander Pharma GmbH, and Wyeth-Pharma GmbH.
(3) Swiss companies: AHP (Schweiz) AG, AstraZeneca AG, Bristol–Myers Squibb AG, Desitin Pharma GmbH, Eli Lilly (Suisse) S.A., Essex Chemie AG, GlaxoSmithKline AG, Janssen-Cilag AG, Lundbeck (Suisse) AG, Mepha Schweiz AG/Teva, MSD Merck Sharp & Dohme AG, Organon AG, Pfizer AG, Pharmacia, Sandoz Pharmaceuticals AG, Sanofi-Aventis (Suisse) S.A., Sanofi Synthelabo SA, Servier SA, SmithKlineBeecham AG, Solvay Pharma AG, Vifor SA, Wyeth AHP (Suisse) AG, and Wyeth Pharmaceuticals AG.
Any applicable disclaimer statements: Dr Bleich: none; Dr Cordes: none since 2017; Dr Degner: none; Dr Druschky: none; Dr. Frieling received speaker’s and consultant’s honoraria from Janssen GmbH and Servier GmbH and works as consultant for the Oberberg GmbH; Dr Greil: none; Dr Grohmann is a project manager of AMSP; Dr Kasper received grants/research support, consulting fees, and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, Celegne GmbH, Eli Lilly, Janssen-Cilag Pharma GmbH, KRKA-Pharma, Lundbeck A/S, Mundipharma, Neuraxpharm, Pfizer, Sanofi, Schwabe, Servier, Shire, Sumitomo Dainippon Pharma Co. Ltd., and Takeda; Hannah Maier: none; Dr Neyazi: none; Dr Schmidt-Kraepelin: none; Dr Stübner: none; Dr Toto is a project manager of AMSP, has been a member of an advisory board for Otsouka, and has received speaker’s honoraria from Janssen Cilag, Lundbeck, Otsouka, and Servier; Tristan Zindler: none.
References
- Bachmann CJ, et al. (2017) International trends in clozapine use: a study in 17 countries. Acta Psychiatr Scand 136:37–51. [DOI] [PubMed] [Google Scholar]
- Bromet EJ, Fennig S (1999) Epidemiology and natural history of schizophrenia. Biol Psychiatry 46:871–881. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Kreyenbuhl J, Zito JM, Lehman A (2002) Relationship of the use of adjunctive pharmacological agents to symptoms and level of function in schizophrenia. Am J Psychiatry 159:1035–1043. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, Himelhoch S, Fang B, Peterson E, Aquino PR, Keller W; Schizophrenia Patient Outcomes Research Team (PORT) (2010) The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull 36:71–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Sweeting M (2007) Review: combination therapy with non-clozapine atypical antipsychotic medication: a review of current evidence. J Psychopharmacol 21:657–664. [DOI] [PubMed] [Google Scholar]
- Chang JS, Ahn YM, Park HJ, Lee KY, Kim SH, Kang UG, Kim YS (2008) Aripiprazole augmentation in clozapine-treated patients with refractory schizophrenia: an 8-week, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 69:720–731. [DOI] [PubMed] [Google Scholar]
- Conley RR, Kelly DL (2001) Management of treatment resistance in schizophrenia. Biol Psychiatry 50:898–911. [DOI] [PubMed] [Google Scholar]
- Correll CU, Frederickson AM, Kane JM, Manu P (2007) Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res 89:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Rummel-Kluge C, Corves C, Kane JM, Leucht S (2009) Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull 35:443–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Rubio JM, Inczedy-Farkas G, Birnbaum ML, Kane JM, Leucht S (2017) Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry 74:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DGPPN e.V. (Hrsg.) für die Leitliniengruppe (15. März 2019). S3-Leitlinie Schizophrenie. Langfassung.https://www.awmf.org/leitlinien/detail/ll/038-009.html20. März 2019.
- Dold M, Li C, Gillies D, Leucht S (2013) Benzodiazepine augmentation of antipsychotic drugs in schizophrenia: a meta-analysis and cochrane review of randomized controlled trials. Eur Neuropsychopharmacol 23:1023–1033. [DOI] [PubMed] [Google Scholar]
- Druschky K, et al. (2018). Seizure rates under treatment with antipsychotic drugs: data from the AMSP project. World J Biol Psychiatry 15:1–10. [DOI] [PubMed] [Google Scholar]
- El-Mallakh RS, McKenzie C (2013) The dopamine D4/D2 receptor antagonist affinity ratio as a predictor of anti-aggression medication efficacy. Med Hypotheses 80:530–533. [DOI] [PubMed] [Google Scholar]
- Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Möller HJ; WFSBP Task Force on Treatment Guidelines for Schizophrenia (2005) World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: acute treatment of schizophrenia. World J Biol Psychiatry 6:132–191. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Heikkinen ME, Olié JP, Landsberg W, Dewaele P, McQuade RD, Loze JY, Hennicken D, Kerselaers W (2010) Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol 13:1115–1125. [DOI] [PubMed] [Google Scholar]
- Freudenreich O, Goff DC (2002) Antipsychotic combination therapy in schizophrenia. A review of efficacy and risks of current combinations. Acta Psychiatr Scand 106:323–330. [DOI] [PubMed] [Google Scholar]
- Galletly C, Castle D, Dark F, Humberstone V, Jablensky A, Killackey E, Kulkarni J, McGorry P, Nielssen O, Tran N (2016) Royal Australian and New Zealand College of psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry 50:410–472. [DOI] [PubMed] [Google Scholar]
- Galling B, Roldán A, Hagi K, Rietschel L, Walyzada F, Zheng W, Cao XL, Xiang YT, Zink M, Kane JM, Nielsen J, Leucht S, Correll CU (2017) Antipsychotic augmentation vs monotherapy in schizophrenia: systematic review, meta-analysis and meta-regression analysis. World Psychiatry 16:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly R, Kotzan JA, Miller LS, Kennedy K, Martin BC (2004) Prevalence, trends, and factors associated with antipsychotic polypharmacy among medicaid-eligible schizophrenia patients, 1998-2000. J Clin Psychiatry 65:1377–1388. [DOI] [PubMed] [Google Scholar]
- Geddes J, Freemantle N, Harrison P, Bebbington P (2000) Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. Bmj 321:1371–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Rochon PA, Herrmann N, Lee PE, Sykora K, Gunraj N, Normand SL, Gurwitz JH, Marras C, Wodchis WP, Mamdani M (2005) Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. Bmj 330:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Study 2013 Collaborators (2015). Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet 386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann R, Engel RR, Rüther E, Hippius H (2004) The AMSP drug safety program: methods and global results. Pharmacopsychiatry 37(Suppl 1):S4–S11. [DOI] [PubMed] [Google Scholar]
- Grohmann R, Engel RR, Möller HJ, Rüther E, van der Velden JW, Stübner S (2014) Flupentixol use and adverse reactions in comparison with other common first- and second-generation antipsychotics: data from the AMSP study. Eur Arch Psychiatry Clin Neurosci 264:131–141. [DOI] [PubMed] [Google Scholar]
- Hálfdánarson Ó, et al. (2017) International trends in antipsychotic use: a study in 16 countries, 2005–2014. Eur Neuropsychopharmacol 27:1064–1076. [DOI] [PubMed] [Google Scholar]
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthøj B, Gattaz WF, Thibaut F, Möller HJ; WFSBP Task Force on Treatment Guidelines for Schizophrenia (2017) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia - a short version for primary care. Int J Psychiatry Clin Pract 21:82–90. [DOI] [PubMed] [Google Scholar]
- Heald A, Livingston M, Yung A, De Hert MA (2017) Prescribing in schizophrenia and psychosis: increasing polypharmacy over time. Hum Psychopharmacol 32 (2). doi: 10.1002/hup.2579. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Yassouridis A, Kellner M, Jahn H, Wiedemann K, Raedler TJ (2013) No effects of antidepressants on negative symptoms in schizophrenia. J Clin Psychopharmacol 33:686–690. [DOI] [PubMed] [Google Scholar]
- Jones PB, Barnes TR, Davies L, Dunn G, Lloyd H, Hayhurst KP, Murray RM, Markwick A, Lewis SW (2006) Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: cost utility of the latest antipsychotic drugs in schizophrenia study (cutlass 1). Arch Gen Psychiatry 63:1079–1087. [DOI] [PubMed] [Google Scholar]
- Joukamaa M, Heliövaara M, Knekt P, Aromaa A, Raitasalo R, Lehtinen V (2006) Schizophrenia, neuroleptic medication and mortality. Br J Psychiatry 188:122–127. [DOI] [PubMed] [Google Scholar]
- Kapur S, Roy P, Daskalakis J, Remington G, Zipursky R (2001) Increased dopamine d(2) receptor occupancy and elevated prolactin level associated with addition of haloperidol to clozapine. Am J Psychiatry 158:311–314. [DOI] [PubMed] [Google Scholar]
- Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB; Schizophrenia Patient Outcomes Research Team (PORT) (2010) The schizophrenia patient outcomes research team (PORT): updated treatment recommendations 2009. Schizophr Bull 36:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman AF, et al. (2004). Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry 161:1–56. [PubMed] [Google Scholar]
- Leslie DL, Rosenheck RA (2002) From conventional to atypical antipsychotics and back: dynamic processes in the diffusion of new medications. Am J Psychiatry 159:1534–1540. [DOI] [PubMed] [Google Scholar]
- Lewis SW, Barnes TR, Davies L, Murray RM, Dunn G, Hayhurst KP, Markwick A, Lloyd H, Jones PB (2006) Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull 32:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223. [DOI] [PubMed] [Google Scholar]
- Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE (2017) What is polypharmacy? A systematic review of definitions. BMC Geriatr 17:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, Swartz MS, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK; CATIE Investigators (2006) Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 163:600–610. [DOI] [PubMed] [Google Scholar]
- McIlwain ME, Harrison J, Wheeler AJ, Russell BR (2011) Pharmacotherapy for treatment-resistant schizophrenia. Neuropsychiatr Dis Treat 7:135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller HJ. (2000) State of the art of drug treatment of schizophrenia and the future position of the novel/atypical antipsychotics. World J Biol Psychiatry 1:204–214. [DOI] [PubMed] [Google Scholar]
- Moore TA, Buchanan RW, Buckley PF, Chiles JA, Conley RR, Crismon ML, Essock SM, Finnerty M, Marder SR, Miller DD, McEvoy JP, Robinson DG, Schooler NR, Shon SP, Stroup TS, Miller AL (2007) The Texas medication algorithm project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry 68:1751–1762. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (NICE) (2014) Psychosis and schizophrenia in adults.Treatment and management. NICE clinical guideline 178. London: National Institute for Health and Clinical Excellence. [Google Scholar]
- Pani L, Villagrán JM, Kontaxakis VP, Alptekin K (2008) Practical issues with amisulpride in the management of patients with schizophrenia. Clin Drug Investig 28:465–477. [DOI] [PubMed] [Google Scholar]
- Pickar D, Vinik J, Bartko JJ (2008) Pharmacotherapy of schizophrenic patients: preponderance of off-label drug use. Plos One 3:e3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehry AA, Potvin S, Elie R, Stip E (2007) Selective serotonin reuptake inhibitor (SSRI) add-on therapy for the negative symptoms of schizophrenia: a meta-analysis. J Clin Psychiatry 68:604–610. [DOI] [PubMed] [Google Scholar]
- Shiloh R, Zemishlany Z, Aizenberg D, Radwan M, Schwartz B, Dorfman-Etrog P, Modai I, Khaikin M, Weizman A (1997) Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry 171:569–573. [DOI] [PubMed] [Google Scholar]
- Sim F, Sweetman I, Kapur S, Patel MX (2015) Re-examining the role of benzodiazepines in the treatment of schizophrenia: a systematic review. J Psychopharmacol 29:212–223. [DOI] [PubMed] [Google Scholar]
- Stahl SM. (2002) Antipsychotic polypharmacy: evidence based or eminence based? Acta Psychiatr Scand 106:321–322. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Grady MM (2004) A critical review of atypical antipsychotic utilization: comparing monotherapy with polypharmacy and augmentation. Curr Med Chem 11:313–327. [DOI] [PubMed] [Google Scholar]
- Stroup TS, Lieberman JA, McEvoy JP, Davis SM, Swartz MS, Keefe RS, Miller AL, Rosenheck RA, Hsiao JK; CATIE Investigators (2009) Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res 107:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup TS, et al. (2019) Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2018.4489. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D, Paton C, Kapur S (2009). The Maudsley prescribing guidelines. 10th ed.Informa Healthcare: London. [Google Scholar]
- Terevnikov V, Joffe G, Stenberg JH (2015) Randomized controlled trials of add-on antidepressants in schizophrenia. Int J Neuropsychopharmacol 18 (9). doi: 10.1093/ijnp/pyv049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P (2011) A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry 168:603–609. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Suokas JT, Suvisaari JM, Haukka J, Korhonen P (2012) Polypharmacy with antipsychotics, antidepressants, or benzodiazepines and mortality in schizophrenia. Arch Gen Psychiatry 69:476–483. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Mittendorfer-Rutz E, Majak M, Mehtälä J, Hoti F, Jedenius E, Enkusson D, Leval A, Sermon J, Tanskanen A, Taipale H (2017) Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry 74:686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington JL, Youssef HA, Kinsella A (1998) Mortality in schizophrenia. Antipsychotic polypharmacy and absence of adjunctive anticholinergics over the course of a 10-year prospective study. Br J Psychiatry 173:325–329. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xia J, Helfer B, Li C, Leucht S (2016) Valproate for schizophrenia. Cochrane Database Syst Rev 11:CD004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AJ. (2008) Treatment pathway and patterns of clozapine prescribing for schizophrenia in New Zealand. Ann Pharmacother 42:852–860. [DOI] [PubMed] [Google Scholar]
- Wu CS, Lin YJ, Liu SK (2011) Benzodiazepine use among patients with schizophrenia in Taiwan: a nationwide population-based survey. Psychiatr Serv 62:908–914. [DOI] [PubMed] [Google Scholar]
- Zink M. (2005) Augmentation of olanzapine in treatment-resistant schizophrenia. J Psychiatry Neurosci 30:409–415. [PMC free article] [PubMed] [Google Scholar]
- Zink M, Englisch S, Meyer-Lindenberg A (2010) Polypharmacy in schizophrenia. Curr Opin Psychiatry 23:103–111. [DOI] [PubMed] [Google Scholar]
- Zink M, Knopf U, Henn FA, Thome J (2004) Combination of clozapine and amisulpride in treatment-resistant schizophrenia–case reports and review of the literature. Pharmacopsychiatry 37:26–31. [DOI] [PubMed] [Google Scholar]
- Zink M, Kuwilsky A, Krumm B, Dressing H (2009) Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomised controlled clinical trial. J Psychopharmacol 23:305–314. [DOI] [PubMed] [Google Scholar]